Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) affects 20–30% of adults with risk factors like obesity and insulin resistance putatively acting through chronic low-grade inflammation. Because periodontitis elicits low-grade inflammation, we hypothesized that it could contribute to NAFLD occurrence.
Objective
To investigate epidemiologic associations between periodontitis and the incidence of NAFLD among 2,623 participants of the Study of Health in Pomerania.
Methods
Periodontitis at baseline was defined as the percentage of sites (0%, <30%, ≥30%) with 1) clinical attachment level (CAL) ≥3mm; 2) probing pocket depth (PD) ≥4mm. Incident NAFLD was defined as a significant increase in liver echogenicity on ultrasound relative to the kidneys, with the diaphragm indistinct OR the echogenic walls of the portal veins invisible.
Results
After a median 7·7 years of follow-up, 605 incident NAFLD cases occurred at a rate of 32·5 cases per 1,000 person-years. Relative to participants without CAL ≥3mm, NAFLD incidence was elevated slightly in participants with <30% of sites affected, and moderately in participants with ≥30% of sites affected (multivariable-adjusted incidence rate ratio= 1.28, 95% CI, 0.84, 1.95 and 1·60, 95% CI, 1·05–2·43) respectively. A similar dose-response relationship was not observed for PD.
Conclusion
History of periodontitis may be a risk factor for NAFLD.
Keywords: Periodontal disease, Hepatic steatosis, Epidemiologic, Population Health, Prospective cohort, Oral-Systemic disease
Introduction
Non-alcoholic fatty liver disease (NAFLD), the excessive infiltration of triglycerides into hepatocytes in the absence of excessive alcohol consumption (Neuschwander-Tetri & Caldwell, 2003) is the most common type of liver disease and the hepatic component of the metabolic syndrome (Kotronen & Yki-Järvinen, 2008; Lazo et al., 2013). It comprises a spectrum of conditions ranging from steatosis, to non-alcoholic steatohepatitis (NASH) with or without fibrosis, to liver cirrhosis and hepatocellular carcinoma (Farrell, George, Hall, & McCullough, 2004). Depending on race/ethnicity and diagnostic modality, NAFLD is estimated to affect 17–33% of adults in the U.S. (Angulo, 2002; Clark, Brancati, & Diehl, 2002; Lazo et al., 2013) and 20–30% worldwide (Bedogni et al., 2005; Bellentani, Bedogni, Miglioli, & Tiribelli, 2004; Neuschwander-Tetri & Caldwell, 2003). NAFLD is associated with higher health care costs (Baumeister et al., 2008) and mortality (Baumeister et al., 2008; Musso, Gambino, Cassader, & Pagano, 2011), the latter attributed to cardiovascular and other liver diseases related complications (Adams et al., 2005; Ong, Pitts, & Younossi, 2008; Soderberg et al., 2010).
Risk factors include obesity and insulin resistance (Angulo, 2002; Neuschwander-Tetri & Caldwell, 2003), the effects of which are thought to be mediated via oxidative stress which contributes to NAFLD initiation (Tilg & Moschen, 2010) and progression (Day & James, 1998; Tilg & Moschen, 2010). Other conditions eliciting systemic inflammatory responses likely contribute to NAFLD occurrence. An example is periodontitis, a chronic oral disease affecting 45% of adults in the U.S. (Eke et al., 2015). It manifests as inflammation of the gums and formation of periodontal pockets in response to pathogenic bacteria that colonizes the tooth surface. Host response include production of endotoxins, lipopolysaccharides (LPS) and proinflammatory cytokines (Gurav & Jadhav, 2011; Yucel-Lindberg & Bage, 2013). In the setting of heightened proinflammatory response, the inflammatory process causes gradual periodontal destruction and loss of attachment between periodontal tissues and the tooth. Bacteremia occurs frequently in individuals with periodontitis (Schenkein & Loos, 2013). Furthermore, sera from individuals affected by periodontitis contain elevated levels of LPS which promotes systemic inflammatory response. In addition to the systemic inflammatory response elicited, periodontitis also worsens glycemic control among diabetics, can impair glucose tolerance among non-diabetics and is linked to insulin resistance (Benguigui et al., 2010; Chapple & Genco, 2013; Demmer, Jacobs, & Desvarieux, 2008; Lalla & Papapanou, 2011; Saito et al., 2004; Stewart, Wager, Friedlander, & Zadeh, 2001; Timonen et al., 2011).
Our objectives were to 1) investigate the relationship between clinical periodontitis at baseline and 2) progression of periodontitis on the subsequent development of NAFLD.
Methods
Data Source and study population
The Study of Health in Pomerania (SHIP) is a population-based cohort sampled from the Western Pomeranian region of Northeastern Germany. Details of the study design and data collection have been described (Hensel et al., 2003; John et al., 2001; Volzke et al., 2011). Briefly, residents of West Pomerania aged 20–79 years in 1996 were sampled using a two-stage stratified cluster design. Communities were selected as part of the first stage and individuals were selected in the second stage after stratifying by age and gender. Baseline examinations (SHIP-0), were conducted between 1997 and 2001. Of 6,265 eligible persons invited, 4,308 participated in SHIP-0 (response rate: 68.8%). Follow-up examinations occurred at approximate 5-year intervals, with the first follow-up (SHIP-1) conducted between 2002 and 2006 and the second (SHIP-2) between 2008 and 2012. A total of 3,300 participated in SHIP-1 and 2,333 participated in SHIP-2. Re-examination participation rates were 76.6% and 70.7% respectively. Ethics approval for this study was obtained from the Institutional Review Board of the University of North Carolina at Chapel Hill.
Exposure assessment and characterization
Dental examiners determined periodontitis status at each study visit using measures of probing pocket depth (PD) and clinical attachment level (CAL). PD, defined as the distance from the free gingival margin to the base of the periodontal pocket, is an indicator of periodontitis at the time of examination. CAL, defined as the distance from the cemento-enamel junction (a fixed landmark on the tooth) to the base of the periodontal pocket, signifies the lifetime history of periodontitis up until the time of the examination.
