Abstract
The potential of bacteriophage therapy for the treatment of pulmonary infections caused by antibiotic-resistant bacteria has been well recognised. The purpose of this study was to investigate the effect of excipients on stabilisation and aerosolisation of spray dried powders of morphologically different phages – PEV podovirus and PEV myovirus. Seven anti-pseudomonal phages were screened against 90 clinical strains of bacterial hosts and three of the phages were selected for formulation study based on the host range. Design of experiments was utilised to assess the effect of different excipients on the stabilisation and aerosolisation of spray dried phages. Both podovirus and myovirus phages were stable in spray dried formulations containing trehalose or lactose and leucine as excipients with less than 1-log10 titre reduction during spray drying, with lactose providing superior phage protection over trehalose. Furthermore, the spray dried phage formulations dispersed in an Osmohaler at 85 L/min produced a high fine particle fraction of over 50 %. The results showed that the phages in this study can form respirable dry powder phage formulations using the same excipient composition. Spray dried various types of lytic phages hold significant potential for the treatment of pulmonary infections.
Keywords: Bacteriophage therapy, biotherapeutics, pulmonary infections, powder aerosols, antibiotic-resistant bacteria
Graphical abstract

INTRODUCTION
The emergence of multiple drug resistance (MDR) bacteria has become a critical problem in the treatment of respiratory infections. Cystic fibrosis patients suffer from chronic bacterial infections by Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenza, and Burkholderia cepacia complex [1]. Chronic lung infection, especially P. aeruginosa shortens the life span of cystic fibrosis patients [2]. Many of these bacteria are intrinsically resistant or have developed resistance to many currently used antibiotics [3, 4]. Additionally, immunocompromised patients in hospital intensive care units are at risk of respiratory infection from MDR bacteria. Despite the efforts to develop new antibiotics to fight emerging antibiotic-resistant bacterial infection, drying pipeline of new antibiotics urges the need for an alternative therapy [5].
Bacteriophage (phage) therapy has been gaining renewed interests for its ability to eradicate MDR bacteria [6]. Phage therapy exploits the lytic life cycle of phages, which causes bacteriolysis followed by subsequent release of progenies. The released phage progenies then target nearby bacteria and the cycle is repeated. Potential advantages of phage therapy over conventional antibiotic treatment are due to the facts that phages are (i) naturally occurring antibacterials with low inherent toxicity, (ii) self-amplifying agents, and (iii) highly specific limiting unnecessary damage to non-targeted bacteria [7, 8]. The activity of phages against MDR bacteria has been shown in in vitro studies [9, 10] as well as their efficacy in animals [10–12] and humans [13–15]. A recent study used intranasal administration of phages to reduce the infective burden and inflammation in a P. aeruginosa lung infection model in mice [11]. In addition, bacterial load and inflammatory response reduction were observed with prophylactic treatment. Other in vitro studies have demonstrated the ability of phages to penetrate and disrupt bacterial biofilms [16], and the synergistic effect of using combinational therapy with antibiotics [17, 18]. The potential use of phages for the treatment of infectious disease is being extensively discussed.
Over the past decade, great efforts have been put into delivering therapeutic dosage forms of phage for treatment of respiratory infections. Successful phage therapy requires phages to remain viable during the production and delivery in aerosolised form so that clinically significant dose can reach the lower airways. Nebulisation has been utilised by earlier studies in Europe for inhalation of liquid phage formulation [19]. More recent studies have confirmed that, depending on the types of nebuliser, phages can withstand the stress and remain viable during nebulisation process with high titre reaching the lower respiratory tract [20–22].
Powder formulations have the potential to provide easy storage, transport and administration with long shelf-life over liquid formulations. Several studies have shown processing liquid phage formulation into dry powders using lyophilisation [23], spray drying [24–26] and spray-freeze drying [25]. Vandenheuvel and colleagues [26] produced highly stable spray dried anti-Pseudomonal Podoviridae phage powders using trehalose as an excipient with less than 1 log10 unit reduction in phage titre. On the other hand, powders containing anti-Staphylococcal phage from Myoviridae family resulted in 2.5 log10 loss. This apparent difference in titre reduction seems to confirm that phage stabilisation is phage dependent. Matinkhoo et al. [24] reported that myovirus phages spray dried with trehalose, leucine and a surfactant resulted in less than 1 log10 unit titre reduction with superior aerosol performance. A more recent study by Leung et al. [25] compared the performance of spray drying with spray freeze drying in producing stable anti-Pseudomonal Podoviridae phage powders using trehalose, mannitol and leucine. Spray drying provided better phage protection over spray freeze drying; however, the three-excipient combination failed to give less than 1 log10 unit titre reduction which is generally considered as a desirable loss incurred during the inhalation process.
For the commercialisation of sprayed dried phage formulations for pulmonary infections, the physical stability of inhalable powders and biological viability of phages are the critical parameters. This study aimed to explore the effect of various excipients on stabilisation and aerosolisation of PEV podovirus and myovirus in spray dried powders, using comprehensive formulation screening, followed by further refinement of excipient composition and ratios within.
MATERIALS AND METHODS
Bacteriophages
Seven bacteriophages comprised of myoviruses and podoviruses, active against P. aeruginosa, were supplied by AmpliPhi Biosciences AU at a high titre of 1010 PFU/mL, stored in phosphate buffered saline (0.01 M phosphate buffer, 0.0027 M KCl and 0.137 M NaCl), with pH adjusted to 7.2). These phages were originally isolated from the sewage treatment plant in Olympia (WA, USA) by the Kutter Lab (Evergreen Phage Lab) using P. aeruginosa dog-ear strain PAV237. This strain was used as a reference bacterial strain to assess phage titre.
Host range and efficiency of plating
In this study, spot test [27] was used to test each phage for host spectrum of activity against 90 clinical and MDR P. aeruginosa isolates collected in Australia. The top-agar plates with the targeted host lawn were prepared by mixing overnight culture of the target host strain (approximately 2 × 108 CFU) with 5 mL of 0.4 % nutrient broth top agar and overlaying onto a 1.5 % nutrient agar plate. Then, 10 μL of a phage stock solution (109 PFU/mL) were spotted on the top agar plate, left to dry for 20 min and incubated at 37°C for 24 h. After incubation, the appearance of the lysis zone was examined for phage-susceptibility. Each phage was tested against each bacterial strain in duplicate. Phages that are active against the clinical isolates were assessed for efficiency of plating by calculating the ratio of the phage titres obtained with the test clinical isolates to those obtained with the host strain, PAV237. The phage titre was determined using plaque assay [25].
Design of experiment
The Taguchi experimental design method was adopted to identify the dominant excipients responsible for phage protection during spray drying. A total of 4 factors were considered for formulations containing trehalose or lactose (Supplementary Table 1) and 5 factors for those containing mannitol (Supplementary Table 2). All factors were evaluated at three levels – L9 orthogonal array for trehalose and lactose, and L27 orthogonal array for mannitol. Taguchi’s ‘smaller-is-better’ criterion (Eq. (1)) was utilised to optimise excipients ratios with least titre loss. For analysis of the results, Minitab® 16 software was used.
Minimise the performance characteristic (‘smaller-is-better’) equation:
where S is signal, N is noise, yi is the characteristic property and n is the number of experimental replicates.
Phage stability in liquid formulation
The stability of phages diluted in excipient solution was tested by adding 10 μL of phage lysate to 990 μL of trehalose, lactose, mannitol, leucine or plurnic F68 at two different concentrations, 50 mM and 0.5 M, except pluronic F68 which was tested at 5% and 15%. The concentrations were determined based on other studies on phage or protein stabilisation in dry powder formulations [23, 24, 26, 28]. Phage viability was tested using plaque assay.
