Abstract
Background
Early life adversity (ELA) is a risk factor for development of gastrointestinal disorders later in life. The underlying mechanisms through which ELA and sex interact to influence disease susceptibility remains poorly understood.
Methods
Utilizing a porcine early weaning stress (EWS) model to mimic ELA, we investigated the long-term effects of EWS on functional diarrhea, ileal permeability, mast cell activity and relationship to enteric ganglia.
Key Results
Juvenile and adult EWS pigs exhibited chronic, functional diarrhea (EWS 43.6% vs LWC 4.8%, p<0.0001), increased intestinal permeability (2 fold increase EWS vs LWC, p<0.0001), and mast cell numbers (at 7 weeks and 20 weeks ~1.6 fold increase EWS vs LWC, p<0.05). Compared with EWS male castrates (Male-C), females EWS pigs exhibited more frequent diarrhea (58.8% vs 29.9%, p=0.0016), and increased intestinal permeability (1–2 fold higher in EWS females, p<0.001). Increased mast cell numbers and their enhanced co-localization with neuronal ganglia were observed in both Male-C and female EWS pigs; however, female pigs exhibited greater release of mast cell tryptase upon activation with c48/80 (~1.5 fold increase, p<0.05), compared with Male-C pigs.
Conclusions and Inferences
These data demonstrate that pigs exposed to ELA exhibit increased vulnerability to functional diarrhea, intestinal permeability and mast cell activity. Further, these studies also showed that EWS female and Male-C pigs exhibited dimorphic responses to EWS with female piglets exhibited greater susceptibility and severity of diarrhea, intestinal permeability and mast cell tryptase release. Together, these findings mimic some of the key pathophysiologic findings in human functional GI disorders (FGIDs) suggesting that the EWS porcine model could be a valuable preclinical translational model for FGID research associated with ELA.
Keywords: Translational Research, Large Animal Model, Intestinal Permeability, Mast Cell, Developmental Origins of Health and Disease, Early Life Adversity, Mast Cell Plexitis
Graphical abstract
Abbreviated abstract: The present study demonstrates that early weaning stress in piglets induces lasting alterations in GI function that mimic some of the key features of human FGID’s including chronic functional diarrhea, intestinal permeability, and enhanced mast cell activity and localization with enteric nerves. Potential sex differences were evident as females pigs exhibited a more severe clinical and pathophysiological EWS phenotype compared with Male-C pigs. Therefore the EWS porcine model holds promise as a pre-clinical translational model for studying the pathophysiology of FGIDs such as IBS-D that are associated with early life adversity.
Introduction
Early life adversity (ELA) is a risk factor in the susceptibility to chronic gastrointestinal (GI) diseases later in life, including functional gastrointestinal disorders (FGIDs) such as irritable bowel syndrome (IBS).(1–4) ELA encompasses many risk factors including physical, emotional, and sexual abuse, which are all known to contribute to development of FGIDs.(2) Further supporting the connection between early life experiences and adult GI health, patients with both FGIDs and a history of ELA experience increased GI symptom severity compared to patients with FGIDs lacking a history of ELA.(5)
The underlying mechanisms linking ELA with increased vulnerability to FGIDs such as IBS remain poorly understood. The primary underlying pathophysiology reported in IBS patients, particularly diarrheal predominant IBS (IBS-D), include increased gut epithelial permeability (6–11) and increased intestinal mast cell numbers and degranulation.(12–18) Furthermore, the association of mast cells with enteric nerves has correlated with increased FGID symptom severity.(12, 19, 20) Increased mast cell numbers and their release of mediators such as histamine and proteases are thought to be responsible for increased intestinal permeability and a central mechanism in abdominal pain reported in IBS patients.(21, 22) Rodent models have been critical to understanding the GI pathophysiology induced by ELA; however, translation of this research to effective therapies in conditions such as human FGID has been slow and unpredictable.(23) In order to facilitate bench to bedside science for FGIDs, a new animal model may be necessary to compliment treatments researched and developed in rodents.
Pigs are recognized as an important translational model for humans, especially for GI research. The ontogeny and at birth developmental stage of the porcine intestinal epithelium, immune system, and enteric nervous system are more similar to human infants than neonatal mouse pups thus making pigs an ideal model to study ELA-associated diseases. (24) Early weaning stress (EWS) has been used previously by our group to mimic ELA in humans and to study the mechanisms of ELA-associated disease. Similar to cases of human early life adversity, EWS in piglets imposes significant psychosocial and environmental stress during a critical window of post-natal development. The stressors associated with EWS in pigs include the abrupt loss of maternal care, littermate separation and commingling with unfamiliar pigs, changes in social status, and introduction to a novel unfamiliar environment. Previously, we have shown that EWS induces activation of the hypothalamic-pituitary-adrenal (HPA) axis, upregulation of intestinal corticotropin releasing factor (CRF), and increased mast cell numbers and activation leading to elevated intestinal permeability (25–27) in pigs at least 9 weeks old. While previous studies have focused on early intestinal barrier responses to EWS up to juvenile stages, the long-term effects of EWS on clinical disease, intestinal permeability, and mast cell activity and localization, and potential sex differences have not been investigated. The objective of the present experiment was to define the influence of EWS on the incidence of diarrhea, intestinal permeability, and intestinal mast cell numbers, activity, and localization in female and Male-C pigs.
Methods
Animals
The University Institutional Animal Care and Use Committee (IACUC) approved all studies (Protocol #09-047-B). Animal protocols were performed as in our previous studies.(28) Yorkshire-duroc cross, female and Male-C piglets (castrated at 9 days of age) were weaned from their sow at 15 days of age (EWS) or 28 days of age (LWC). Weaned pigs were housed in pens with Tenderfoot® flooring (3.7 m × 2.7 m, n = 6 pigs per pen) in the same environmentally controlled room. All pigs were offered ad libitum access to the same diet and water. The diets were formulated to meet or exceed the nutrient requirements of all pigs in the study.(29) Upon arrival, all pigs were evaluated by a licensed veterinarian and no clinical evidence consistent with common enteric diseases (e.g. reduced feed intake, depressed activity, hypothermia/huddling) were observed.
At 7 and 20 weeks of age, representing juvenile and adulthood stages, respectively, n = 12 pigs/weaning age group (6 females, 6 Male-C) were euthanized via captive bolt penetration and intestinal tissues were immediately harvested for experiments, and prepared for Ussing chambers analysis or stored at −80°C for subsequent biochemical analyses.
Fecal Scoring
Fecal scores were recorded on 12 early weaned and 12 late weaned pigs (equal number of Male-C and females between groups) for a 4 week period between 16–20 weeks of age at 1500–1700 h. To evaluate stool form, pigs were rectally palpated every 2–3 days at the same time of day to stimulate defecation. The resulting bowel movements from each pig following rectal palpation were scored by a trained individual blinded to experimental treatments using The Bristol Stool Form Scale. Scores ≥6 were considered diarrhea based on the description of the scoring system and what has been used to determine diarrhea in humans (30). The percentage of days in diarrhea were calculated by counting the number of times a pig was scored with ≥6 divided by the total number of days scored. Bowel movements rated as 7 on the Bristol Stool Form Scale were considered severe diarrhea.
Histology evaluation of intestinal tissues
Distal ileum sections were collected at euthanasia and placed directly in 10% neutral buffered formalin. After 24 hours, samples were removed and placed in 70% ethanol for long-term storage. Transverse sections of ileum were embedded in paraffin for and stained with hematoxylin and eosin. A board certified veterinary pathologist (KJW) read the slides (n=6 for each weaning age group and balanced by sex) to evaluate differences in inflammatory cell infiltrate or epithelial cell morphology between EWS and LWC pigs.