These measurements were made around teeth other than 3rd molars in two dental quadrants (a selected quadrant and its ipsilateral quadrant). PD and CAL were recorded at four sites per tooth: the mesio-buccal, mid-buccal, disto-buccal and mid-lingual or mid-palatal. Measurements were not made when teeth were missing or landmarks could not be determined. The maximum number of sites was 56 per study participant.
To characterize periodontitis at baseline, two person-level classifications were created: 1) the proportion of sites with CAL ≥3mm (0%, <30%, ≥30%); 2) the proportion of sites with PD ≥4mm (0%, <30%, ≥30%). Participants with no teeth (i.e. edentulous) were included as a separate exposure category, under the premise that reasons for tooth-loss probably included some prior experience of periodontitis (Burt & Eklund, 2005). In addition, participants’ mean CAL and mean PD at baseline were also modeled separately.
Progression of periodontitis was computed as the 5-year change in mean CAL between SHIP-0 and SHIP-1. This calculation used only those periodontal sites that were present at both visits (Beck & Elter, 2000). The PCP11 periodontal probe was used for periodontal measurements at SHIP-0, while the PCP2 probe was used at SHIP-1. Thus, mean CAL values were adjusted to minimize biases from digit preferences as described elsewhere (Holtfreter, Alte, Schwahn, Desvarieux, & Kocher, 2012). Because it is measured longitudinally, change in CAL is regarded as the cardinal sign of destructive periodontitis (Beck & Elter, 2000).
Outcome assessment and characterization
Abdominal sonography was performed by trained physicians using a 7.5 MHz transducer (Vingmed VST Gateway, Santa Clara CA). Levels of serum transaminases i.e. markers of hepatic inflammation were determined by analyzing blood samples stored at −80C using standardized procedures (Hitachi 704; Roche, Mannheim, Germany). The presence of fatty liver was assessed using hepatic ultrasound and serum transaminase-alanine aminotransferase (ALT) at baseline (SHIP-0); ALT at SHIP-1; ALT and hepatic ultrasound at SHIP-2. A positive finding on ultrasound was defined as a significant increase in liver echogenicity relative to the kidneys, with the diaphragm indistinct OR the echogenic walls of the portal veins invisible (Baumeister et al., 2008; Williams et al., 2011).
NAFLD case-classification was based on a combination of ultrasound findings and ALT levels in the absence of other causes of liver diseases as previously described (Clark, Brancati, & Diehl, 2003). NAFLD cases were those with a positive finding on ultrasound or ALT above the sex-specific upper threshold of normal defined for this study population i.e. >0.57 μmol/sl for men and >0.4 μmol/sl for women (equivalent to >34.2 U/L for men and 24 U/L for women). For SHIP-1, only ALT values identified incident NAFLD, thus necessitating a strong reliance on the exclusion criteria described below in identifying ‘true’ cases (Clark et al., 2003). ALT instead of AST was chosen because ALT is primarily found in the liver, it’s a more specific marker of hepatocellular injury with levels persisting longer than those of AST after an injury.
Study exclusions
At baseline, individuals (n=604) who reported excessive alcohol consumption (see Appendix Materials and Methods) were excluded. Also excluded were participants self-reporting the following hepatic steatosis-promoting medications: tamoxifen, amiodarone or methotrexate (n=18) (Angulo, 2002; Osman, Osman, & Ahmed, 2007); participants with a doctor’s diagnosis of hepatitis B or C in the past year (n=17), or detectable levels of the corresponding antigen (n=15) or antibody (n=22) in blood samples. Participants with steatosis on ultrasound with or without elevated ALT levels (n=1,265) were excluded as prevalent NAFLD cases. Lastly, individuals (n=14) with missing dental examination data were excluded. Some participants were ineligible for multiple reasons.
Covariates
Baseline covariates include confounders identified after analyzing a directed acyclic graph (Greenland, Pearl, & Robins, 1999) and risk factors for NAFLD. Age was self-reported and was modeled using restricted quadratic splines, thereby allowing for non-linear relationships between age and NAFLD, and hence better adjustment for potential confounding effects of age than is the case if age was modeled using categories or a linear parameter. Gender was reported as male or female. Alcohol was adjusted for to minimize any residual effect of alcohol and was modeled using restricted quadratic splines. Waist circumference was measured in centimeters and modeled using restricted quadratic splines. BMI was categorized into underweight/normal (<25 kg/m2), overweight (25–30 kg/m2) and obese (>30 kg/m2). Education was used as a marker of socio-economic position and was categorized into <10, 10 and >10 years of formal education. Diabetes was based on self-reported physician’s diagnosis or antidiabetic treatment or non-fasting glucose levels ≥11.1 mmol/l or glycated haemoglobin (HbA1c) concentration ≥6.5%. Self-reported smoking was categorized as former, never and current. Physical activity was based on self-reported number of hours per week of moderate activity.
Statistical Analysis
Control of confounding
Confounding control was accomplished by inverse probability of exposure weights (IPEW) (Cole & Hernan, 2008) that included the following confounders and NAFLD risk factors as variables: age, waist circumference, BMI, alcohol, education, smoking, diabetes and physical activity (see Appendix Materials and Methods).
Controlling for censoring due to Loss-to-Follow-up (LTFU)
The overall LTFU was 40%. Given this magnitude, LTFU may be informative to the extent of biasing study findings if there are differential losses between exposure groups or with respect to the outcome. To minimize potential biases from LTFU, inverse probability of censoring weights (Howe, Cole, Lau, Napravnik, & Eron, 2016) were created with the following variables that predicted dropping out of study with p <0.05: age, gender, smoking, alcohol, PD ≥4mm, and ALT (see Appendix Materials and Methods).
Outcome models
Weighted Poisson regression estimated incidence rate (IR), incidence rate ratio (IRR) and incidence rate difference (IRD) of NAFLD with 200 bootstrap resamples (Nevitt & Hancock, 2001) estimating the corresponding standard errors and 95% confidence intervals. In addition, confounding and censoring due LTFU adjusted cumulative risk of NAFLD for each exposure groups were estimated and results are presented graphically.
