Abstract
Increasing evidence points to a central role for immune dysregulation in autism spectrum disorder (ASD). Several ASD risk genes encode components of the immune system and many maternal immune system-related risk factors — including autoimmunity, infection and fetal reactive antibodies — are associated with ASD. In addition, there is evidence of ongoing immune dysregulation in individuals with ASD and animal models of this disorder. Recently, several molecular signalling pathways have been identified that link immune activation to ASD phenotypes, including pathways downstream of cytokines, hepatocyte growth factor receptor (MET), MHCI molecules, microglia and complement factors. These findings indicate that the immune system is a point of convergence for various ASD-related genetic and environmental risk factors.
Autism spectrum disorder (ASD) arises during the early years of life with a heterogeneous presentation and is diagnosed based on impairments in social skills and communication, repetitive behaviour and narrow and intense interests1. The estimated prevalence of ASD has recently skyrocketed: in 1992, it was estimated that 1 in 500 children in the USA had ASD, but by 2007 this figure had been adjusted to 1 in 110, and current estimates have reached the alarming level of 1 in 68 US children and 1 in 42 boys2. Although the wider diagnostic criteria for, and enhanced public awareness of, ASD have surely contributed to this increase, these factors cannot account for all, and in some estimates most, of this rise in prevalence3. This implies that one or more factors in our environment have increased the likelihood of children to develop ASD. Consistent with this idea, recent reports have suggested that the environment may have a much larger role in causing ASD than had been initially proposed4,5.
Although there is a long list of diverse environmental factors that contribute to ASD6, most of these converge on alterations in immune responses during prenatal or early postnatal development (FIG. 1). The immune system is designed to reflect environmental changes and predict future ones as a defensive strategy. The genetic composition and initial programming of the immune system in utero and shortly after birth7,8 determines how much environmental insult the immune system can buffer during the lifetime of each individual. This buffering is important not only for general health but also for neural processing, owing to the pervasive and dynamic cross-talk that occurs between the immune and nervous systems. Indeed, immune status can have profound effects on brain development and cognition (BOX 1) and alterations in immune signaling can, in different contexts, induce helpful, homeostatic or harmful effects.
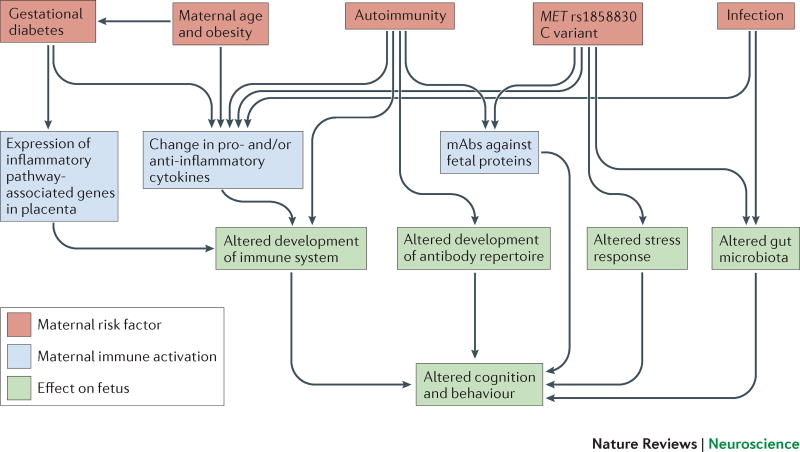
Figure 1. ASD risk factors during pregnancy converge on maternal immune system activation.
Maternal autoimmunity, infection during pregnancy, maternal age and obesity, gestational diabetes, and maternal MET variant rs1858830 ‘C’ allele are all associated with a higher incidence of ASD. These risk factors (red boxes) cause maternal immune activation (MIA) (blue boxes), which manifests as changes in the maternal peripheral cytokine milieu, generation of IgG maternal autoantibodies (mAbs) that are reactive to fetal proteins and activation of inflammatory pathway genes within the placenta. Based on findings in animal models, MIA is sufficient to induce long-lasting changes in brain development, gut microbiota, immune and endocrine systems of the developing fetus (green boxes).
Box 1 | Immune system influences on cognition and behavior.
In the past 10 years, it has become increasingly clear that immune status influences cognition and behaviour. Immune cells, especially T-cells, have roles in many aspects of brain development and function, in addition to mediating the response to disease249,250. This is perhaps best illustrated in mice with severe combined immunodeficiency (SCID) and nude mice, which are deprived of all lymphocytes and T-cells, respectively. These animals exhibit impairments in hippocampal neurogenesis and learning and memory, as well as increased repetitive behaviours and anxiety251,252. Remarkably, replenishment of the immune system by adoptive transfer of wild-type splenocytes or by bone marrow reconstitution improves the learning ability of SCID and nude mice in several learning tasks and ameliorates repetitive behaviours131,253,254, suggesting that the defects are not caused by lifelong immune deficiency but rather by ongoing depletion of immune cells. Interestingly, increased anxiety is not rescued by wild-type reconstitution, suggesting it has a developmental aetiology and is a lasting behavioural consequence of impaired immunity252. CD4+ T-cells mediate the pro-cognitive effects 255 indirectly through effects at meningeal spaces rather than through infiltration into the CNS256. When mice are exposed to learning tasks, T-cells home to the meninges and become activated, acquiring a TH2-like phenotype (regarded as anti-inflammatory) and expressing high levels of IL-4, which causes myeloid cells in the meninges to become skewed to an M2 (also anti-inflammatory) phenotype256. Preventing this T-cell migration to the meninges, or genetic deletion of IL-4, results in a pro-inflammatory, M1 skewing of meningeal myeloid cells and deficits in learning and memory256. Conversely, reconstituting wild-type mice with T cells from IL-4 knockout mice results in learning and memory deficits131. Thus, CD4+ T cells, which clearly regulate brain immune status, normal cognition and emotional behavior, may have important roles in causing and/or contributing to ASD and clearly represent an important potential therapeutic target for ASD.
Like ASD, childhood disorders of the immune system such as asthma, life-threatening food allergies and autoimmune disorders have reached epidemic levels over the past two decades9,10. As this time frame is too short for genetic changes at a population level to have had an appreciable impact on the prevalence of these conditions, these increases must have an environmental catalyst. It is hypothesized that the rise in childhood immune disorders reflects the exposure to an increasing number of environmental stressors during critical periods of development that results in disease expression in individuals with a vulnerable genetic background11. This multiple hit model for immune disorders is also hypothesized in the aetiology of ASD6,12. Although accumulating evidence indicates that immune dysregulation increases the risk for, and contributes to the pathophysiology of, ASD, this does not mean that immune responses to vaccines cause ASD. In fact, recent studies have debunked the myth of a link between ASD and early childhood vaccines13. Despite the unequivocal and compelling research behind the conclusion that vaccines do not cause ASD, society is increasingly at risk of preventable devastating diseases — including whooping cough, measles and tuberculosis — because of the unsubstantiated fear of vaccinations. For these reasons alone, it is critical to disseminate and improve our understanding of immune and environmental contributions to ASD.
In this Review, we provide an overview of the evidence that maternal and postnatal immune dysregulation play a part in the aetiology and pathophysiology of ASD. First, we examine evidence from genetic studies that indicates an association between mutations in immune genes and ASD. Next, we discuss the epidemiological, clinical and animal studies implicating prenatal immune system contributions to ASD, including autoimmunity, fetal reactive antibodies and maternal immune activation (MIA). Then, we review the evidence for chronic immune perturbations in individuals with ASD. Finally, we present the molecular mechanisms that might underlie the link between immune activation and ASD phenotypes, focusing on common molecular pathways that might be targeted by future, novel therapeutic interventions.
Genetic studies
Early estimates of the heritability of ASD of around 90%14,15, and even recently revised estimates of between 38 and 54%, strongly suggest that genetic mutations are a major cause of ASD4,16. However, no single gene has been identified to date that substantially increases the risk of developing ASD17. Instead, ASD seems to be associated with a large number of genetic mutations, including possibly hundreds of rare causal variants and over one hundred common variants of small effect18,19. Much insight into the genetic pathways that are altered in ASD has come from studies of syndromic disorders with a high incidence of ASD, including fragile X syndrome (FXS), Rett syndrome, tuberous sclerosis (TSC), neurofibromatosis type 1 and PTEN macrocephaly. These syndromes are caused by mutations in single genes (fragile X mental retardation protein 1 (FMR1), methyl-CpG-binding protein 2 (MECP2), TSC1 or TSC2, neurofibromin 1 (NF1) and PTEN, respectively) and account for 5–7% of ASD cases20–22. Recent reports have also highlighted the strong contribution of copy number variants (CNVs) to ASD, accounting for 7–20% of cases of this disorder23–25.
Importantly, several ASD-associated genes encode components of the immune system (TABLE 1). One of the immune genes most associated with ASD is MET, which encodes hepatocyte growth factor receptor26. A MET variant that includes a common G-to-C single nucleotide polymorphism (SNP) in its promoter (the rs1858830 ‘C’ allele) is associated with decreased MET signalling in the temporal lobe and ASD27–29. This variant leads to disrupted expression of multiple downstream targets of the MET signalling cascade28. Post-mortem transcriptome analysis of individuals with ASD also shows reduced MET expression in the temporal lobe, suggesting that reduced MET levels may indeed cause ASD phenotypes27,30,31. Consistent with this idea, individuals with ASD and the MET ‘C’ allele exhibit reduced structural and functional connectivity in temporoparietal lobes in comparison with both ASD individuals without the MET variant and control individuals31. Moreover, structural MRI in ASD individuals with the MET ‘C’ allele shows decreased cortical thickness in several brain regions that are associated with socio-communicative function32. Interestingly, MECP2, which is implicated in Rett syndrome, regulates MET transcription and gene expression of MET is notably reduced in the temporal lobe of females with Rett syndrome in the absence of the MET ‘C’ allele33, suggesting that reduced MET expression may contribute to the ASD-related pathophysiology in this syndrome.
Table 1.
ASD-associated genes with roles in the immune system and CNS
| Gene | CNS function of encoded protein |
Immune function of encoded protein |
Refs* |
|---|---|---|---|
| CD99L2 (CD99 molecule-like 2 region) | Unknown | Homophilic adhesion molecule involved in leukocyte extravasation | 46 |
| JARID2 (Jumonji, AT rich interactive domain 2) | Transcriptional repressor involved in neural tube fusion | Transcriptional repressor regulating hematopoiesis | 46 |
| TPO (thyroid peroxidase) | Necessary for proper brain development. | Enzyme producing thyroid hormones. | 46 |
| MET (hepatocyte growth factor receptor) | Mediates migration of neuronal precursor and excitatory synapse formation. | Receptor for hepatocyte growth factor that promotes differentiation and proliferation of hematopoietic cells, and exerts broad anti-inflammatory effects. | 26 |
| MIF (macrophage migration inhibitory factor) | Unknown | Inflammatory cytokine regulating innate immune response to bacterial pathogens | 55 |
| PRKCB1 (protein kinase C, Beta 1) | Implicated in circadian rhythms, and learning and memory. | Serine/threonine-specific protein kinase that mediates B-cell activation, T-cell migration, antigen presenting cell function and cytokine release. | 55 |
| HLA-A2 (human leukocyte antigen A2 allele) | Negatively regulates synapse formation in developing brain. | MHC class I molecule that is expressed on all nucleated cells to identify them as ‘self’ to patrolling immune cells; regulates cellular immune response to intracellular pathogens. | 47 |
| HLA-DRβ1 (human leukocyte antigen, DRβ1 allele) | Unknown | MHC class II molecule that is expressed on antigen-presenting cells (dendritic, monocytes, macrophages and microglia); initiates cellular immune response to extracellular pathogens. | 47 |
| C4B (complement component 4B) | May be involved in complement-mediated synaptic pruning. | Complement cascade protein that is involved in clearing pathogens and cellular debris. | 55 |
| IL1RAPL1 (interleukin 1 receptor accessory protein-like 1) | Involved in pre- and post-synaptic formation through trans-synaptic adhesion interactions. | Interleukin 1 family receptor with unknown immune function. | 60 |
| EIF4E (eukaryotic translation initiation factor, 4E) | Promotes translation of many ASD-associated genes with synaptic roles. | Part of a multi-subunit complex downstream of mTOR; initiates cap-dependent translation. | 20 |
| FMR1 (fragile X mental retardation 1) | Regulates translation of many ASD-associated genes with synaptic roles | RNA-binding protein involved in translational control; forms a complex with EIF4E to suppress translation. | 20 |
| NF1 (neurofibromin 1) | Negatively regulates Ras-mediated neurotransmitter and neurotrophin signaling. | Tumor suppressor; GTPase-activating protein that negatively regulates the Ras oncogene signal transduction pathway (the Ras pathway feeds into the mTOR pathway, and Ras mediates cytokine and growth factor signaling in the immune system). | 21 |
| PTEN (phosphate and tensin homologue) | Regulates neuronal metabolic processes and the mTOR pathway. | Tumor suppressor that acts as a phosphatase that inhibits PI3K signaling (the PI3K pathway also integrates cytokine, neurotransmitter and growth factor signalling) and is an upstream regulator of the mTOR pathway. | 20 |
| TSC1 and TSC2 (tuberous sclerosis 1 and 2) | Negatively regulates mTOR pathway. Induces neuroprotective autophagy in response to glucose deprivation and stroke. | Tumor suppressors that, when in complex with eachother, have GTPase activity and negatively regulate the mTOR pathway. | 20 |
Citations are reviews; for an updated list of references for genetic associations, see: https://gene.sfari.org. ASD, autism spectrum disorder; EIF4E, eukaryotic translation initiation factor 4E; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3 kinase.
