Abstract
BACKGROUND
Black race is associated with prostate cancer (PC) diagnosis and poor outcome. Previously, we found black men undergoing radical prostatectomy (RP) in equal access hospitals had increased biochemical recurrence (BCR) risk, but recurrences were equally aggressive as white men. We examined the association between race and long-term outcomes after RP.
METHODS
We analyzed data on 1665 (37%) black and 2791 (63%) white men undergoing RP. Using Cox models, we tested the association between race and BCR, BCR with PSA doubling time <9 months (aggressive recurrence, PSADT), metastases, PC-specific death, and overall death.
RESULTS
At 102-months median follow-up, 1566 (35%) had BCR, 217 (6%) aggressive recurrence, 193 (4%) metastases, and 1207 (27%) died, of which 107 (2%) from PC. White men were older, had lower pre-operative PSA, lower biopsy and pathological grade, more capsular penetration, but less seminal vesicle invasion and positive margins versus black men (all p<0.05). Black men had more recent surgery year (p<0.001). On univariable analysis, black race was associated with increased BCR (p=0.003) and overall death (p=0.017). On multivariable analysis, black race was not associated with BCR (HR:1.07, p=0.26), PSADT (HR:1.14, p=0.42), metastasis (HR:1.24, p=0.21), PC-specific death (HR:1.03, p=0.91), or overall death (HR:1.03, p=0.67).
CONCLUSIONS
Among men undergoing RP at equal access centers, though black men had increased BCR risk, they had similar aggressive recurrence, metastasis, and PC-specific death risk versus white men, and BCR risk was similar after controlling for risk parameters. Longer follow-up is needed to confirm these findings.
Keywords: Prostate cancer, race, radical prostatectomy, biochemical recurrence, PSADT, prostate cancer-specific death
INTRODUCTION
Black men in the United States have the highest prostate cancer (PC) incidence and mortality compared to other races.1 The reasons for this remain controversial, but are likely multifactorial.2 There are clear racial differences in socioeconomic status, which some have argued are a major contributor to the poorer outcomes among black men.3,4 While undoubtedly socioeconomic differences are important, there are also racial differences in genetics that could contribute.5,6 Further evaluating the contribution of each of these factors remains an important endeavor.
A different approach to studying race and PC is to study men from equal access centers. As such, the VA health system, the largest health care delivery system in the US, is ideally poised for such study due to its equal access nature and the high percentage of black men. Using the Shared Equal Access Regional Cancer Hospital (SEARCH) database, we previously studied men undergoing radical prostatectomy (RP) and found black race was associated with increased BCR risk, but similar PSA doubling times (PSADT) at recurrence.7 Given PSADT is correlated with risk of PC-specific death,8 this would suggest that among men in equal access medical centers all treated with surgery, race would not impact PC death. However, at the time of our prior analyses, long-term cancer control data were not available. Indeed, there are only a limited number of studies on racial differences in long-term outcomes among men treated with radical prostatectomy (RP), which were limited by either being small and including only black men (n=222),9 only examining older men (age >65 years), not controlling for PSA values,10 or had short follow-up (4-year median follow-up).11 With continued follow-up, we now have long-term cancer control data (metastasis and PC death) and overall survival (OS) data. Herein we report the early findings of the association between race and long-terms outcomes after RP from SEARCH, which to our knowledge is the largest study to date examining race and the risk of metastases, PC-specific death, and OS following RP in black men with equal access to care.
MATERIALS AND METHODS
Patient population
After obtaining Institutional Review Board approval, we combined data from patients undergoing RP between 1988–2016 at the VA Hospitals in West Los Angeles, San Diego, and Palo Alto, California; Augusta, Georgia; and Durham and Asheville, North Carolina into SEARCH.12
Patients treated with preoperative androgen deprivation or radiation were excluded. Of 5,515 patients, we excluded men with missing data for race or whose race was other (n=199), men with missing data for biopsy grade group (n=487), pathological grade group (n=48), pre-operative PSA (n=79), margins (n=39), capsular penetration (n=93), seminal vesicle (n=19), or no PSA or overall follow-up (n=95), resulting in a study population of 4,456.