Multiple imputation
Multiple imputation was performed for missing data using chained equations (White, Royston, & Wood, 2011). The following variables were imputed: transaminases (ALT, AST, GGT), alcohol, smoking, BMI and waist circumference. Because approximately 40% of transaminase values were missing at SHIP-1 (Appendix Table 1), a total of 40 datasets were imputed using 500 between imputation iterations. Trace plots (Appendix Figure 1) assessed how the imputation algorithm performed, while kernel density plots (Appendix Figure 2) assessed deviation of imputed values from observed. Statistical tests were 2-sided and p <0.05 was considered nominally statistically significant. Analyses (including multiple imputation) were conducted in SAS v.9.4 (SAS Institute, Cary NC), across 40 imputed datasets. The results from each imputed dataset were summarized using Rubin’s rule (Rubin, 1987) into an overall estimate accounting for both within and between imputation variances.
Results
Of the 2,330 participants with baseline PD measurements, 766 (32.8%) had no sites with PD ≥4mm (periodontally-healthy), 1,293 (49.3%) had up to 30% of sites affected (moderate PD-periodontitis) and 271 (10.3%) had ≥30% sites affected (extensive PD-periodontitis). Of the 2,233 participants with baseline CAL measurements, 258 (11.6%) were periodontally-healthy, 767 (34.3%) had moderate CAL-periodontitis and 1,208 (54.1%) had extensive CAL-periodontitis. There were slightly more female than male participants (59% vs. 41%). The median age at baseline was 46 years (IQR: 33–62) and the 293 edentulous participants were on average older than participants in the other exposure groups (Table 1).
TABLE 1.
Baseline characteristics according to the proportion of periodontal sites exhibiting clinical attachment level ≥3mm or probing pocket depth ≥4mm among adults free of Non-alcoholic Fatty Liver Disease (NAFLD) in the Study of Health in Pomerania (SHIP), 1997–2012
|
|
|
Proportion of sites with CAL ≥3mm (n=2,233)
|
Proportion of sites with PD ≥4mm (n=2,330)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n=2,623) | Edentulous (n=293) | No site (n=258) | <30% (n=767) | ≥30% (n=1,208) | P Value | No site (n=766) | <30% (n=1,293) | ≥30% (n=271) | P Value | |
|
|
|
|
|
|||||||
| Age (yrs.) | 46 (33, 62) | 71 (65, 76) | 26 (23, 31) | 34 (27, 42) | 54 (42, 63) | <0.001 | 34 (27, 52) | 45 (34, 58) | 54 (44, 64) | <0.001 |
| Male sex | 1,074 (41) | 145 (49) | 94 (36) | 259 (34) | 531 (44) | 0.9 | 272 (36) | 524 (41) | 133 (49) | 0.5 |
| Smoking | ||||||||||
| Non-smoker | 1,002 (38) | 108 (37) | 101 (39) | 291 (38) | 460 (38) | 0.02 | 313 (41) | 489 (38) | 92 (34) | <0.001 |
| Former smoker | 809 (31) | 125 (43) | 69 (27) | 213 (28) | 372 (31) | 214 (28) | 403 (31) | 67 (25) | ||
| Current Smoker | 801 (31) | 58 (20) | 88 (34) | 263 (34) | 367 (31) | 239 (31) | 394 (31) | 110 (41) | ||
| missing | 11 | 2 | 9 | 7 | 2 | |||||
| BMI (Kg/m2) | ||||||||||
| Normal | 1,152 (44) | 86 (29) | 176 (68) | 430 (56) | 423 (35) | 0.003 | 417 (55) | 565 (44) | 84 (31) | 0.2 |
| Overweight | 1,020 (39) | 138 (47) | 61 (24) | 238 (31) | 546 (45) | 260 (34) | 500 (39) | 122 (45) | ||
| Obese | 445 (17) | 69 (24) | 21 (8) | 97 (13) | 235 (20) | 88 (12) | 223 (17) | 65 (24) | ||
| missing | 6 | 2 | 4 | 1 | 5 | |||||
| Diabetes mellitus | 392 (15) | 88 (30) | 15 (6) | 60 (8) | 205 (17) | 0.001 | 82 (11) | 168 (13) | 54 (20) | 0.001 |
| Waist cir a (cm) | 85 (75, 94) | 91 (83, 98) | 75 (69, 85) | 79 (72, 89) | 88 (79, 96) | <0.001 | 80 (71, 89) | 84 (76, 94) | 91 (80, 99) | <0.001 |
| Education (yrs.) | ||||||||||
| <10 | 260 (10) | 245 (84) | 17 (7) | 94 (12) | 514 (43) | <0.001 | 143 (19) | 393 (31) | 146 (55) | <0.001 |
| 10 | 578 (22) | 34 (12) | 172 (67) | 486 (64) | 501 (42) | 419 (55) | 663 (52) | 105 (39) | ||
| >10 | 1,768 (68) | 12 (4) | 69 (27) | 184 (24) | 178 (15) | 204 (27) | 223 (17) | 16 (6) | ||
| missing | 17 | 2 | 3 | 15 | 14 | 4 | ||||
| Standard drinks b | 4 (1, 10) | 1 (0, 5) | 5 (1, 10) | 5 (2, 11) | 4 (1, 10) | <0.001 | 5 (2, 10) | 5 (1, 10) | 3 (0, 9) | <0.001 |
| Physical activity c | 3 (2, 5) | 4 (3, 6) | 3 (2, 5) | 3 (2, 5) | 4 (2,5) | <0.001 | 3 (2, 5) | 3 (2, 5) | 4 (2, 5) | <0.001 |
Data are presented as No. (%) or Median (lower quartile, upper quartile)
waist circumference
Number of standard drinks (beer, wine or liquor) in the past 30 days
Number of hours of moderate physical activity in the past week
P-values for continuous variables are based on the Kruskal-Wallis test (the non-parametric equivalent of the one-way Anova) that tests if the distribution of each variable is similar against the alternative that they differ only with respect to the median. And the chi square test for differences in proportions of categorical variables
After a median follow-up of 7.7 years (IQR: 2.5–10.6), 588 NAFLD cases were identified during 17,973.2 person-years of follow-up among the edentulous and participants with baseline CAL measurements and 605 NAFLD cases accrued during 18,595.1 person-years of follow-up among the edentulous and participants with baseline PD measurements. Approximately 40% of study participants were lost to follow-up with edentulous participants having the highest proportion of losses at 74% (Table 2).