Mast Cell Staining and Counting
Ileum was collected from EWS and LWC pigs 7 at 20 weeks of age and fixed in neutral buffered formalin and processed as mentioned above. Slides were immunohistochemically labeled by Michigan State University’s Investigative Histopathology Laboratory (East Lansing, MI) with Mast Cell Tryptase Antibody (FL-275) (sc-32889, Santa Cruz Biotechnology, Dallas, TX) at 1:300 dilution. Detection of tryptase was performed using secondary anti-rabbit-on-Farma HRP-Polymer for 30min at RT. Toluidine blue staining was performed on 4% PFA fixed sections that had been embedded in Tissue Tek OCT compound. 10 μm sections were stained in 0.5% Toluidine blue at 0.5 pH for 30 min. Mucosal mast cells were counted in 10 random fields per subject and corrected for lamina propria area using ImageJ (U.S. NIH, Bethesda, MD). Submucosal (SMP) and myenteric plexi (MP) were identified by morphology (collection of cells with large nucleus and large nucleolus) and confirmed with S100 immunohistochemical labeling. Plexus-associated mast cells were defined as mast cells that were adjacent to enteric plexi without any other cell or cell structure in between. For the SMP counts, the outer and inner SMP of the pig were included in the total SMP counts. Counts were performed on n=12 EWS and LWC pigs with 5–6 Male-C and females per group (for tryptase staining) and confirmed on n=6 early and late weaned pigs with 3 males and 3 females per group with Toluidine ble staining. Images and quantification was performed by an individual blinded to experimental treatments.
Ileal mucosal permeability measurements on Ussing Chambers
Ileum was harvested from each animal at 7 and 20 weeks of age. Following euthanasia, ileal segments were opened lengthwise along the anti-mesenteric border. In oxygenated (95% O2, 5% CO2) porcine ringer solution (154 Na+ mM, 6.3 K+ mM, 137 Cl− mM, 0.3 H2PO3 mM, 1.2 Ca2+ mM, 0.7 Mg2+ mM, 24 HCO3− at pH 7.4) at 37°C, the seromuscular layer was removed from the tissue by blunt dissection. Tissue free of Peyer’s patches was then mounted in a 0.3cm2 aperture on Ussing chambers (Physiologic Instruments, Inc., Sand Diego, CA or World Precision Instruments, Sarasota, FL) as described in previous studies.(28, 31) The tissue was bathed in porcine ringer’s solution on each side of the tissue. The serosal bathing solution contained 10 mM glucose that was balanced with 10 mM mannitol on the mucosal side. Bathing solutions were oxygenated (95% O2, 5% CO2) and maintained at 37°C. The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Tissues were maintained in the short-circuited state, except for brief intervals to record the open-circuit PD. Transepithelial electrical resistance (TER; Ω•cm2) was calculated from the spontaneous PD and short-circuit current (Isc). After a 30-min equilibration period on Ussing chambers, TER was recorded at 1-min intervals over a 60-minute period and then averaged to derive the basal TER values for a given animal.
Mucosal-to-serosal fluxes of FITC Dextran and 3H-labeled mannitol
Permeability studies were performed as described in previous experiments.(31, 32) Mucosal-to-serosal fluxes of (FITC)-dextran 4kDa (FD4) and 3H mannitol (180 Da) (Sigma, St. Louis, MO) were used to assess ileal permeability. After tissue was equilibrated for 15 minutes on Ussing chambers, 0.25mM FD4 and 0.2 mCi ml−1 of 3H mannitol were added to the mucosal side of Ussing chamber-mounted tissues. FD4 flux was run on Physiologic Instruments Ussing Chambers and radioisotope flux was performed on WPI Ussing Chamber system. The probes were allowed to equilibrate for 15 min after which standards were taken from the mucosal and serosal side of each chamber and a 90-min flux period was established by taking 50 μl samples for FD4 and 500 μl samples for 3H mannitol from the serosal compartment at the beginning and end of the 90-min flux period. Presence of FD4 was measuring using Ex/Em readings at 488/525 on a fluorescent plate reader. The presence of and 3H was established by measuring β-emission in a liquid-scintillation counter (model 1219 Rack Beta, LKB Wallac, Perkin Elmer Life and Analytical Sciences, Boston, MA). Unidirectional FD4 and 3H mannitol mucosal-to-serosal fluxes were evaluated by determining the net appearance of and FD4 and 3H over time in the serosal bathing solution on a chamber over time and presented as μmol (cm2) −1 h−1 unit area basis. Data are presented as the Fold change in EWS pigs relative to their respective, sex matched LWC pigs, such that EWS Male-C paracellular probe flux was measured relative to LWC Male-C and EWS females paracellular probe flux was measured relative to LWC female.
Ex vivo ileal mast cell activation experiments
Mast cell activation experiments were performed with ileal mucosal explants from 20 week old EWS and LWC pigs Ileum. Tissues were pinned out and stripped of seromuscular layer as in Ussing chamber experiment. An approximately, 1cm × 3cm strip of mucosa was cut from an area free of Peyer’s patch and placed in 3mL room air bubble RPMI and allowed to equilibrate for 15 minutes. At 15 minutes, 10ug mL−1 of the mast cell degranulating drug c48/80 (Sigma Aldrich, St. Louis, MO) was added, and the samples were allowed to incubate for 30 minutes. Tissues were removed, blotted dry, and weighed. Supernatants were set on ice and spun at 300xg at 4°C for 15 minutes. Supernatant was aliquoted and stored at −70°C, and sample were thawed on ice prior to tryptase activity quantification. Tryptase activity was quantified using a kinetic assay with a thiobenzyl ester substrate, and activity was standardized based on tissue weight.(33)
Clinical pathology and enteric infectious disease panel
Following euthanasia, ileum and colon samples were collected from pigs at 20 weeks and flash frozen on liquid nitrogen and stored at −80°C. Three EWS female and two LWC females samples of ileum and colon were packaged on dry ice and shipped to the Veterinary Diagnostic Laboratory at Iowa State University’s College of Veterinary Medicine in Ames, Iowa to screen for diarrheal enteric pathogens, including Brachyspira hampsonii, Brachyspira hyodysenteriae, Lawsonia intracellularis, and Salmonella spp using qPCR. Samples were considered negative if the cycle threshold exceeded 40.
Statistical Analysis
Data was analyzed with a Two Way ANOVA with post hoc Fisher’s Least Significant Difference test where appropriate to determine effects of weaning, sex, or interaction and any specific difference between groups using GraphPad Prism version 6 for Windows, (GraphPad Software, San Diego CA USA). Fecal Scoring data was analyzed using SAS University Edition for Windows, SAS Institute, Cary NC USA, and a generalized linear mixed model with logistical regression and least square means was employed to determine interactions between weaning and sex.