Study outcomes
BCR was defined as a single PSA >0.2ng/ml, two concentrations at 0.2ng/ml, or secondary treatment for an elevated postoperative PSA. A total of 84 white (3.0%) and 58 black men (3.5%) received adjuvant treatment for an undetectable PSA and were censored as non-recurrent at the time of adjuvant treatment (chi-squared p-value=0.43). For BCR, patients were censored at the time of last known PSA. For patients who received secondary treatments after BCR, we included all data without censoring as this was considered salvage therapy and not adjuvant. A total of 1,147 men received salvage treatment after BCR, of which 487 were black (29.3%), and 660 were white (23.7%) (Chi-squared, p<0.001).
PSADT at recurrence was calculated by dividing the natural log of 2 (0.693) by the slope of the linear regression line of the natural log of PSA over time. To calculate PSADT, patients must have had ≥2 PSA values, separated by ≥3 months, within 2 years after recurrence. All PSA values within the first 2 years after recurrence were used. For patients beginning salvage hormone or radiation therapy within this time, only values before salvage therapy were used. Patients with PSADT <0 (i.e., no increase/decline in PSA) or >120 months (n=178) were assigned a PSADT of 120 months for ease of calculations.
We a priori defined aggressive recurrence as a recurrence with a PSADT <9 months, based upon the high PC-specific mortality risk seen in a prior study.8 Patients without recurrence were censored as not having an aggressive recurrence at time of last PSA. Patients with a BCR and PSADT ≥9 months were censored as not having an aggressive recurrence at the time of recurrence, as these men were at low risk of PC death in a prior study.8 Patients with recurrence but incalculable PSADT (n=439) were unclassifiable and were excluded from aggressive recurrence analyses.
Metastases were identified typically by bone scan or computer tomography (CT) imaging performed as per clinician discretion. Equivocal scans were considered negative as they do not change treatment management unless confirmed positive by a secondary imaging modality or biopsy. Patients without metastases were censored either at death or at last known contact.
Date and cause of death was ascertained via VA electronic medical records. Patients were censored at date of last known contact. Men who died with metastatic, progressive, castrate-resistant PC without another obvious cause of death were considered to have died of PC.
Statistical Analysis
We explored differences in clinicopathological characteristics by race using rank sum test for continuous variables and chi-squared test for categorical variables. Time to BCR, aggressive recurrence, PC-specific death, and OS were compared between races using Kaplan-Meier plots and log-rank tests. To test differences in progression for black vs. white, we used a Cox proportional hazards model adjusted for age (continuous), year of surgery (continuous), center (categorical), biopsy grade group (ordinal: 1, 2, 3, 4, 5) and pre-operative PSA (continuous, logarithmically transformed). In a separate model, in addition to pre-operative characteristics, we further adjusted for the pathological characteristics of margins, capsular penetration, seminal vesicle invasion, lymph nodes (categorical: positive, negative, not performed), and pathological grade group (1, 2, 3, 4, 5). In the latter model we did not adjust for biopsy grade group given the inclusion of pathological grade group. Given BMI data were missing on 345 men (8%) and there was no statistical difference between BMI of black and white patients (Table 1), we explored whether including these men altered our results. Specifically, analyses were conducted among all men as well as only men with available BMI data adjusting for BMI. When limited to those with BMI data, results were unchanged. Therefore, we concluded BMI was not a confounder in our analysis and therefore we included men with missing BMI data and did not adjust for BMI.
Table 1.