Table 2.
Disposition of 2,623 adults free of NAFLD with measured periodontal status at baseline, followed for a median of 7.7 years in the Study of Health in Pomerania, 1997–2012
|
|
Proportion of sites with CAL ≥3mm (n=2,233)
|
Proportion of sites with PD ≥4mm (n=2,330)
|
|||||
|---|---|---|---|---|---|---|---|
| Edentulous (n=293) | No site (n=258) | <30% (n=767) | ≥30% (n=1,208) | No site (n=766) | <30% (n=1,293) | ≥30% (n=271) | |
|
|
|
|
|||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
|
|
|
|
|||||
| Total PY | 1,716.3 | 1,942.0 | 5,715.3 | 8,599.6 | 5,687.1 | 9,466.2 | 1,725.5 |
| NAFLD cases | 30 (10.4) | 57 (21.9) | 203 (26.5) | 298 (24.6) | 182 (23.8) | 335 (25.9) | 58 (21.5) |
| Dropout | 216 (73.7) | 93 (36.1) | 226 (29.5) | 457 (37.8) | 255 (33.3) | 433 (33.5) | 142 (52.3) |
Number of cases and person-years were the averages from 40 rounds of multiple imputation
PY-person-years
CAL-clinical attachment level; PD-probing pocket depth
The unadjusted incidence rate of NAFLD was slightly elevated in the two CAL-periodontitis groups compared to periodontally-healthy participants (Table 3), although the IRRs were imprecisely estimated. However, upon adjusting for confounders and censoring, there was a dose-response relationship in the respective IRRs and IRDs. For instance, the IRR comparing participants with moderate CAL-periodontitis to periodontally-healthy participants was 1.28 (95% CI, 0.84–1.95, P=0.2) while for extensive CAL-periodontitis the estimate was 1.60 (95% CI, 1.05–2.43, P=0.03). The corresponding IRDs were 5.49 additional cases per 1,000 person-years (95% CI, −2.53–13.5) and 11.9 additional cases per 1,000 person-years (95%CI, 4.09–19.6, P=0.03) respectively (Table 3). NAFLD rate was also elevated among edentulous participants relative to the periodontally-healthy, although the increase was not significantly different from the null, adjusted IRR= 1.37 (95%CI, 0.26–7.15, P=0.7). Similar tendencies were seen for PD-periodontitis, although there was no dose-response relationship in the fully-adjusted analysis (Table 3). Qualitatively, similar inferences for CAL and PD were obtained from complete case analysis (no data imputation), although the corresponding NAFLD rates were smaller (Appendix Table 2).
Table 3.
Relationship between baseline periodontitis status and the incidence of NAFLD after a median follow-up of 7.7 years among participants of the Study of Health in Pomerania, 1997–2012
| Proportion of sites with clinical attachment level ≥3mm (n=2,526) | Proportion of sites with Probing pocket depth ≥4mm (n=2,623) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Rate | IRR (95% C.I) | P Value | IRD (95% C.I) | P Value | Rate | IRR (95% C.I) | P Value | IRD (95% C.I) | P Value | |
|
|
|
|||||||||
| Unadjusted | ||||||||||
| Edentulous | 17.6 | 0.61 (0.36, 1.02) | 0.1 | −11.4 (−21.3, −1.59) | 0.02 | 17.6 | 0.55 (0.34, 0.87) | 0.02 | −14.5 (−22.3, −6.65) | 0.0003 |
| No site | 29.0 | 1 [Reference] | NA | 0 [Reference] | NA | 32.1 | 1 [Reference] | NA | 0 [Reference] | NA |
| <30% of sites | 35.5 | 1.22 (0.88, 1.70) | 0.2 | 6.46 (−2.56, 15.5) | 0.2 | 35.3 | 1.10 (0.90, 1.35) | 0.3 | 3.28 (−2.72, 9.28) | 0.3 |
| ≥30% of sites | 34.6 | 1.19 (0.86, 1.65) | 0.3 | 5.56 (−2.98, 14.1) | 0.2 | 33.7 | 1.05 (0.75, 1.47) | 0.8 | 1.64 (−8.20, 11.5) | 0.7 |