Results
Early weaned pigs exhibit chronic, relapsing functional diarrhea
In the present study we used the Bristol Stool Form Scale to assess the consistency of stool produced by pigs. Our use of the Bristol Stool Form Scale was not to directly compare the nature of pig feces with that of human feces, but to provide a more detailed description and comparison of the stools produced by EWS and LW pigs. Analysis of fecal scores conducted over 4-week period, showed that EWS pigs exhibiting diarrhea 43.6%±4.0% of the time whereas LWS pigs exhibited diarrhea 4.80%±1.7% of the time Figure 1a (p<0.0001). When combining LWC and EWS data, there were no statistical differences in fecal scores between Female and Male-C groups (All Male-C, 14.4%±3.2%, All Females 18.8%±4.8%, p=0.441, Figure 1B) indicating that there were no inherent differences in fecal scores between Male-C and females pigs. However, 2-Way ANOVA analysis revealed that EWS female pigs exhibited diarrhea more frequently compared with EWS Male-C pigs (EWS-female, 58.8% ± 5.31% vs. EWS-Male-C 29.9% ± 5.0%, p=0.0016, Figure 1C). In addition, EWS female pigs had significantly more days with fecal scores equal to 7 (extremely liquid diarrhea), compared to EWS Male-C pigs (Figure 1D). Representative bi-daily fecal scores for EWS Male-C pigs (Figure 1E) and females (Figure 1F) showed the episodic nature of fecal score patterns. Together these data demonstrated that while female and Male-C pigs both exhibited increased fecal scores in response to EWS, EWS female pigs exhibited more frequent and severe diarrhea compared with EWS Male-C pigs.
Figure 1. Influence of early weaning stress in pigs on diarrhea frequency and intestinal histopathology.
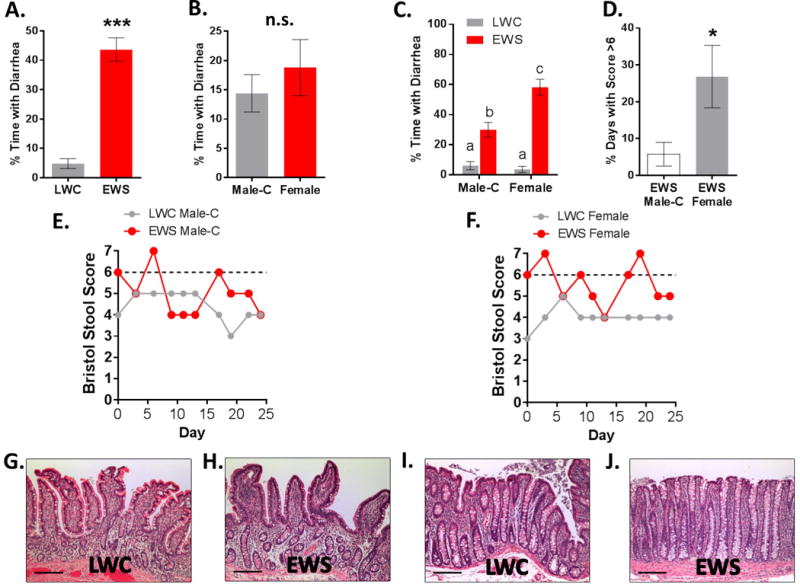
Stool scores were conducted in pigs between 16–20 weeks of age using the Bristol Stool Scale Scoring System. A) % of days with diarrhea (stool score ≥ 6) in LWC and EWS pigs, B) Comparison of Male-C and female pigs (combined LWC and EWS pig data) C) % of days with diarrhea for Male-C and female pigs; D) % of days with severe diarrhea (stool score ≥ 7) in EWS Male-C and females. E&F) Representative Bristol Stool Form Scores over a 25-d period (dotted line indicating threshold for diarrhea). G–H) Representative H&E stained sections of ileum mucosa. I–J) Representative H&E stained sections of colonic mucosa. Scale bar = 100 μM. Data (A–D) are means ± standard error. Student’s T-test (A,B, and D) *** p<0.001, * p<0.05; Letters “a, b, c” (Panel C) indicate statistical significance between groups by Two way ANOVA with Fisher’s LSD post hoc test. LWC = Late Weaned Control, EWS = Early Weaning Stress, Male-C = male castrated pigs. LWC=Late.
Histopathological analysis of ileal and colonic mucosa evaluated by a board certified veterinary pathologist (KJW) blinded to experimental groups, revealed no histopathological differences associated with early weaning or sex with regards to lamina propria cellularity (inflammation), presence of epithelial abnormalities, or edema. (Figure 1G–J). Intestinal samples were determined to be negative (CT values >40), for major porcine bacterial enteric pathogens (Brachyspira hyodysenteriae, Brachyspira hampsonii, Salmonella spp, and Lawsonia intracellularis) (Supplemental Table S1). No differences in rectal temperature, or peripheral blood leukocyte counts as determined by complete blood count analysis were detected between the groups (Supplemental Figure S1). Together, these findings provide evidence that the diarrhea in EWS pigs was functional in nature rather than from infectious or inflammatory causes.
EWS induces heightened and persistent elevations in ileal permeability in female pigs compared with Male-C pigs
In the present study, both Male-C and female EWS pigs exhibited increased ileal permeability (measured as increases in FD4 flux rate) relative to LWC pigs at 7 weeks of age. Female EWS pigs exhibited significantly greater FD4 flux rates (2 fold increase over female LWC) compared with Male-C pigs (1.5 fold increase over Male-C LWC) (p<0.0001; Figure 2A). At 20 weeks of age, there were no significant differences between EWS and LWC pigs with regards to FD4 flux rate (Figure 2B); however, measurements using the smaller paracellular probe, 3H mannitol, demonstrated an increased ileal permeability in female EWS pigs but not Male-C pigs (p<0.05; Figure 2C). Transepithelial electrical resistance (TER), a measurement predominantly reflecting paracellular ion permeability, showed that EWS pigs exhibited reduced TER at 7 weeks of age (p=0.018) compared with LWC pigs; with a significant difference between EWS Male-C and LWC Male-C. (Figure 2D). While TER differences between LWC and EWS pigs were not different at 20 weeks of age (2-WAY ANOVA, p=0.15), Female EWS pigs exhibited a lower ileal TER compared with Male-C EWS pigs (Figure 2E).
Figure 2. Influence of early weaning stress in pigs on ileal permeability.
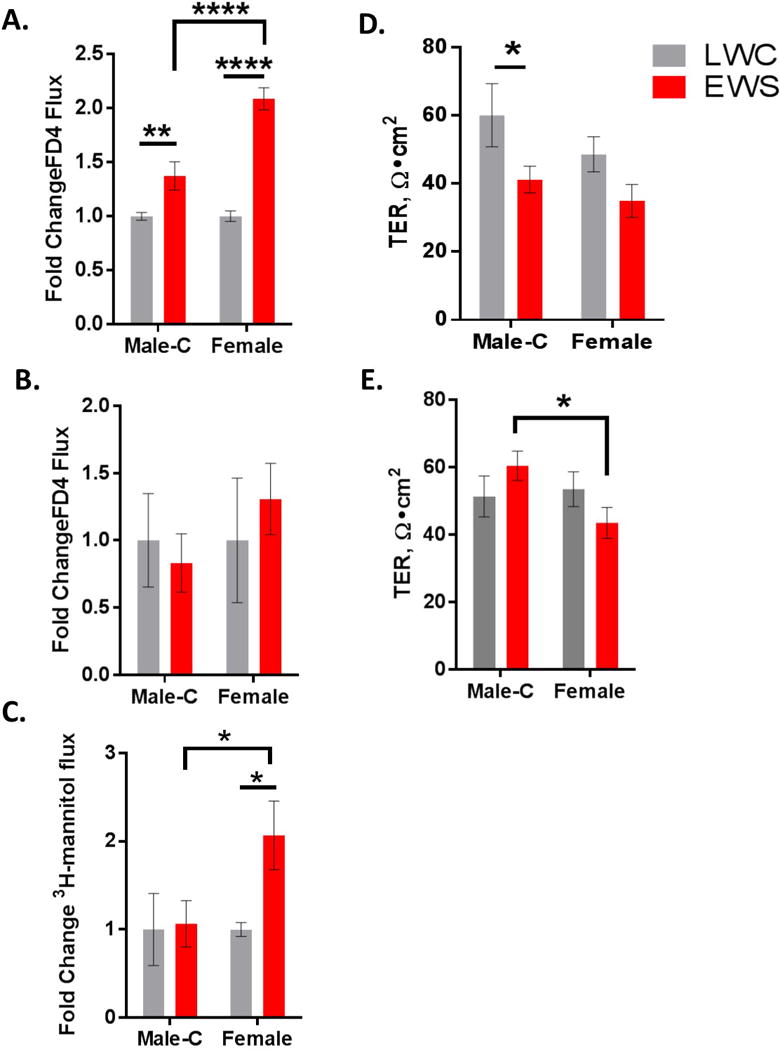
Ileal mucosa from pigs were mounted on Ussing Chambers for evaluation of permeability. A) FD4 flux in 7 week old Male-C and female pigs, data presented as fold change relative to respective to LWC control. B) FD4 flux in 20 week old Male-C and female pigs, C) 3H-mannitol flux in 20 week old Male-C and female pigs. D) Ileal transepithelial resistance (TER) in 7 week old Male-C and female pigs. E) Ileal TER in 20 week old Male-C and female pigs. Data presented are means ± standard error (n=6/experimental group). Two way ANOVA with Fisher’s LSD post hoc test for panels A–E. ****p<0.0001, **p<0.01,*p<0.05. LWC=Late Weaned Control. EWS=Early Weaning Stress, Male-C = male castrated pigs.