Spray Drying
The liquid feed was composed of 30 mL of excipient solution in ultra-pure water and 0.3 mL of phage suspension (109 pfu/mL) with pH adjusted to 7.4. The phage viability in the feed solution was assessed prior to spray drying using plaque assay. The resulting phage-excipient suspension was spray dried using a Büchi 290 spray dryer coupled with a conventional two-fluid nozzle for atomisation. The suspension was fed at a constant feed rate 1.8 mL/min and an atomising airflow of 742 L/h with an aspiration rate of 35 m3/h. The drying inlet air was heated to 60 °C and the outlet temperature ranged between 40–41 °C. Dried powder after passing through the cyclone was collected in a vial. A small amount of powder was resuspended in PBS to give a concentration of 50 mg/ml and the phage titre was assessed using plaque assay. Spray dried powders were stored over silica beads at 20 °C before use.
Formulation Screening Criteria
Fig. 1 shows the system we developed for screening the phage formulations (Fig 1). Screening Criteria Level 1 involved powder analysis by optical microscopy and laser diffraction. Using optical microscopy, the morphology, approximate size and the solid state (amorphous or crystalline) of powders were assessed. Particle size distribution was measured using laser diffraction. Powders having particle size (D50) greater than 5 μm were discarded from the list as they are less likely to be inhalable. Powders that passed Screening Criteria Level 1 proceeded to Level 2: plaque assay, followed by scanning electron microscopy (SEM). Formulations that met the target specification for titre loss (<1.0 log) were imaged with SEM for more detailed morphology. Screening Criteria Level 3 involved aerosol performance testing. An Osmohaler™ and reduced Next Generation Impactor coupled with a validated weighing method were utilised as an initial screening tool, followed by full characterisation using a multi-stage liquid Impinger (MSLI). Target specification for fine particle fraction (FPF) is 40%.
Figure 1.
Schematic diagram of formulation development stages and screening criteria.
Particle size distribution
Laser diffraction (Mastersizer 2000, Malvern Instruments Ltd., UK) was used to measure the particle size distribution of spray dried formulations. Powders were loaded onto Scirocco 2000 dry powder module (Malvern Instruments, UK) and dispersed using compressed air at 4.0 bars. Volumetric diameters (D10, D50, and D90) and span ((D90–D10)/D50) were reported. All measurements were performed in triplicate.
Scanning electron microscopy (SEM)
The particle morphology of the spray dried powders was analysed using SEM (Zeiss Ultra Plus, Carl Zeiss NTS GmbH, Oberkochen, Germany). Samples were mounted on a carbon tape and sputter coated with 15 nm of gold using a K550X sputter coater (Quorum Emitech, Kent, UK)
X-ray powder diffraction (XRD)
XRD pattern of the samples were recorded using an X-ray diffractometer (Model D5000; Siemens, Munich, Germany) under ambient conditions. Samples were spread on glass slides and subjected to Cu Kα radiation at 30 mA and 40 kV. The scattered intensity was collected by a detector for a 2θ range of 5–40° at an angular increment rate of 0.04° 2θ / s.
Dynamic vapour sorption (DVS)
DVS (DVS-Intrinsic, Surface Measurement Systems, London, UK) was used to analyse the moisture sorption profile of the powders. For each sample, 5 mg of powder was exposed to a dual moisture ramping cycle of 0 to 60% relative humidity (RH). The criterion for equilibrium moisture content at each RH was defined as dm/dt of < 0.02% per minute.
Screening of formulation dispersion performance using reduced Next Generation Impactor
The formulations were initially screened for their in vitro aerosolisation performance using a reduced Next Generation Impactor (Copley, Nottingham, UK) with an NGI induction port. A paper filter was placed on a filter holder assembly in the top of stage 3. Powders (30 ± 3 mg) were loaded in size 3 hydroxypropyl methylcellulose (HPMC) capsules (Capsugel, NSW, Australia) and dispersed through a low resistance Osmohaler™ (0.021 kPa1/2 min/L) at a flow rate of 85 L/min (the maximum flow rate that could be generated) for 2.8 s. The cut-off diameters of stages 1 and 2 of the NGI at 85 L/min were 6.68 μm and 3.72 μm, respectively. Particles smaller than 3.72 μm were captured on the filter. Device, capsule, mouthpiece adapter, stage 1, stage 2 and filter were weighed before and after dispersion to determine the amount of powder on each component. NGI induction port was rinsed with 3 mL of 50% ethanol, of which 1 mL was aliquoted to a pre-weighed beaker and left to evaporate using a hotplate. Once dry, the beaker was weighed to determine amount of powder deposited on the induction port. The dispersion was carried out in duplicate for each formulation.
Dispersion using multi-stage liquid impinger
The in vitro aerosol performance of the formulations which passed the initial NGI screening (described above) was assessed using multi-stage liquid impinger (MSLI) followed by chemical assay (by HPLC) or plaque assay. In brief, powder samples (30 mg each) were loaded to size 3 HPMC capsules in a humidity controlled box (RH 13–18%) and dispersed using an Osmohaler™at 100 L/min for 2.4 s. PBS and ultra-pure water were used as the collecting solvent to determine the deposition profiles of the phage and the sugar excipient, respectively. The cut-off diameters of the MSLI stages 1–4 at 100 L/min are 10.1, 5.3, 2.4 and 1.32 μm, respectively. FPF was defined as the mass fraction of particles ≤ 5.0 μm with respect to the loaded dose.
HPLC chemical assay
The deposition of trehalose, lactose or sucrose in the inhaler, capsule, adaptor, throat and each part of the MSLI was determined using a HPLC system (Model LC-20; Shimadzu, Japan) with RI detection. The HPLC system consisted of a CBM-20A controller, LC-20AT pump, RID-10A RI detector, SIL-20A HT auto-sampler, Agilent Hi-Plex Ca Ligand Exchange Columns (300 × 7.7 mm, 8 μm; Phenomenex, Torrance, CA), and LCSolution software. The chromatographic conditions were as follows: injection volume 50 μL; oven temperature 85 °C; mobile phase ultra-pure water; flow rate 0.6 mL/min.
RESULTS
Host range
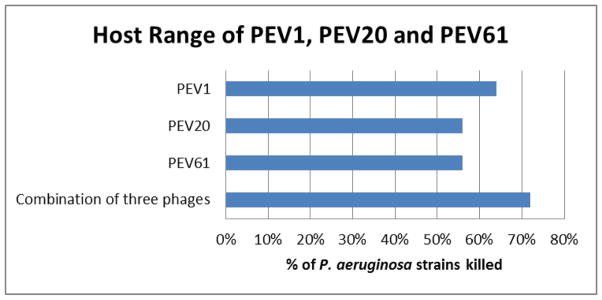
Out of the seven PEV phages tested for bactericidal activity against 90 P. aeruginosa strains for assessment of the host range, three phages, namely PEV1, PEV20 and PEV61, killed combination over 70% of P. aeruginosa clinical and MDR strains with high killing efficiency (Fig. 2). Hence, the three phages were selected for the formulation work.
Figure 2.
Host range of seven PEV phages against various clinical MDR P. aeruginosa strains. Host range was tested using standard spot test.
Phage viability in liquid formulations
The effect of excipients on the viability of PEV1, PEV20 and PEV61 in liquid was examined over 30 min at room temperature. All three phages were found to be stable (<1.0 log10 loss) in the presence of high and low concentrations of excipients including trehalose, lactose, mannitol, glycine, leucine, pluronic F68 and PEG3000 (Table 1). Of the three phages, PEV20 was more vulnerable when formulated with different excipients. PEV1 and PEV61 resulted in less than 0.1 log10 loss whereas PEV20 titre was reduced by up to 0.8 log10 loss in liquid formulation. The extent of titre loss for all three phages was within the target specification of 1.0 log10 loss. These excipients were used for spray drying of the phages.
Table 1.