Many of the ASD-related phenotypes that are associated with decreased MET signalling in the brain may result from deficits in the key functions that MET has in brain development (described below). However, MET could also cause these changes in neural circuitry and function indirectly through its ability to negatively regulate immune responsiveness34,35 and gastrointestinal homeostasis36,37. Increased MET signalling ameliorates disease pathogenesis in several models of classic systemic inflammatory diseases, including multiple sclerosis and systemic lupus erythematosis (SLE) among others38–42. Moreover, during pregnancy, the MET ‘C’ allele is associated with decreased production of the cytokine interleukin (IL)-1043, preventing the important gestational increase in IL-10 that is responsible for maternal immune suppression and tolerance to the developing fetus44 (FIG. 1). The MET ‘C’ allele is also enriched in mothers with another immune-related risk factor for ASD — maternal antibodies that are reactive to fetal brain proteins43 (discussed below). Finally, the MET ‘C’ allele may interact with environmental factors to increase the risk for ASD. Indeed, the MET ‘C’ allele and exposure to high levels of air pollution during prenatal development act synergistically to increase risk of this disorder45.
In addition to variants in MET, variants in the human leukocyte antigen (HLA) locus also confer risk for neurodevelopmental disorders46,47. The HLA locus encodes the MHC genes in humans, which are involved in myriad immune functions and comprise three classes. Although the limited scale of the genetic studies of MHC in relation to ASD do not allow any definitive associations to be made, variants in all three classes of MHC genes have been reported to enhance ASD risk, namely the MHC class I HLA-A2 haplotype, the MHC class II DRβ1 alleles and the complement C4B null allele in the MHC class III region47. As with many other ASD-related genes, the HLA associations are not specific to ASD; they are also associated with other neurodevelopmental disorders and autoimmune disorders, including rheumatoid arthritis and SLE, which occur at higher rates in the relatives of individuals with ASD48. The combination of a specific DRβ1*11 allele with a family history of autoimmune disorders increases the odds ratio for the association of this allele with ASD49. Similarly, ASD-associated deficiencies in a complement gene — C4 — within the MHC class III region50,51 are also among the strongest genetic risk factors for SLE52 and individuals with idiopathic ASD exhibit a 34% decrease in plasma levels of C4b53. These ASD-linked deficits in complement are thought to activate autoimmune responses directly, owing to the production of autoantibodies against excess cellular debris that is typically removed in a complement-dependent manner, and indirectly, through the chronic immune activation that is caused by recurrent and persistent bacterial infections54.
Finally, mutations in genes for several members of the IL-1 cytokine receptor family are also associated with ASD. Two recent exome sequencing studies of individuals with ASD found synonymous SNPs in the gene encoding the IL-1β decoy receptor IL-1R2 (IL-1 receptor type 2)55,56. The functional consequences of these SNPs have so far not been assessed but synonymous SNPs can affect protein folding and messenger RNA splicing, stability and structure57,58. A rare ASD-associated mutation has also been identified in a gene encoding another IL-1 family receptor, IL1RAPL1 (interleukin-1 receptor accessory protein-like 1)59,60. This mutation was originally identified in a screen for genes that are related to X-linked intellectual disability61. ASD-associated mutations, CNVs and somatic mosaics have subsequently been discovered in this gene, most of which result in a loss of function62. Together, these findings indicate that mutations in a wide array of immune genes contribute to ASD.
Maternal immune contributions
Autoimmune disorders
Although genetic mutations appear to contribute a sizeable amount of risk for ASD, recent estimates suggest that between 50 and 60% of the risk for ASD is unaccounted for, which implies that environmental factors or gene–environment interactions contribute substantially to the risk for this disorder4,16. In addition to mutations in immune genes, autoimmune disorders, allergies and asthma strongly associate with the families of children with ASD48. Estimates indicate that there is up to a 50% increase in the odds of an ASD diagnosis among children who have a parent who has had an autoimmune disorder48,63. Although maternal immune disorders such as rheumatoid arthritis, SLE and type-1 diabetes account for the largest portion of this increased risk for ASD in offspring, paternal immune disorders and a general familial history of immune disorders in the absence of maternal immune conditions also appear to contribute, suggesting a heritable component for this autoimmune association48,64. Indeed, autoimmune disorders are over-represented in the ASD population65. However, such disorders also increase the risk of intellectual disability and several other neurodevelopmental disorders, suggesting that immune disorders generally increase the vulnerability of the developing brain to developmental defects66. The strong contribution of maternal immune status to ASD risk could involve genetic factors and environmental influences on the developing fetus. Similar to the situation caused by the MET ‘C’ allele, mothers with autoimmune disorders fail to develop an anti-inflammatory immune system profile that typically occurs during pregnancy67 (FIG. 1). Although the relative contributions of these genetic and environmental factors are yet to be determined, animal models show a causal link between activation of the maternal immune system and altered neurodevelopment (see below).
Fetal-brain-reactive antibodies
The association of maternal autoimmune disorders with ASD in offspring may be mediated by the passive transfer to the fetus of maternal IgG antibodies (mAbs) that exhibit reactivity to self-proteins in the mother or child. In typical development, passive immunity protects the fetus and early neonate from infection until the child’s adaptive immune system has matured. These fetal mAbs could theoretically enter the fetal brain during early gestation because the blood–brain barrier (BBB) is not mature until the postnatal period68. Bolstering this hypothesis, women with rheumatoid arthritis or SLE are more than four times as likely as women without these disorders (10.5% versus 2.6%) to harbor peripheral antibodies with reactivity to neurons from the brains of fetal and adult mice69. Moreover, 53% of mothers of a child with ASD who test positive for anti-brain antibodies also have anti-nuclear antibodies — an indicator of latent autoimmunity— versus 13.4% of mothers of an ASD child without anti-brain antibodies69. Thus, autoimmunity in pregnant women, even at a clinically undetected level, may be associated with the production of maternal antibodies that can reach the fetal brain and potentially perturb fetal brain development.
A functional role for such maternal autoantibodies has recently been demonstrated by several groups. Injecting NMDA receptor (NMDAR)-specific autoantibodies derived from the blood of patients with SLE into a pregnant mouse alters brain development and impairs cognitive behaviour in the offspring70. Antibody clones recognizing the NR2A and NR2B NMDAR subunits appear to be a major cause of the behavioural changes. Interestingly, although 30–60% of individuals with SLE possess NMDAR-specific autoantibodies, each antibody clone has unique physiological effects. Some antibodies have potent co-agonist properties, leading to increased calcium influx through NMDARs and eventually neuronal death71, whereas other clones with slightly different epitopes are not pathogenic and yet others cross-react with C1q, a component of the complement cascade72. Remarkably, the effects of these anti-NMDAR antibodies are sex-specific in mice: they cause death through apoptosis of NR2A-expressing neurons, which are enriched in the brainstem of female, but not male, fetuses73. A female-specific vulnerability to these antibodies could result in increased rates of resorption of female fetuses leading to an increase in the proportion of male offspring, similar to the male skewing seen in ASD. Additionally, female fetuses with these antibodies that are carried to term would be expected to show more profound deficits. This prediction may fit with the recurrent observation that a subset of girls with ASD show more social communication impairments and lower cognitive abilities than boys with matched low IQs74. Collectively, these results suggest mechanisms whereby maternal autoimmuntity can impair brain development in offspring in a sex-specific way.
Fetal-brain-reactive antibodies have also been found in approximately 11% of mothers of children with ASD in the absence of any evidence of autoimmunity. These antibodies recognize fetal brain proteins of 37, 39 and 73kD and may confer specific pathophysiology (such as abnormal brain enlargement, self-injurious behavior and greater language deficits) depending on the antibody clone75. Several studies suggest that these mAbs are ASD-specific, since these antibodies did not detect proteins of these sizes in mothers of unaffected children75. However, it is currently unknown whether these ASD-specific mAbs are sufficient to cause ASD. Further studies are needed to determine the risk of developing ASD in subsequent children born to mothers harboring ASD-specific antibodies and therefore, whether these banding patterns could be used as an ASD biomarker.
Seven proteins — lactate dehydrogenase A and B (LDH), Y-Box-binding protein 1 (YBX1), guanine deaminase (cypin), stress-induced phosphoprotein 1 (STIP1), collapsin response mediator protein 1 (CRMP1) and CRMP2 — have recently been reported to be targeted by these antibodies. These proteins are immuexpressed in the developing brain, where they have roles in growth cone motility, dendritic morphology, cellular stress and metabolism, and/or transcription regulation76. Disrupting the function of any of these proteins could alter brain development, thereby contributing to ASD pathogenesis. Indeed, injecting pregnant non-human primates with mAbs from mothers of ASD children (mAbs-ASD) induces ASD-like behavioural changes in offspring, including reduced reciprocal interactions and inappropriate approach behaviours77,78. Additionally, these offspring have larger brains especially in the frontal lobes, which may be consistent with the aberrant white matter tracts found in a subset of young children with ASD78–81. Similar species-specific aberrant social behaviours in offspring are caused by injecting pregnant mice with human IgG-ASD82. Moreover, a single low dose intraventricular injection at mid-gestation of IgG-ASD in mice causes increases in repetitive behaviours and alterations in social approach behaviour83. Thus, the presence of maternally derived brain-directed autoantibodies in early development is associated with a higher incidence of ASD and specific pathophysiology. Ongoing research is aimed at determining if these fetal antibodies exert their functions by acting directly on their protein targets in the brain and which targets are causal for specific ASD phenotypes.
Maternal infection
In addition to maternal antibodies, acute immune activation that is caused by maternal infection during specific periods of gestation also contributes to ASD risk in offspring84. Maternal infection was first associated with ASD following the observation that ASD incidence increased from 0.05% to 8–13% in children of mothers that were exposed to the 1964 rubella pandemic during pregnancy85–87. Numerous single-case studies have also associated ASD with a variety of parasitic, bacterial and viral prenatal infections including toxoplasmosis, syphilis, varicella, cytomegalovirus, mumps and herpes simplex virus infection84. This diversity of infectious agents suggests that general immune activation during gestation, rather than a specific immune disorder or virus, underlies the link with ASD. Consistent with this idea of heightened immune activity and infection, several recent reports have described elevated levels of pro-inflammatory cytokines in the amniotic fluid of mothers of children with ASD88,89.