Clinical characteristics stratified by race (N=4456)
| White | Black | p value* | |
|---|---|---|---|
| No. of patients (%) | 2791 (63%) | 1665 (37%) | |
| Age in years at surgery, Median (IQR) | 64 (60, 67) | 61 (56, 65) | <0.001† |
| Year of surgery, Median (IQR) | 2005 (1999, 2010) | 2007 (2002, 2011) | <0.001† |
| PSA ng/mL, Median (IQR) | 6.2 (4.6, 9.3) | 7.0 (5.0, 10.9) | <0.001† |
| Body mass index kg/m2, Median (IQR)^ | 28.0 (25.4, 31.0) | 28.1 (25.1, 31.6) | 0.741† |
| Biopsy grade group, no. (%) | <0.001 | ||
| 1 | 1425 (51) | 753 (46) | |
| 2 | 677 (24) | 484 (29) | |
| 3 | 320 (11) | 202 (12) | |
| 4 | 259 (9) | 170 (10) | |
| 5 | 110 (4) | 46 (3) | |
| Clinical Stage, no. (%) | <0.001 | ||
| T1 | 1386 (55) | 1117 (69) | |
| T2 and above | 1150 (45) | 491 (31) | |
| Pathological grade group, no. (%) | <0.001 | ||
| 1 | 964 (35) | 450 (27) | |
| 2 | 988 (35) | 734 (44) | |
| 3 | 438 (16) | 302 (18) | |
| 4 | 216 (8) | 110 (7) | |
| 5 | 185 (6) | 69 (4) | |
| Extracapsular extension, no. (%) | 582 (21) | 300 (18) | 0.022 |
| Seminal vesicle invasion, no. (%) | 264 (9) | 193 (23) | 0.023 |
| Positive surgical margins, no. (%) | 1003 (36) | 756 (45) | <0.001 |
| Lymph node involvement, no. (%) | 0.288 | ||
| Negative | 1774 (64) | 1075 (65) | |
| Positive | 50 (2) | 39 (2) | |
| Node dissection not performed | 967 (35) | 551 (33) |
p value assessed by chi squared test unless otherwise specified
p value assessed by rank sum test
BMI was available on 4111 men
All statistical analyses were performed using STATA 14.2 (Stata Corp., College Station, TX).
RESULTS
A total of 37% of men were black (n=1,665). Black men were younger at surgery (p<0.001), underwent RP more recently (p<0.001), had higher PSA values (p<0.001), higher biopsy grade group (p<0.001) and pathological grade group (p<0.001), were less likely to have capsular penetration (p=0.022), but were more likely to have positive surgical margins (p<0.001) and seminal vesicle invasion (p=0.023, table 1).
BCR
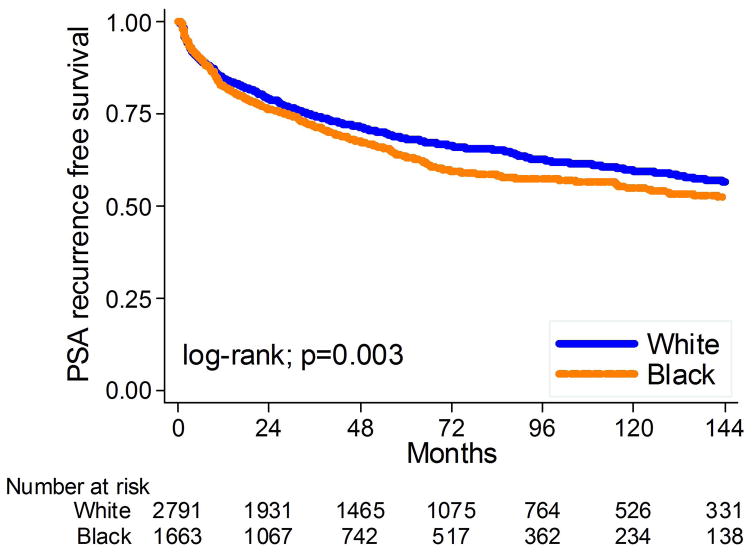
Mean and median PSA follow-up for men without BCR were 80 and 70 months, respectively. During this time, 1,566 men (35%) developed BCR – 1,244 due to an elevated PSA and 322 due to secondary treatment for a rising PSA but that was below the recurrence threshold. Median time from surgery to recurrence among men with BCR was 18.0 months and median time from surgery to secondary treatment was 13.4 months. Overall, black men were significantly more likely to develop BCR (log-rank, p=0.003; Figure 1). When men who recurred due to secondary treatment were excluded, results were unchanged. After adjusting for either multiple clinical or multiple clinical and pathological characteristics, the association between black race and BCR was attenuated and no longer significant (HR: 1.04, 95%CI 0.93–1.16, p=0.49; HR: 1.07, 95%CI 0.95–1.20, p=0.26, respectively, table 2).
Figure 1.
Kaplan-Meier estimates of time to PSA recurrence stratified by race at surgery.
Table 2.