Titre loss of PEV1, PEV20 and PEV61 when exposed to high and low concentrations of different excipients in liquid suspension
| Excipient | Concentration | Titre loss (log10)
|
||
|---|---|---|---|---|
| PEV1 | PEV20 | PEV61 | ||
|
| ||||
| Trehalose | 50 mM | 0.1 | −0.7 | 0.0 |
| 0.1 M | 0.1 | −0.5 | 0.0 | |
| 0.5 M | 0.1 | −0.8 | −0.1 | |
|
| ||||
| Lactose | 50 mM | 0.0 | 0.0 | −0.1 |
| 0.1 M | 0.0 | −0.1 | 0.0 | |
| 0.5 M | 0.7 | 0.0 | 0.0 | |
|
| ||||
| Mannitol | 50 mM | 0.0 | −0.7 | −0.1 |
| 0.5 M | 0.0 | −0.7 | −0.1 | |
|
| ||||
| Glycine | 50 mM | 0.1 | −0.7 | −0.1 |
| 0.5 M | 0.0 | −0.8 | −0.1 | |
|
| ||||
| Leucine | 50 mM | 0.0 | −0.7 | −0.1 |
| 0.5 M | −0.1 | −0.7 | 0.0 | |
|
| ||||
| PEG3000 | 50 mM | 0.0 | 0.0 | 0.0 |
| 0.5 M | 0.0 | −0.4 | −0/1 | |
|
| ||||
| Pluronic F68 | 5% | 0.2 | −0.7 | −0.1 |
| 15% | 0.4 | −0.2 | 0.0 | |
Phage viability in powder formulations
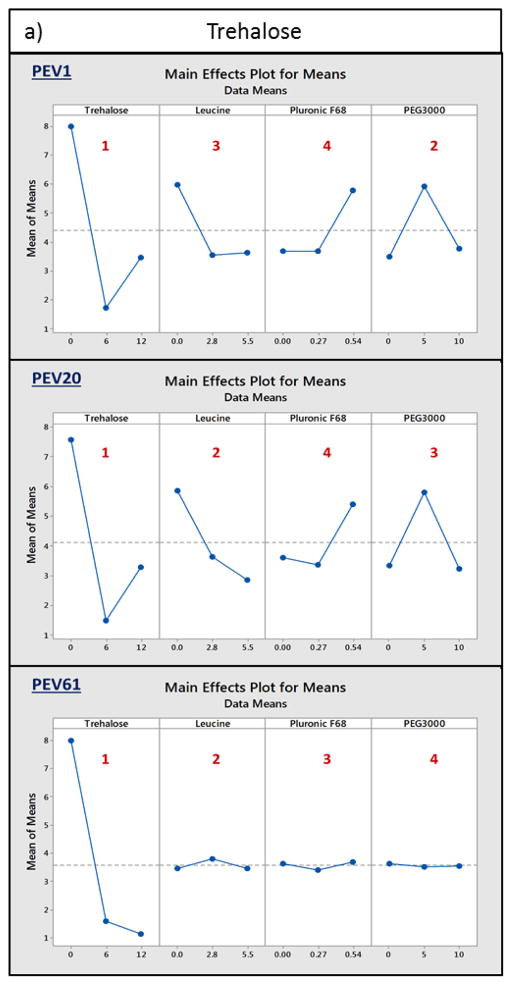
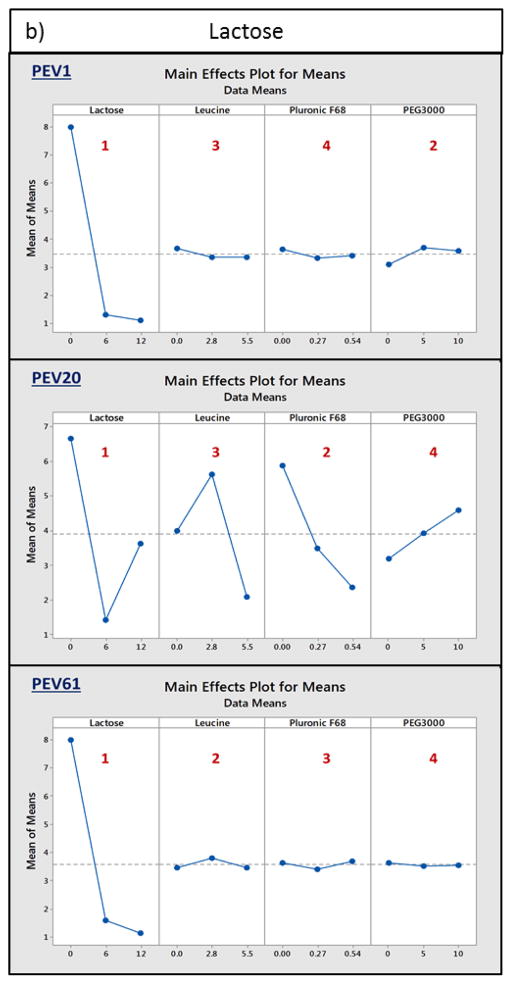
Taguchi experimental design was employed to assess the role of excipients in phage stabilisation in dry powder formulations. The three phages were individually spray dried with either trehalose or lactose, together with leucine, pluronic F68 and PEG3000, followed by plaque assay to determine the titre loss. The results showed sugar as the most important excipient for retaining the biological activity in spray dried powders for all three phages (Fig. 3). Majority of PEV1, PEV20 and PEV61 phages spray dried with 12 mg/mL trehalose or lactose resulted in less than log10 1.5 titre loss (Table 2). In the absence of the sugars, spray dried phages were unable to maintain their bioactivity during the spray drying process, resulting in log10 6.7 loss or complete titre reduction. Formulations that showed significant reduction in phage titre despite high concentration of sugars were those without leucine. In absence of leucine, a significant amount of powder was adhered to the cyclone during spray drying. Insufficient amounts of PEV1 and PEV20 formulations were collected for those spray dried with 12mg/mL trehalose, 0.54% pluronic F68 and 5mg/mL PEG3000 for biological assay. Overall, the results revealed that sugar and leucine were the key determinants for the biological stability of PEV1, PEV20 and PEV61 phages in the spray dried formulations. Hence, the effect of different ratios of sugar and leucine on phage stabilisation in the formulations was further investigated.
Figure 3.
Main effects plots for means. PEV1, PEV20 and PEV61 were individually spray dried with either a) trehalose or b) lactose, together with leucine, pluronic F68 and PEG1000. Each excipient is ranked from 1 to 4 depending on the significance of their role in phage stabilisation (rank 1 being the most significant).
Table 2.
Formulation information according to the Taguchi experimental design, and log titre loss of PEV1, PEV20 and PEV61 after spray drying.
| Formulation | Sugar (mg/mL) | Leucine (mg/mL) | Pluronic F68 (%,v/v) | PEG3000 (mg/mL) | Titre loss (log10) | |||
|---|---|---|---|---|---|---|---|---|
| Trehalose | lactose | PEV1 | PEV20 | PEV61 | ||||
| F1 | 0 | 0 | 0 | 0 | −8.0 a | −8.0a | −8.0a | |
| F2 | 0 | 2.8 | 0.27 | 5 | −8.0 | −8.0a | −8.0a | |
| F3 | 0 | 5.5 | 0.54 | 10 | −8.0 | −6.7 | −8.0 | |
| F4 | 6 | 0 | 0.27 | 10 | −2.0 | −1.6 | −1.3 | |
| F5 | 6 | 2.8 | 0.54 | 0 | −1.4 | −1.5 | −2.0 | |
| F6 | 6 | 5.5 | 0 | 5 | −1.8 | −1.4 | −1.5 | |
| F7 | 12 | 0 | 0.54 | 5 | −8.0b | −8.0b | −1.1 | |
| F8 | 12 | 2.8 | 0 | 10 | −1.3 | −1.4 | −1.4 | |
| F9 | 12 | 5.5 | 0.27 | 0 | −1.1 | −0.5 | −0.9 | |
| F10 | 0 | 0 | 0 | 0 | −8.0a | −8.0a | −8.0a | |
| F11 | 0 | 2.8 | 0.27 | 5 | −8.0a | −8.0a | −8.0a | |
| F12 | 0 | 5.5 | 0.54 | 10 | −8.0a | −4.0 | −8.0a | |
| F13 | 6 | 0 | 0.27 | 10 | −1.5 | −1.8 | −1.3 | |
| F14 | 6 | 2.8 | 0.54 | 0 | −0.8 | −0.9 | −2.0 | |
| F15 | 6 | 5.5 | 0 | 5 | −1.6 | −1.6 | −1.5 | |
| F16 | 12 | 0 | 0.54 | 5 | −1.5 | −2.2 | −1.1 | |
| F17 | 12 | 2.8 | 0 | 10 | −1.3 | −8.0 | −1.4 | |
| F18 | 12 | 5.5 | 0.27 | 0 | −0.5 | −0.7 | −0.9 | |
Formulation not spray dried
Not enough powders for the plaque assay.
