Abstract
Autoimmune diseases including multiple sclerosis predominantly affect females. Although high levels of sex hormones, particularly estrogen (E2), can reduce proinflammatory immune responses, it remains unclear if a lack of endogenous sex hormones might affect treatment with exogenous sex hormones. Pretreatment with E2 almost completely prevents intact female and male mice from developing clinical and histological signs of experimental autoimmune encephalomyelitis (EAE) by promoting various regulatory immune cell phenotypes. To evaluate the effects of exogenous estrogen in the absence of endogenous sex hormones, the current study compared EAE severity and the emergence of different immunoregulatory cell populations after E2 pretreatment of ovariectomized (OVX) female versus male mice. We found that E2 equally protected both OVX females and males from EAE over a 21 day observation period concomitant with reduced total cell numbers in spleen and spinal cord (males only), but enhanced percentages of CD19+CD5+CD1dhi, CD19+CD138+CD44hi and CD19+Tim-1+ Breg cells, CD8+CD122+ Treg cells and CD11b+206+ARG-1+ anti-inflammatory M2-like monocytes/macrophages in both groups. In contrast, E2 decreased the percentage of CD4+CD25+FoxP3+ Treg cells in OVX females but increased these Treg cells in males and intact female mice. These data suggest that with the exception of CD4+CD25+FoxP3+ Treg cells, E2 protection against EAE promotes highly overlapping immunoregulatory subsets in OVX females and males.
Keywords: Estrogen, EAE, Regulatory B cells, Sex Differences, Ovariectomized, Anti-inflammatory macrophages
Introduction
Autoimmune diseases disproportionately affect women compared to men. Approximately 80% of all autoimmune diseases are diagnosed in women (Jacobson et al. 1997). Multiple sclerosis (MS) is no different, with women at a rate of 3:1, or 75%, compared to men (Confavreux et al. 1998). It is known that male and female immune systems function differently, with the female immune system responding and clearing pathogens better than male immune systems (vom Steeg and Klein 2016). Additionally, females have more robust response to vaccines compared to males (Klein et al. 2010). However, this highly responsive immune system in females leads to an increase in immune attacks on self-tissues resulting in autoimmune diseases. Testosterone is known to promote an anti-inflammatory immune state (Roberts et al. 2001), while low levels of estrogen promote proinflammatory immune states. Higher levels of estrogen, however, can promote a more anti-inflammatory response (Straub 2007). This is thought to be why women with autoimmune diseases, especially MS, experience a decrease in relapses during pregnancy due to elevated levels of estrogen (Confavreux et al. 1998). This observation has led to research into how high dose estrogen can protect female mice from experimental autoimmune encephalomyelitis (EAE), a mouse model of MS.
Pregnancy levels of 17β-estradiol (E2) protect female mice from developing EAE, as do middle range levels of E2 (Bebo et al. 2001; Offner and Polanczyk 2006; Polanczyk et al. 2006; Wang et al. 2009). E2 also regulates the immune system by increasing regulatory B and T cells (Benedek et al. 2016; Polanczyk et al. 2005). Treatment with E2 does not change the frequency of splenic B cells but it does shift the frequency of multiple regulatory B cell (Breg) subsets, including B10, Tim-1+, and plasmablasts, (Benedek et al. 2016) all of which produce IL-10 and can modulate T cell function (Ding et al. 2011; Iwata et al. 2011; Matsumoto et al. 2014; Matsushita et al. 2010). B10 cells also up regulate the expression of programmed death ligand 1 (PD-L1) which is critical for E2 dependent protection from EAE (Bodhankar et al. 2013; Zhang et al. 2015). In addition to increasing regulatory lymphocytes, E2 treatment also increases anti-inflammatory macrophages (Benedek et al. 2017). Male C57BL/6 mice are also protected from EAE when pretreated with E2 (Matejuk et al. 2005).