Multivariate adjusted association between black race and risk of various outcomes after radical prostatectomy in the SEARCH database
| Adjusted for only pre-operative characteristics* | Adjusted for pre- and post-operative characteristics** | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| BCR | 1.04 | (0.93, 1.16) | 0.495 | 1.07 | (0.95, 1.20) | 0.257 |
| Aggressive PSA recurrence | 1.13 | (0.83, 1.53) | 0.439 | 1.14 | (0.83, 1.55) | 0.423 |
| Metastasis | 1.21 | (0.87, 1.67) | 0.255 | 1.24 | (0.89, 1.72) | 0.212 |
| Prostate cancer specific death | 1.00 | (0.61, 1.64) | 0.999 | 1.03 | (0.62, 1.69) | 0.912 |
| Overall death | 1.02 | (0.90, 1.17) | 0.725 | 1.03 | (0.90, 1.18) | 0.670 |
p value assessed by Cox model adjusted for age, BMI, year of surgery, center, pre-operative PSA, and biopsy Gleason score
p value assessed by Cox model adjusted for age, BMI, year of surgery, center, pre-operative PSA, positive margins, extracapsular extension, seminal vesicle invasion, lymph node metastasis, and pathological Gleason score
Note: out of 4456 patients, there were 1566 BCR, 217 aggressive PSA recurrence, 193 distance metastases, 107 deaths of prostate cancer and 1207 of all-cause deaths.
Aggressive recurrence (PSADT)
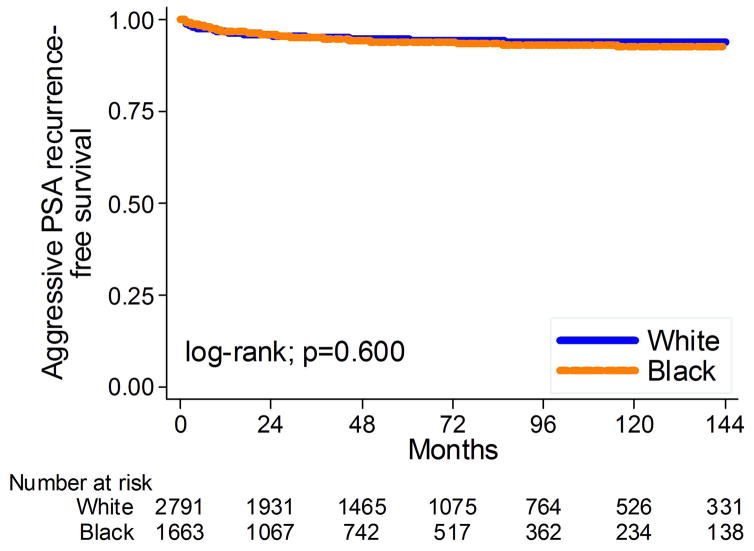
Of 1,329 men who recurred, 322 did so due to secondary treatment and thus did not have a calculable PSADT and 439 of the remaining 1244 (35%) had an incalculable PSADT and were excluded from this analysis. Thus, of 3695 men, 217 (6%) had an aggressive recurrence. Overall, black men had similar risk of aggressive BCR as white men (log-rank, p=0.600; Figure 2). This lack of association between race and aggressive recurrence remained unchanged after adjusting for multiple clinical or clinical and pathological characteristics (HR: 1.13, 95%CI 0.83–1.53, p=0.44; HR: 1.14, 95%CI 0.83–1.55, p=0.42, respectively, table 2).
Figure 2.
Kaplan-Meier estimates of time to aggressive PSA recurrence stratified by race at surgery.