Powders with a high lactose content resulted in superior phage stabilisation (Table 4). PEV1 and PEV20 and PEV61 showed a log10 – loss of 0.3–0.4 when spray dried with 20 mg/mL lactose and 5 mg/mL leucine, or 17 mg/mL lactose and 8 mg/mL leucine. Interestingly, higher lactose concentration (23 mg/mL) was not beneficial for further retaining phage bioactivity. Decreasing the lactose:leucine ratio led to increased log10 titre loss of all three phages. Phage PEV1, PEV20 and PEV61 formulations spray dried with 10 mg/mL lactose showed > 1 log10 titre loss. Similar trend was observed for formulations containing trehalose and leucine. Minimal titre loss was observed for phage formulations spray dried with 17 mg/mL trehalose and 8 mg/mL leucine and the magnitude of titre loss increased with decreased trehalose concentration. PEV20 and PEV61 showed 0.9 and 0.7 log10 loss, respectively, when spray dried with high trehalose:leucine ratio. On the other hand, sorbitol and leucine failed to protect phage activity in spray dried formulations. Phage formulations spray dried with 20 mg/mL sorbitol and 5 mg/mL leucine formed sticky paste-like powders upon spray drying, unsuitable for inhalation delivery, which was reversed by increasing the leucine content, but at the expense of phage survival (>log10 3 loss).
Table 4.
Particle size distributions of formulations spray dried with PEV1, PEV20 and PEV61.
| Formulation | PEV1 | PEV20 | PEV61 | |||
|---|---|---|---|---|---|---|
| D50 (μm) | Span | D50 (μm) | Span | D50 (μm) | Span | |
| F1 | Formulation not spray dried: feed solution contains phage alone (no excipient) | |||||
| F2 | 1.66 ± 0.04 | 1.48 ± 0.14 | Not spray dried: PEV1-F2 resulted in complete titre reduction | |||
| F3 | 1.89 ± 0.05 | 1.49 ± 0.01 | 1.83 ± 0.02 | 1.47 ± 0.00 | 1.91 ± 0.02 | 1.49 ± 0.08 |
| F4 | 2.82 ± 0.02 | 2.27 ± 0.17 | 2.69 ± 0.03 | 1.70 ± 0.06 | 2.86 ± 0.05 | 1.79 ± 0.13 |
| F5 | 1.98 ± 0.02 | 1.67 ± 0.12 | 1.85 ± 0.08 | 1.77 ± 0.08 | 1.78 ± 0.05 | 1.78 ± 0.04 |
| F6 | 1.97 ± 0.03 | 1.74 ± 0.08 | 1.69 ± 0.02 | 1.56 ± 0.02 | 1.79 ± 0.01 | 1.57 ± 0.02 |
| F7 | Not enough powders were collected for analysis | |||||
| F8 | 1.90 ± 0.07 | 1.55 ± 0.02 | 1.85 ± 0.01 | 1.54 ± 0.03 | 1.89 ± 0.03 | 1.64 ± 0.07 |
| F9 | 1.80 ± 0.05 | 1.62 ± 0.05 | 1.78 ± 0.01 | 1.58 ± 0.00 | 1.75 ± 0.02 | 1.60 ± 0.01 |
| F10 | Formulation not spray dried: feed solution contains phage alone (no excipient) | |||||
| F11 | Formulation not spray dried: same excipient composition as F3 | |||||
| F12 | Formulation not spray dried: same excipient composition as F4 | |||||
| F13 | 3.33 ± 0.17 | 2.17 ± 0.04 | 4.50 ± 0.07 | 2.13 ± 0.02 | 4.66 ± 0.23 | 2.25 ± 0.13 |
| F14 | 1.52 ± 0.01 | 1.47 ± 0.09 | 1.56 ± 0.05 | 1.54 ± 0.15 | 1.56 ± 0.01 | 1.54 ± 0.01 |
| F15 | 1.78 ± 0.02 | 1.53 ± 0.01 | 1.85 ± 0.03 | 1.57 ± 0.06 | 1.94 ± 0.05 | 1.59 ± 0.03 |
| F16 | Not enough powders collected for analysis | |||||
| F17 | 1.81 ± 0.01 | 1.56 ± 0.01 | 1.88 ± 0.01 | 1.62 ± 0.08 | 1.96 ± 0.03 | 1.64 ± 0.04 |
| F18 | 1.69 ± 0.03 | 1.56 ± 0.02 | 1.76 ± 0.01 | 1.57 ± 0.01 | 1.80 ± 0.01 | 1.59 ± 0.02 |
Hence, sorbitol was found to be a poor phage stabiliser and thus, was not further pursued. In a separate study, mannitol, in combination with glycine, was explored using a 5-factor, 3-level Taguchi experimental design. Of these, 27 representative formulations were subjected to spray drying and powder characterisation. Viable phages were detected in only four out of 27 spray dried formulations, of which significant titre loss was observed (data not shown). The results showed mannitol did not help stabilise the PEV phages in powder formulations.
Particle morphology
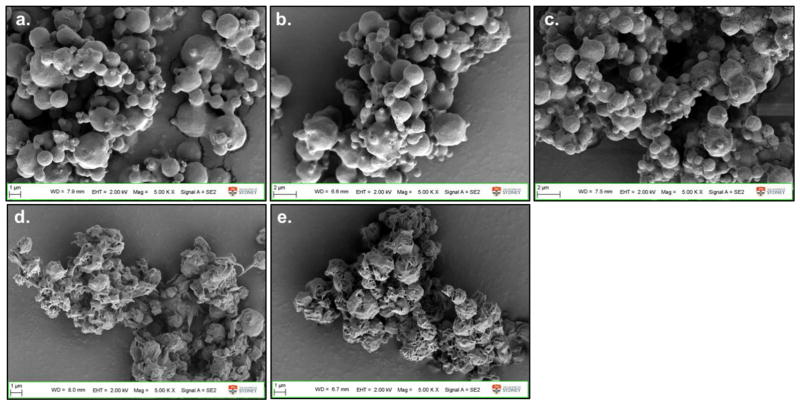
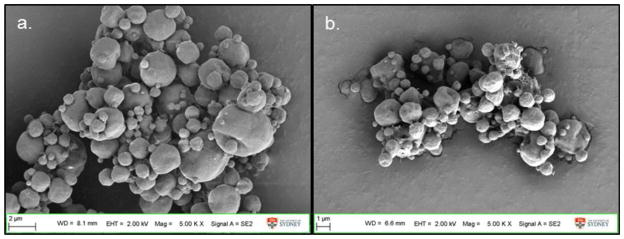
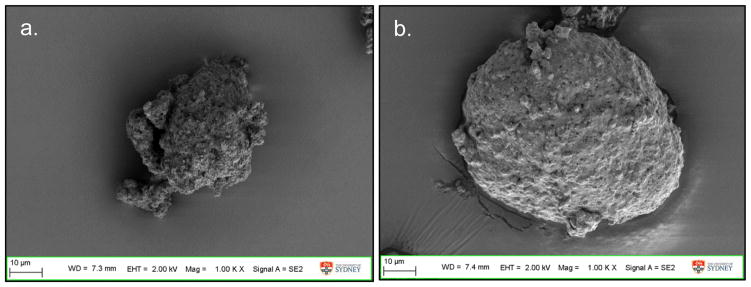
The particle morphology of formulations containing the same excipient composition stayed the same irrespective of the phage. Phages PEV1, PEV20 or PEV61 spray dried with 12 mg/mL trehalose, 2.8 mg/mL leucine and 10 mg/mL PEG3000 (formulation 8) all formed spherical particles with smooth surfaces (Fig. 4a, b and c). Phage PEV1 or PEV20 formulations spray dried with 6 mg/mL lactose, 2.8 mg/mL leucine and 0.54 % (v/v) pluronic F68 formed corrugated particles with rough surfaces (Fig. 4d and e). Formulations spray dried with high concentration of lactose or trehalose with leucine formed spherical particles below 3 μm (Fig. 5). Without leucine, particles formed large irregular agglomerates unsuitable for inhalation (Fig. 6).