The goal of this study was to determine if the mechanism by which male mice are equally protected from EAE by middle range E2 pre-treatment was similar as that in female mice and to see if endogenous estrogen was necessary for protection in female mice. To avoid estrogen fluctuation during cycling in females, E2 pre-treatment was carried out in ovariectomized (OVX) female mice during EAE. Male and OVX female mice were protected from developing EAE when pretreated with E2. Additionally E2 increased splenic regulatory B cells and anti-inflammatory macrophages. Differential effects on splenic immune cell populations and the expression of PD-L1 on B cells were dependent on sex. The frequency of CD4 regulatory T cells (Treg) was increased in both male and intact female E2 treated mice but there was a decrease in the frequency of CD4 Treg cells in OVX female mice treated with E2 during EAE.
Methods
Animals
Male, female, and ovariectomized (OVX) female C57BL/6 mice (8–10 weeks old) were purchased from The Jackson Laboratory (Sacramento, CA). In order to allow complete recovery form operation ovariectomized females were housed for two weeks before starting any procedure. All mice were housed in climate controlled settings at the Animal Resource Facility at the VAPHCS and kept on a 12 h light/dark cycle. Mice were given food and water ad libitum. This study was conducted in accordance with the NIH guidelines for the use of experimental animals and the VAPORHCS Animal Care and Use Committee approved protocols.
Hormone treatment and induction of EAE
Male, OVX female, and intact female C57BL/6 mice were implanted subcutaneously with 2.5mg/60-day release 17β-estradiol pellets (Innovative Research of America, Sarasota, FL) or sham treated (control) one week prior to immunization with 200μg mouse MOG-35–55 peptide (PolyPeptide Laboratories, San Diego, CA) in 200μg Complete Freund’s adjuvant (Incomplete Freund’s adjuvant (IFA, Sigma-Aldrich, St. Louis, MO)) containing heat-killed Mycobacterium tuberculosis (Mtb, Difco, Detroit, MI) subcutaneously at four sites on the flanks. Mice were immunized at four sites on the flanks and received intraperitoneal injections of pertussis toxin (Ptx, List Biologicals, Campbell, CA) 75ng on the day of immunization and 200ng two days later.
All mice were monitored daily for clinical signs of EAE disease progression and scored using the following scale: 0 = normal; 1 = limp tail or mild hind limb weakness; 2 = moderate hind limb weakness or mild ataxia; 3 = moderately severe hind limb weakness; 4 = severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5 = paraplegia with no more than moderate forelimb weakness; 6 = paraplegia with severe forelimb weakness or severe ataxia or moribund condition. Mice were scored daily and evaluated for incidence of disease, day of disease onset, day of maximal clinical signs (peak), and total disease score over the course of the experiment (cumulative disease index, CDI). Mean ± SEM were calculated for these parameters for each experimental group.
Leukocyte isolation from the spleen and spinal cord
Spleens and spinal cords were collected from mice 21 days post-immunization. Spleens were processed into single cell suspensions by passing the spleen through a 100μm nylon mesh (BD Falcon, Bedford, MA) into RPMI 1640. Red blood cells were lysed with 1× red cell lysis buffer (eBioscience, Inc., San Diego, CA). After being washed with RPMI 1640 the cells were counted on a Cellometer Auto T4 cell counter (Nexcelom, Lawrence, MA). Cells were centrifuged and resuspended in staining buffer (PBS with 0.1% NaN3 and 1% bovine serum albumin) for staining.
Spinal cords were passed through a 100μm nylon mesh (BD Falcon) and washed with RPMI 1640. Pelleted cells were resuspended in 80% Percoll (GE Healthcare, Pittsburgh, PA) and overlaid with 40% Percoll to establish a density gradient and centrifuged at 1600 rpm for 30 min as previously described (Campanella et al. 2002). Leukocytes were collected from the resultant interface, counted, and resuspended in staining buffer for staining for FACS analysis.