EWS induces increased ileal and colonic mast cell numbers and tryptase release
We examined the ileum mucosa of both LWC and EWS pigs at 7 and 20 weeks of age for numbers of tryptase-positive mast cells. A significant increase in tryptase-positive mast cells was observed in the ileal mucosa of EWS pigs, compared with LWC pigs at both 7 (Figure 3A, 3Aii–3Aiii; by 1.8 fold) and 20 weeks (Figure 3B, 3Bii–3Biii by 1.6 fold) of age. Comparisons between Male-C pigs and Female groups revealed that EWS Male-C pigs had a greater number of tryptase-positive mast cells relative to respective LWC controls while no differences were observed between LWC and EWS Female pigs (Figure Ai and Bi). Because tryptase is an intracellular MC mediator which the cellular composition can be influence by activation or regulation by external stimuli such as LPS,(34) we wanted to confirm increased mast cell numbers in EWS pigs using Toluidine blue (T. blue) staining which identifies all mast cell granules. In line with tryptase cell counts, the number of T. blue-positive mast cells was elevated in EWS pig mucosa, with a significant difference between EWS Male-C and LWC Male-C pigs (Fig 3C–Ci). A significant increase in mast cell numbers in colonic mucosa of EWS pigs was also observed at 7 weeks of age Male-C EWS pigs exhibiting the highest tryptase-positive cell counts (Fig 3D, Di.). At 20 weeks of age, there was a trend (p=0.08) for higher numbers of colonic mast cells in EWS pigs (Supplemental Figure 2).
Figure 3. Influence of early weaning stress in pigs on ileal and colonic mast cell numbers.
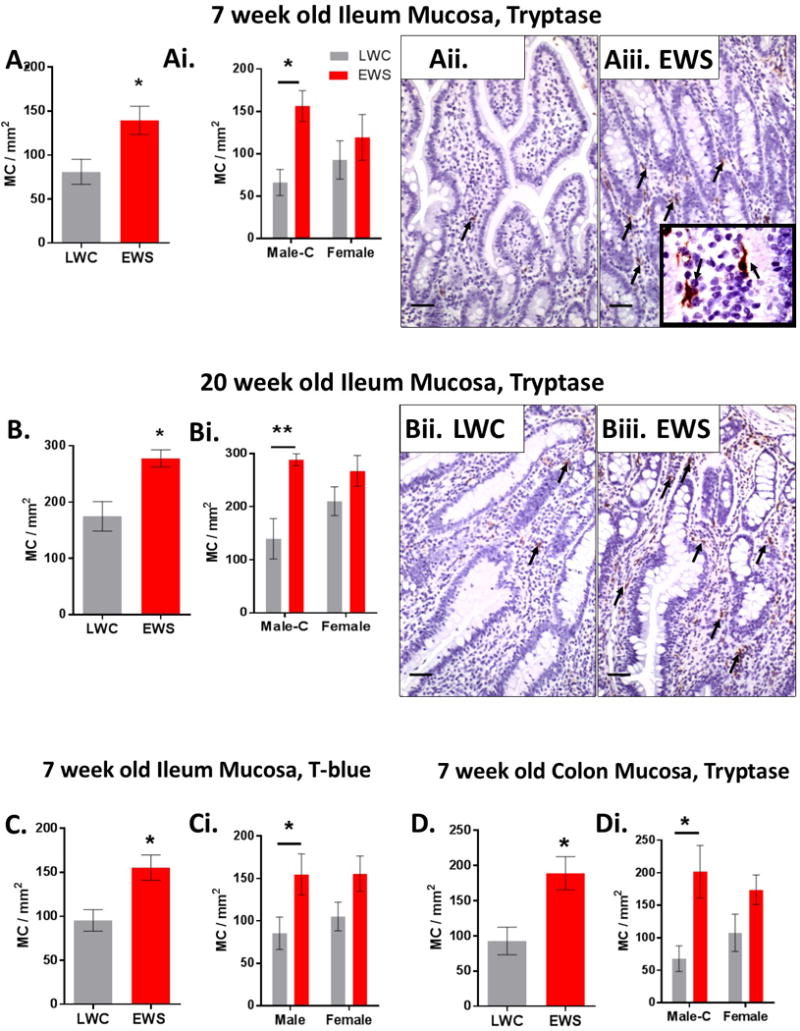
Ileum sections from pigs were stained for mast cell tryptase and tryptase stained cells were quantified to determine mast cell numbers. A) Ileal mast cell (MC) numbers in 7 week old LWC and EWS pigs. Ai) Ileal mast cell numbers in 7 week old Male-C and female pigs. Aii) LWC ileum mucosa and Aiii) EWS ileum mucosa (inset is 100x image demonstrating tryptase-positive mast cell morphology). Arrows indicate red stained tryptase positive mast cells. B) Ileal mast cell numbers in 20 week old pigs. Bi) Ileal mast cell numbers 20 week old Male-C and female pigs. Bii) LWC ileum mucosa and Biii) EWS ileum mucosa. Arrows indicate red staining mast cells. C) Ileal mast cell numbers determined by Toluidine blue staining in 7 week old pigs. Ci) Ileal mast cell numbers determined by Toluidine blue staining in 7 week old Male-C and female pigs. D) Colonic mast cell numbers determined in 7 week old pigs. Di) Colonic mast cell numbers in 7 week old Male-C and female pigs. Data presented are means ± standard error (n=6/experimental group) Two way ANOVA with Fisher’s LSD post hoc test. **p<0.01,*p<0.05. LWC=Late Weaned Control. EWS=Early Weaning Stress, Male-C = male castrated pigs. All images 20x magnification. Scale bar = 50 μm. LWC=Late Weaned Controls. EWS=Early Weaning Stress.