Figure 4.
SEM images of phage (a) PEV1, (b) PEV20, and (c) PEV61 formulation spray dried with 12 mg/mL trehalose, 2.8 mg/mL leucine and 10 mg/mL PEG3000 (formulation 8), phage (d) PEV1, and (e) PEV61 formulation spray dried with 6 mg/mL lactose, 2.8 mg/mL leucine and 0.54% (v/v) pluronic F68 (formulation 14).
Figure 5.
SEM images of powders spray dried from formulations containing high concentration of lactose or trehalose. Phage formulation spray dried with 12 mg/mL of sugar a) lactose or b) trehalose, 5.5 mg/mL of leucine and 0.27 mg/mL of pluronic F68.
Figure 6.
SEM images of powders spray dried from formulations without leucine. Phage formulation spray dried with 12 mg/mL of sugar a) lactose or b) trehalose, 5 mg/mL of PEG1000 and 0.27 mg/mL of pluronic F68.
For formulations containing sugar and leucine, those with high trehalose (Fig. 7a) or lactose (Fig. 7f) formed smooth spherical particles with size of 2 μm. Increasing in leucine content led to formation of irregular platelet-shaped particles with rough surfaces (Fig 7). Again, the presence of phages with different morphologies had no effect on the particle morphology.
Figure 7.
SEM images of formulations. The codes “23TH2LC” denotes 23 mg/mL trehalose with 2 mg/mL leucine; “23LT2LC” denotes 23 mg/mL lactose with 2 mg/mL leucine and so on.
Particle size distribution
The particle size (D50) values of the spray dried powders were less than 2 μm, except those without leucine (Table 4). PEV1, PEV20 and PEV61 phage formulations spray dried with 6 mg/mL trehalose, 10 mg/mL PEG3000 and 2.7 μg/mL pluronic F68 (i.e., Formulation F4) showed very similar D50 values of 2.8 μm, 2.7 μm and 2.9 μm, respectively. When trehalose was replaced by lactose (i.e., Formulation F13), D50 was increased to 3.3 μm, 4.5 μm and 4.7 μm for the PEV1, PEV20 and PEV61 formulations, respectively. Powders with very low yield were insufficient to be sized.
The particle size (D50) of all the spray dried powders only containing sugar and leucine were less than 2.2 μm (Table 5). Decreasing trehalose and increasing leucine ontent caused a slight increase in the particle size. Phage formulations (F19) spray dried with 23 mg/mL trehalose gave 1.87–1.89 μm sized particles, whereas 14 mg/mL trehalose gave 2.02–2.03 μm sized particles (i.e., Formulation F22). However, the reversed effect was observed when the trehalose concentration reached 10 mg/mL with 15 mg/mL leucine. The same effect was detected for formulations containing lactose and leucine. No significant differences in the size of the powders were observed across different phage types.
Table 5.
Particle size distributions of formulations spray dried with PEV1, PEV20 and PEV61
| Formulation | PEV1 | PEV20 | PEV61 | |||
|---|---|---|---|---|---|---|
| D50 (μm) | Span | D50 (μm) | Span | D50 (μm) | Span | |
| F19 | 1.89 ± 0.03 | 1.66 ± 0.04 | 1.88 ± 0.00 | 1.64 ± 0.00 | 1.87 ± 0.03 | 1.70 ± 0.05 |
| F20 | 1.97 ± 0.03 | 1.64 ± 0.06 | 1.83 ± 0.03 | 1.70 ± 0.08 | 1.87 ± 0.03 | 1.63 ± 0.01 |
| F21 | 1.97 ± 0.02 | 1.61 ± 0.00 | 1.98 ± 0.01 | 1.60 ± 0.01 | 2.01 ± 0.03 | 1.64 ± 0.06 |
| F22 | 2.02 ± 0.00 | 1.65 ± 0.01 | 2.03 ± 0.05 | 1.64 ± 0.01 | 2.02 ± 0.01 | 1.66 ± 0.00 |
| F23 | 1.86 ± 0.01 | 1.58 ± 0.01 | 1.82 ± 0.02 | 1.58 ± 0.02 | 1.81 ± 0.06 | 1.58 ± 0.02 |
| F24 | 2.02 ± 0.00 | 1.63 ± 0.01 | 2.00 ± 0.00 | 1.63 ± 0.01 | 1.94 ± 0.00 | 1.62 ± 0.00 |
| F25 | 1.91 ± 0.04 | 1.65 ± 0.05 | 1.87 ± 0.02 | 1.65 ± 0.02 | 1.90 ± 0.02 | 1.64 ± 0.00 |
| F26 | 2.04 ± 0.01 | 1.60 ± 0.01 | 2.05 ± 0.03 | 1.64 ± 0.07 | 1.99 ± 0.01 | 1.60 ± 0.01 |
| F27 | 2.13 ± 0.02 | 1.69 ± 0.01 | 2.13 ± 0.02 | 1.69 ± 0.02 | 2.09 ± 0.01 | 1.66 ± 0.01 |
| F28 | 1.95 ± 0.00 | 1.59 ± 0.01 | 1.88 ± 0.01 | 1.56 ± 0.00 | 1.88 ± 0.06 | 1.59 ± 0.02 |
Initial phage stability and aerosol performance screening
Spray dried phage formulations that exhibited superior phage stability and aerosol performance in the initial screening are summarised in Table 6. Phage content in these formulations spray dried using feed solution (phage-excipient suspension) at 107 pfu/mL ranged 104 – 105 PFU/mg. The loading amount of phage in formulation P1 TH17LC8 (0.2 log10 titre loss) and P20 LT14LC11 (0.9 log10 titre loss) was 2 × 105 pfu/mg and 4 × 104 pfu/mg, respectively. Phage formulation with least titre loss These formulations were subjected to full powder characterisation, including XRD, DVS, and in vitro aerosol performance using MSLI followed by HPLC chemical and biological assays. FPFloaded was used to express the proportion of particles below 5.0 μm.
Table 6.
List of formulations that exhibited good phage protection and superior aerosol performance during the initial screening using reduced Next Generation Impactor.
| Formulation Name | Formulation composition (mg/mL) | Log10 loss | FPF (%) | |||
|---|---|---|---|---|---|---|
| Trehalose | Lactose | Leucine | Pluronic F68 | |||
| P1 TH17LC8 | 17 | 8 | 0.2 | 58 | ||
| P1 LT17LC8 | 17 | 8 | 0.3 | 57 | ||
| P20 LT17LC8 | 17 | 8 | 0.4 | 72 | ||
| P61 LT14LC11 | 14 | 11 | 0.4 | 52 | ||
| P20 LT23LC2 | 23 | 2 | 0.5 | 75 | ||
| P61 LT17LC8 | 17 | 8 | 0.5 | 71 | ||
| P1 LT12LC5.5PF27 | 12 | 5.5 | 0.0027 | 0.5 | 61 | |
| P20 TH12LC5.5PF27 | 12 | 5.5 | 0.0027 | 0.5 | 55 | |
| P1 LT14LC11 | 14 | 11 | 0.6 | 72 | ||
| P20 LT20LC5 | 20 | 5 | 0.7 | 56 | ||
| P61 LT23LC2 | 23 | 2 | 0.7 | 82 | ||
| P1 LT23LC2 | 23 | 2 | 0.7 | 67 | ||
| P20 LT12LC5.5PF27 | 12 | 5.5 | 0.0027 | 0.7 | 59 | |
| P61 LT20LC5 | 20 | 5 | 0.9 | 54 | ||
| P20 LT14LC11 | 14 | 11 | 0.9 | 80 | ||
FPF: fine particle fraction
All the spray dried phage formulations showed FPF values of over 50% (Table 7), with those containing 0.0027 mg/mL of Pluronic F68 giving the best aerosol performance (FPF range of 65–67%). No other notable difference or trend in FPF was found among the formulations.