Flow cytometry
For flow cytometry, cells were placed in staining buffer at a concentration of 1×106 cells/ml for splenocytes and 2×105 cell/ml for spinal cords. Cells were blocked with rat anti-mouse CD16/CD32 Mouse BD Fc Block™ (BD Bioscience, San Jose, CA) and then incubated, protected from light, with various combinations of fluorescently tagged antibodies. To assess cell survival, 7-aminoactinomycin D (7AAD) viability dye was used in some samples. For intracellular and FoxP3 staining, the samples were fixed with 4% paraformaldehyde and washed. FoxP3 staining was done using the fixation/permeabilization reagents per the manufacturer’s instructions (eBioscience). For intracellular staining, cells were resuspended in 1× permeabilization buffer (BD Bioscience) and incubated with antibodies or isotype controls. The samples were then run on a BD AccuriTM C6 (BD Bioscience) under a four color (FITC, PE, PerCP/PECy5.5, and APC) fluorescence flow cytometry analysis.
The following antibodies were used: CD4 (GK1.5), PDL1 (M1H5), CD11b (M1/70), CD19 (1D3), CD1d (1B1), CD23 (B3B4), TIM1 (RMT1-4), CD138 (281-2), CD25 (PC61), CD86 (GL1), CD21 (7G6), CD206 (CO68C2), CD8 (53-6.7), CD122 (TM-β1) (BD Biosciences), CD44 (1M7), FoxP3 (FJK-16s) (eBioscience), CD9 (MZ3), CD5 (53-7.3) (Biolegend), and PE-ARG1 (R&D Systems, Minneapolis, MN).
Statistics
Data were analyzed using Prism software (GraphPad Software, La Jolla, CA) using Mann-Whitney U test for determining significance for disease course. Splenocyte and spinal cord cell counts were analyzed using an ANOVA with a Bonferroni’s post-hoc test. All other data were analyzed using a Student’s t-test. A p value of ≤0.05 was considered significant. Data are represented by mean ± standard error of the mean (SEM) and all analyses were done blinded.
Results
E2 protects male and OVX female mice from EAE
E2 pretreatment protected intact female C57B/6 mice, as previously reported (Benedek et al. 2016). Male and OVX female C57B/6 mice were also significantly protected from EAE when E2 was administered 7 days prior to the induction of EAE compared to sham treated controls (Fig. 1a, p<0.05). There was also a significant decrease in the number of leukocytes present in the spinal cords of male mice treated with E2 compared to sham (Fig 1b, p<0.001). OVX female mice treated with E2 had lower leukocyte counts in the spinal cords that did not reach significance compared to OVX sham treated mice. Additionally, E2 treatment significantly decreased splenocytes counts in male and OVX female mice compared to sham treated male or OVX female mice, respectively, (Fig 1c, p<0.05) and sham treated OVX female mice had significantly lower splenocyte counts compared to sham treated male mice (p<0.05).
Figure 1. E2 pretreatment protects male and OVX mice from EAE.
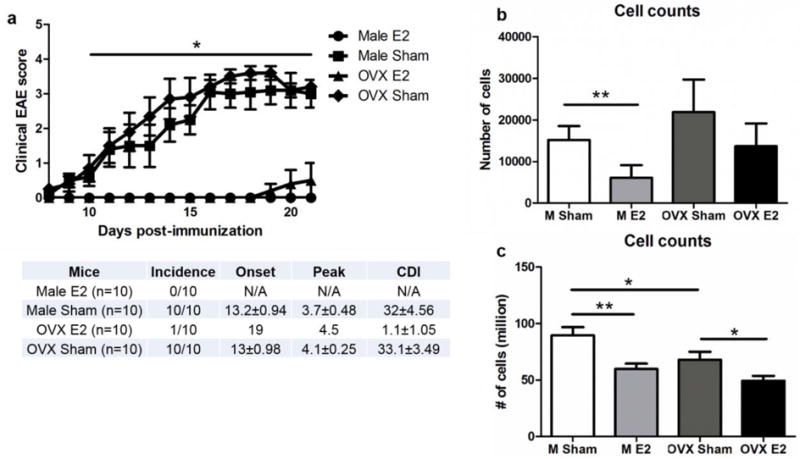
a) Disease course for male (n=10) and ovariectomized (OVX) female (n=10) mice E2 treated and sham treated with E2 treatment significantly improving disease course in both groups p<0.05. b) Spinal cord cell leukocyte counts are significantly decreased in E2 treated males p<0.001. c) Splenocyte counts are significantly decreased in E2 treated males and OVX females. Sham treated OVX female mice have significantly fewer splenocytes than male sham treated mice *p<0.05, **p<0.001. Panel describes incidence, onset, peak and cumulative disease index for each group.