| Adjusteda | ||||||||||
| Edentulous | 29.0 | 1.31 (0.27, 6.39) | 0.7 | 6.81 (−4.36, 18.0) | 0.2 | 28.7 | 0.92 (0.23, 3.64) | 0.9 | −2.58 (−12.0, 6.89) | 0.6 |
| No site | 22.1 | 1 [Reference] | NA | 0 [Reference] | NA | 31.3 | 1 [Reference] | NA | 0 [Reference] | NA |
| <30% of sites | 31.4 | 1.42 (0.96, 2.10) | 0.1 | 9.23 (0.45, 18.0) | .04 | 33.2 | 1.06 (0.86, 1.31) | 0.6 | 1.90 (−4.04, 7.83) | 0.5 |
| ≥30% of sites | 30.8 | 1.39 (0.94, 2.06) | 0.1 | 8.68 (0.38, 17.0) | .04 | 22.3 | 0.71 (0.49, 1.04) | 0.1 | −8.97 (−16.7, −1.22) | 0.02 |
| Adjustedb | ||||||||||
| Edentulous | 23.1 | 1.36 (0.33, 5.56) | 0.6 | 6.12 (−3.37, 15.6) | 0.2 | 23.0 | 0.89 (0.27, 2.97) | 0.8 | −2.72 (−11.1, 5.69) | 0.5 |
| No site | 17.0 | 1 [Reference] | NA | 0 [Reference] | NA | 25.7 | 1 [Reference] | NA | 0 [Reference] | NA |
| <30% of sites | 24.5 | 1.45 (0.92, 2.27) | 0.1 | 7.59 (0.26, 14.9) | 0.04 | 34.9 | 1.36 (1.01, 1.83) | 0.05 | 9.15 (3.59, 14.7) | 0.001 |
| ≥30% of sites | 29.4 | 1.74 (1.10, 2.74) | 0.02 | 12.5 (5.47, 19.5) | 0.0005 | 18.6 | 0.72 (0.48, 1.09) | 0.1 | −7.13 (−14.1, −0.12) | 0.05 |
| Adjustedc | ||||||||||
| Edentulous | 27.2 | 1.37 (0.26, 7.15) | 0.7 | 7.32 (−3.31, 17.9) | 0.2 | 25.2 | 0.91 (0.25, 3.34) | 0.9 | −2.47 (−11.3, 6.39) | 0.6 |
| No site | 19.9 | 1 [Reference] | NA | 0 [Reference] | NA | 27.6 | 1 [Reference] | NA | 0 [Reference] | NA |
| <30% of sites | 25.4 | 1.28 (0.84, 1.95) | 0.2 | 5.49 (−2.53, 13.5) | 0.2 | 42.3 | 1.53 (1.00, 2.35) | 0.05 | 14.6 (8.87, 20.4) | <0.0001 |
| ≥30% of sites | 31.8 | 1.60 (1.05, 2.43) | 0.03 | 11.9 (4.09, 19.6) | 0.003 | 21.3 | 0.77 (0.44, 1.33) | 0.3 | −6.34 (−13.7, 1.02) | 0.1 |
All estimates were averages from 40 rounds of multiple imputation combined using Rubin’s rule and the variance a function of the within and between completed dataset variances.
IRR-Incidence rate ratio; IRD-incidence rate difference
Rates and IRD are expressed per 1,000 person-years
Adjustment variables: age, sex, waist circumference, BMI, physical activity, alcohol consumption, education, smoking and diabetes
Adjusted for confounders only, using Inverse probability of exposure weights
Adjusted for confounders and censoring due to loss-to-follow-up (LTFU)
Adjusted for confounders and censoring from any reason (including LTFU, end of follow-up, excessive alcohol consumption, hepatitis diagnosis in past year, use of hepatotoxic medications)
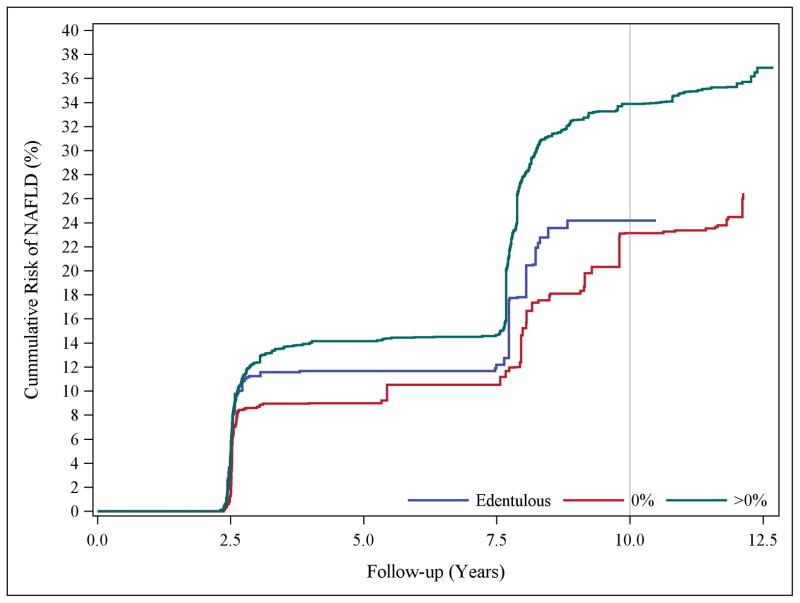
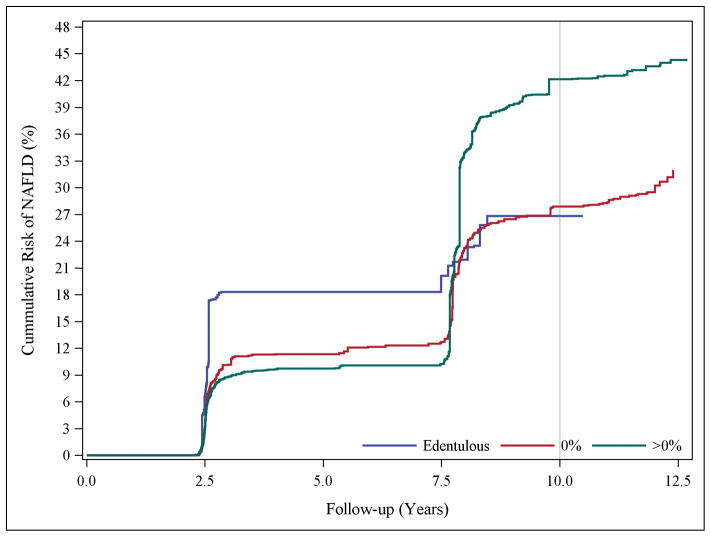
To ensure adequate control for smoking, data analysis was restricted to non-smokers; the results were consistent with those above. For instance, the confounding and censoring-adjusted IRR comparing participants with moderate and extensive CAL-periodontitis to periodontally-healthy participants were 1.11 (95% CI, 0.56–2.21, P=0.7) and 1.72 (95% CI, 0.92–3.21, P=0.09) respectively (results not shown). Irrespective of the proportion of sites affected, having periodontitis increased the cumulative risk of NAFLD (Figure 1a). For PD classification, the edentulous group had a greater risk earlier during follow-up, however, the risk among participants with PD-periodontitis rose sharply over the follow-up period (Figure 1b).