Metastasis
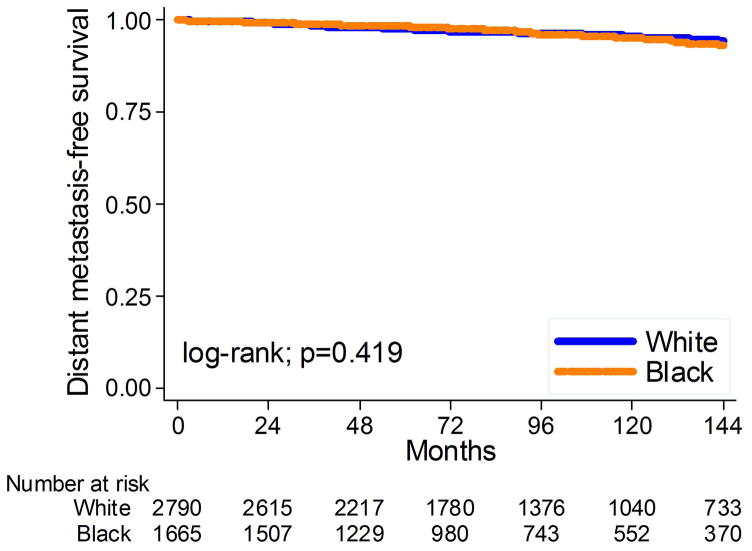
The mean and median overall follow-up among those without metastases were 103 and 93 months, respectively. During that time, 193 men (4.3%) developed metastases. Overall, black men had similar risk of metastases as white men (log-rank, p=0.42; Figure 3). This lack of association between race and metastases remained unchanged after adjusting for multiple clinical or clinical and pathological characteristics (HR: 1.21, 95%CI 0.87–1.67, p= 0.26; HR: 1.24, 95%CI 0.89–1.72, p=0.21, respectively, table 2).
Figure 3.
Kaplan-Meier estimates of time to metastasis stratified by race at surgery.
PC-specific and OS
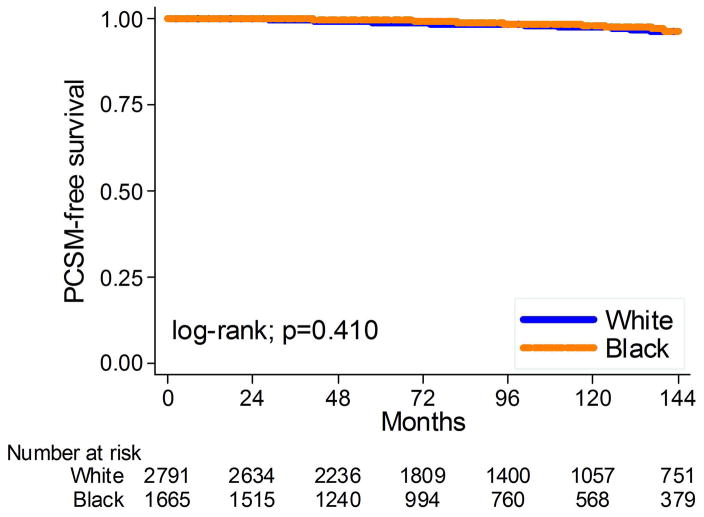
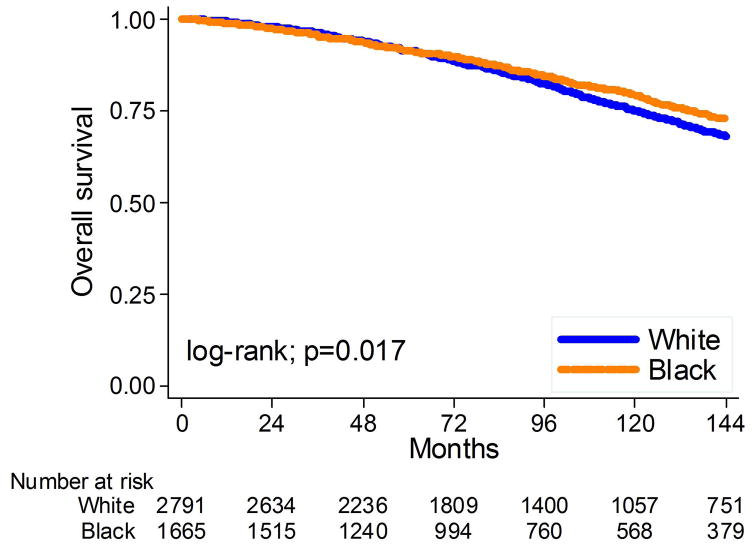
The mean and median overall follow-up among those alive at last follow-up were 102 and 91 months, respectively. During that time, 1207 men (27%) died: 107 (2%) from PC. Overall, black race was not related to PC-specific death (log-rank, p=0.410; Figure 4) but was associated with decreased risk of OS (log-rank, p=0.017; Figure 5). After adjusting for clinical or clinical and pathological characteristics, black race remained unrelated to PC death (HR: 1.00, 95%CI 0.61–1.64, p=0.99; HR: 1.03, 95%CI 0.62–1.69, p=0.91, respectively, table 2) and the relationship between race and OS became null (HR: 1.02, 95%CI 0.90–1.17, p=0.73; HR: 1.03, 95%CI 0.90–1.18, p=0.67, respectively, table 2).