E2 regulates splenic immune cell populations differently in male and female mice
Splenocyte populations of CD4+ T cells, CD19+ B cells, and CD11b+ monocytes/macrophages vary depending on sex and E2 treatment during EAE. The frequency of B cells (CD19+) was unchanged with E2 treatment during EAE in intact (Benedek et al. 2016) and OVX females (Fig 2a) compared to E2 treated male mice that had a significance increase in the frequency of B cells (CD19+) compared to sham treated males (Fig 2b, p<0.05). Female mice, intact (Benedek et al. 2016) or OVX (Fig 2a, p<0.05), treated with E2 had a significant decrease in the frequency of CD4+ T cells. The frequency of CD4+ T cells was unchanged in male mice treated with E2 compared to sham treated mice (Fig 2b). Unlike B cells and T cells, the frequency of CD11b+ (monocytes/macrophages) was significantly decreased in E2 treated OVX females and males (Fig 2a, p<0.05 and Fig 2b, p<0.0001), respectively, whereas intact female mice showed no significant difference in the frequency of CD11b+ cells between E2 and sham treated mice (Benedek et al. 2016).
Figure 2. Differential effects of E2 on splenic immune cell populations in male and OVX mice.
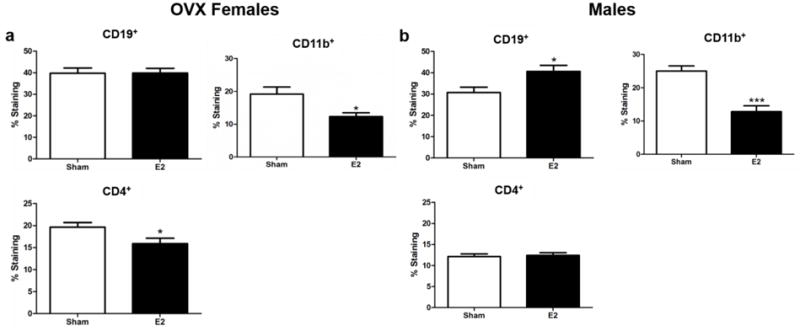
a) OVX female mice (n=10) frequency of splenic CD19+, CD11b+, and CD4+ cells. b) Male mice (n=10) frequency of splenic CD19+, CD11b+, and CD4+ cells. *p<0.05, ***p<0.0001.
Regulatory B cell subtypes are increased in E2 treated male and OVX female mice
E2 is known to upregulate various subtypes of Breg cells in the spleen of intact female mice (Benedek et al. 2016). These cells include B10 (CD19+ CD5+ CD1dhi), Tim-1+ B cells (CD19+ TIM-1+), and plasmablasts (CD19+ CD138+ CD44hi) all of which produce IL-10 and can suppress the effects of T cells (Ding et al. 2011; Iwata et al. 2011; Matsumoto et al. 2014; Matsushita et al. 2010). Both OVX female (Fig 3a) and male mice (Fig 3b) had a significant increase in the frequency of B10 (p<0.0001), Tim-1 B cells (p<0.001 and p<0.05 respectively) and plasmablasts (p<0.05 and p<0.0001, respectively) in E2 treated mice compared to sham treated mice. In addition to secreting IL-10, B10 cells also up regulate the expression of PD-L1 which is important in E2 protection against EAE (Bodhankar et al. 2013; Zhang et al. 2015). Both intact females (Benedek et al. 2016) and OVX females treated with E2 had a significantly increased frequency of CD19+ PD-L1hi cells in the spleen compared to sham treated mice (Fig 3a, p<0.0001). There was no difference in frequency of CD19+ PD-L1hi cells in E2 or sham treated male mice (Fig 3b).
Figure 3. E2 effects on regulatory B cells.