Figure 1.
Panel A, Confounding and censoring adjusted cumulative risk curves of NAFLD occurrence according to the proportion of periodontal sites with clinical attachment level of ≥3mm (edentulous, 0%, >0%) at baseline. Panel B, Confounding and censoring adjusted cumulative risk curves of NAFLD occurrence according to the proportion of periodontal sites with probing pocket depth of ≥4mm (edentulous, 0%, >0%) at baseline.
The mean CAL at baseline was 2.4 (SD: 1.8) per participant while the corresponding mean PD was 2.5 (SD: 0.7) per participant. The adjusted IRR of NALFD for each 3mm increase in mean CAL, was 1.11 (95% CI, 0.92–1.34, P=0.3) and 1.28 (95% CI, 0.63–2.57, P=0.5) for each 4mm increase in mean PD (Table 4).
Table 4.
Relationship between mean clinical attachment level, mean pocket depth at baseline and mean change in clinical attachment level with the incidence of NAFLD among participants of the Study of Health in Pomerania, 1997–2012
| Incidence Rate Ratio IRR (95% CI)
|
||||
|---|---|---|---|---|
| Unadjusted | P Value | Adjusted a | P Value | |
|
|
|
|||
| Baseline periodontitis | ||||
| Mean CAL | 0.99 (0.85, 1.16) | 0.9 | 1.11 (0.92, 1.34) | 0.3 |
| Mean PD | 1.02 (0.58, 1.80) | 0.9 | 1.28 (0.63, 2.57) | 0.5 |
| Progression of periodontitis | ||||
| Participant with baseline CAL <3mm (n=1,091) | ||||
| ≥2mm (Yes v. No) | 0.72 (0.32, 1.63) | 0.5 | 0.44 (0.15, 1.30) | 0.1 |
| ≥1mm (Yes v. No) | 0.95 (0.63, 1.42) | 0.6 | 0.88 (0.61, 1.28) | 0.5 |
| Participants with baseline CAL ≥3mm (n=372) | ||||
| ≥2mm (Yes v. No) | 2.32 (1.00, 5.37) | 0.05 | 2.07 (0.96, 4.58) | 0.06 |
| ≥1mm (Yes v. No) | 1.78 (1.02, 3.11) | 0.04 | 1.55 (0.90, 2.65) | 0.1 |
All estimates were averages from 40 rounds of multiple imputation combined using Rubin’s rule and the variance a function of the within and between completed dataset variances
Estimates are for each 3mm increase in mean CAL at baseline, or 4mm increase in mean PD at baseline
Progression of periodontitis is the mean difference in CAL between baseline and first follow-up visits
Adjustment variables: age, sex, waist circumference, BMI, physical activity, alcohol consumption, education, smoking and diabetes. Interaction P=0.05
Confounders and censoring-adjusted estimates
Among 1,463 eligible participants, progression of periodontitis between SHIP-0 and SHIP-1, was observed for 253 (17.3%) participants at a threshold of ≥1mm increase in mean CAL, and for 69 (4.7%) participants at a threshold of ≥2mm increase in mean CAL. There was no meaningful difference in the IR of NAFLD according to periodontitis progression using either threshold. However, there was a significant statistical interaction between CAL at baseline and periodontitis progression (P=0.05). That is, among participants with CAL ≥3mm at baseline, the adjusted IRR of NAFLD comparing participants with mean change in CAL of ≥2mm to participants with <2mm was of 2.07 (95% CI, 0.96–4.58, P=0.06) (Table 4).
Discussion
Summary of current findings
Our results were consistent with a greater incidence rate of NAFLD among participants with a history of periodontitis (i.e. CAL-periodontitis) compared to participants with a healthy periodontium. In contrast, a weaker and inconsistent association was observed between PD-periodontitis and incidence of NAFLD, with the estimate been imprecise and contrary to expectation among participants with ≥30% of sites with PD ≥4mm, possibly due to the relatively small number of participants in this stratum. Progression of periodontitis measured over five years was also associated with greater incidence of NAFLD, although only among participants with a relatively extensive history of periodontitis at baseline.
Summary of previous findings
Evidence to date of an association between periodontitis and NAFLD comes from experimental animal models (Tomofuji et al., 2007; Yoneda et al., 2012) and a cross-sectional clinic-based study (Yoneda et al., 2012). Mice randomized to a high fat diet and Porphyromonas gingivalis (a potent periodontal pathogen) compared to those randomized to a high fat diet alone (Tomofuji et al., 2007; Yoneda et al., 2012), had significant increase in body and liver weight, and elevated ALT (Yoneda et al., 2012). Substituting P. gingivalis with Streptococcus mutans (a dental caries pathogen), had no effect on mice body or liver weight. A clinic based study of biopsy-confirmed NAFLD found more NAFLD cases than non-cases to have detectable P.gingivalis levels and a 3-month periodontal therapy led to subsequent reductions in elevated transaminases (Yoneda et al., 2012). To the extent that persistent infection with periodontal pathogens accelerates destruction of periodontal tissues, these findings support biologic plausibility of this study’s finding that extensive attachment loss predicts an increased rate of NAFLD.
Possible biologic mechanisms
The clearer dose-response association between CAL (as compared to PD) and NAFLD suggests that a history of periodontitis matters most in predicting NAFLD in this population. This finding is consistent with an underlying hyper-inflammatory trait (Shaddox et al., 2010), that increases the risk of initiation and progression of periodontitis and subsequently to a heightened inflammatory response. Unlike the acute inflammatory response to injury, sustained ‘low-grade’ or chronic inflammation (Hotamisligil, 2006), is non-beneficial, although it engages similar sets of molecules and signaling pathways. This ‘low-grade’ inflammation is central to the pathogenesis of obesity related insulin resistance, an NAFLD precursor (Hotamisligil, 2006). Increased serum levels of LPS and TNF-α associated with P. gingivalis infection can demonstrably initiate and worsen insulin resistance (Santos Tunes, Foss-Freitas, & Nogueira-Filho, 2010). Therefore, ‘low-grade’ inflammation and exacerbation of insulin resistance also likely links periodontitis to NAFLD.