Figure 4.
Kaplan-Meier estimates of time to prostate cancer-specific death by race at surgery.
Figure 5.
Kaplan-Meier estimates of time to overall death stratified by race at surgery.
DISCUSSION
Black men have increased PC incidence and mortality versus white men. The exact reasons for this are not clear. One key issue relates to access to care. If black men have the same access to care and receive the same treatment, regardless of socioeconomic status and other disease related factors, can they expect similar outcomes? Herein, we addressed the subgroup of men diagnosed with PC, who have equal access to care within the VA system, and elected to undergo RP. A previous study on this population from our group found black men undergoing RP had a slightly higher recurrence risk, though the PSADT at recurrence (a measure of disease aggressiveness) was similar in black and white men. Limited data are available on endpoints of critical importance in this population including risk of metastases, PC-specific survival, and OS following RP. To address this, we queried the SEARCH database, a database from multiple equal access VA centers consisting of 4,456 patients (37% black, 63% white) treated with RP during the PSA-era. We found black men had higher recurrence rates and correspondingly higher rates of salvage therapy. However, when adjusting for disease severity, we found no difference in the risk of recurrence, aggressive recurrence, metastases, PC-specific survival, and OS between the groups. These data suggest that with equal access to care, black men still present with worse disease resulting in higher recurrence rates, but when this worse disease at diagnosis is accounted for, black men can expect equally effective and long-term results following RP, though longer follow-up is needed to confirm these findings.
To our knowledge this is the largest study to date examining race and the risk of metastases, PC-specific death, and OS following RP in black men with equal access to care. A prior study of 222 intermediate and low-risk patients (65% black) from the New York Harbor VA treated by RP, found that PC-specific survival did not differ by race.9 While in-line with our results, these findings were based upon 4 metastatic events and 2 PC deaths, preventing any meaningful conclusions. Similarly, our findings are consistent with limited data from non-equal access medical centers as well. An analysis of men who underwent RP in the SEER-Medicare database between 1991 and 2009, found no racial differences in PC-specific or all-cause mortality.10 This study was limited by only examining older men (age >65 years), not controlling for PSA values, and controlling for tumor grade using AJCC grading wherein 98% of patients had grade 2 or 3 vs. our approach of using the commonly used Grade Grouping. Finally, data from John Hopkins, which included 15,993 (91%) white and 1,634 (9%) black men undergoing RP from 1992–2013 found that long-term outcomes, including metastasis free survival and PC-specific survival did not differ by race.11 However, results are limited by a 4-year median follow-up. Thus, the preponderance of the literature to date concludes that when detected early enough to be a surgical candidate and treated aggressively with RP, black men have similar long-term outcomes as white men.
Interestingly, both the study from the New York Harbor VA and Johns Hopkins found that despite no differences in PC death risk, black men were more likely to have a recurrence. Indeed, we found similar results, albeit this was not significant after multivariable analysis. Moreover, multiple other studies have found similar results,13–15 though some found no differences in recurrence by race.16,17 In trying to put these two disparate pieces of information together (higher recurrence rates, but similar long-term outcomes), we propose several possible explanations. First, black men received secondary treatments at a higher rate, which may have resulted in improved long-terms and thus matching those of white men. Second, black men may respond better to secondary treatments such as salvage radiation or hormonal therapy. There are some data suggesting tumors in black men are more hormonally driven (i.e. higher expression of the androgen receptor),18,19 which in theory may make them more hormonally sensitive. Third, biochemical recurrence is not a surrogate for PC death as many men with recurrence will not die of their disease.8 Thus, perhaps recurrences in black men, while higher in numbers, are less aggressive on the whole leading to an overall equal number of PC deaths. This is supported by our current finding that black men were no more likely to develop an aggressive recurrence. Finally, all the studies examining PC death after RP (ours included) are underpowered to detect modest associations between race and PC death. While the hazard ratios for PC death were actually under one in our study suggesting there is no evidence for even a slightly increased risk, we cannot confidently exclude a modest association positive. Thus, further studies with longer follow-up and more patients are needed.