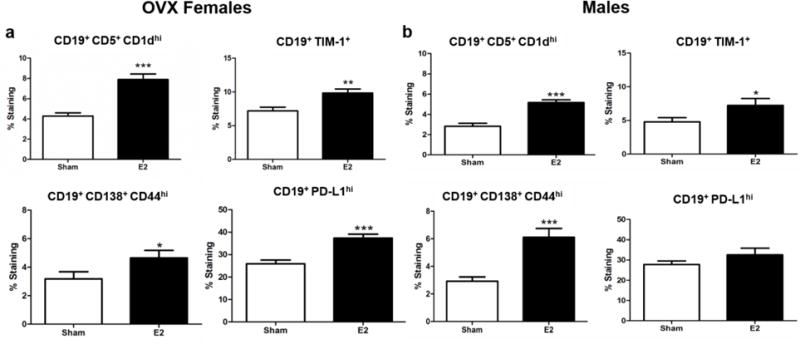
a) OVX female mice (n=10) frequency of splenic CD19+ CD5+ CD1dhi, CD19+ TIM-1+, CD19+ CD138+ CD44hi, and CD19+ PD-L1hi cells. b) Male mice (n=10) frequency of splenic CD19+ CD5+ CD1dhi, CD19+ TIM-1+, CD19+ CD138+ CD44hi, and CD19+ PD-L1hi cells. *p<0.05, **p<0.001, ***p<0.0001.
Regulatory CD4 T cells are upregulated in E2 treated male and female mice but not in OVX female mice, while E2 increased CD8 regulatory cells
Sex hormones can regulate the expression of FoxP3, the transcription factor responsible for CD4 Treg cell differentiation (Walecki et al. 2015). E2 treatment of intact female (Online Resource 1a, p<0.05) and male mice (Fig 4b, p<0.05) prior to EAE resulted in a significant increase in the frequency of splenic CD4+CD25+FoxP3+ cells. OVX mice had a significant decrease in the frequency of CD4+CD25+FoxP3+ cells (Fig 4a, p<0.05). Both OVX females and males had a significant increase in the frequency of CD8+CD122+ Treg cells (Fig 4a, p<0.05 and Fig 4b, p<0.05). There was no difference in the frequency of CD8+CD122+ T cells in intact female mice treated with E2 (Online Resource 1b).
Figure 4. The different responses of Treg cells in male and OVX mice to E2 treatment.
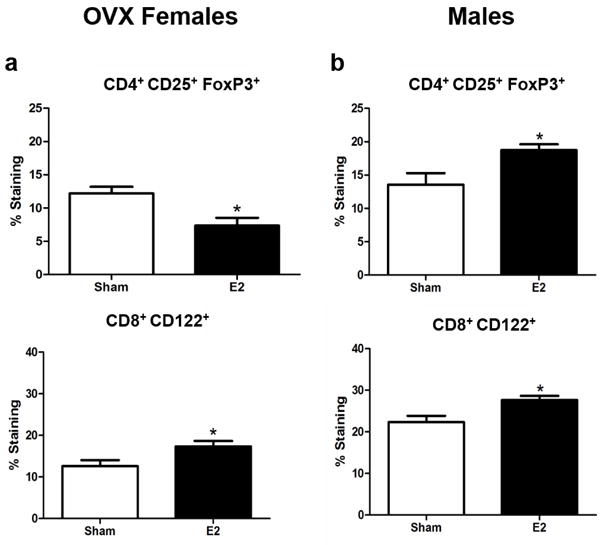
a) OVX female mice (n=10) frequency of splenic CD4+ CD25+ FoxP3+, CD4 Treg, and CD8+ CD122+, CD8 Treg cells. b) Male mice (n=10) frequency of splenic CD4+ CD25+ FoxP3+, CD4 Treg, and CD8+ CD122+, CD8 Treg cells. *p<0.05.
E2 upregulates anti-inflammatory macrophages in male and OVX female mice
It has been previously demonstrated that E2 treatment of intact female mice can induce a significant increase in the anti-inflammatory macrophage markers CD206 and Arg-1 (Benedek et al. 2017). Similarly both OVX female (Fig 5a) and male (Fig 5b) mice treated with E2 displayed a significant increase in the frequency of CD206 (p<0.05 and p<0.0001, respectively) and Arg-1 (p<0.05 and p<0.0001, respectively) compared to sham treated mice.
