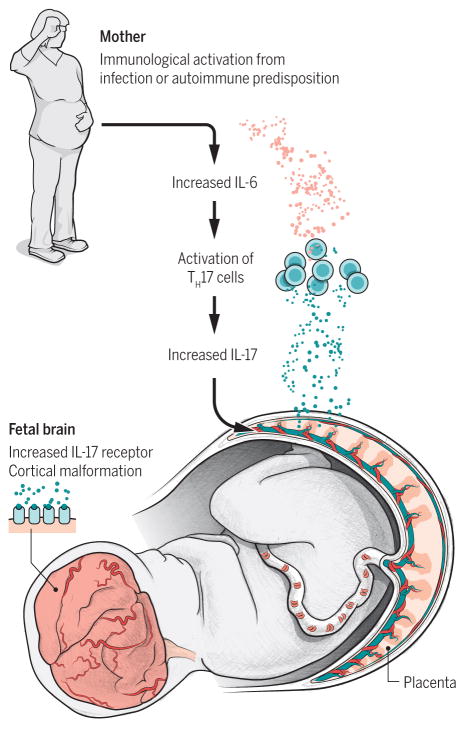
The alarming headlines of an epidemic of microcephaly likely caused by Zika virus have captured everyone’s attention. While few people have heard of an association between infection during preg-nancy and changes in brain development, epidemiologists have known about this connection for many years. Moreover, mounting evidence suggests that maternal immune activation (MIA) alone is sufficient to alter brain development and may be causally linked to autism spectrum disorder (ASD)(1-3). How could the maternal immune system, which normally serves to protect mother and child from environ-mental insults, cause changes in brain development? On page XX of this Issue, Choi et al. (4) uncover an important component of this immune pathway—a critical signal from a special class of cells in the mother’s immune system, called Th17 cells, that alters brain development in her fetal offspring (Figure 1). These findings have exciting implications for developing new treatments to prevent ASD caused by maternal infection.
Figure 1. A model for the role for Th17 cells and IL-17 in infection-induced ASD.
MIA, and possibly a predisposition for autoimmunity, leads to an increase in Th17 cells in maternal serum and IL-17a, which crosses the placenta and increases IL-17 receptor levels in the brains of offspring leading to ASD-related cortical and behavioral abnormalities in offspring.
How does the mother’s immune system, which has limited access to the fetal compartment, al-ter brain development? Cytokines, immune system signaling molecules that are generated in response to infection, can cross the placental boundary and are critical for MIA to change brain development and behavior in offspring. One of the first cytokines elevated in the serum of pregnant mice following im-mune activation is interleukin-6 (IL-6) and this elevation is necessary and sufficient for MIA to alter brain development and behavior in offspring (5). Choi et al. (this issue) have identified another cytokine, IL-17, that is necessary and sufficient to mediate the effects of MIA (4). But this isn’t just another cytokine to add to the steadily growing list of factors implicated in MIA. Choi and colleagues convincingly show, using several genetic models and targeted blockade, that IL-17 acts downstream of IL-6 in offspring fol-lowing MIA. While previous studies identifying cytokines involved in MIA raised more questions than they answered, Choi et al present an impressive series of experiments demonstrating the role for IL-17 in mediating the effects of MIA in offspring. They go on to identify, for the first time, the cellular source of the cytokine. The culprit in this case is secreted by Th17 cells in the mother’s serum, where it crosses the placenta and acts in the brains of offspring to increase IL-17 receptor levels, further increasing IL-17 signaling in the fetal brain. This sequence of events is required for MIA to cause cortical malformations and the three core behavioral abnormalities associated with ASD (4).
Although IL-17 and the cells that produce it, Th17 cells, have been implicated in ASD through both genetic associations and observations of elevated IL-17 in the serum of children (6-8), there was no evidence for a role for Th17 cells in MIA until the Choi et al. study in this issue (4). Th17 cells protect against bacterial and fungal pathogens, especially at muscosal surfaces like the gut, and are an impor-tant part of an inflammatory response to infection (9). Th17 cells may also be critical for the develop-ment of autoimmunity and have been implicated in numerous animal models of autoimmune disorders (10). Interestingly, Choi et al. used a specific mouse line (C57BL/6) from Taconic that has a propensity for Th17 polarization due to a distinct microbiota signature and is the mouse line of choice for generating autoimmune models of EAE and obesity-induced diabetes (11). This choice may have produced a more relevant and robust phenotype compared to other C57BL/6 mice since these Taconic C57 mice have a different immune system than C57 mice lacking Th17-inducing microbiota, which are often used to gen-erate the MIA model (from Jackson Labs and the C57/J from Charles River). The latter C57 mouse lines express barely detectable levels of Th17 cells (9, 11-13). This altered baseline immune response may explain the surprising, long-lasting increase in IL-1β in maternal serum following poly I:C injection in the mice in this study compared to the transient increase found in C57/J mice (14). Importantly, polyI:C injection to induce MIA does not induce Th17 cells in a mouse line that typically does not express Th17 cells (12), as it does in the Taconic mice reported in the Choi et al study in this issue (4). Thus, fu-ture research should determine if induction of Th17 cells is critical for the effects of MIA regardless of background or whether it specifically mediates the effects of MIA in mice with Th17 cell polarization. The latter possibility may be of even greater interest since it is consistent with epidemiological studies implicating a family history of autoimmunity in ASD (1) and would imply that infection in pregnant women with elevated Th17 cells might lead to a greater likelihood of ASD in offspring.
With their discovery that injection of a function-blocking antibody to IL-17 following MIA induc-tion decreases the severity of some ASD behaviors in offspring (4), Choi et al. raise the possibility that pharmacological intervention of the IL-17 pathway could prevent ASD caused by maternal infection. It is important to note, however, that the subset of pregnant mothers who experience a strong infection and will go on to have a child with ASD is incredibly low. Moreover, cytokines are sneakily versatile—just when you think you know what they’re up to, they turn out to be integral to some unrelated process. Thus, inhibiting IL-17 in infected mothers may have equally detrimental consequences as the infection itself, since many cytokines play important roles in typical brain development (1). The study by Choi et al suggests that women with Th17 skewing may have a much higher risk that ultimately warrants interven-tion. In this regard, alternative approaches to dampen Th17 cell differentiation, such as using vitamin D and/or retinoic acid (a metabolite of vitamin A)(10), or by manipulating intestinal microbiota (11,15), will be exciting avenues of investigation for years to come.
References
- 1.Estes ML, McAllister AK. Nature Reviews Neuroscience. 2015;16:469. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atladottir HO, et al. Journal of autism and developmental disorders. 2010;40:1423. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 3.Atladottir HO, et al. Pediatrics. 2012;130:e1447. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi GB, et al. Science. 2016;XX [Google Scholar]
- 5.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. J Neurosci. 2007;27:10695. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Ayadhi LY, Mostafa GA. J Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K, et al. PLoS One. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Zwaag B, et al. PLoS One. 2009;4:e5324. doi: 10.1371/journal.pone.0005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov II, et al. Cell Host Microbe. 2008;4:337. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedoya SK, Lam B, Lau K, Larkin J., III Clinical and Developmental Immunology. 2013;2013:986789. doi: 10.1155/2013/986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov II, et al. Cell. 2009;139:485. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Brain Behav Immun. 2011;25:863. doi: 10.1016/j.bbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, et al. Nature. 2014;510:152. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer U, et al. J Neurosci. 2006;26:4752. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao EY, et al. Cell. 2013;155:1451. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]