Previously in juvenile (9 weeks of age) EWS pigs, we demonstrated that mast cell activity and protease expression were increased in EWS and contributed to increased intestinal permeability (31) To gain further insight into the chronicity of this response, we conducted ex vivo mast cell stimulation experiments with EWS and LWC ileum (20 week old pigs) and measured tryptase release in supernatants. Stimulation of ileal explants with the mast cell secretagogue, c48/80 induced a release of tryptase which tended to be higher in EWS pigs compared to later weaned controls (p=0.09) (Figure 4A). Comparison between Male-C and Female groups revealed that Female EWS ileum exhibited a greater (by ~ 1.6-Fold) release of tryptase compared with Female LWC and Male-C pigs (Figure 4B). Given that intestinal mast cell numbers were similar between female and Male-C EWS pigs, these data suggest that Female pigs exposed to EWS released more tryptase upon activation.
Figure 4. Influence of early weaning stress on ileal mast cell tryptase release.
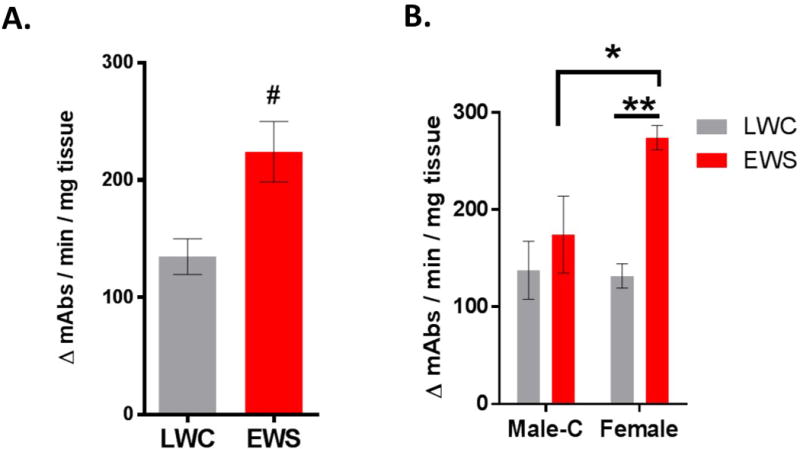
Ileal mucosal explants from 20 week old pigs were treated with secretagogue c48/80 and tryptase was measured in the supernatant via a substrate-based tryptase activity assay. A) EWS pigs tend to release more mast cell tryptase when treated with c48/80. B) EWS female ileum sections released more mast cell tryptase into supernatants compared LWC females and EWS Male-C pigs. Two Way ANOVA with Fisher’s LSD post hoc test (*p<0.01); (**p<0.005). LWC=Late Weaned Controls. EWS=Early Weaning Stress, Male-C = male castrated pigs
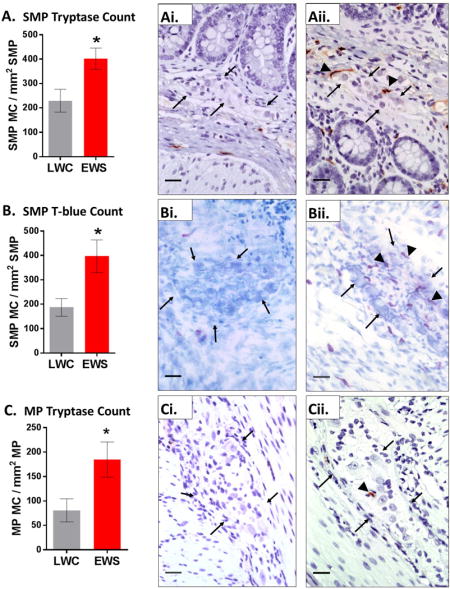
Mast cells infiltrate enteric nerve ganglia in EWS pigs
Previous studies in IBS patients have reported mast cells to be in increased association with the peripheral enteric nervous system.(12, 19) Additionally, in other FGIDs such as functional dyspepsia, mast cells have been found in have increased associations with submucosal plexus.(35) Therefore, we next examined whether EWS in pigs increased the number of intestinal mast cells associated with the SMP and MP. At the 7 week time point, ileum from EWS pigs exhibited increased numbers of tryptase-positive mast cells (Fig 5A–Aii.) and T-blue mast cells (Figure 5B–Bii). Increased tryptase positive mast cell numbers in the MP were also found in EWS pigs compared to LWC pigs (Fig 5C–Cii). The increased association of mast cells with enteric plexi was found in both Female and Male-C (data not shown).
Figure 5. Influence of early weaning stress on the numbers of enteric ganglia-associated mast cells in pigs.
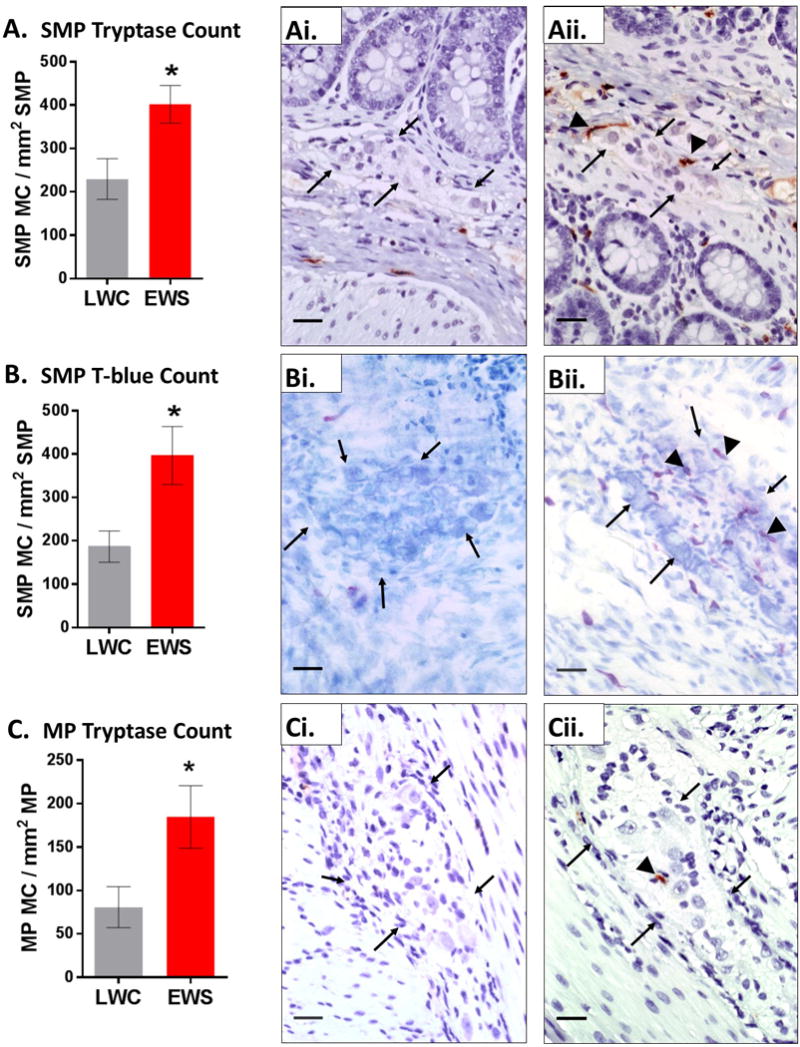
Ileal mucosa/submucosa segments from 7 week old EWS and LWC pigs (Male-C and female pigs combined within each weaning experimental group) were stained for mast cell tryptase or T-blue and the number of mast cells (MC) co-localized with neuronal ganglia in the submucosal plexus (SMP) and myenteric plexus (MP) were quantified. A) SMP-associated MCs (tryptase-positive) in LWC and EWS pigs Ai) LWC ileum SMP tryptase stained, Aii) EWS ileum SMP tryptase stained. B) SMP-associated MCs (T.blue stained) in LWC and EWS pigs. Bi) LWC ileum SMP, T-blue stained. Bii) EWS ileum SMP, T-blue stained. C) MP-associated MCs (tryptase-positive) in LWC and EWS pigs Ci) LWC ileum MP tryptase stained, Cii) EWS ileum MP tryptase stained. B) SMP-associated MCs (T.blue stained) in LWC and EWS pigs. Images are 40x. Scale bar = 25 μm. Black arrows indicated periphery of ganglia. Black arrowheads identify mast cells. Data (A–C) are means ± standard error. Students T-test (* p<0.05) LWC=Late Weaned Controls. EWS=Early Weaning Stress.