Figure 5. E2 promotes anti-inflammatory splenic macrophages in males and OVX mice.
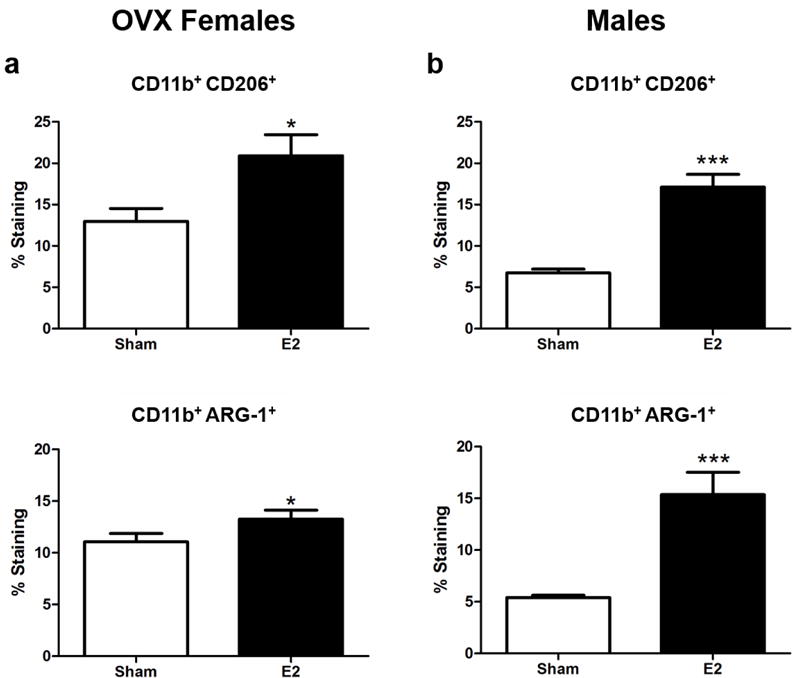
a) OVX female mice (n=10) frequency of splenic CD11b+ CD206+ and CD11b+ ARG-1+ cells. b) Male mice (n=10) frequency of splenic CD11b+ CD206+ and CD11b+ ARG-1+ cells. *p<0.05, ***p<0.0001.
Discussion
Sex hormones can influence the state of the immune system and this causes female and male immune systems to respond differently to immune challenges from pathogens and in autoimmune diseases. While autoimmune diseases, like MS, affect women at a higher rate, men are still diagnosed with these types of diseases including MS. Despite testosterone having anti-inflammatory effects on the immune system it still does not protect men from developing MS or male mice from developing EAE. Moreover, administration of exogenous testosterone protects young male mice from EAE but does not protect middle aged mice (Matejuk et al. 2005). The proinflammatory effects of low levels of estrogen (Straub 2007) is one of the reasons why women are more susceptible to MS and other autoimmune diseases. However, pregnancy, levels of estrogen are protective in women (Confavreux et al. 1998; Straub 2007) and female mice (Bebo et al. 2001; Offner and Polanczyk 2006; Polanczyk et al. 2006; Wang et al. 2009). Additionally, high 5mg doses of E2 protect young and middle aged male mice from EAE (Matejuk et al. 2005). The current data demonstrate pretreatment with a middle range dosage of E2, 2.5mg, protects male and OVX female mice from EAE just as it protects intact female mice (Bebo et al. 2001; Benedek et al. 2016).
Male mice have a significant decrease in total leukocyte counts in the spinal cord compared to sham treatment. There was a similar trend in OVX female mice which did not reach significance. This suggests one way E2 protects against EAE is by reducing inflammation in the spinal cord from infiltrating leukocytes, whereas in the spleen there is a significant decrease in the total splenocyte counts in both E2 treated groups, male and OVX females, compared to their respective sham control group. Even sham treated OVX female mice had significantly lower splenocyte counts compared to male sham treated mice. This could indicate OVX female mice had more leukocytes released from the spleen than male mice or that male mice had increased leukocyte proliferation during EAE than OVX females. Intact female mice demonstrated no difference in splenocyte counts between E2 and sham groups (data not shown).