Definitive evidence that maternal infection during pregnancy is a risk factor for ASD in offspring was obtained through more recent studies of the Danish health registry90. Data from more than a million children born between the years of 1980 and 2005 revealed an almost 3-fold increase in the rate of ASD in children born to mothers who were hospitalized for viral infection in the first trimester and in children of women who experienced an episode of fever lasting a week or more before gestational week 3290,91. These data point to a specific temporal window of immune activation during the first trimester of fetal development and the association with a specific duration of fever suggests that an immune activation threshold must be surpassed to confer risk of developing ASD. It is important to note that most maternal infections that fall within this time-window and are above this threshold do not lead to ASD in offspring, suggesting that the immune status of the mother and the immuno-genetic background of the developing child may both be critical factors in determining outcome. In support of this hypothesis, a recent study using a mouse model of MIA found that the degree of maternal immune response (as measured by weight loss and tumor necrosis factor (TNF) serum levels) to prenatal immune challenge with poly(I:C), a viral mimic, is positively associated with the severity of sensorimotor gating deficits in the offspring92. Finally, like maternal autoimmune disorders, maternal infection during pregnancy has been associated with other developmental disorders as well, including schizophrenia and mood disorders93. It has been proposed that the specific combination of gestational week, type of immune activation (viral versus bacterial versus chronic) and the duration or intensity of activation may determine which disorder manifests in offspring12.
Studies using several animal models of maternal infection during pregnancy support the association of MIA with ASD phenotypes. These models include influenza infection, viral and bacterial mimics (poly I:C and lipopolysaccharide, respectively) and specific cytokines, such as IL-2 and IL-6. These studies support the idea that the timing of MIA and the type of antigen that is used for immune challenge can lead to overlapping yet distinct phenotypes, including behavioural outcomes and transcriptome signatures in the developing brains of offspring94. Poly(I:C) injection at mid-gestation in mice and non-human primates generates offspring that display all (in mice) or some (in non-human primates) of the three core behavioural symptoms of ASD95–97. Both mouse and non-human primate MIA offspring also exhibit deficiencies in sensorimotor gating and increased anxiety, co-morbidities observed in a subset of individuals with ASD. In addition, mouse MIA offspring show localized aberrations in Purkinje cells98, similar to the localized deficits in cerebellar Purkinje cells that have been reported in many postmortem ASD cases99. These MIA-induced ASD-like behaviours and neuropathologies in offspring appear to be caused by altered levels of maternal cytokines, including IL-2, IL-6 and IL-10. Both a single injection of IL-6 at mid-gestation or low-dose IL-2 injections daily between gestational days 12 and 16 in mice are sufficient to induce ASD-like behavioural changes and neuropathologies in offspring, including deficits in sensorimotor gating, increased anxiety and stereotypical behaviour, aberrant social interactions, and increased serum pro-inflammatory cytokines95,100. Maternal cytokines also appear to be necessary for the ASD-like phenotypes in offspring because poly(I:C) injections in mid-gestation on an IL-6 knockout background, co-administration of an IL-6 function-blocking antibody and poly(I:C), or overexpression of the anti-inflammatory cytokine IL-10 by macrophages, prevent the MIA-induced changes in gene expression and behaviour95,101. Despite several considerations in interpreting findings from animal models of ASD (BOX 2), these results provide strong support for the involvement of MIA with ASD in offspring.
Box 2 | Considerations for interpreting results from animal models of ASD.
Despite the excitement surrounding the relevance of the MIA model for ASD, there are several caveats that must be considered in interpreting results from this model. First, it is important to note that MIA models a single environmental risk factor amid a sea of implicated genetic and environmental susceptibilities for ASD and therefore should not be expected to capture all of the diverse phenotypes across the autism spectrum. Second, MIA is a shared environmental risk factor for a wide range of neuropsychiatric and degenerative disorders that manifest at distinct time-points during life. Therefore, MIA models also should not be expected to express pathophysiology exclusive to ASD. It is important to note that these caveats are not exclusive to the MIA models, but rather are applicable to, monogenetic animal models of ASD as well. Like some of the MIA models, most of the genetic ASD models fail to recapitulate the full pathophysiology observed in individuals with ASD. In some instances, genetic models either show no overt pathology or demonstrate behaviours that are opposite to those characterizing ASD257,258. Moreover, many of the genes that were initially thought to be exclusively linked to ASD have turned out to be shared risk factors for other neuropsychiatric disorders, particularly schizophrenia. Rather than undermining their relevance to ASD, the caveats to preclinical ASD animal models could be embraced and used experimentally to test hypotheses and develop molecular models for the cause of different forms of ASD and other disorders. These models provide a reductive platform from which we can build more complex models such as pairing MIA with specific genetic backgrounds or later-life immune insults. For example, pairing a low dose of poly(I:C) at gestation day 9 with chronic mild stress at adolescence unmasked schizophrenia-like behaviours and biomarkers in mice259. In the future, similar pairings of MIA with other ASD risk factors may parse the phenotypic heterogeneity of ASD into subtypes of this condition, as well as other disorders, reflecting specific combinations of genetic and environmental insults during particular developmental periods of susceptibility.
Chronic immune changes in ASD
Peripheral changes
In addition to immune dysregulation in families, and especially mothers, there is ample evidence of ongoing immune dysfunction in the peripheral immune system and the brain of individuals with ASD102. Like their relatives, individuals with ASD have an increased incidence of autoimmune disorders, allergy and asthma65,103. A subset of individuals with ASD also have autoantibodies that are reactive to CNS self-proteins, including serotonin receptors104, glial fibrillary acidic protein104, myelin basic protein105–107, and unidentified targets in the basal ganglia, prefrontal cortex, cingulate gyrus and cerebellum104,105,107–112. Because unaffected siblings of people with ASD harbor similar autoantibody profiles107,113, brain-directed autoantibodies may not be generally predictive of ASD and may instead represent secondary autoimmune processes or evidence of a previous CNS injury. Nevertheless, a recent study associated specific autoantibodies recognizing cerebellar targets of 45 and 65 kDa with ASD diagnosis, impaired behavioural scores, and lower cognitive and adaptive function in comparison to children without the antibodies114. Interestingly, the maternally derived anti-brain autoantibodies discussed above have a much higher degree of association with ASD diagnosis and specific pathophysiology than patients’ own autoantibodies. This discrepancy may represent a critical difference in the timing of exposure, with insults during gestation having greater pathogenic impact than the limited potential for exposure after the BBB is formed and brain architecture is more developed.
In addition to the presence of autoantibodies, many reports have identified changes in cytokine levels in the blood of individuals with ASD who are over 2 years of age. These changes include increases in IL-1β, IL-6, IL-8, IL-12p40 and granulocyte-macrophage colony-stimulating factor (GM-CSF), which are generally considered to be ‘pro-inflammatory’, and decreases in IL-10 and transforming growth factor-β (TGF-β), which are generally considered to be ‘anti-inflammatory’115–120. The lower levels of TGF-β in these children with ASD are associated with less adaptive behaviours and worse behavioural symptoms118. Elevations in IL-1β, IL-6, IL-8, IL-12p40 are specifically associated with a regressive form of ASD and more impaired stereotypical behaviours, and elevations in the chemokines C-C motif chemokine 2 (CCL2), CCL5 and eotaxin are associated with higher aberrant behaviour scores and more impaired development120. These changes in peripheral cytokine levels may be developmentally regulated since measurements at earlier ages differ in both the cytokines altered and the direction of their change in ASD. For example, several cytokines were decreased (GM-CSF, interferon-γ (IFN-γ), IL-2, IL-4 and IL-6) in neonatal blood samples from individuals who were later diagnosed with ASD in a large recent longitudinal study using the Danish Newborn Screening Biobank121. Importantly, similar to the situation for peripheral autoantibodies and ASD, peripheral cytokine profiles in individuals with ASD and their unaffected siblings are similar122, suggesting that these changes alone may not be sufficient to cause ASD.
In addition to altered cytokines, evidence exists for impaired immune cell function and responsiveness to immune challenge in ASD. Natural killer (NK) cells from individuals with ASD are defective in their normal function of lysing infected cells when challenged123,124. Monocytes isolated from individuals with ASD also exhibit impaired responses to challenge: they secrete excess pro-inflammatory cytokines following challenge with ligands for Toll-like receptor 4 (TLR-4; a receptor that is responsive to bacterial pathogens) but show reduced production of these same cytokines when challenged with ligands for TLR-9 (a receptor that is responsive to viral pathogens)124,125. Similarly, circulating levels of CD4+ T cell populations are low, biased towards an anti-inflammatory (TH2) profile and exhibit a dysfunctional response to stimulation in individuals with ASD126–130. Studies trying to associate particular cellular immuno-phenotypes with symptom severity have produced seemingly contradictory results. For example, T-cell skewing to a TH2 phenotype (which is considered ‘anti-inflammatory’ and found in a subset of the ASD population) has been associated with better cognitive and adaptive behaviour126,131, but in another study, elevated levels of the classic TH2 cytokine IL-4 have been associated with greater impairments in non-verbal communication119. Despite these ambiguities, sufficient evidence indicates that general abnormalities in peripheral immunity are a common feature in the ASD population.
Studies in mice have provided further support for an association between peripheral immune changes and ASD. The offspring of MIA mice exhibit peripheral immune abnormalities. For example, T-cells from adult MIA offspring secrete excess pro-inflammatory cytokines when challenged and show a bias toward TH1 and TH17 phenotypes100,132,133. In addition, myeloid cell populations from these animals are elevated and produce increased levels of IL-12p40 and CCL3, which is consistent with a pro-inflammatory immune profile132,134. Collectively, these findings suggest that MIA induces an irregular immune phenotype that persists into adulthood in rodents, as in ASD, but is distinct in nature from the T-cell response profile in ASD. However, comparing results from animal studies using a single model (MIA) with ASD in humans resulting from a wide range of aetiologies may be misleading. Moreover, cross-species comparisons from the current literature are not informative because of the often limited number of cytokines assayed in human samples, the wide range of ages, co-morbidities, and therapies within the patient population, and the challenge in comparing postnatal ages between rodents and humans. Nevertheless, these studies do clearly show that an environmental risk factor for ASD causes long-lasting immune dysregulation that is associated with ASD-like phenotypes in rodents. Importantly, future studies with larger numbers of individuals with ASD are needed to determine if there is a consistent peripheral cytokine signature that is diagnostic for ASD or even negatively associated with ASD expression in unaffected siblings.
Immune changes in the CNS
It is often assumed that changes in cytokine levels in the blood from individuals with ASD reflect changes in cytokine levels in the brain. The findings of some studies are consistent with this idea, reporting elevations in GM-CSF, IL-6, IL-8, TNF-α and IFN-γ levels in the frontal cortex and in IL-6, TGF-β and MCP-1 levels in the anterior cingulate gyrus and cerebellum115,135,136. As further evidence of potential neuroinflammation, microglia in the dorsolateral prefrontal cortex exhibit increased MHCII expression (a marker of activation), activated morphology (amoeboid shape), and increased density137,138. However, other results contradict these findings: some studies have shown decreases or no change in sensitive markers of CNS immune activation — including quinolinic acid, neopterin and biopterin — in the cerebrospinal fluid (CSF) of individuals with ASD139–141. Similarly, increases in soluble TNF receptors that blunt the inflammatory response in the CSF and serum from individuals with ASD141. Future studies defining the roles for ASD-associated changes in cytokines as mediators of neuroinflammation, as dynamic adaptive responses to peripheral changes, or simply as growth factors or neuromodulators will be critical to explain these contradictory findings.
In mouse models of MIA, offspring with ASD-like behaviours and neuropathology also exhibit long-lasting changes in cytokine levels in the brain. A broad spectrum of cytokines are elevated in the fetal brain hours after poly(I:C) injection in pregnant mice142,143 and many of these cytokines remain chronically altered in the brains of offspring throughout postnatal development and into adulthood144. Although cytokines are generally increased at birth and in the adult brain, levels of many cytokines are decreased in frontal cortical regions during peak periods of synaptogenesis and plasticity144. These changes do not correlate with changes in serum cytokine levels and are not accompanied by changes in BBB permeability or immune cell infiltration into the brain parenchyma. Thus, these results do not fit the classic definition of neural inflammation and suggest caution in interpreting changes in cytokines at one time-point as indicative of an inflammatory process (BOX 3)145. Understanding how a discrete event of immune activation during pregnancy causes ongoing and dynamic dysregulation of immune molecules in the brains of offspring is one of the most important areas for future research in this field.