Another plausible pathway is created by increased permeability in gut epithelia induced by swallowed P.gingivalis, potentially leading to an alteration in the gut microbial composition (Arimatsu et al., 2014). Given that the liver is constantly exposed to gut-derived factors through the portal vein, resident liver cells become activated by proinflammatory factors like LPS with subsequent production of cytokines and, reactive oxygen species (ROS) that contribute to liver injury (Imajo, Yoneda, Ogawa, Wada, & Nakajima, 2014). Regarding NAFLD pathogenesis, the ‘two-hits’ (Day & James, 1998) theory attributes the ‘first-hit’ to steatosis secondary to insulin resistance and, the ‘second-hit’ to gut derived bacteria endotoxins which promote the inflammation that enhances disease progression. A likely source of gut-derived bacterial endotoxin is periodontal pathogen derived LPS given that an average 107 copies of periodontal bacteria are found in a mL of saliva (Saygun et al., 2011). In experimental animal models, oral administration of P.gingivalis led to changes in the gut microbiota leading to metabolic endotoxemia, a precursor for metabolic disorders (Arimatsu et al., 2014). While the current study lacked microbiologic data, it was noteworthy that progression of periodontitis was associated with NAFLD only among participants with relatively extensive history of periodontitis. One possible explanation is that progressive loss of periodontal attachment elicits systemic responses only when progression is occurring at deep periodontal sites that are more likely anaerobic and capable of eliciting systemic inflammation.
Clinical and Public Health implications
This is the first large-scale epidemiologic study to have demonstrated an association between history of periodontitis and subsequent incidence rate of NAFLD. Additionally, there was evidence that incidence of periodontitis predicted incidence of NAFLD, at least among people with a history of extensive CAL. Relative effect estimates were small-to-modest, although given the high prevalence of both periodontitis and NAFLD, the associations, if replicated in future studies, have population-wide implications for periodontitis as a modifiable risk factor for NAFLD.
Strengths and Limitations
Study limitations include potential misclassification of NAFLD status. While ultrasound is used to assess liver diseases in epidemiologic settings with reported sensitivity of 85% (95% CI: 80%–89%) and specificity of 93% (95% CI: 87%–97%), it is only able to detect disease if upwards of 20% of liver cells are affected (Hernaez et al., 2011). Another limitation is the reliance on ALT for identifying NAFLD at SHIP-1 given ALT is not always elevated when NAFLD is present. Therefore, if ALT is differentially under or overestimated according to periodontitis status then estimates are likely biased but the direction of bias is hard to predict. If non-differential, then estimates are likely biased towards the null.
Strengths include the prospective design and the ability to minimize temporal ambiguity by ensuring exposure preceded outcome. The in-depth characterization of the cohort enabled an extensive assessment of relevant confounding variables, which permitted adjustments not only for confounders but also an assessment of the impact of censoring due to loss to follow-up on study findings.
Conclusions
NAFLD prevalence is on the rise both in the U.S. and around the world. This investigation is the first to implicate history of periodontitis as an independent risk factor contributing to NAFLD incidence in a population-based sample. If these findings are replicated in future studies, it would support interventions to control periodontitis would have benefits in reducing NAFLD, for which there are no approved pharmacologic interventions, and which is a disease that is difficult to prevent via other lifestyle modifications alone.
Supplementary Material
Clinical Relevance.
Scientific rationale for the study
Non-alcoholic Fatty Liver Disease is common and it is associated with high health care costs. Periodontitis is associated with NAFLD risk factors like obesity and insulin resistance while the evidence linking periodontitis to NAFLD comes from one cross-sectional clinic based study in humans and several experimental mice models.
Principal findings
This investigation provides new information of a longitudinal nature, linking periodontitis to NAFLD in a large population based setting.
Practical implications
Management of periodontal infection should be considered in individuals suspected of or at risk for NAFLD.
Acknowledgments
Source of funding
The Study of Health in Pomerania is part of the Community Medicine Research network of the University of Greifswald, Germany, and funded by the Federal Ministry of Education and Research (Grants number: 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs and the Social Ministry of the Federal State of Mecklenburg-West Pomerania.
Support for this work was provided by the National Institutes of Health/National Institute of Dental and Craniofacial Research (Grant number: R03DE025652-01A1).
Footnotes
Conflicts of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Contributor Information
Aderonke A. Akinkugbe, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC
Gary D. Slade, Department of Dental Ecology, University of North Carolina at Chapel Hill, Chapel Hill, NC
A. Sidney Barritt, Department of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Stephen R. Cole, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC
Steven Offenbacher, Department of Periodontology, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Astrid Petersmann, Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, Greifswald Germany.
Thomas Kocher, Unit of Periodontology, Department of Restorative Dentistry, Periodontology, Endodontology, and Preventive and Pediatric Dentistry, University Medicine Greifswald, Greifswald, Germany.
Markus M. Lerch, Department of Medicine A, University Medicine Greifswald, Greifswald Germany
Julia Mayerle, Department of Medicine, Ludwig-Maximilians University, Munich, Germany.
Henry Völzke, Institute for Community Medicine, University Medicine Greifswald, Greifswald Germany.
Gerardo Heiss, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Birte Holtfreter, Unit of Periodontology, Department of Restorative Dentistry, Periodontology, Endodontology, and Preventive and Pediatric Dentistry, University Medicine Greifswald, Greifswald, Germany.