Discussion
Early life adversity and environmental challenges during postnatal development represent a significant risk factor for chronic GI diseases in adult life (1–3); however, the mechanisms remain poorly understood. Here we show that, ELA induced by EWS in piglets, induces lasting disturbances in ileal barrier function, mast cell numbers, mast cell-nerve association, and functional diarrhea. This study also revealed that female pigs exposed to EWS exhibited a higher incidence and severity of diarrhea, heightened and persistent illeal barrier defects, and increased mast cell tryptase release, compared with Male-C pigs.
ELA in piglets induces chronic, relapsing functional diarrhea
In the present study, piglets exposed to ELA through EWS developed a chronic, relapsing functional diarrhea that persisted into adulthood. The frequency of diarrhea in the EWS pigs was high, as male and female pigs had chronic relapsing diarrhea approximately 40% of the time. Moreover, EWS female pigs presented more frequently with severe diarrhea, compared with Male-C pigs. The pathophysiologic mechanisms driving the diarrhea in EWS are not completely understood. In line with the present findings, we have previously demonstrated that EWS pigs exhibited increased diarrheal disease in response to a live oral challenge with enterotoxigenic E. coli. (36) In addition, more recently we showed that EWS pigs exhibited a persistent, heightened sensitivity to neural-evoked secretomotor responses mediated largely by an upregulated cholinergic nervous system (28) that was more severe in EWS female pigs compared with Male-C pigs. Additional contributing factors contributing to diarrhea in EWS pigs could be the heightened excitability of intestinal mast cells. Mast cell activation and release of mediators including histamine and proteases can activate enteric secretomotor neurons (37) and (or) induces intestinal barrier defects (38, 39) as observed in EWS pigs leading to enhanced intestinal secretion and diarrhea. Together, findings in the present study and our previous studies suggest that EWS leads to developmental alterations in the ENS (e.g. upregulated cholinergic system) and immune system (e.g. mast cell activation) leading to a hypersensitivity to intestinal secretory stimuli which in turn could manifest as chronic functional diarrhea or heightened sensitivity to later life stress.
While the link between ELA and chronic diarrheal disease in humans is poorly defined, FGID’s associated with ELA such as IBS exhibit chronic alterations in bowel habits. As stated above, both Male-C and female EWS pigs exhibited diarrhea but females exhibited more frequent bouts and severe diarrhea compared with Male-C pigs. In human IBS, men report more diarrhea predominant IBS (IBS-D), while females more commonly present with constipation-predominant (IBS-C) diarrhea. (40). However, women reporting childhood abuse were equally at risk for developing adult IBS-D or IBS-C.(41) Therefore the timing and nature of the stress could potentially dictate whether an individual will develop diarrhea vs constipation. Currently, the IBS literature is ambiguous on how the age at which abuse occurs, type of abuse, severity of abuse, and duration or times of abuse influence the development one bowel habit over the other (IBS-D vs IBS-C). It seems plausible that some combination of abuse events and environmental factors may influence development of one IBS subtype over another. Therefore, EWS female pigs could more closely resemble a subset of IBS patients who have experienced ELA event.
ELA in piglets induces lasting increases in ileal permeability in female pigs
Intestinal barrier defects marked by increased intestinal permeability is a central pathophysiologic mechanism of GI disease and results in increased translocation of novel food and bacteria antigens. (42–44) Subsequent exposure of antigens to the immune and enteric nervous systems can initiate mucosal inflammation, neuronal hypersensitization and clinical symptoms of abdominal pain and altered bowel habits as seen in IBS (45). In the present study, Male-C and female pigs in the LWC group exhibited a similar ileal permeability suggesting no inherent differences between Male-C and female pigs. Male-C and female pigs subjected to EWS exhibited increased ileal permeability relative to their LWC controls in the adolescent time point (7 weeks of age); however, the severity of the permeability defect in EWS pigs was greater in female pigs at 7 and 20 weeks. In comparison with the present study, human females tend to have increased intestinal permeability when exposed to acute stressors.(46, 47) In line with these findings, our previous studies in mice showed that, compared with adult intact males, female mice exhibited greater ileal permeability in response to acute restraint stress. (48) As the pigs aged and approached sexual maturity in the present study, EWS females continued to exhibit heightened ileal permeability while Male-C EWS pig did not. These data suggest that Male-C pigs may have recovered from EWS-induced permeability disturbances while female EWS pigs did not. The reason for the persistence in ileal permeability defects in EWS females but not Male-C pigs is unclear but may be related to the increased mast cell activity (increased mast cell tryptase release) observed in female EWS pigs, compared with Male-C pigs. We and others have shown previously that mast cell proteases such as tryptase and chymase induce intestinal permeability (38, 39). Also in line with the current findings, our recent studies showed that despite having similar numbers of tissue mast cells, female mice released more mast cell mediators in response to psychological restraint stress and passive systemic anaphylaxis and exhibited increased intestinal permeability compared with intact male mice.(48) Another potential factor contributing to persistent ileal permeability in EWS females could be the increased cholinergic tone and acetylcholine release which was demonstrated in our previous study.(49) Given the female-specific response, potential estrogen effects are also a consideration as estrogen has been shown to both positively and negatively modulate intestinal barrier properties. (50–52) However, given there were no differences in intestinal permeability between LWC Male-C and LWC female pigs at the 7 (prepubertal) or 20 week (pubertal) time point, the role of postnatal estrogens, while possible, does not fit with our the present findings. Experiments with ovariectomized female pigs would provide a more definitive conclusion regarding its role in the heightened and persistent intestinal barrier defects in EWS females.
While functional bowel disorders such as IBS are associated with increased intestinal permeability, the focus has been predominantly on the large intestine (6–11). However, through the use of oral sugar studies in IBS patients, the small intestines have been identified as source of increased intestinal permeability with the small intestine found to be the most significant source of permeability in IBS-D patients after controlling for other confounding factors.(11) Therefore, our observations in the ileum in the present study might be relevant to study the pathophysiology of human GI diseases associated with increased small intestinal permeability.