Despite the finding that long-terms outcomes after RP are similar, black men continue to present at earlier ages and with more aggressive disease, leading to an overall increased risk of PC death at the population level.20 The reasons for black men having an increased PC incidence and mortality at the population level remain unclear and are likely multifactorial.21 However, the fact that even when diagnosed early and treated equally in an equal access medical center, black men presented at younger ages and had higher recurrence rates suggests potential biological differences by race. At the molecular level, possible causes include genetics, differences in androgen levels and androgen receptor biology, dietary factors and lifestyle factors affecting PC carcinogenesis pathways, such as smoking.22 Powell et al.23 recently found no difference between black and white men in PC incidence or extent at autopsy and suggested specific factors in black men somehow trigger the transformation from latent to aggressive PC. Recent data suggest aggressive PC in black men may be related to the presence of the Broad11934905 risk allele which is unique to AAM and is present on chromosome 8q24 (a known PC risk loci).24 Moreover, we found several aggressive PC markers including Ki67, androgen receptor, and alpha-methyl-acyl-racemase (AMACR) were more highly expressed among black men.18 Finally, differences in inflammatory genes expression in the tumors between black and white men have been noted.25,26 Indeed, there are genetic differences in inflammatory-related genes between races: black men have lower expression of DARC, a Duffy antigen/receptor for chemokines such as CXCL1 and CXCL8, on endothelial cells and erythrocytes.27 Loss of DARC is hypothesized to be selected for in men of African ancestry as it leads to malaria resistance. Regarding inflammation, DARC acts as a sink for cytokines and thus loss of DARC leads to higher systemic cytokines.28 All this evidence further supports the hypothesis that PC may biologically be different in black and white men.
The present study has several limitations. The number of events at the various endpoints (metastasis, OS, and PC-specific survival) was modest. Thus, we were relatively underpowered to detect these later events and thus we cannot exclude a weak to modest association between race and long-term outcomes. Likewise, given the long natural history of PC and our modest ~8.5 year mean follow-up, it is possible results may potentially change with longer follow-up and larger sample sizes. Our study only evaluated men undergoing RP and thus represents a selection bias of men who are healthy enough to undergo surgery and excludes men who present with metastatic disease who are not typically offered surgery. Future studies should address progression endpoints in black men undergoing other treatments such as radiotherapy. Nonetheless, our approach to analyze only surgical patients creates a more homogenous cohort wherein we can better ask do equal men, when treated equally, have equal outcomes. Moreover, our study still represents the largest to date evaluating metastasis and death in black men undergoing RP with equal access to care. Further studies from other institutions are required to verify our findings.
In conclusion, among black and white men undergoing RP at the multiple equal access centers of the SEARCH database, we found black men were at increased BCR risk in univariate but not multivariable analysis, consistent with our prior results.10 However, black men were not at increased risk of aggressive recurrence or the longer-term outcomes of metastasis, PC-specific death, or overall death compared to white men. As the number of events was small, even longer follow-up will be required to confirm these findings. Whether similar results will be seen at non-equal access centers remains to be determined.
Acknowledgments
This work was supported by the Department of Veterans Affairs, the Department of Defense, Prostate Cancer Research Program (SJF), NIH R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), Georgia Cancer Coalition (MKT). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Dept. of Defense.
Footnotes
CONFLICTS OF INTEREST STATEMENT: NONE
AUTHOR CONTRIBUTIONS STATEMENT: SJF, WJA, MKT, CLA, CJK, MRC, ACV contributed to the formulation of overarching research goals and aims, writing, reviewing and editing of the manuscript; SJF, WJA, MKT, CLA, CJK, MRC provided the resources (patients and data); LEH, SJF, ACV conducted the study and performed the analysis; SJF, WJA, MKT secured funding for this study.