Table 7.
In vitro aerosol dispersion results using multi-stage liquid impinger
| Formulation Name | Average FPF (%) | Recovery (%) |
|---|---|---|
| P1 LT23LC2 | 55.6 ± 1.6 | 97.0 ± 4.2 |
| P1 LT17LC8 | 62.8 ± 6.0 | 100.5 ± 3.5 |
| P1 LT14LC11 | 54.0 ± 4.0 | 92.0 ± 4.2 |
| P1 LT12LC5.5PF27 | 63.2 ± 0.3 | 92.5 ± 0.7 |
| P1 TH17LC8 | 46.3 ± 1.0 | 89.5 ± 0.7 |
| P20 LT23LC2 | 52.8 ± 1.4 | 93.5 ± 0.7 |
| P20 LT20LC5 | 57.7 ± 1.8 | 94.5 ± 0.7 |
| P20 LT17LC8 | 58.2 ± 7.4 | 97.0 ± 1.4 |
| P20 LT14LC11 | 52.2 ± 0.2 | 92.0 ± 2.8 |
| P20 LT12LC5.5PF27 | 62.2 ± 4.7 | 93.0 ± 1.4 |
| P20 TH12LC5.5PF27 | 52.1 ± 2.2 | 91.5 ± 0.7 |
| P61 LT23LC2 | 52.5 ± 0.8 | 94.5 ± 0.7 |
| P61 LT20LC5 | 55.2 ± 0.6 | 95.0 ± 4.2 |
| P61 LT17LC8 | 56.7 ± 1.5 | 91.0 ± 1.4 |
| P61 LT14LC11 | 48.7 ± 1.5 | 91.5 ± 0.7 |
FPF: fine particle fraction
Based on the biological assay, the lung doses of spray dried PEV1, PEV20 and PEV61 formulation were 3.4 × 105 – 5.7 × 106 PFU, 1.2 × 105 PFU – 5.9 × 106, and 9.6 × 105 – 5.7 × 106 PFU, respectively (Fig. 8). The highest lung dose was achieved with the formulation which was spray dried from the solution containing 17 mg/mL of lactose and 8 mg/mL of leucine for PEV1 and PEV61, and 20 mg/mL of lactose and 5 mg/mL of leucine for PEV20.
Figure 8.
Lung dose of different spray dried (a) PEV1, (b) PEV20, and (c) PEV61 formulations determined by in vitro aerosolisation followed by biological assay.
XRD
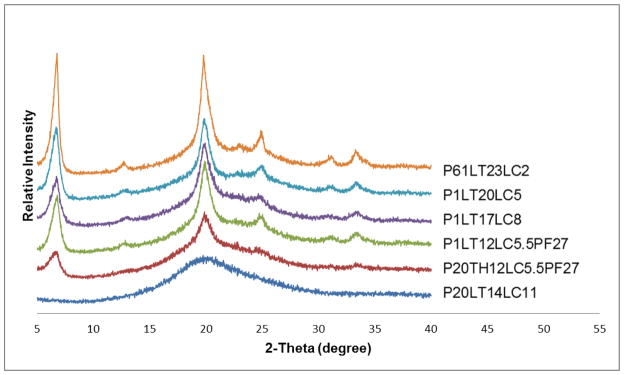
Phage formulations spray dried with high lactose (23 mg/mL) and low leucine (2 mg/mL) concentrations showed a broad peak at 20°, indicating the powder was mainly amorphous (Fig. 9). With addition of more leucine (5 mg/mL), the diffraction peak at 20° became more distinct with additional peaks appearing around 6°, 25°, 31° and 34°, suggesting increased crystallinity of the powder. With further increase in leucine concentration, the aforementioned diffraction peaks became sharper and an additional peak was detected around 12°. Signal peak at 20° belongs to alpha-lactose monohydrate [29], whereas those at 6°, 12°, 25°, 31° and 34° belong to crystalline leucine [30]. Phage formulation spray dried from solution containing 17 mg/mL trehalose and 8 mg/mL leucine gave sharper diffraction peaks than those with 17 mg/mL lactose and 8 mg/mL leucine. Presence of different phages PEV1, PEV20 and PEV61 had no effect on x-ray diffraction pattern.
Figure 9.
X-ray powder diffraction patterns of spray dried phage formulations.
DVS
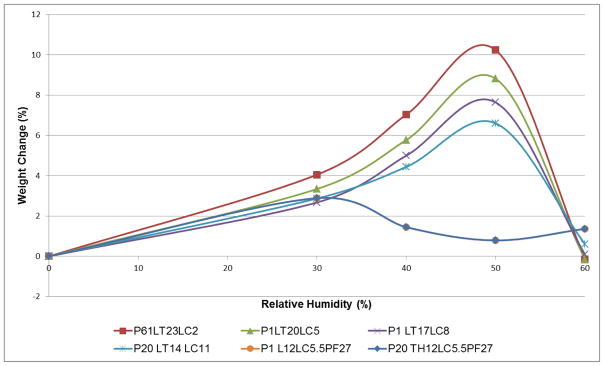
Based on the excipient composition, representative formulations were selected to study moisture uptake behaviour using DVS analysis. DVS results for formulations containing the same excipient ratios but different phage type were consistent. All the phage formulations with lactose and leucine were recrystallized at 50% RH irrespective of the ratio of the excipients (Fig. 10). Phage formulations spray dried with 12 mg/mL trehalose or lactose, 5.5 mg/mL leucine and 0.0027mg/mL pluronic F68 showed recrystallization at 30% RH.
Figure 10.
First sorption cycle data from dynamic vapour sorption (DVS) isotherm for representative phage spray dried formulations.
DISCUSSION
An extensive dry powder phage formulation screening (Supplementary Table 3), followed by further refinement was conducted in this study for three phages with efficient killing activity against clinical and MDR strains. Using the Taguchi experimental design, several GRAS type (i.e. generally regarded as safe) excipients were identified as being responsible for phage stabilisation in spray dried powders (Table 2 & 3). Sugars including lactose and trehalose played a vital role in protecting and stabilising phages from temperature and shear stress during spray drying process, whereas mannitol failed to provide sufficient protection. Lactose and trehalose are commonly used as a protein stabiliser [24, 25, 31]. In particular, trehalose is known for its exceptional phage stabilising properties [32] and its ability to protect biomaterials from thermal stress and dehydration [33, 34]. Protein protection is generally believed to be via the mechanism of water replacement and/or vitrification [35]. Sugars such as trehalose and lactose provide structural and conformational stability by forming direct hydrogen bonds or vitrifying the phages in glass thereby limiting the mobility. This study showed that lactose provides superior phage protection over trehalose.
Table 3.
Formulation information and log titre loss of PEV1, PEV20 and PEV61 after spray drying.
| Formulation | Trehalose (mg/mL) | Lactose (mg/mL) | Leucine (mg/mL) | Titre loss (log10) | ||
|---|---|---|---|---|---|---|
| PEV1 | PEV20 | PEV61 | ||||
| F19 | 23 | 2 | −1.1 | −0.9 | −0.7 | |
| F20 | 20 | 5 | −0.5 | −0.9 | −0.7 | |
| F21 | 17 | 8 | −0.2 | −1.1 | −1.0 | |
| F22 | 14 | 11 | −1.3 | −1.5 | −1.6 | |
| F23 | 10 | 15 | −1.1 | −2.2 | −1.6 | |
| F24 | 23 | 2 | −0.7 | −0.5 | −0.7 | |
| F25 | 20 | 5 | −0.4 | −0.4 | −0.3 | |
| F26 | 17 | 8 | −0.3 | −0.4 | −0.4 | |
| F27 | 14 | 11 | −0.6 | −0.9 | −0.5 | |
| F28 | 10 | 15 | −1.4 | −1.1 | −1.5 | |
Use of lactose over trehalose as an excipient for pulmonary application provides significant advantage as lactose is FDA-approved for inhalation products. Despite the safety acceptance of lactose in pulmonary application, the use of lactose in protein drug and biologic formulations has been debatable because of its reducing properties. Lactose has been portrayed as a poor phage stabiliser responsible for significant titre reduction in spray dried powders by damaging the integrity of the phage particles [36]. Our study shows that lactose, in combination with leucine, can protect and stabilise the phages during the spray drying process, and form partially-crystalline and partially-amorphous (Fig. 9) inhalable particles (Table 4 & 5). In addition, lactose shows comparable phage-protection as trehalose which is a non-reducing sugar. Lack of phage protection in lactose-only formulations in a previous study [26] could be explained by hygroscopic nature of amorphous lactose in spray dried powders. Amorphous powders are thermodynamically unstable, with a high risk of recrystallization when exposed to moisture because water acts as a plasticizer thereby lowering its Tg. At a relative humidity of 37 %, the Tg of amorphous lactose is close to room temperature [37]. Unless the production process and storage are maintained below 37 % RH, lactose-only phage spray dried formulation will quickly recrystallize, potentially destroying the embedded phages. Besides phage inactivation, recrystallization will cause formation of solid bridges between the particles, making the powder difficult to disperse. Hence, the extent of amorphous properties of spray dried phage powders and moisture content would be critical in determining the fate of phage survival and the dispersibility of the powder.