ELA in pigs induces an increase in intestinal mast cell numbers and localization to SMP and MP
In the present study, piglets that were exposed to EWS exhibited increased intestinal mast cell numbers in the ileum (at 7 and 20 weeks of age) and colon (at 7 weeks of age). In comparison, humans with IBS-D have been found to have increased mast cell numbers in the ileum.(15, 18) Similarly, rodent models have shown that NMS stress results in a persistently elevated increase in intestinal mast cell numbers (53). In addition, the present studies revealed that in EWS pigs, mast cells were more closely associated with enteric neuronal ganglia, suggesting an enhanced neuro-immune communication between mast cells and nerves as a result of EWS in pigs. An enhanced intestinal mast cell-nerve co-localization has been demonstrated in IBS patients, correlating with severity of abdominal pain.(12) In human IBS, female patients were shown to exhibit a higher number of mast cells compared with male IBS patients. (14) In the present study Male-C EWS pigs but not EWS females were statistically different from their respective LWC. While these data imply that EWS Male-C pigs exhibited increased mast cell numbers in response to EWS while EWS females did not, caution should be taken with this conclusion as other factors may have influenced the numbers of mast cells. First, although the increased in mast cell numbers induced by EWS was statistically different Male-C pigs, EWS Male-C and female EWS pigs had similar numbers of tryptase-positive mast cells. The slightly higher number of mast cells in LWS female pigs might have influenced the ability to detect statistical differences (via a 2 Way ANOVA) between LWC and EWS females. Second, mast cell numbers were based upon tryptase and T-blue staining which are both intracellular mast cell granule stains. Therefore, any potential differences in the mediator composition or content between Male-C and female pigs could have influenced mast cell counts. For example, an enhanced released of tryptase could have resulted in less tryptase-stained cells thus leading to an interpretation of less mast cell numbers. In support of this explanation, Male-C and female EWS exhibited comparable numbers of tryptase and T-blue-positive mast cells, but EWS females released greater amounts of tryptase upon c48/80 stimulation. Therefore, an enhanced release of MC tryptase in EWS females pigs could have artificially reduced the number of mast cells in the intestinal tissues in female pigs. We have previously shown that mast cell tryptase and chymase expression can differ significantly in chronically stressed pigs (54) and that female mast cells obtained from rat and mice contain higher amounts of intracellular mediators at baseline (non-stressed conditions) compared with sexually mature intact males (48) suggesting the composition of mediators in female and male mast cells are different and can change in response to stress.
The underlying mechanism for enhanced ileal tryptase release in female EWS pigs remains to be elucidated. Given the present findings and our previous studies in mice and rats, female mast cells possess the ability to synthesize, store and release more mast cell mediators compared with male MCs (48), the enhanced tryptase release could be a result of increased tryptase content in female EWS mast cells. In addition differences in adult sex hormones, such as increased estrogen in female pigs or reduced androgens in Male-C pigs could have contributed to these changes. Unfortunately we did not stage the estrous cycle in female pigs in the present study and therefore we cannot conclude the influence estrous or sex hormone levels on enhanced mast cell activity or numbers. While previous in vitro experiments showed that application of estrogens to cultured mast cells can induce mast cell degranulation(55), very little is known about the influence of estrogens on mast cell activity in vivo. If high estrogen levels during the estrous cycle were influencing the ileal mast cell tryptase response observed in the present study, we would have anticipated an elevated mast cell tryptase released in LWC females compared with LWC Male-C pigs, which was not observed in the present study. In mice, we showed that the elevated mast cell mediator levels and release observed in adult female mice were not influenced by different stages of the estrous cycle. (48)
Comparisons between female and Male-C pigs in their response to EWS: potential role of biological sex factors
In the present study we observed that diarrhea, intestinal permeability and mast cell activity were increased in both EWS Male-C and female pigs; however, EWS females exhibited a more severe EWS phenotype. It is known that biological sex is an important determinant in the susceptibility to stress-related GI disorders such as IBS with females at increased risk by up to 2–3 fold (1, 3, 40, 56–59). Furthermore, recent studies indicated that females, but not males with IBS have a greater prevalence of ELA suggesting a potential interaction between ELA and biological sex in IBS. (4, 5). The precise mechanisms underlying sex differences in human FGID’s such as IBS remain poorly understood. In general, biological sex differences are determined in large part by sex hormones and (or) chromosomal (XX vs XY) factors. Furthermore, sex differences arise from organizational period events (e.g. perinatal gonadal androgen surge in males) which influence early sexual differentiation and prepare the system for later life activational effects of adult sex hormones upon puberty.(60) In the present study, we compared the responses of female pigs with gonectomized (castrated) male pigs (Male-C), which lack the influence of postnatal gonadal androgens. We understand that Male-C pigs are not representative of an intact male and should not be interpreted or compared with intact females as such; however, the dimorphic responses observed between Male-C pigs and females in response to EWS at both prepubertal and adult stages imply that sex factors are likely influencing the response to EWS in this porcine model. However, studies where intact male boars are compared with Male-C and female pigs are required to conclusively define the nature of the dimorphic response observed in the present work. Relevant to this proposed study is a working paradigm in sex biology that suggests that gonadal hormones and sex chromosomes (XX vs XY) can have compensatory, counteracting functions resulting in sexual monomorphism (both sexes having similar phenotype).(61) Therefore, it is possible that intact EWS Male-C pigs would exhibit a similar disease phenotype with EWS females. A monomorphic phenotype in this case however would not imply sexual equivalence because the present studies revealed that Male-C and females are inherently biologically different in their response to EWS. Instead a monomorphic response would suggest that the route by which male and female EWS pigs achieved a similar EWS disease phenotype is via different mechanisms (e.g. gonadal androgens would be involved in creating the disease phenotype in male pigs which would not be the case in females). This could have potential implications for sex-specific therapeutic interventions (61). Furthermore, with comparison to a Male-C pig, a monomorphic response to EWS in intact male and female pigs would implicate testosterone as a detrimental component; however, in male IBS patients, there is evidence of a negative correlation between testosterone levels and visceral pain (62), suggesting that testosterone might be protective. Therefore, it is also possible that use of intact EWS males might have resulted in even larger differences compared to EWS females. In summary, the present study revealed dimorphic responses to EWS between Male-C and female pigs with female pigs exhibiting heightened sensitivity and GI disease. Future studies comparing intact males will be required to determine the nature and mechanisms of this difference.
In summary, the present study demonstrates that EWS in piglets induces lasting alterations in GI function that mimic some of the key features of human FGID’s including chronic functional diarrhea, intestinal permeability, and enhanced mast cell activity and localization with enteric nerves. Potential sex differences were evident as females pigs exhibited a more severe clinical and pathophysiological EWS phenotype compared with Male-C pigs. Therefore the EWS porcine model holds promise as a pre-clinical translational model for studying the pathophysiology of FGIDs such as IBS-D that are associated with ELA.
Supplementary Material
Supplemental Figure 1. WBC and rectal temperatures of EWS and LWC piglets.
Distal colon sections from 20 week old pigs were stained for mast cell (MC) tryptase and tryptase-stained cells were quantified to determine MC numbers. A) Colon MC numbers in LWC and EWS pigs, B) Colon MC numbers in Male-C and female LWC and EWS pigs. Data are means ± standard error. Student’s T-test (A) and Two way ANOVA with Fisher’s LSD post hoc test (B) were conducted. LWC = Late Weaned Control, EWS = Early Weaning Stress, Male-C = male castrated pigs.
CT values >40 indicate non-detectable genetic products of these pathogens. LWC=Late Weaned Controls. EWS=Early Weaning Stress.
Key Points.
Early life adversity (ELA) is risk factor for functional gastrointestinal disorders (FGID) later in life.
Using a porcine early weaning stress (EWS) model of ELA, female and Male-C pigs exposed to EWS exhibited a greater incidence of functional diarrhea, increased intestinal permeability, and increased mast cell numbers and localization to enteric ganglia.
Compared with Male-C pigs, female pigs exhibited a more chronic and severe diarrhea, and heightened ileal permeability and mast cell tryptase release.
Results here highlight the clinical and pathophysiologic responses to ELA in a large animal model which could be relevant to human FGID’s
Acknowledgments
This work was supported by grants from the National Institutes of Health HD072968 (to AJM), DK097462 (to AJM), R03 DK097462 (to AJM) OD011070 (T32 support for EM).