Box 3 | Detecting neuroinflammation.
Inflammation in the body is a protective, organized and adaptive response to invading pathogens260 that is rigorously defined by four hallmarks: elevations in pro-inflammatory cytokines, activation of macrophages, recruitment of leukocytes to sites of inflammation, and local tissue damage. Inflammation begins with an abrupt rise in pro-inflammatory cytokines, followed by a gradual rise in anti-inflammatory cytokines, which limits damage to secondary tissues. Classically, neuroinflammation occurs when the nervous system is exposed to infection or trauma that is accompanied by breaches in the blood–brain barrier (BBB). Under these conditions, microglia and astrocytes adopt a reactive phenotype (gliosis) and proliferate and perpetuate cellular and molecular responses that are aimed at removing infected or damaged tissue. Prolonged gliosis can recruit peripheral leukocytes, amplify the initial tissue damage, and thereby cause neurodegeneration in the surrounding healthy tissue.
In the past 10 years, since our ability to measure cytokine levels and microglial morphology in the brain has become routine, the definition of neuroinflammation has grown increasingly murky to the point that the presence of any single hallmark of classic inflammation is now sufficient to define a disease as being ‘inflammatory’. Reports of elevated levels of pro-inflammatory cytokines in the post-mortem brains of individuals with ASD, in particular, have led to the hypothesis that chronic neuroinflammation plays a part in ASD pathogenesis. However, numerous studies cited as supporting this hypothesis have assessed only a handful of pro-inflammatory cytokines (typically IL-1β, IL-6, TNF-α and IFN-γ) without reporting any other hallmark of neuroinflammation119,135,141. Individual cytokines function as part of a larger homeostatic network of both pro- and anti-inflammatory, as well as regulatory, cytokines in which each factor can influence the synthesis and action of the other factors261. Thus, the impact of these cytokine networks on immune cells and tissues cannot be inferred from examining the levels of individual cytokines262. Determining whether or not inflammatory conditions predominate requires assessing (at the very least) the levels of a wide range of cytokines as well as the accompanying expected cellular signs of inflammation, such as microglial activation. However, despite the increasing numbers of studies measuring microglial activation, this classification remains subjective and represents a range of morphologies and states that change with developmental age and are only beginning to be understood.
Defining neurological and psychiatric diseases as inflammatory requires even more rigorous assessment within the brain because immune molecules and cells in the CNS are involved in physiological processes that can be mistaken for pathogenesis263,264. Currently, there is no consensus in the ASD field as to which criteria must be met to satisfy the label ‘neuroinflammation’, despite the widespread assumption that any inflammatory process is detrimental and leads to degeneration. Given the abundance of immune signalling that occurs under physiological conditions in the developing and mature brain, some of which is described in this Review, choosing a definitive set of criteria that constitutes a pathological state will prove challenging265. Nevertheless, it is especially important in the case of ASD to define those immune mediators that serve adaptive roles, and those that are pathological, to better understand the mechanisms underlying this disorder and to tap into the exciting potential of targeting those functions for the development of new neural-immune therapies to treat ASD in the future.
In addition to altered cytokine levels, the brains of individuals with ASD also show changes in the expression of genes encoding proteins that were initially discovered in the immune system. A weighted gene co-expression network analysis comparing the expression of more than 30,000 genes in post-mortem autistic and control brains revealed two distinct networks — each comprising more than 400 genes — that are disrupted in the brains of individuals with ASD30. One of the networks of genes is associated with synaptic function and is down-regulated, whereas the other module contains immune-related genes and is up-regulated in ASD. Taken together, these results indicate ongoing dysregulation of the immune system and altered expression of immune molecules in the CNS in ASD and in mouse models of MIA. How these changes relate to the pathophysiology observed in these individuals and animal models is still unclear. Since the immune system is primarily tasked with tissue repair and homeostatic processes, these alterations could represent compensatory responses to dysfunctional network activity and cellular stress. Studies addressing the temporal dynamics of these central alterations with age and whether they are linked to the ongoing and dynamic immune changes in the periphery are important areas for future research.
A role for microbiota?
Adding to the complexity of the neural–immune axis is the recent focus on the gut as an important nexus for nervous–immune–endocrine system interactions. The initial colonization of the gut is dominated by maternal microbiota during birth and this formative colonization assists in priming the developing immune system and directs immune homeostasis146–148. Children with ASD have excessive levels of Clostridium spp and Desulfovibrio spp in their gut microbiome149–151 and this imbalance could influence peripheral immune responses, potentially contributing to the observed abnormalities in immune cell composition and function in these individuals. In fact, recent studies suggest that gut microbiota may act as a ‘tuning fork’ for the immune system. The T-cell repertoire (defined as the various sub-types — each with their own effector properties — and myriad T-cell receptor clones within these sub-types) in particular, is highly influenced by the composition of gut microbiota152,153. Certain microbiota signatures in the gut inhibit the differentiation of brain-supportive T cell populations, whereas administration of Bacteriodes fragilis (B. fragilis) restores the proper balance of T cell populations in mice154.
The idea that therapies directed at altering the gut microbiota may regulate immune function and rescue ASD-related phenotypes has received support from animal models of ASD. Offspring from the poly (I:C) mouse model of MIA exhibit gastrointestinal changes that are also found in some humans with ASD155,156, including increased intestinal permeability and abnormal intestinal cytokine profiles157. These MIA offspring also have abnormal microbiota signatures, most significantly in elevated levels of Clostridia spp, paralleling findings in humans with ASD150,158. Remarkably, oral treatment with B. fragilis restores intestinal permeability and serum cytokine levels, rectifies microbiota imbalances, ameliorates stereotypic and anxiety-like behaviour, improves sensorimotor gating, and increases the number and duration of ultrasonic vocalizations in these mice157. Interestingly, B. fragilis has no effect on the deficits in sociability in these offspring. Future studies will determine if social behaviors are resistant to probiotic therapy, whether B. fragilis also reduces the underlying neuropathology, and whether it does so through normalizing immune dysregulation in offspring.
Potential mechanisms
As our understanding and appreciation of neural-immune crosstalk continues to grow, there is an increasing focus on determining how immune dysregulation might alter brain connectivity and function to contribute to ASD-like phenotypes. In the past 10 years, immune molecules on neurons or glia in the brain have been demonstrated to regulate every stage of brain development and function159,160. The body repurposes immune cells, immune molecules and their receptors for widely divergent tasks in both region- and developmental-stage specific manners. Alterations in the expression of these immune molecules in the brain, by genetic mutations or as a result of environmental risk factors, can lead to transient and/or lasting changes in brain development and function.
Cytokines
Findings from both epidemiological studies and animal models indicate that cytokine imbalances can disturb fetal development and/or chronically impair brain function161. Cytokines and their receptors are expressed by neurons and glia throughout development and regulate a diverse array of physiological processes in brain development, plasticity and function159. For example, IL-1β and its receptors have important roles in a wide range of processes, including neurogenesis and synapse formation and plasticity, from early prenatal CNS development to postnatal development and adulthood162–168. Moreover, a mouse model mimicking the ASD-associated null mutation in IL1RAPL1 exhibits reduced cortical synapse density, an enhanced ratio of excitation to inhibition in the amygdala, and deficits in associative memory168,169. These findings are similar to those found in Mecp2-null mice, a model of Rett syndrome169,170. IL-1β functions within a tight homeostatic range; deviations above or below physiological levels lead to impairments in long-term potentiation and synaptic plasticity — two key molecular processes thought to underlie learning and memory171. Several other pro-inflammatory cytokines, including TNF, have similar diverse roles in early brain development and postnatal synaptic plasticity172,173. IFN-γ also negatively regulates homeostatic processes during experience-dependent plasticity in the visual system174. Collectively, these findings suggest that increases or decreases in cytokines owing to genetic mutations or downstream of MIA could have deleterious effects on brain development and function independent of any role in inflammation (BOX 3 and FIG. 2).
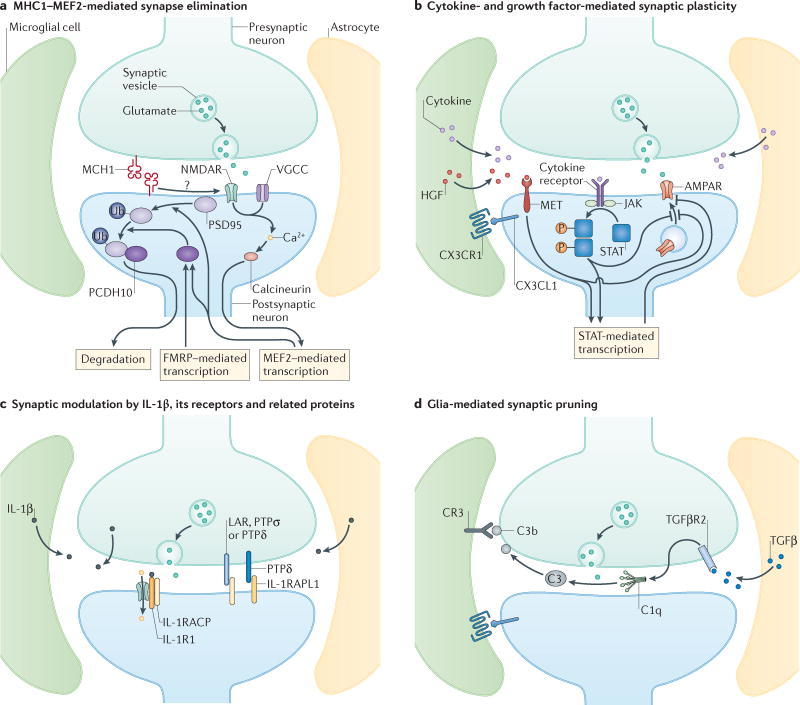
Figure 2. Immune molecules at glutamatergic synapses.
At the ‘quad-partite’ synapse214, pre- and postsynaptic neurons, astrocytes and microglia communicate using immune mediators, many of which are altered in individuals with ASD. Each panel represents molecular pathways that are used by immune molecules at the synapse to regulate synapse formation and/or plasticity. a | MHCI molecules are located at synapses, where they act through calcineurin to activate MEF2 transcription factors to negatively regulate synapse strength and density. The MHCI-dependent activation of MEF2 requires calcium influx through NMDARs and voltage-gated calcium channels (VGCCs). MEF2 acts in concert with FMRP to stimulate the ubiquitination of PSD-95 and increased association with protocadherin 10, which then chaperons PSD-95 to proteosomes. b | In general, cytokines released by astrocytes, microglia, and/or neurons bind to their specific receptors and activate JAK/STAT which regulates signalling at the synapse and alters transcription, leading to negative regulation of AMPA receptor (AMPAR) expression either through inhibiting new insertion or increasing internalization. Growth factors, especially HGF, are also thought to be secreted from glial cells into the synaptic cleft, where they bind and activate the MET receptor, which negatively regulates AMPAR expression possibly through STAT-mediated transcription. Chemokines are also presented by neurons and bind to receptors on glial cells, depicted here for the interaction between CX3C chemokine receptor 1 (CX3CR1) on microglia and its ligand, CX3CL1, secreted by neurons or expressed in a tethered form on the neuronal cell surface. CX3CR1-CX3CL1 signaling is required for the migration of sufficient numbers of microglia into the brain in early development, and synaptic plasticity under physiological conditions. c | IL-1β exerts distinct effects at the synapse. Binding to IL-1R1 recruits the IL-1 accessory receptor (IL-1AcP), which increases NMDAR signaling. Unbound IL-1RAcP acts as a trans-synaptic adhesion molecule through its interactions with pre-synaptic PTPσ, PTPδ and LAR. The IL-1R accessory-like receptor 1 (IL-1RAPL1) also acts as a synaptic organizer binding to pre-synaptic PTPδ. These trans-synaptic interactions exert multiple effects on synapse formation and plasticity. d | Astrocyte-secreted TGF-β binds to neuronal TGFβRII (placed here presynaptically due to findings at the neuromuscular junction), which induces neuronal secretion of the complement protein C1q. C1q initiates the complement cascade leading to cleavage of C3 into C3b, which binds to synaptic surfaces. Microglial expressed complement receptor 3 recognizes tagged synapses and initiates synaptic pruning at a subset of synapses. CX3CR1-CX3CL1 is also required for microglia-mediated synaptic pruning in early development, and spine elimination and formation in mature circuits. While currently unknown, local neuron-microglia signaling through CX3CR1-CX3CL1 may serve as an instructive signal for complement-mediated synaptic pruning.