While splenocyte counts were reduced in both male and OVX female mice after E2 treatment there were still sex dependent effects on different leukocyte populations. Consistent with previously published data on intact female mice (Benedek et al. 2016), OVX female mice had a significant decrease in the frequency of CD4+ T cells and showed no difference in the frequency of CD19+ B cells. Male mice however, showed a significant increase in the frequency of CD19+ B cells and no difference in the frequency of CD4+ T cells. The frequency of CD11b+ cells in male and OVX female mice treated with E2 were significantly decreased compared to sham treated mice. Intact female mice demonstrated no difference at 21 days post immunization in the frequency of CD11b+ cells in E2 treated compared to sham treated mice (Benedek et al. 2016). However, at both 8 and 14 days post immunization, the frequency of CD11b+ cells was significantly decreased in intact female mice treated with E2 compared to sham treated mice. The male and OVX female mice were studied at 21 days post immunization.
Despite the difference in the frequency of CD19+ B cells between the sexes, E2 treatment resulted in all groups showing a significant increase in the frequency of the different Breg subsets in the spleen. Intact females (Benedek et al. 2016), male, and OVX female mice treated with E2 had a significant increase in the frequency of B10, (CD19+ CD5+ CD1dhi), Tim-1+ B cells (CD19+ TIM-1+), and plasmablasts (CD19+ CD138+ CD44hi) in the spleen compared to sham treated mice. This suggests E2 alone is enough to induce the differentiation of these three different Breg cell populations, all of which produce IL-10 and modulate T cell function (Ding et al. 2011; Iwata et al. 2011; Matsumoto et al. 2014; Matsushita et al. 2010), during EAE. B cells are known to be important for E2 protection against EAE, as female μMt−/− mice, mice lacking B cells, are not protected from EAE when pretreated with E2 (Bodhankar et al. 2011). Even the presence of B cells alone is not enough for E2 to protect against EAE. PD-L1 needs to be present on B cells in order for female mice to be protected from EAE. B cells from PD-L1−/− mice did not restore E2 protection in μMt−/− mice while B cells from PD-L2−/− mice did restore the protective effects of E2 in μMt−/− mice (Bodhankar et al. 2013). Intact female mice had a significant increase in the frequency of CD19+ PD-L1hi cells in E2 treated mice compared to sham treated mice. OVX female mice also had a significant increase in the frequency of CD19+ PD-L1hi cells and there was no difference in the frequency of CD19+ PD-L1hi cells in male mice. This suggests there are sex differences in the way E2 mediates protection during EAE as female mice appear to require PD-L1 expression on B cells while male mice are still protected without increasing the frequency of PD-L1 on B cells. It may be that the protection seen in male mice is mediated more by IL-10 than by direct cell to cell contact through PD-L1 interactions with programmed death receptor 1 (PD-1).
Bregs are known to secrete IL-10 and have also been shown to influence microglia polarization by secreting IL-4, resulting in more anti-inflammatory microglia (Bodhankar et al. 2015b). The frequency of anti-inflammatory splenic macrophages (CD11b+ CD206+ or CD11b+ Arg-1+) is significantly increased in E2 treated intact females (Benedek et al. 2017), male and OVX females during EAE compared to sham treated mice. These data suggest the increase in anti-inflammatory macrophages is E2 dependent regardless of sex. There is a positive feedback loop that is established between Breg cells and anti-inflammatory macrophages as both cell types can increase numbers of the other cell type (Benedek et al. 2017). It is possible that E2 sets off this positive feedback loop during EAE in both sexes and without the presence of endogenous estrogen.