Dr. Adam Moeser is the Matilda R. Wilson Endowed Chair of Large Animal Clinical Sciences, Michigan State University.
We would like to thank Michigan State University’s Histopathology Laboratory Technicians Kathy Joseph and Amy Porter for developing a mast cell staining protocol in pig tissue.
List of Abbreviations
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- FGID
functional gastrointestinal disorder
- ELA
early life adversity
- EWS
early weaning stress
- LWC
late wean control
- ENS
enteric nervous system
- SMP
submucosal plexus
- MP
myenteric plexus
- MC
mast cell
- NMS
neonatal maternal separation
Footnotes
Competing Interests: All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. The authors have no competing interests
Author Contributions:
CSP (performed research, analyzed data, and prepared manuscript). JEM (performed research, reviewed manuscript), LLE (performed research, reviewed manuscript), KDB (performed research, analyzed data, reviewed manuscript), EM (performed research, prepared and reviewed manuscript), MPD (performed research, analyzed data, reviewed manuscript), KJW (analyzed histopathology slides, reviewed manuscript). AJM (designed study, prepared and reviewed manuscript).
References
- 1.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 2.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang L, Toner BB, Fukudo S, et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 4.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2012;10:385–390.e381. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanuri N, Cassell B, Bruce SE, et al. The impact of abuse and mood on bowel symptoms and health-related quality of life in irritable bowel syndrome (IBS) Neurogastroenterol Motil. 2016;28:1508–1517. doi: 10.1111/nmo.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. The American Journal of Gastroenterology. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 7.Gecse K, Roka R, Sera T, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2012;85:40–46. doi: 10.1159/000333083. [DOI] [PubMed] [Google Scholar]
- 8.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. American journal of physiology Gastrointestinal and liver physiology. 2011;301:G919–928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. The Journal of pediatrics. 2008;153:646–650. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 11.Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. 2014;40:288–297. doi: 10.1111/apt.12829. [DOI] [PubMed] [Google Scholar]
- 12.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 13.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Rhee PL, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71–78. doi: 10.1111/j.1440-1746.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 16.Matricon J, Meleine M, Gelot A, et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 17.Martinez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 18.Weston AP, Biddle WL, Bhatia PS, Miner PB., Jr Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–1595. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- 19.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011.e1004. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci. 2003;18:204–210. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome American journal of physiology Gastrointestinal and liver physiology. 2012;303:G775–785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 22.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 23.Holschneider DP, Bradesi S, Mayer EA. The role of experimental models in developing new treatments for irritable bowel syndrome. Expert review of gastroenterology & hepatology. 2011;5:43–57. doi: 10.1586/egh.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohl CS, Medland JE, Moeser AJ. Early Life Stress Origins of Gastrointestinal Disease: Animal Models, Intestinal Pathophysiology, and Translational Implications. American journal of physiology Gastrointestinal and liver physiology. 2015 doi: 10.1152/ajpgi.00206.2015. ajpgi 00206 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeser AJ, Klok CV, Ryan KA, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G173–181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 26.Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G413–421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 27.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medland JE, Pohl CS, Edwards LL, et al. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol Motil. 2016;28:1317–1329. doi: 10.1111/nmo.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research Council (U.S.) Nutrient requirements of swine. 10th. Washington, D.C.: National Academy Press; 1998. Subcommittee on Swine Nutrition. rev. edn. [Google Scholar]
- 30.Palsson OS, Baggish J, Whitehead WE. Episodic nature of symptoms in irritable bowel syndrome. Am J Gastroenterol. 2014;109:1450–1460. doi: 10.1038/ajg.2014.181. [DOI] [PubMed] [Google Scholar]
- 31.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. American journal of physiologyGastrointestinal and liver physiology. 2010;298:G352–363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflammatory bowel diseases. 2013;19:712–719. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson DA. Human mast cell proteases: activity assays using thiobenzyl ester substrates. Methods Mol Biol. 2006;315:193–202. doi: 10.1385/1-59259-967-2:193. [DOI] [PubMed] [Google Scholar]
- 34.Kirshenbaum AS, Swindle E, Kulka M, Wu Y, Metcalfe DD. Effect of lipopolysaccharide (LPS) and peptidoglycan (PGN) on human mast cell numbers, cytokine production, and protease composition. BMC Immunol. 2008;9:45. doi: 10.1186/1471-2172-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cirillo C, Bessissow T, Desmet AS, Vanheel H, Tack J, Vanden Berghe P. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol. 2015;110:1205–1215. doi: 10.1038/ajg.2015.158. [DOI] [PubMed] [Google Scholar]
- 36.McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PloS one. 2013;8:e59838. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett KE. Immune-related intestinal chloride secretion. III. Acute and chronic effects of mast cell mediators on chloride secretion by a human colonic epithelial cell line. J Immunol. 1991;147:959–964. [PubMed] [Google Scholar]
- 38.Groschwitz KR, Ahrens R, Osterfeld H, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PloS one. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther. 2010;32:738–755. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 42.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 43.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 44.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Current opinion in pharmacology. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alonso C, Guilarte M, Vicario M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172.e161. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Alonso C, Guilarte M, Vicario M, et al. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neurogastroenterol Motil. 2012;24:740–746. e348–749. doi: 10.1111/j.1365-2982.2012.01928.x. [DOI] [PubMed] [Google Scholar]
- 48.Mackey E, Ayyadurai S, Pohl CS, DC S, Li Y, Moeser AJ. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016;7:60. doi: 10.1186/s13293-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medland JE, Pohl CS, Edwards LL, et al. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621–626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 52.Diebel ME, Diebel LN, Manke CW, Liberati DM. Estrogen modulates intestinal mucus physiochemical properties and protects against oxidant injury. The journal of trauma and acute care surgery. 2015;78:94–99. doi: 10.1097/TA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 53.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–590. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Song Z, Kerr KA, Moeser AJ. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS One. 2017;12:e0171617. doi: 10.1371/journal.pone.0171617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaitsu M, Narita S, Lambert KC, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Molecular immunology. 2007;44:1977–1985. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 57.Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99:409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- 58.Talley NJ, Zinsmeister AR, Melton LJ., 3rd Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am J Epidemiol. 1995;142:76–83. doi: 10.1093/oxfordjournals.aje.a117548. [DOI] [PubMed] [Google Scholar]
- 59.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e714. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 60.Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32:2096–2104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnold AP. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Exp Neurol. 2014;259:2–9. doi: 10.1016/j.expneurol.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Houghton LA, Jackson NA, Whorwell PJ, Morris J. Do male sex hormones protect from irritable bowel syndrome? Am J Gastroenterol. 2000;95:2296–2300. doi: 10.1111/j.1572-0241.2000.02314.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. WBC and rectal temperatures of EWS and LWC piglets.
Distal colon sections from 20 week old pigs were stained for mast cell (MC) tryptase and tryptase-stained cells were quantified to determine MC numbers. A) Colon MC numbers in LWC and EWS pigs, B) Colon MC numbers in Male-C and female LWC and EWS pigs. Data are means ± standard error. Student’s T-test (A) and Two way ANOVA with Fisher’s LSD post hoc test (B) were conducted. LWC = Late Weaned Control, EWS = Early Weaning Stress, Male-C = male castrated pigs.
CT values >40 indicate non-detectable genetic products of these pathogens. LWC=Late Weaned Controls. EWS=Early Weaning Stress.