What mechanisms downstream from cytokines affect brain development? In the periphery, numerous cytokines signal through the janus kinase–signal transducer and activation of transcription (JAK–STAT) pathway, which comprises three mammalian JAK and seven STAT proteins175. Receptor binding activates the kinase function of JAK, which recruits and phosphorylates STAT. Upon STAT dimerization, the complex translocates to the nucleus, where it regulates the transcription of genes involved in cell growth, differentiation and function, and immune genes including those encoding MHCI molecules. Although it is unknown whether the JAK–STAT pathway works in similar ways in the brain, recent reports suggest that JAK–STAT signalling is important for normal brain functions and that cytokines regulate this signalling in neurons. For example, STAT1 is upregulated in the visual cortex following long-term mononuclear deprivation, where it regulates AMPA receptor surface expression and synaptic function174,176. Moreover, IFN-γ enhances STAT1 expression, which reduces visual cortical plasticity174. STAT1 is also implicated in spatial learning177 and STAT1 and STAT3 have roles in hippocampal plasticity178,179. Although JAK–STAT signalling in the brain has just begun to be explored, it may represent an important therapeutic target for pathogenic cytokine-mediated alterations in brain development and function, like those described above for ASD.
MET
In addition to regulating immunity, MET has essential roles in the brain throughout development. MET is expressed throughout the neocortex, hippocampus180, cerebellum181 and brainstem182, where it is enriched in excitatory pre- and post-synaptic compartments183. MET signalling induced by hepatocyte growth factor (HGF) increases the levels and clustering of synaptic proteins184,185, increases the number of dendritic spines186, modulates hippocampal synaptic function185,187 and enhances hippocampal long-term potentiation188. Moreover, decreases in MET expression confer local hyperconnectivity, which is a putative hallmark of ASD pathophysiology189. Consistent with these results, reduced MET expression, as seen in ASD, alters key neurodevelopmental processes and is associated with structural and functional alterations in ASD26. Further investigation is needed to determine if targeting downstream MET signalling modulates circuit activity and ameliorates the impairments in socio-communicative function associated with decreased MET expression31.
MHCI molecules
MHCI molecules have been implicated in ASD through genetic associations (described above) and as a downstream effector of MIA190. MHCI molecules are found on all nucleated cells in the body, where they mediate the adaptive and innate immune responses. They are also present throughout the CNS of many mammalian species in neurons and glial cells191,192. In cortical pyramidal neurons, MHCI is present both pre- and post-synaptically at glutamatergic synapses, where its surface expression is tightly modulated by activity, therefore allowing it to regulate synaptic plasticity191,193–199. During CNS development, MHCI controls axonal and dendritic outgrowth, negatively regulates the initial establishment of cortical connections, and promotes synapse elimination during activity-dependent refinement of connections in the developing visual system190,192. Interestingly, poly(I:C)-induced MIA in mice causes a dramatic increase in the expression of MHCI molecules on cortical neurons from newborn offspring. Dissociated neurons from MIA offspring also exhibit a profound deficit in their ability to form glutamatergic synapses. Remarkably, normalizing MHCI levels in cultured MIA neurons prevents the MIA-induced decrease in synapse density190. Thus, changes in MHCI signalling may be a common mechanism through which genetic mutations in MHC genes and exposure to maternal infection could alter the establishment of connectivity in the developing brains of offspring. Whether changes in MHCI act downstream of genetic mutations in other immune or non-immune ASD-linked genes, utilize convergent ASD-linked signaling hubs at the glutamatergic synapse, or contribute to ASD-related behaviours are active areas of investigation.
MEF2 transcription factors
Although myocyte enhancer factor 2 (MEF2) transcription factors are not classic immune molecules, they appear to mediate the effects of immune dysregulation on synaptic connectivity, are also associated with ASD risk through genetic mutations, and act as central molecular hubs downstream of several other ASD risk factors. MEF2 transcription factors regulate gene expression in an activity-dependent manner, affecting the expression of many proteins that regulate synaptic plasticity and function during neural development200,201. Interestingly, MEF2C haploinsufficiency syndrome is a recently discovered neurodevelopmental disorder that is characterized by autism-like behaviours, intellectual disability, high rates of epilepsy and abnormal movements202. MEF2 negatively regulates the establishment of hippocampal connections201 and mediates the effects of MHCI on cortical connectivity in normal brain development and the MIA-induced deficit in synapse formation in newborn neurons190. In the genome-wide transcriptional profiling study of ASD that was mentioned previously30, the MEF2 splicing factor ataxin-2-binding protein 1 (A2BP1; also known as RBFOX1) — previously implicated in ASD203,204 — was identified as a central hub within the network of downregulated genes associated with synaptic function. Moreover, in an integrative functional genomic analysis of ASD-associated genes, two co-expressed networks upregulated during early fetal development and during late fetal–early postnatal development showed binding site enrichment for two isoforms of MEF2 (MEF2A and MEF2C)205 that are predicted to drive the transcriptional co-regulation of both processes. In addition, MEF2 interacts with FMRP to regulate glutamatergic synaptic function206 and this interaction controls the expression of protocadherin 10 (Pcdh10), which is another ASD-linked gene and is necessary for MEF2-mediated activity-dependent synapse elimination207. Together, these findings suggest that ASD that is associated with immune dysregulation during gestation, as well as idiopathic and syndromic forms of ASD, may converge on a molecular pathway with MEF2 as a hub208. Identifying new therapeutic targets within the signalling cascades upstream and downstream of MEF2 should be a priority for ASD research owing to their potential efficacy in treating a wide range of disorders on the autism spectrum.
Microglia and complement
Microglia and complement may — like cytokines, MHCI and MEF2 — also mediate the effects of both environmental and genetic ASD risk factors. Activated microglia are present in increased numbers and with an altered distribution in postmortem brain tissue, especially the prefrontal cortex, in a subset of ASD cases115,137,209,210. Although microglia are best known for their role in clearing debris following injury, they have recently been shown to have important roles in the normal brain211. During development, microglia phagocytize debris from naturally-occurring cell death, secrete trophic factors like insulin-like growth factor I (IGF-1) and TGFβ, regulate neurogenesis through phagocytosis of neural precursor cells and participate in activity-dependent elimination of synaptic connections212–214. Deficits in microglia, such as those seen in the CX3C chemokine receptor 1 (Cx3cr1) knockout mice, lead to increased densities of immature synapses in the cerebral cortex, deficits in functional connectivity across brain regions, and ASD-like behaviours215,216.
Components of the complement cascade may mediate some of these microglial functions. In the peripheral immune system, the complement pathway mediates clearance of cellular debris and increases the degree of antibody binding to circulating bacteria and infected cells, thereby enhancing their destruction. Intriguingly, the complement C4 gene lies within the MHC region and deficiencies in C4 are associated with ASD50,128,217. Complement deficiency is also strongly linked to autoimmune disorders, especially SLE218. In the brain, the complement protein C1q is secreted by neurons219. Although many of the details of the complement cascade in the brain are unknown, C1q typically complexes with other C1 proteins to activate the C3 convertase, which cleaves C3 into opsonizing fragments in the periphery220. One of these fragments, C3b, is thought to tag weak synapses in the CNS. These tagged synapses are subsequently pruned through phagocytosis by microglia expressing the complement receptor 3 (CR3)221. Thus, early immune insults have the potential to diminish or enhance microglia-mediated synaptic pruning. Astrocyte-dependent secretion of TGF-β regulates C1q expression and deposition in the lateral geniculate nucleus222. Because TGF-β is increased in the CSF and postmortem brain tissue from individuals with ASD115, it is possible that these increases of TGF- β in ASD brains could cause and/or reflect compensatory or pathology-inducing alterations in synaptic pruning. Importantly, microglia, like their macrophage relatives in the periphery, can be primed early in development223. Depending on the developmental trigger, altered priming can impair or enhance reactions to subsequent immune challenges224,225. Whether, and how, this immune priming contributes to ASD phenotypes remains an important area for future research.
Perspective
A common pathway?
The results reviewed here suggest that many of the diverse immune contributions to ASD — including dysregulated signalling through MET, cytokines, MHCI and microglia–complement — share the downstream effect of regulating synapse formation and elimination, thereby controlling synaptic function and plasticity in the developing and mature brain. Although research into the molecular mechanisms used by these immune ASD risk factors is in its infancy, one common signaling hub — MEF2 —has already been identified (FIG. 3). Importantly, this hub also mediates the effects of mutations in MEF2 and FMR1 in contributing to ASD-related phenotypes.
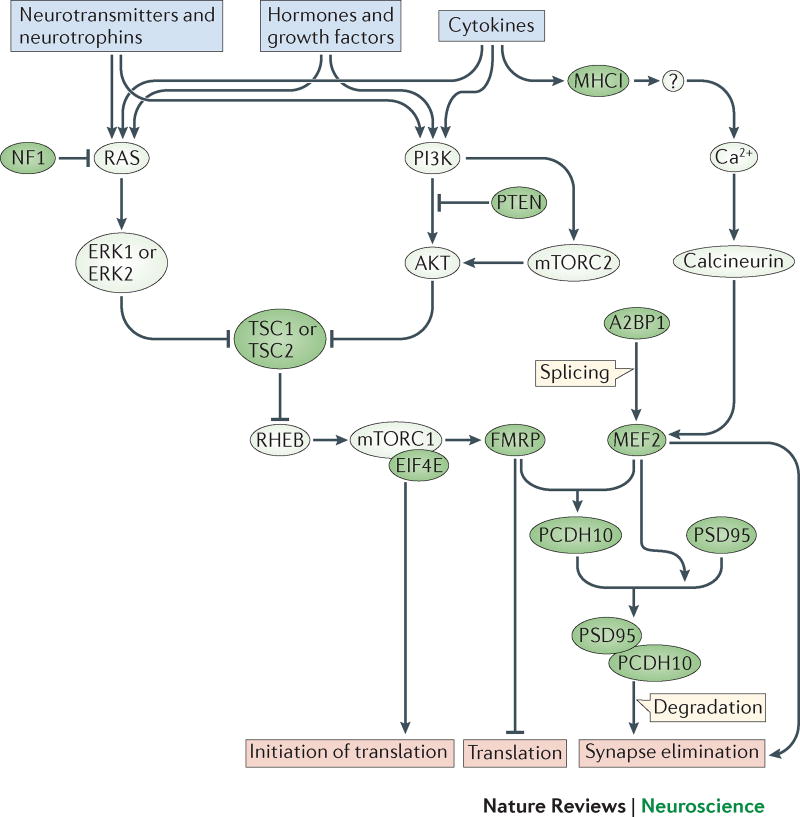
Figure 3. Synaptic immune signaling converges on mTOR.
Immune and neuronal receptor signaling activate molecular pathways, which feed into the mTOR pathway and activate MEF2-dependent transcriptional regulation. These central signalling pathways may become dysregulated through genetic mutations or environmental exposures associated with ASD and thereby alter neural development and function. mTOR activity regulates numerous processes including protein synthesis, mitochondrial function, lipid synthesis, cell growth and proliferation, synaptic plasticity, neurogenesis, neuronal cell death, ion channel expression and cytoskeletal dynamics. Importantly, mTORC1 regulates the synthesis of glutamatergic receptors and protein products, including SHANK, SAPAP, neuroligins, AMPA and NMDA receptor subunits, many of which are genetically associated with ASD. Mutations in the genes that cause most of the syndromic forms of ASD—FMR1, NF1, PTEN, and TCS1/2—disrupt components of the mTOR signaling pathway. The MEF2 transcription factor is also implicated in ASD. MEF2C haploinsufficiency syndrome is characterized by ASD-like behaviors, perhaps through the function of MEF2 in regulating transcription during synapse formation and elimination. MEF2 also likely regulates the expression of cytokine receptors in a positive or negative feedback loop, since the promoters of some cytokine receptors (like Il1rapl1, for example) contain a MEF2 binding motif. MEF2 is also a target of the splicing regulator A2BP1—the central gene in a synaptic module identified in a transcriptome analysis of brain tissue from individuals with ASD. Green fill indicates protein products of genes associated with ASD.