Treg cells have a controversial role in mediating protection from EAE. Previous studies have demonstrated CD4 Treg knock out mice are still protected from EAE with E2 treatment (Subramanian et al. 2011). Other studies have shown estrogen increases CD4 Treg cells (CD4+ CD25+ FoxP3+) in intact female mice during EAE (Polanczyk et al. 2005). Even OVX female mice treated with E2 after the induction of EAE demonstrated some protection from EAE and an increased frequency of CD4 Treg cells (Haghmorad et al. 2016). Interestingly, intact female and male mice had a significant increase in the frequency of CD4 Treg cells in E2 pretreated mice compared to sham pretreatment, but OVX female mice had a significant decrease in the frequency of CD4 Treg cells in E2 pretreated mice compared to sham pretreated mice. This suggests other factors in addition to E2 are necessary to increase the frequency of CD4 Treg cells and these are absent in OVX female mice. One reason for the difference in the previous findings in OVX female mice is the time when E2 was administered (eg, within 30 min after the induction of EAE compared (Haghmorad et al. 2016) to 7 days prior to the induction of EAE). Another subset of Treg cells are CD8 Treg cells (CD8+ CD122+) which are protective after stroke (Bodhankar et al. 2015a). Male and OVX female mice had a significant increase in the frequency of CD8 Treg cells with E2 pretreatment compared to sham pretreatment. There was no difference in the frequency of CD8 Treg cells in intact female mice pretreated with E2 compared to sham.
These data suggest middle range E2 pretreatment prior to EAE protects both sexes and is not dependent on endogenous estrogen. Some of the protective effects previously observed in intact female mice, like the effect on Breg cells and anti-inflammatory macrophages, are strongly dependent on E2 treatment as the effects were present regardless of sex. Other immune modulating properties of E2 appear to be dependent on sex since intact and OVX female mice had similar responses and male mice had different responses, particularly with PD-L1 expression on B cells. While other effects, like changes in CD4 Treg cells, seem to involve factors other than E2, (eg. progesterone or estriol), intact female and male mice responded similarly and OVX female mice had an opposing response. This study highlights how E2 pretreatment can protect female and male mice, although the way in which E2 modulates the immune response in each sex is similar in some responses and different in others. In the end, each set of cells results in protection from EAE and should be studied further as potential therapeutics could arise from studying which cell types are most important in protecting each sex from MS. Additionally this study focuses only on the effect of E2 on immune function and not the differential effect between the sexes that E2 could have on neural cells in protecting the CNS from MS.
Supplementary Material
Acknowledgments
Funding: This study was funded by the National Institute of Neurological Disorders and Stroke grant RO1 NS080890 (HO).
This work was supported by the National Institute of Neurological Disorders and Stroke grant RO1 NS080890 (HO). This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, Portland, OR. The contents do not represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the VA Portland Health Care System. This article does not contain any studies with human participants performed by any of the authors.
References
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Benedek G, et al. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2016;293:45–53. doi: 10.1016/j.jneuroim.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Zhang J, Nguyen H, Kent G, Seifert H, Vandenbark AA, Offner H. Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. J Neuroimmunol. 2017;305:59–67. doi: 10.1016/j.jneuroim.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Lapato A, Vandenbark AA, Murphy SJ, Saugstad JA, Offner H. Regulatory CD8(+)CD122 (+) T-cells predominate in CNS after treatment of experimental stroke in male mice with IL-10-secreting B-cells. Metab Brain Dis. 2015a;30:911–924. doi: 10.1007/s11011-014-9639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Galipeau D, Vandenbark AA, Offner H. PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. J Clin Cell Immunol. 2013;4:143. doi: 10.4172/2155-9899.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis. 2015b;30:1515–1529. doi: 10.1007/s11011-015-9714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltrama M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Ding Q, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghmorad D, et al. Medium-dose estrogen ameliorates experimental autoimmune encephalomyelitis in ovariectomized mice. J Immunotoxicol. 2016;13:885–896. doi: 10.1080/1547691X.2016.1223768. [DOI] [PubMed] [Google Scholar]
- Iwata Y, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005;174:2387–2395. doi: 10.4049/jimmunol.174.4.2387. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–347. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Steeg LG, Klein SL. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walecki M, et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell. 2015;26:2845–2857. doi: 10.1091/mbc.E14-08-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H. IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice. J Neuroimmunol. 2015;285:129–136. doi: 10.1016/j.jneuroim.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