References
- 1.Ries LAG, Eisner MP, Kosary CL, et al., editors. SEER cancer statistics review, 1973–1997. Bethesda, MD: National Cancer Institute; 2000. [Google Scholar]
- 2.Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. 2005;62:243. doi: 10.1002/pros.20052. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert KL, Ray R, Siddiqi A, et al. Visible and Invisible Trends in Black Men’s Health: Pitfalls and Promises for Addressing Racial, Ethnic, and Gender Inequities in Health. Annu Rev Public Health. 2016;37:295–311. doi: 10.1146/annurev-publhealth-032315-021556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorday W, Sadrzadeh H, de Koning L, et al. Association of sociodemographic factors and prostate-specific antigen (PSA) testing. Clin Biochem. 2014;47:164–9. doi: 10.1016/j.clinbiochem.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Lillard JW, Jr, Singh R. Molecular basis for prostate cancer racial disparities. Front Biosci (Landmark Ed) 2017;22:428–450. doi: 10.2741/4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd Elmageed ZY1, Moroz K, Srivastav SK, et al. High circulating estrogens and selective expression of ERβ in prostate tumors of Americans: implications for racial disparity of prostate cancer. Carcinogenesis. 2013;34:2017–23. doi: 10.1093/carcin/bgt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton RJ, Aronson WJ, Presti JC, Jr, et al. Race, biochemical disease recurrence, and prostate-specific antigen doubling time after radical prostatectomy: results from the SEARCH database. Cancer. 2007;110:2202–9. doi: 10.1002/cncr.23012. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:43. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber D, Levy EB, Schwartz D, et al. Impact of race in a predominantly African-American population of patients with low/intermediate risk prostate cancer undergoing radical prostatectomy within an equal access care institution. Int Urol Nephrol. 2014;46:1941–6. doi: 10.1007/s11255-014-0773-3. [DOI] [PubMed] [Google Scholar]
- 10.Schmid M, Meyer CP, Reznor G, et al. Racial Differences in the Surgical Care of Medicare Beneficiaries with Localized Prostate Cancer. JAMA Oncol. 2016;2:85–93. doi: 10.1001/jamaoncol.2015.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faisal FA, Sundi D, Cooper JL, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology. 2014;84:1434–41. doi: 10.1016/j.urology.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd JC, Banez LL, Aronson WJ, et al. Preoperative predictors of blood loss at the time of radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2009;12:264. doi: 10.1038/pcan.2009.6. [DOI] [PubMed] [Google Scholar]
- 13.Schroeck FR, Sun L, Freedland SJ, et al. Race and prostate weight as independent predictors for biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 2008;11:371–6. doi: 10.1038/pcan.2008.18. [DOI] [PubMed] [Google Scholar]
- 14.Yamoah K, Deville C, Vapiwala N, et al. African American men with low-grade prostate cancer have increased disease recurrence after prostatectomy compared with Caucasian men. Urol Oncol. 2015;33:70.e15–22. doi: 10.1016/j.urolonc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–45. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 16.Moses KA, Chen LY, Sjoberg DD, et al. Black and White men younger than 50 years of age demonstrate similar outcomes after radical prostatectomy. BMC Urol. 2014;11:14:98. doi: 10.1186/1471-2490-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett WW, Cooperberg MR, Sadetsky N, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005;66:1060–5. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Moreira DM, Jayachandran J, et al. Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men. Prostate Cancer Prostatic Dis. 2011;14:262–5. doi: 10.1038/pcan.2011.18. [DOI] [PubMed] [Google Scholar]
- 19.Koochekpour S, Buckles E, Shourideh M, et al. Androgen receptor mutations and polymorphisms in African American prostate cancer. Int J Biol Sci. 2014;10:643–51. doi: 10.7150/ijbs.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016 CA:a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 21.Ziehr DR, Mahal BA, Aizer AA, et al. Income inequality and treatment of African American men with high-risk prostate cancer. Urol Oncol. 2015;33:18.e7–13. doi: 10.1016/j.urolonc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. 2005;62:243–52. doi: 10.1002/pros.20052. [DOI] [PubMed] [Google Scholar]
- 23.Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitman EJ, Pomerantz M, Chen Y, et al. Prostate cancer risk allele specific for African descent associates with pathologic stage at prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19:1–8. doi: 10.1158/1055-9965.EPI-08-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 26.Powell IJ, Dyson G, Land S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer epidemiology, biomarkers & prevention. 2013;22:891–7. doi: 10.1158/1055-9965.EPI-12-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS genetics. 2009;5:e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novitzky-Basso I, Rot A. Duffy antigen receptor for chemokines and its involvement in patterning and control of inflammatory chemokines. Frontiers in immunology. 2012;3:266. doi: 10.3389/fimmu.2012.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]