In addition to convergence on MEF2 signalling, it is likely that many immune risk factors will converge on the most common intracellular signaling hub for ASD-risk genes — the mammalian target of rapamycin (mTOR). mTOR has been implicated as a common pathway in ASD from numerous studies of monogenic forms of ASD226. mTOR is a serine/threonine protein kinase that controls many aspects of neural development as well as the function and plasticity of the mature brain227,228. This pathway integrates signals from growth factors and hormones, neurotransmitters, cytokines, and stress mediators and involves cross-talk between two intracellular signaling cascades — RAS–ERK and phosphoinositide 3-kinase–AKT— that are essential in immune and neuronal function. In the immune system, mTOR integrates signals from the immune microenvironment to direct immune cell metabolism, differentiation and function, and is important for mounting an adaptive immune response229. Alterations in mTOR signalling seem to underlie several common monogenic forms of ASD, including FXS, TSC, Rett syndrome, PTEN macrocephaly and neurofibromatosis type 1, and syndromes with autistic-like features, such as MEF2C haploinsufficiency and 9q34.3 deletion syndrome. Interestingly, IGF-1, which ameliorates some behavioural and neuropathologies of Rett and 22q13 deletion syndrome, and is secreted by microglia, activates mTOR230.
Although most of the immune risk factors for ASD discussed above have not yet been directly studied for their effects on mTOR, there is some evidence to date that is consistent with the hypothesis that immune dysregulation may converge on this central signalling pathway. For example, MET translates extracellular signals into mTOR activation; thus, the ASD-related MET C variant is predicted to lead to hypoactive mTOR signalling231. Moreover, many cytokine receptors found at the synapse typically activate mTOR signaling in the periphery, potentially causing phenotypes similar to monogenic forms of ASD. Although it is unknown whether mTOR is activated in response to cytokines in the brain, the signalling components for mTOR are present in neurons and are activated by a growing list of growth factors, guidance molecules and neurotransmitters227. mTOR is thus a focal point to integrate immune signalling in the brain, changes in cytokine levels due to MIA, postnatal environmental insults or genetic mutations in immune genes, and ongoing immune dysregulation throughout life. Determining if, and how, the immune contributions discussed in this review converge on this common mTOR pathway will be critical for determining how pervasive mTOR signalling is across the diverse forms of ASD and for the development of new therapeutics that target mTOR in the future.
Therapeutics
One of the most exciting implications of the discovery that immune dysregulation may contribute to ASD is the possibility that agents targeting immune function could alleviate some of the symptoms associated with this spectrum of disorders. One of the most effective, albeit drastic, therapeutic approaches for restoring immune function is bone marrow transplantation. Remarkably, transplantation to reconstitute the brain with wild-type microglia reverses somatic phenotypes, neuropathological changes, and behavioural abnormalities in two mouse models of ASD — MeCP2−/y model of Rett syndrome232 and the MIA model132. Full immune system reconstitution from control animals also rescues peripheral immune abnormalities in MIA offspring, including deficits in regulatory T-cells and a disproportionate increase in CD4+ memory T cells with an inflammatory profile previously implicated in learning and memory deficits131,132 (BOX 3). On the basis of these animal studies, clinical trials using strategies to reconstitute immune cells in individuals with ASD have been initiated233,234. However, a recent study failed to find any benefit of wild-type microglia in three rodent models of Rett syndrome including the model used in the original study235. The reason for these divergent findings is currently unclear, but the therapeutic benefits of transplantation must be confirmed before performing such a drastic procedure in individuals with Rett syndrome.
Another class of potential therapies targeting immune function is anti-inflammatory or immunosuppressive agents. To date, therapies in this category have focused on decreasing the assumed ongoing inflammation in the periphery and brain of individuals with ASD. Consistent with this idea, minocycline, a broad-spectrum antibiotic that has immunosuppressive properties, corrects synaptic abnormalities, heightened anxiety and social deficits in Fmr1 knockout mice236,237. In a randomized double-blind, placebo-controlled trial in children and adolescents with FXS, three months of minocycline treatment improved anxiety and mood-related behaviours238. Although antibiotic treatment may also improve symptoms in idiopathic forms of ASD239–241, ASD with regressive features may be resistant to minocycline therapy242. The exact immunomodulatory mechanisms of minocycline are unknown; however, it may exert its effects through a combination of suppressing cytokine signalling, enhancing neurotrophic factor secretion and suppressing mTOR signalling243. Minocycline could relieve ASD-related phenotypes directly through altering neural function or indirectly through ameliorating ongoing immune dysfunction or through regulating the intestinal microbiota241.
Despite their potential, treatments focused on preventing or ameliorating neuroinflammation in ASD should be considered with caution because the immune dysregulation associated with ASD may not always be pro-inflammatory. As discussed above, mouse models of MIA exhibit decreased levels and function of many types of immune molecules in the brains of offspring during postnatal development, which is the opposite of the widely assumed inflammatory processes present in ASD144. Moreover, many of the immune molecules associated with ASD play important parts in brain development, function and plasticity. Thus, altered levels of these immune molecules in ASD may alter brain development through defects in their normal function in addition to, or even rather than, causing inflammation. Finally, it is important to consider the possibility that limited periods of neuroinflammation could be an adaptive response to allow the brain to cope with ASD-related deficits145. Studies in humans indicate that fever improves many ASD symptoms, especially deficits in communication and increases in repetitive behaviours244–246. Although these effects have not been directly tested in animal models, several studies suggest that fever temporarily corrects chronic mitochondrial dysfunction — a frequently observed co-morbidity seen in ASD — through alterations in purinergic signalling247,248. The mechanisms underlying these paradoxical effects of fever are important areas of ongoing research since they may well reveal new therapeutic targets for ASD. If inflammation is indeed adaptive, as implied by these reports, then treating individuals with anti-inflammatory agents could have unintended detrimental consequences. Nevertheless, despite the possible complexity in their roles in ASD, immune molecules provide a new and important set of targets for drug discovery in the future.
Key Points.
Genetic and environmental risk factors for ASD suggest that dysfunction of the immune system may contribute to the development of this disorder.
Maternal immune dysfunction due to autoimmune disease, infection or immunogenetics may alter common molecular signaling pathways in the developing brain, increasing the likelihood of ASD
Individuals with ASD exhibit chronic changes in immune system function that may represent disease-related pathophysiology, beneficial compensation, or a combination of both
ASD-related changes in the expression of immune molecules in the brain are not always indicative of neural inflammation, even though they may be detrimental to brain development and function
Many immune molecules are expressed in the brain at synapses and their signaling may converge on several intracellular signaling hubs, such as MEF2 and mTOR, that also mediate idiopathic and syndromic forms of ASD
Immune molecules provide a new and important set of targets for development of new therapeutics for ASD
Acknowledgments
The authors would like to thank members of the McAllister Lab for ongoing discussions about the topics covered in this review, especially Dr. Bradford M. Elmer. M. L. E. has been supported by a Dennis Weatherstone Predoctoral Fellowship from Autism Speaks (#7825), the Letty and James Callinan and Cathy and Andrew Moley Fellowship from the ARCS Foundation, and a Dissertation Year Fellowship from the University of California Office of the President. A.K.M. is supported by NINDS R01-NS060125-05, NIMH P50-MH106438-01, a grant from the Simons Foundation (SFARI #321998), and a grant from the UC Davis Research Investments in Science and Engineering Program.
Glossary
- Maternal immune activation (MIA)
Animal models of prenatal immune challenge by stimulation of the maternal immune system with viral or bacterial mimics, live antigens or inflammatory cytokines.
- Fetal-brain-reactive antibodies
Maternally-derived IgG antibodies that can cross the placenta and bind to fetal brain proteins.
- Maternal IgG antibodies (mAb)
Immunoglobulin G antibodies that pass through the placenta during the third trimester and enter fetal circulation, where they persist at high titer levels for several months after birth.
- Human leukocyte antigen (HLA)
Gene locus that encodes the human versions of three different classes of MHC proteins.
- Complement
A system of plasma proteins that attack extracellular pathogens, assist in pathogen and cellular debris clearage by phagocytes, and facilitate synaptic pruning in the brain.
- Single-nucleotide polymorphisms (SNPs)
The most common form of genetic variation due to nucleotide substitutions.
- Copy number variants (CNVs)
Deletions or duplications of chromosomal segments leading to phenotypic diversity among individuals.
- Autoimmune disorders
Disorders where the immune system attacks normal substances and tissues of the body.
- Polyinosinic:polycytidylic acid (Poly(I:C))
Mismatched double-stranded RNA that acts as a viral mimic.
- The blood-brain barrier (BBB):
A selectively permeable network of endothelial cells, pericytes, and astrocytes separating the circulating blood from the brain extracellular fluid, which begins forming in the first trimester and is fully formed by birth. Infection, disease and certain drugs can increase the permeability of the BBB.
- Gut microbiota
A diverse set of microorganisms that inhabit the gut and shape host immune function.
- Phagocytosis
The engulfment of extracellular pathogens or cellular debris by certain immune cells including microglia.
Biographies
Myka Estes is currently a PhD student in Kim McAllister’s lab at the University of California, Davis where she studies neuro-immune mechanisms in the initial establishment of cortical connections under healthy and pathological conditions. She has received a Dennis Weatherstone Predoctoral Fellowship from Autism Speaks, the Letty and James Callinan and Cathy and Andrew Moley Scholarship from the ARCS Foundation, and a Dissertation Year Fellowship from the University of California Office of the President.
A. Kimberley McAllister is Associate Director of the Center for Neuroscience and Professor of Neurology and Neurobiology, Physiology, and Behavior at UC Davis. The research in her lab focuses on elucidating the molecular mechanisms of synapse formation, elimination and dynamics during multiple stages of development. Her laboratory also studies the role for immune molecules on neurons in cortical development and as mediators of environmental risk factors for neuropsychiatric disorders.
Footnotes
Competing interests statement: The authors declare no competing interests.