Leucine as a hydrophobic amino acid is often used in DPI formulations to improve powder flowability and dispersibility. In addition, leucine can also impart stability against moisture for spray dried inhalable powders stored at elevated RH [30, 38]. In the absence of leucine, spray dried phage powders formed large agglomerates unsuitable for inhalation (Fig. 6). During the evaporation stage of spray drying, leucine, being hydrophobic with lower aqueous solubility than other water soluble ingredients, would become supersaturated earlier at the surface of the droplet and form a crystalline shell [39]. Increase in leucine content led to formation of particles with corrugated and irregular surface structures (Fig. 7). These features provide lower cohesive forces and reduce aerodynamic diameter leading to enhanced dispersibility [38, 40, 41]. Our results have shown improved FPF with the presence of leucine, and increasing the leucine content in phage formulation beyond 32 % (w/w) did not affect the in vitro aerosol performance (Table 7). This is probably due to plateauing of the surface concentration of leucine when the concentration exceeded 20% (v/v) in the feed concentration [30]. Instead, the addition of Pluronic F68, a surfactant, further enhanced the FPF of phage formulation
Since our results showed that amorphous lactose and trehalose are necessary for stable spray dried phage formulation, adequate moisture protection becomes critical. Leucine provides primary protection, both biological and physicochemical, against moisture during the manufacturing process. Without leucine, the collected spray dried powders are large agglomerates, sticky paste-like texture, with significant phage titre reduction. However, leucine is unable to provide continued protection when the powder is exposed to moisture. Phage formulations containing sugar and leucine are prone to recrystallization and subsequent phage inactivation at 50% RH; increased leucine concentration beyond a certain level do not offer enhanced moisture protection (Fig. 10). Provided storage at low RH (<22% RH) can be achieved (e.g., in heat-sealed aluminium foil or under vacuum), the formulation would likely have sufficient physical and biological stability for ≥ 12 months [32]. However, long-term safety concerns arising from pulmonary delivery of large amount of leucine and sugar remain to be addressed.
In addition to moisture exposure from the external environment, the moisture content in the particles could impact long term stability of the phage formulations. Use of low inlet temperature is critical for retaining phage bioactivity during spray drying, which in turn may also lead to increased residual moisture content in the powder. A recent work from our group has assessed the stability of phage PEV2 spray dried powders at low inlet temperature of 60°C [32]. The biological and physicochemical stability of the formulations were maintained after storage at 0 and 22% relative humidity at 4°C for 12 months. However, storage at 60% RH storage resulted in complete inactivation of phages after 3 months. Hence, we expect that the stability of our phage formulations will be maintained when stored at low relative humidity.
In the present study, the excipients were capable of stabilizing PEV myovirus and PEV podovirus in spray dried powders. No major differences in titre loss and aerosol performance were detected in podovirus PEV1 and PEV61, and myovirus PEV20 when the same excipient composition was used (Table 2 & 3). Vandenheuvel et al. [26] reported that phage survival during the production is phage dependent, where some phages are more susceptible to inactivation compared to others. During spray dyring, podovirus LUZ19 suffered less than 1 log10 titre reduction whereas myovirus Romulus resulted in more than 2.5 log10 loss. Additionally, formulation containing LUZ19 had larger particle size and wider size distribution compared to those containing Romulus. Interestingly, our study showed that both myovirus and podovirus types of PEV phage had similar changes in biological activities upon spray drying with similar particle size and aerosol performance, indicating that the structural differences in phage morphology has a minimal effect on the level of stabilization and physicochemical properties. These differences could be due to combined effects of different innate phage characteristics and spray drying conditions. High inlet temperature (85 °C) used by Vandenheuvel et al. could have resulted in loss of infectivity of myovirus Romulus. Additionally, phage Romulus could be sensitive to shear stress from atomizing pressure, where delicate structure of the myovirus could have lost infectious capacity. The long tail structure of myovirus can contract or the capsid head can detach under stress, and consequently, lose their infectivity [42, 43]. The short-tailed podovirus may potentially be more robust and have their structure and infectivity maintained.
Powder phage formulation has been explored by several groups [24–26], yet the research is still at infancy. This study has provided insight into optimal excipient combination for stabilization of phages in spray dried formulations with superior aerosol performance. We have uncovered that both PEV myovirus and podovirus can be successfully spray dried using the same excipient combinations. Phage cocktail is a preferred treatment scheme for phage therapy to minimize the development of phage-resistant bacteria. Hence, this study has shown potential for producing phage cocktail spray dried powders comprising various lytic phages. Further exploration and stability testing is required in determining the optimal excipient balance for phage cocktail formulations. Additionally, the exact mechanisms by which the phage is stabilized by excipients in the dry state are yet to be elucidated. Greater understanding of the underlying mechanism will allow rapid and cost-effective formulation screening by actively selecting excipients that will enhance phage stabilization. This will dramatically reduce the time and cost involved in developing dry powder phage formulation once suitable phages with therapeutic potential are identified
CONCLUSION
Three PEV phages effective against a wide range of P. aeruginosa clinical isolates were identified and selected for spray drying. Inhalable phage spray dried formulations containing various amounts of different GRAS-type excipients were screened for phage protection and in vitro aerosol performance. A Taguchi experimental design with funnelling approach was employed to gain perspective into which excipients are critical for phage stability. We found that lactose, an approved excipient for inhalation, provides excellent phage stabilization. Furthermore, simple formulation of lactose or trehalose, with leucine is sufficient for providing phage protection and good aerosol performance regardless of the phage morphology and during spray drying process. In summary, we have identified a combination of excipients capable of protecting two PEV podoviruses and one PEV myovirus phage for generation of respirable dry powder formulations, which may have significant potential in treating lung infections caused by MDR P. aeruginosa infections.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI121627 (H-K C. and J.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. J.L. is an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellow. The authors would like to thank Professor Jonathan Iredell at The Westmead Institute for providing clinical strains for phage host range testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hassett DJ, Korfhagen TR, Irvin RT, Schurr MJ, Sauer K, Lau GW, Sutton MD, Yu H, Hoiby N. Pseudomonas aeruginosa biofilm infections in cystic fibrosis: insights into pathogenic processes and treatment strategies. Expert Opin Ther Targets. 2010;14:117–130. doi: 10.1517/14728220903454988. [DOI] [PubMed] [Google Scholar]
- 2.McCallum SJ, Corkill J, Gallagher M, Ledson MJ, Hart CA, Walshaw MJ. Superinfection with a transmissible strain of Pseudomonas aeruginosa in adults with cystic fibrosis chronically colonised by P aeruginosa. Lancet. 2001;358:558–560. doi: 10.1016/s0140-6736(01)05715-4. [DOI] [PubMed] [Google Scholar]