References Cited
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. New England Journal of Medicine. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Lida T, Yamazaki K. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Scientific Reports. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister SE, Volzke H, Marschall P, John U, Schmidt CO, Flessa S, Alte D. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134:85–94. doi: 10.1053/j.gastro.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Beck JD, Elter JR. Analysis strategies for longitudinal attachment loss data. Community Dentistry and Oral Epidemiology. 2000;28:1–9. doi: 10.1034/j.1600-0528.2000.280101.x. [DOI] [PubMed] [Google Scholar]
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Bedogni G, Miglioli L, Tiribelli C. The epidemiology of fatty liver. European Journal of Gastroenterology and Hepatology. 2004;16:1087–1093. doi: 10.1097/00042737-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Benguigui C, Bongard V, Ruidavets JB, Chamontin B, Sixou M, Ferrieres J, Amar J. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. Journal of Clinical Periodontology. 2010;37:601–608. doi: 10.1111/j.1600-051X.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- Burt BA, Eklund SA. Dentistry, Dental Practice and the Community. 6. Elsevier Inc; 2005. [Google Scholar]
- Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Periodontology. 2013;84:S106–112. doi: 10.1902/jop.2013.1340011. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. American Journal of Gastroenterology. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American Journal of Epidemiology. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–1379. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of Periodontology. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell GC, George J, Hall PdlM, McCullough AJ. Overview: an introduction to NASH and related fatty liver disorders. Blackwell; Oxford: 2004. [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Gurav A, Jadhav V. Periodontitis and risk of diabetes mellitus. Journal of Diabetes. 2011;3:21–28. doi: 10.1111/j.1753-0407.2010.00098.x. [DOI] [PubMed] [Google Scholar]
- Hensel E, Gesch D, Biffar R, Bernhardt O, Kocher T, Splieth C, Born G, John U. Study of Health in Pomerania (SHIP): a health survey in an East German region. Objectives and design of the oral health section. Quintessence International. 2003;34:370–378. [PubMed] [Google Scholar]
- Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter B, Alte D, Schwahn C, Desvarieux M, Kocher T. Effects of different manual periodontal probes on periodontal measurements. Journal of Clinical Periodontology. 2012;39:1032–1041. doi: 10.1111/j.1600-051X.2012.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection Bias Due to Loss to Follow Up in Cohort Studies. Epidemiology. 2016;27:91–97. doi: 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo K, Yoneda M, Ogawa Y, Wada K, Nakajima A. Microbiota and nonalcoholic steatohepatitis. Seminars in Immunopathology. 2014;36:115–132. doi: 10.1007/s00281-013-0404-6. [DOI] [PubMed] [Google Scholar]
- John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, Haertel U, Hense HW, Haerting J, Willich S, Kessler C. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Sozial und Praventivmedizin. 2001;46:186–194. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arteriosclerosis, Thrombosis and Vascular Biology. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nature Reviews Endocrinology. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. American Journal of Epidemiology. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Annals of Medicine. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Nevitt J, Hancock G. Performance of Bootstrapping Approaches to Model Test Statistics and Parameter Standard Error Estimation in Structural Equation Modeling. Structural Equation Modeling: A Multidisciplinary Journal. 2001;8:353–377. [Google Scholar]
- Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. Journal of Hepatology. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Osman KA, Osman MM, Ahmed MH. Tamoxifen-induced non-alcoholic steatohepatitis: where are we now and where are we going? Expert Opinion on Drug Safety. 2007;6:1–4. doi: 10.1517/14740338.6.1.1. [DOI] [PubMed] [Google Scholar]
- aRubin DB. Multiple Imputation for Nonresponse in Surveys. 1987. [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Koga T. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. Journal of Dental Research. 2004;83:485–490. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- Santos Tunes R, Foss-Freitas MC, Nogueira-Filho GaR. Impact of periodontitis on the diabetes-related inflammatory status. Journal (Canadian Dental Association) 2010;76:a35. [PubMed] [Google Scholar]
- Saygun I, Nizam N, Keskiner I, Bal V, Kubar A, Açıkel C, Serdar M, Slots J. Salivary infectious agents and periodontal disease status. Journal of Periodontal Research. 2011;46:235–239. doi: 10.1111/j.1600-0765.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. Journal of Periodontology. 2013;84:S51–69. doi: 10.1902/jop.2013.134006. [DOI] [PubMed] [Google Scholar]
- Shaddox L, Wiedey J, Bimstein E, Magnuson I, Clare-Salzler M, Aukhil I, Wallet SM. Hyper-responsive phenotype in localized aggressive periodontitis. Journal of Dental Research. 2010;89:143–148. doi: 10.1177/0022034509353397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. Journal of Clinical Periodontology. 2001;28:306–310. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- Timonen P, Suominen-Taipale L, Jula A, Niskanen M, Knuuttila M, Ylostalo P. Insulin sensitivity and periodontal infection in a non-diabetic, non-smoking adult population. Journal of Clinical Periodontology. 2011;38:17–24. doi: 10.1111/j.1600-051X.2010.01642.x. [DOI] [PubMed] [Google Scholar]
- Tomofuji T, Ekuni D, Yamanaka R, Kusano H, Azuma T, Sanbe T, Tamaki N, Yamamoto T, Watanabe T, Miyauchi M, Takata T. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. Journal of Periodontololgy. 2007;78:1999–2006. doi: 10.1902/jop.2007.070056. [DOI] [PubMed] [Google Scholar]
- Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Jünger M, Mayerle J, Kraft M, Lerch MM, Dörr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Gläser S, Ewert R, Fietze I, Penzel T, Dören M, Rathmann W, Haerting J, Hannemann M, Röpcke J, Schminke U, Jürgens C, Tost F, Rettig R, Kors JA, Ungerer S, Hegenscheid K, Kühn JP, Kühn J, Hosten N, Puls R, Henke J, Gloger O, Teumer A, Homuth G, Völker U, Schwahn C, Holtfreter B, Polzer I, Kohlmann T, Grabe HJ, Rosskopf D, Kroemer HK, Kocher T, Biffar R, John U, Hoffmann W. Cohort profile: the study of health in Pomerania. International Journal of Epidemiology. 2011;40:294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, Murata S, Tohnai I, Sumida Y, Shima T, Kuboniwa M, Umemura K, Kamisaki Y, Amano A, Okanoue T, Ooshima T, Nakajima A. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterology. 2012;12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel-Lindberg T, Bage T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Reviews in Molecular Medicine. 2013;15:e7. doi: 10.1017/erm.2013.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.