References
- 1.King BH, Navot N, Bernier R, Webb SJ. Update on diagnostic classification in autism. Curr Opin Psychiatry. 2014;27:105–109. doi: 10.1097/YCO.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. In: Baio J, editor. Morbidity and Mortality Weekly Report. Vol. 63. Naitonal Center on Birth Defects and Developmental Disabilities, CDC; 2014. pp. 1–21. [PubMed] [Google Scholar]
- 3.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20:84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallmayer J, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg RE, et al. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med. 2009;163:907–914. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- 6.Pessah IN, et al. Immunologic and neurodevelopmental susceptibilities of autism. Neurotoxicology. 2008;29:532–545. doi: 10.1016/j.neuro.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverstein AM. The most elegant immunological experiment of the XIX century. Nat Immunol. 2000;1:93–94. doi: 10.1038/77874. [DOI] [PubMed] [Google Scholar]
- 9.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 10.Akinbami LJ, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 11.Thorburn AN, Macia L, Mackay CR. Diet, Metabolites, and “Western-Lifestyle” Inflammatory Diseases. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Stamou M, Streifel KM, Goines PE, Lein PJ. Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol Teratol. 2013;36:3–16. doi: 10.1016/j.ntt.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623–3629. doi: 10.1016/j.vaccine.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 14.Bailey A, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 15.Steffenburg S, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandin S, et al. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devlin B, Scherer SW. Genetic architecture in autism spectrum disorder. Curr Opin Genet Dev. 2012;22:229–237. doi: 10.1016/j.gde.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Gaugler T, et al. Most genetic risk for autism resides with common variation. Nature genetics. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg JM, Geschwind DH. Autism genetics: searching for specificity and convergence. Genome Biol. 2012;13:247. doi: 10.1186/gb-2012-13-7-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santini E, Klann E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Science signaling. 2014;7:re10. doi: 10.1126/scisignal.2005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franz DN, Weiss BD. Molecular therapies for tuberous sclerosis and neurofibromatosis. Current neurology and neuroscience reports. 2012;12:294–301. doi: 10.1007/s11910-012-0269-4. [DOI] [PubMed] [Google Scholar]
- 22.Schaaf CP, Zoghbi HY. Solving the autism puzzle a few pieces at a time. Neuron. 2011;70:806–808. doi: 10.1016/j.neuron.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Marshall CR, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Roak BJ, State MW. Autism genetics: strategies, challenges, and opportunities. Autism Res. 2008;1:4–17. doi: 10.1002/aur.3. [DOI] [PubMed] [Google Scholar]
- 25.Luo R, et al. Genome-wide transcriptome profiling reveals the functional impact of rare de novo and recurrent CNVs in autism spectrum disorders. Am J Hum Genet. 2012;91:38–55. doi: 10.1016/j.ajhg.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Y, Huentelman M, Smith C, Qiu S. MET receptor tyrosine kinase as an autism genetic risk factor. International review of neurobiology. 2013;113:135–165. doi: 10.1016/B978-0-12-418700-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell DB, et al. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Ann Neurol. 2007;62:243–250. doi: 10.1002/ana.21180. [DOI] [PubMed] [Google Scholar]
- 28.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson PB, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2:232–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- 30.Voineagu I, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudie JD, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedrick A, et al. Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism Res. 2012;5:434–439. doi: 10.1002/aur.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer JT, et al. Transcriptional regulation of the MET receptor tyrosine kinase gene by MeCP2 and sex-specific expression in autism and Rett syndrome. Translational psychiatry. 2013;3:e316. doi: 10.1038/tp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutella S, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 35.Okunishi K, et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 36.Tahara Y, et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther. 2003;307:146–151. doi: 10.1124/jpet.103.054106. [DOI] [PubMed] [Google Scholar]
- 37.Ido A, Numata M, Kodama M, Tsubouchi H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J Gastroenterol. 2005;40:925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- 38.Okunishi K, et al. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J Immunol. 2007;179:5504–5513. doi: 10.4049/jimmunol.179.8.5504. [DOI] [PubMed] [Google Scholar]
- 39.Futamatsu H, et al. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ Res. 2005;96:823–830. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
- 40.Kuroiwa T, et al. Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft-versus-host disease. Arthritis Res Ther. 2006;8:R123. doi: 10.1186/ar2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh K, et al. Ameliorating effect of hepatocyte growth factor on inflammatory bowel disease in a murine model. Am J Physiol Gastrointest Liver Physiol. 2005;288:G729–735. doi: 10.1152/ajpgi.00438.2004. [DOI] [PubMed] [Google Scholar]
- 42.Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 43.Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Translational psychiatry. 2011;1:e48. doi: 10.1038/tp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volk HE, et al. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2014;25:44–47. doi: 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsiao EY. Immune dysregulation in autism spectrum disorder. International review of neurobiology. 2013;113:269–302. doi: 10.1016/B978-0-12-418700-9.00009-5. [DOI] [PubMed] [Google Scholar]
- 47.Needleman LA, McAllister AK. The major histocompatibility complex and autism spectrum disorder. Dev Neurobiol. 2012;72:1288–1301. doi: 10.1002/dneu.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keil A, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010;21:805–808. doi: 10.1097/EDE.0b013e3181f26e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostafa GA, Shehab AA, Al-Ayadhi LY. The link between some alleles on human leukocyte antigen system and autism in children. J Neuroimmunol. 2013;255:70–74. doi: 10.1016/j.jneuroim.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Warren RP, Burger RA, Odell D, Torres AR, Warren WL. Decreased plasma concentrations of the C4B complement protein in autism. Arch Pediatr Adolesc Med. 1994;148:180–183. doi: 10.1001/archpedi.1994.02170020066011. [DOI] [PubMed] [Google Scholar]
- 51.Warren RP, et al. Increased frequency of the null allele at the complement C4b locus in autism. Clin Exp Immunol. 1991;83:438–440. doi: 10.1111/j.1365-2249.1991.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–692. doi: 10.1038/nrrheum.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mostafa GA, Shehab AA. The link of C4B null allele to autism and to a family history of autoimmunity in Egyptian autistic children. J Neuroimmunol. 2010;223:115–119. doi: 10.1016/j.jneuroim.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 54.Careaga M, Ashwood P. Autism spectrum disorders: from immunity to behavior. Methods in molecular biology. 2012;934:219–240. doi: 10.1007/978-1-62703-071-7_12. [DOI] [PubMed] [Google Scholar]
- 55.O’Roak BJ, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nature genetics. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nature reviews. Genetics. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- 58.Parmley JL, Hurst LD. How do synonymous mutations affect fitness? Bioessays. 2007;29:515–519. doi: 10.1002/bies.20592. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi H, Craig AM. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends in neurosciences. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhat SS, et al. Disruption of the IL1RAPL1 gene associated with a pericentromeric inversion of the X chromosome in a patient with mental retardation and autism. Clin Genet. 2008;73:94–96. doi: 10.1111/j.1399-0004.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 61.Bahi N, et al. IL1 receptor accessory protein like, a protein involved in X-linked mental retardation, interacts with Neuronal Calcium Sensor-1 and regulates exocytosis. Hum Mol Genet. 2003;12:1415–1425. doi: 10.1093/hmg/ddg147. [DOI] [PubMed] [Google Scholar]
- 62.Piton A, et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum Mol Genet. 2008;17:3965–3974. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- 63.McDougle CJ, et al. Toward an immune-mediated subtype of autism spectrum disorder. Brain research. 2014 doi: 10.1016/j.brainres.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 64.Atladottir HO, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 65.Kohane IS, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One. 2012;7:e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 67.Iaccarino L, et al. Polarization of TH2 response is decreased during pregnancy in systemic lupus erythematosus. Reumatismo. 2012;64:314–320. doi: 10.4081/reumatismo.2012.314. [DOI] [PubMed] [Google Scholar]
- 68.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9:449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18:1171–1177. doi: 10.1038/mp.2013.101. [DOI] [PubMed] [Google Scholar]
- 70.Lee JY, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Jacobi AM, Wang T, Diamond B. Pathogenic autoantibodies in systemic lupus erythematosus are derived from both self-reactive and non-self-reactive B cells. Mol Med. 2008;14:675–681. doi: 10.2119/2008-00066.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franchin G, et al. Anti-DNA antibodies cross-react with C1q. J Autoimmun. 2013;44:34–39. doi: 10.1016/j.jaut.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L, et al. Female mouse fetal loss mediated by maternal autoantibody. J Exp Med. 2012;209:1083–1089. doi: 10.1084/jem.20111986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:329–340. e321–323. doi: 10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Archives of neurology. 2012;69:693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braunschweig D, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Translational psychiatry. 2013;3:e277. doi: 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin LA, et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bauman MD, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Translational psychiatry. 2013;3:e278. doi: 10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ben Bashat D, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 80.Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 2012;12:148. doi: 10.1186/1471-2377-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolff JJ, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singer HS, et al. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Camacho J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. 2014;266:46–51. doi: 10.1016/j.bbr.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chess S. Autism in children with congenital rubella. Journal of autism and childhood schizophrenia. 1971;1:33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- 86.Chess S. Follow-up report on autism in congenital rubella. Journal of autism and childhood schizophrenia. 1977;7:69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- 87.Swisher CN, Swisher L. Letter: Congenital rubella and autistic behavior. The New England journal of medicine. 1975;293:198. [PubMed] [Google Scholar]
- 88.Abdallah MW, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14:528–538. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- 89.Goines PE, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atladottir HO, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of autism and developmental disorders. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 91.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130:e1447–1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Missault S, et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 94.Knuesel I, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nature reviews. Neurology. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 95.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bauman MD, et al. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biological psychiatry. 2014;75:332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi L, et al. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in neurosciences. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 100.Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Annals of the New York Academy of Sciences. 2007;1107:118–128. doi: 10.1196/annals.1381.013. [DOI] [PubMed] [Google Scholar]
- 101.Meyer U, et al. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 102.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mostafa GA, Al-Ayadhi LY. The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J Neuroimmunol. 2013;261:77–81. doi: 10.1016/j.jneuroim.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatric neurology. 1997;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 105.Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7:97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- 106.Vojdani A, et al. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J Neuroimmunol. 2002;129:168–177. doi: 10.1016/s0165-5728(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 107.Singer HS, et al. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 108.Singh VK, Singh EA, Warren RP. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biological psychiatry. 1997;41:753–755. doi: 10.1016/S0006-3223(96)00522-7. [DOI] [PubMed] [Google Scholar]
- 109.Singh VK, Rivas WH. Prevalence of serum antibodies to caudate nucleus in autistic children. Neuroscience letters. 2004;355:53–56. doi: 10.1016/j.neulet.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 110.Silva SC, et al. Autoantibody repertoires to brain tissue in autism nuclear families. J Neuroimmunol. 2004;152:176–182. doi: 10.1016/j.jneuroim.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 111.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Annals of the New York Academy of Sciences. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 112.Wills S, et al. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morris CM, Zimmerman AW, Singer HS. Childhood serum anti-fetal brain antibodies do not predict autism. Pediatric neurology. 2009;41:288–290. doi: 10.1016/j.pediatrneurol.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 114.Goines P, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 116.Molloy CA, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 117.Okada K, et al. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 118.Ashwood P, et al. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ashwood P, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ashwood P, et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abdallah MW, et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 2012;252:75–82. doi: 10.1016/j.jneuroim.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 122.Napolioni V, et al. Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. Journal of neuroinflammation. 2013;10:38. doi: 10.1186/1742-2094-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warren RP, Foster A, Margaretten NC. Reduced natural killer cell activity in autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:333–335. doi: 10.1097/00004583-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 124.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Warren RP, Margaretten NC, Pace NC, Foster A. Immune abnormalities in patients with autism. Journal of autism and developmental disorders. 1986;16:189–197. doi: 10.1007/BF01531729. [DOI] [PubMed] [Google Scholar]
- 126.Ashwood P, et al. Altered T cell responses in children with autism. Brain Behav Immun. 2011;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85:106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 128.Warren RP, Yonk J, Burger RW, Odell D, Warren WL. DR-positive T cells in autism: association with decreased plasma levels of the complement C4B protein. Neuropsychobiology. 1995;31:53–57. doi: 10.1159/000119172. [DOI] [PubMed] [Google Scholar]
- 129.Stubbs EG, Crawford ML. Depressed lymphocyte responsiveness in autistic children. Journal of autism and childhood schizophrenia. 1977;7:49–55. doi: 10.1007/BF01531114. [DOI] [PubMed] [Google Scholar]
- 130.Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29:12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- 131.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mandal M, et al. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun. 2013;33:33–45. doi: 10.1016/j.bbi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 134.Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun. 2014;38:220–226. doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei H, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. Journal of neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morgan JT, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 138.Tetreault NA, et al. Microglia in the cerebral cortex in autism. Journal of autism and developmental disorders. 2012;42:2569–2584. doi: 10.1007/s10803-012-1513-0. [DOI] [PubMed] [Google Scholar]