- 3.Seed KD, Dennis JJ. Isolation and characterization of bacteriophages of the Burkholderia cepacia complex. FEMS Microbiol Lett. 2005;251:273–280. doi: 10.1016/j.femsle.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Mandsberg LF, Ciofu O, Kirkby N, Christiansen LE, Poulsen HE, Hoiby N. Antibiotic resistance in Pseudomonas aeruginosa strains with increased mutation frequency due to inactivation of the DNA oxidative repair system. Antimicrob Agents Chemother. 2009;53:2483–2491. doi: 10.1128/AAC.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 6.Burrowes B, Harper DR, Anderson J, McConville M, Enright MC. Bacteriophage therapy: potential uses in the control of antibiotic-resistant pathogens. Expert Rev Anti Infect Ther. 2011;9:775–785. doi: 10.1586/eri.11.90. [DOI] [PubMed] [Google Scholar]
- 7.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olszak T, Zarnowiec P, Kaca W, Danis-Wlodarczyk K, Augustyniak D, Drevinek P, de Soyza A, McClean S, Drulis-Kawa Z. In vitro and in vivo antibacterial activity of environmental bacteriophages against Pseudomonas aeruginosa strains from cystic fibrosis patients. Appl Microbiol Biotechnol. 2015;99:6021–6033. doi: 10.1007/s00253-015-6492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alemayehu D, Casey PG, McAuliffe O, Guinane CM, Martin JG, Shanahan F, Coffey A, Ross RP, Hill C. Bacteriophages phiMR299–2 and phiNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. MBio. 2012;3:e00029–00012. doi: 10.1128/mBio.00029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabary R, Singh C, Morales S, Bush A, Alshafi K, Bilton D, Alton EW, Smithyman A, Davies JC. Antipseudomonal Bacteriophage Reduces Infective Burden and Inflammatory Response in Murine Lung. Antimicrob Agents Chemother. 2016;60:744–751. doi: 10.1128/AAC.01426-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 13.Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage Therapy. Antimicrob Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulakvelidze A, Kutter E. 14 Bacteriophage Therapy in Humans. Bacteriophages: biology and applications. 2004:381. [Google Scholar]
- 15.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 16.Parasion S, Kwiatek M, Gryko R, Mizak L, Malm A. Bacteriophages as an alternative strategy for fighting biofilm development. Pol J Microbiol. 2014;63:137–145. [PubMed] [Google Scholar]
- 17.Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PloS one. 2017;12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P, Entenza JM, Resch G, Que Y-A. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis. 2016:jiw632. doi: 10.1093/infdis/jiw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoe S, Semler DD, Goudie AD, Lynch KH, Matinkhoo S, Finlay WH, Dennis JJ, Vehring R. Respirable bacteriophages for the treatment of bacterial lung infections. J Aerosol Med Pulm Drug Deliv. 2013;26:317–335. doi: 10.1089/jamp.2012.1001. [DOI] [PubMed] [Google Scholar]
- 20.Sahota JS, Smith CM, Radhakrishnan P, Winstanley C, Goderdzishvili M, Chanishvili N, Kadioglu A, O'Callaghan C, Clokie MR. Bacteriophage Delivery by Nebulization and Efficacy Against Phenotypically Diverse Pseudomonas aeruginosa from Cystic Fibrosis Patients. J Aerosol Med Pulm Drug Deliv. 2015;28:353–360. doi: 10.1089/jamp.2014.1172. [DOI] [PubMed] [Google Scholar]
- 21.Cooper CJ, Denyer SP, Maillard JY. Stability and purity of a bacteriophage cocktail preparation for nebulizer delivery. Lett Appl Microbiol. 2014;58:118–122. doi: 10.1111/lam.12161. [DOI] [PubMed] [Google Scholar]
- 22.Golshahi L, Seed KD, Dennis JJ, Finlay WH. Toward modern inhalational bacteriophage therapy: nebulization of bacteriophages of Burkholderia cepacia complex. J Aerosol Med Pulm Drug Deliv. 2008;21:351–360. doi: 10.1089/jamp.2008.0701. [DOI] [PubMed] [Google Scholar]
- 23.Merabishvili M, Vervaet C, Pirnay JP, De Vos D, Verbeken G, Mast J, Chanishvili N, Vaneechoutte M. Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization) PloS one. 2013;8:e68797. doi: 10.1371/journal.pone.0068797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matinkhoo S, Lynch KH, Dennis JJ, Finlay WH, Vehring R. Spray-dried respirable powders containing bacteriophages for the treatment of pulmonary infections. J Pharm Sci. 2011;100:5197–5205. doi: 10.1002/jps.22715. [DOI] [PubMed] [Google Scholar]
- 25.Leung SS, Parumasivam T, Gao FG, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK. Production of inhalation phage powders using spray freeze drying and spray drying techniques for treatment of respiratory infections. Pharm Res. 2016;33:1486–1496. doi: 10.1007/s11095-016-1892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenheuvel D, Singh A, Vandersteegen K, Klumpp J, Lavigne R, Van den Mooter G. Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections. Eur J Pharm Biopharm. 2013;84:578–582. doi: 10.1016/j.ejpb.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Garbe J, Wesche A, Bunk B, Kazmierczak M, Selezska K, Rohde C, Sikorski J, Rohde M, Jahn D, Schobert M. Characterization of JG024, a pseudomonas aeruginosa PB1-like broad host range phage under simulated infection conditions. BMC Microbiol. 2010;10:301–301. doi: 10.1186/1471-2180-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajmera A, Scherlieβ R. Stabilisation of proteins via mixtures of amino acids during spray drying. Int J Pharm. 2014;463:98–107. doi: 10.1016/j.ijpharm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Thomas NR, Shumway LS, Hansen LD. Quantitative X-ray diffraction determination of alpha-lactose monohydrate and beta-lactose in chocolate. J Food Sci. 2009;74:C513–518. doi: 10.1111/j.1750-3841.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, Mao S, Chan HK. L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm. 2016;102:132–141. doi: 10.1016/j.ejpb.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Bosquillon C, Lombry C, Préat V, Vanbever R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J Countrol Release. 2001;70:329–339. doi: 10.1016/s0168-3659(00)00362-x. [DOI] [PubMed] [Google Scholar]
- 32.Leung SS, Parumasivam T, Gao FG, Carter EA, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK. Effects of storage conditions on the stability of spray dried, inhalable bacteriophage powders. Int J Pharm. 2017;521:141–149. doi: 10.1016/j.ijpharm.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowe JH, Carpenter JF, Crowe LM. The role of vitrification in anhydrobiosis. Annu Rev Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- 34.Grasmeijer N, Stankovic M, de Waard H, Frijlink HW, Hinrichs WL. Unraveling protein stabilization mechanisms: vitrification and water replacement in a glass transition temperature controlled system. Biochim Biophys Acta. 2013;1834:763–769. doi: 10.1016/j.bbapap.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WL. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur J Pharm Biopharm. 2017;114:288–295. doi: 10.1016/j.ejpb.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Vandenheuvel D, Singh A, Vandersteegen K, Klumpp J, Lavigne R, Van den Mooter G. Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections. Eur J Pharm Biopharm. 2013;84:578–582. doi: 10.1016/j.ejpb.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Roos YH. Importance of glass transition and water activity to spray drying and stability of dairy powders. Lait. 2002;82:475–484. [Google Scholar]
- 38.Chen L, Okuda T, Lu XY, Chan HK. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100:102–115. doi: 10.1016/j.addr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Boraey MA, Hoe S, Sharif H, Miller DP, Lechuga-Ballesteros D, Vehring R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol water cosolvent system. Powder Technology. 2013;236:171–178. [Google Scholar]
- 40.Chew NY, Tang P, Chan HK, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res. 2005;22:148–152. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- 41.Tang P, Chan HK, Raper JA. Prediction of aerodynamic diameter of particles with rough surfaces. Powder Technology. 2004;147:64–78. [Google Scholar]
- 42.Arisaka F, Engel J, Klump H. Contraction and dissociation of the bacteriophage T4 tail sheath induced by heat and urea. Prog Clin Biol Res. 1981;64:365–379. [PubMed] [Google Scholar]
- 43.Steele PR. Morphological manifestations of freezing and thawing injury in bacteriophage T4Bo. J Hyg (Lond) 1976;77:119–127. doi: 10.1017/s0022172400055595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.