- 139.Tani Y, Fernell E, Watanabe Y, Kanai T, Langstrom B. Decrease in 6R-5,6,7,8-tetrahydrobiopterin content in cerebrospinal fluid of autistic patients. Neuroscience letters. 1994;181:169–172. doi: 10.1016/0304-3940(94)90586-x. [DOI] [PubMed] [Google Scholar]
- 140.Komori H, et al. Cerebrospinal fluid biopterin and biogenic amine metabolites during oral R-THBP therapy for infantile autism. Journal of autism and developmental disorders. 1995;25:183–193. doi: 10.1007/BF02178503. [DOI] [PubMed] [Google Scholar]
- 141.Zimmerman AW, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatric neurology. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 142.Arrode-Bruses G, Bruses JL. Maternal immune activation by poly I:C induces expression of cytokines IL-1beta and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. Journal of neuroinflammation. 2012;9:83. doi: 10.1186/1742-2094-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meyer U, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Estes ML, McAllister AK. Alterations in immune cells and mediators in the brain: it’s not always neuroinflammation! Brain Pathology. 2014 doi: 10.1111/bpa.12198. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS biology. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salzman NH. Microbiota-immune system interaction: an uneasy alliance. Current opinion in microbiology. 2011;14:99–105. doi: 10.1016/j.mib.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Applied and environmental microbiology. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of medical microbiology. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 151.Finegold SM. Desulfovibrio species are potentially important in regressive autism. Medical hypotheses. 2011;77:270–274. doi: 10.1016/j.mehy.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 152.Gaboriau-Routhiau V, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 153.Ishikawa H, et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008;153:127–135. doi: 10.1111/j.1365-2249.2008.03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 155.de Magistris L, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. Journal of pediatric gastroenterology and nutrition. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 156.Coury DL, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;2(130 Suppl):S160–168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 157.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;18:260–262. doi: 10.1016/j.anaerobe.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 159.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 160.Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de la Mano A, et al. Role of interleukin-1beta in the control of neuroepithelial proliferation and differentiation of the spinal cord during development. Cytokine. 2007;37:128–137. doi: 10.1016/j.cyto.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 163.Gambino F, et al. IL1RAPL1 controls inhibitory networks during cerebellar development in mice. Eur J Neurosci. 2009;30:1476–1486. doi: 10.1111/j.1460-9568.2009.06975.x. [DOI] [PubMed] [Google Scholar]
- 164.Valnegri P, et al. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPdelta and RhoGAP2. Hum Mol Genet. 2011;20:4797–4809. doi: 10.1093/hmg/ddr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoshida T, Mishina M. Zebrafish orthologue of mental retardation protein IL1RAPL1 regulates presynaptic differentiation. Mol Cell Neurosci. 2008;39:218–228. doi: 10.1016/j.mcn.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 166.Yoshida T, et al. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase delta. J Neurosci. 2011;31:13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yoshida T, et al. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J Neurosci. 2012;32:2588–2600. doi: 10.1523/JNEUROSCI.4637-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pavlowsky A, et al. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr Biol. 2010;20:103–115. doi: 10.1016/j.cub.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 169.Houbaert X, et al. Target-specific vulnerability of excitatory synapses leads to deficits in associative memory in a model of intellectual disorder. J Neurosci. 2013;33:13805–13819. doi: 10.1523/JNEUROSCI.1457-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moretti P, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 172.Bernardino L, et al. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- 173.Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology. 2014;78:13–22. doi: 10.1016/j.neuropharm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 174.Nagakura I, Van Wart A, Petravicz J, Tropea D, Sur M. STAT1 Regulates the Homeostatic Component of Visual Cortical Plasticity via an AMPA Receptor-Mediated Mechanism. J Neurosci. 2014;34:10256–10263. doi: 10.1523/JNEUROSCI.0189-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 176.Tropea D, et al. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nature neuroscience. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- 177.Tai DJ, Hsu WL, Liu YC, Ma YL, Lee EH. Novel role and mechanism of protein inhibitor of activated STAT1 in spatial learning. The EMBO journal. 2011;30:205–220. doi: 10.1038/emboj.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feng J, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nature neuroscience. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nicolas CS, et al. The Jak/STAT pathway is involved in synaptic plasticity. Neuron. 2012;73:374–390. doi: 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Judson MC, Bergman MY, Campbell DB, Eagleson KL, Levitt P. Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain. The Journal of comparative neurology. 2009;513:511–531. doi: 10.1002/cne.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ieraci A, Forni PE, Ponzetto C. Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15200–15205. doi: 10.1073/pnas.222362099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu HH, Levitt P. Prenatal expression of MET receptor tyrosine kinase in the fetal mouse dorsal raphe nuclei and the visceral motor/sensory brainstem. Developmental neuroscience. 2013;35:1–16. doi: 10.1159/000346367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eagleson KL, Milner TA, Xie Z, Levitt P. Synaptic and extrasynaptic location of the receptor tyrosine kinase met during postnatal development in the mouse neocortex and hippocampus. The Journal of comparative neurology. 2013;521:3241–3259. doi: 10.1002/cne.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tyndall SJ, Walikonis RS. The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell cycle. 2006;5:1560–1568. doi: 10.4161/cc.5.14.2918. [DOI] [PubMed] [Google Scholar]
- 185.Nakano M, et al. Hepatocyte growth factor promotes the number of PSD-95 clusters in young hippocampal neurons. Experimental neurology. 2007;207:195–202. doi: 10.1016/j.expneurol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 186.Kawas LH, Benoist CC, Harding JW, Wayman GA, Abu-Lail NI. Nanoscale mapping of the Met receptor on hippocampal neurons by AFM and confocal microscopy. Nanomedicine : nanotechnology, biology, and medicine. 2013;9:428–438. doi: 10.1016/j.nano.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 187.Lim CS, Walikonis RS. Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cellular signalling. 2008;20:825–835. doi: 10.1016/j.cellsig.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Akimoto M, et al. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128:155–162. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 189.Qiu S, Anderson CT, Levitt P, Shepherd GM. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J Neurosci. 2011;31:5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elmer BM, Estes ML, Barrow SL, McAllister AK. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. J Neurosci. 2013;33:13791–13804. doi: 10.1523/JNEUROSCI.2366-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 192.Elmer BM, McAllister AK. Major histocompatibility complex class I proteins in brain development and plasticity. Trends in neurosciences. 2012;35:660–670. doi: 10.1016/j.tins.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee H, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16999–17004. doi: 10.1073/pnas.1006087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Glynn MW, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nature neuroscience. 2011;14:442–451. doi: 10.1038/nn.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fourgeaud L, et al. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22278–22283. doi: 10.1073/pnas.0914064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huh GS, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nelson PA, et al. MHC class I immune proteins are critical for hippocampus-dependent memory and gate NMDAR-dependent hippocampal long-term depression. Learning & memory. 2013;20:505–517. doi: 10.1101/lm.031351.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Flavell SW, et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 202.Paciorkowski AR, et al. MEF2C Haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics. 2013;14:99–111. doi: 10.1007/s10048-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Martin CL, et al. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2007;144B:869–876. doi: 10.1002/ajmg.b.30530. [DOI] [PubMed] [Google Scholar]
- 204.Babatz TD, Kumar RA, Sudi J, Dobyns WB, Christian SL. Copy number and sequence variants implicate APBA2 as an autism candidate gene. Autism Res. 2009;2:359–364. doi: 10.1002/aur.107. [DOI] [PubMed] [Google Scholar]
- 205.Parikshak NN, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zang T, et al. Postsynaptic FMRP bidirectionally regulates excitatory synapses as a function of developmental age and MEF2 activity. Mol Cell Neurosci. 2013;56:39–49. doi: 10.1016/j.mcn.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tsai NP, et al. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151:1581–1594. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Morgan JT, et al. Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain research. 2012;1456:72–81. doi: 10.1016/j.brainres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 210.Suzuki K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA psychiatry. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- 211.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature reviews. Neuroscience. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 212.Butovsky O, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 213.Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 216.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 217.Odell D, et al. Confirmation of the association of the C4B null allelle in autism. Human immunology. 2005;66:140–145. doi: 10.1016/j.humimm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 218.Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40:560–566. doi: 10.1080/08916930701510673. [DOI] [PubMed] [Google Scholar]
- 219.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 220.Gasque P. Complement: a unique innate immune sensor for danger signals. Molecular immunology. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 221.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nature neuroscience. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 223.Bilbo SD, Frank A. Beach award: programming of neuroendocrine function by early-life experience: a critical role for the immune system. Hormones and behavior. 2013;63:684–691. doi: 10.1016/j.yhbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nature reviews. Neuroscience. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 225.Streit WJ, Xue QS. Life and death of microglia. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 226.Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nature neuroscience. 2013;16:1537–1543. doi: 10.1038/nn.3546. [DOI] [PubMed] [Google Scholar]
- 227.Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Frontiers in molecular neuroscience. 2014;7:28. doi: 10.3389/fnmol.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kassai H, et al. Selective activation of mTORC1 signaling recapitulates microcephaly, tuberous sclerosis, and neurodegenerative diseases. Cell reports. 2014;7:1626–1639. doi: 10.1016/j.celrep.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 229.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annual review of immunology. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ricciardi S, et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum Mol Genet. 2011;20:1182–1196. doi: 10.1093/hmg/ddq563. [DOI] [PubMed] [Google Scholar]
- 231.Qin S, et al. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. The Journal of clinical investigation. 2010;120:3617–3628. doi: 10.1172/JCI41531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sharma A, et al. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem cells international. 2013;2013:623875. doi: 10.1155/2013/623875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lv YT, et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. Journal of translational medicine. 2013;11:196. doi: 10.1186/1479-5876-11-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang J, et al. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature. 2015;521:E1–4. doi: 10.1038/nature14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bilousova TV, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. Journal of medical genetics. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 237.Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain research. 2012;1439:7–14. doi: 10.1016/j.brainres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 238.Leigh MJ, et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. Journal of developmental and behavioral pediatrics : JDBP. 2013;34:147–155. doi: 10.1097/DBP.0b013e318287cd17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Posey DJ, et al. A pilot study of D-cycloserine in subjects with autistic disorder. Am J Psychiatry. 2004;161:2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 240.Sandler RH, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. Journal of child neurology. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 241.Ramirez PL, Barnhill K, Gutierrez A, Schutte C, Hewitson L. Improvements in Behavioral Symptoms following Antibiotic Therapy in a 14-Year-Old Male with Autism. Case reports in psychiatry. 2013;2013:239034. doi: 10.1155/2013/239034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pardo CA, et al. A pilot open-label trial of minocycline in patients with autism and regressive features. Journal of neurodevelopmental disorders. 2013;5:9. doi: 10.1186/1866-1955-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jung HJ, et al. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1alpha. Archives of biochemistry and biophysics. 2014;545:74–82. doi: 10.1016/j.abb.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 244.Sullivan RC. Why do autistic children…? Journal of autism and developmental disorders. 1980;10:231–241. doi: 10.1007/BF02408474. [DOI] [PubMed] [Google Scholar]
- 245.Cotterill RM. Fever in autistics. Nature. 1985;313:426. doi: 10.1038/313426c0. [DOI] [PubMed] [Google Scholar]
- 246.Curran LK, et al. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120:e1386–1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- 247.Naviaux JC, et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Translational psychiatry. 2014;4:e400. doi: 10.1038/tp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Naviaux JC, et al. Antipurinergic therapy corrects the autism-like features in the Fragile X (Fmr1 knockout) mouse model. Mol Autism. 2015;6:1. doi: 10.1186/2040-2392-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology. 2014;141:340–344. doi: 10.1111/imm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rattazzi L, et al. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Translational psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 254.Ron-Harel N, et al. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuvenation Res. 2008;11:903–913. doi: 10.1089/rej.2008.0755. [DOI] [PubMed] [Google Scholar]
- 255.Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav Immun. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun. 2011;25:379–385. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Baudouin SJ. Heterogeneity and convergence: the synaptic pathophysiology of autism. Eur J Neurosci. 2014;39:1107–1113. doi: 10.1111/ejn.12498. [DOI] [PubMed] [Google Scholar]
- 258.Patterson PH. Modeling autistic features in animals. Pediatric research. 2011;69:34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Giovanoli S, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 260.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Elsevier/Saunders; Philadelphia: 2012. [Google Scholar]
- 261.Schmitz ML, Weber A, Roxlau T, Gaestel M, Kracht M. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochimica et biophysica acta. 2011;1813:2165–2175. doi: 10.1016/j.bbamcr.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 262.Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cellular and molecular biology. 2001;47:695–702. [PubMed] [Google Scholar]
- 263.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 264.Schwartz M, Shechter R. Protective autoimmunity functions by intracranial immunosurveillance to support the mind: The missing link between health and disease. Mol Psychiatry. 2010;15:342–354. doi: 10.1038/mp.2010.31. [DOI] [PubMed] [Google Scholar]
- 265.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nature reviews. Neuroscience. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]