Abstract
Background and Aim
Extended-release naltrexone (XR-NTX) blocks the effects of opioids for 4 weeks; however, starting treatment can be challenging because it requires 7 to 10 days of abstinence from all opioids. In the present study we identified patient and treatment characteristics that were associated with successful induction onto XR-NTX.
Methods
144 unemployed heroin-dependent adults who had recently undergone opioid detoxification completed self-report measures and behavioral tasks before starting an outpatient XR-NTX induction procedure. Employment-based reinforcement was used to promote opioid abstinence and adherence to oral naltrexone during the induction. Participants were invited to attend a therapeutic workplace where they earned wages for completing jobs skills training. Participants who had used opioids recently were initially invited to attend the workplace for a 7-day washout period. Then those participants were required to provide opioid-negative urine samples and then take scheduled doses of oral naltrexone to work and earn wages. Participants who had not recently used opioids could begin oral naltrexone immediately. After stabilization on oral naltrexone, participants were eligible to receive XR-NTX and were randomized into one of four treatment groups, two of which were offered XR-NTX. Binary and multiple logistic regressions were used to identify characteristics at intake that were associated with successfully completing the XR-NTX induction.
Results
58.3% of participants completed the XR-NTX induction. Those who could begin oral naltrexone immediately were more likely to complete the induction than those who could not (79.5% vs. 25.0%). Of 15 characteristics, 2 were independently associated with XR-NTX induction success: legal status and recent opioid detoxification type. Participants who were not on parole or probation (vs. on parole or probation) were more likely to complete the induction (OR [95% CI] = 2.5 [1.1–5.7], p = .034), as were those who had come from a longer-term detoxification program (≥ 21 days) (vs. a shorter-term [< 21 days]) (OR [95% CI] = 7.0 [3.0–16.6], p < .001).
Conclusions
Our analyses suggest that individuals recently leaving longer-term opioid detoxification programs are more likely to complete XR-NTX induction. Individuals on parole or probation are less likely to complete XR-NTX induction and may need additional supports or modifications to induction procedures to be successful.
Keywords: heroin, opioids, extended-release naltrexone, induction
1. Introduction
Rising rates of overdose and opioid use disorder caused by nonmedical prescription opioid use and heroin are significant public health issues in the United States (Han, Compton, Jones, & Cai, 2015; Jones, Logan, Gladden, & Bohm, 2015). Although underutilized, medication-assisted treatment including methadone (agonist), buprenorphine (partial agonist), and naltrexone (antagonist) is critical to and effective in managing the chronic relapsing nature of opioid use disorder (Connery, 2015; Substance Abuse and Mental Health Service Administration [SAMHSA], 2014). Methadone and buprenorphine are the most commonly used medications to treatment opioid use disorder; however, naltrexone is preferred by some patients (Uebelacker, Bailey, Herman, Anderson, & Stein, 2016) and may be more suitable for certain situations because it blocks the subjective and physiological effects of opioids (Bigelow, Preston, Schmittner, Dong, & Gastfriend, 2012), cannot produce lethal overdose or be abused, does not have special prescribing regulations (SAMHSA, 2015), and can be delivered as a monthly injection (extended-release naltrexone; XR-NTX) to address poor compliance associated with its oral formulation (Brooks et al., 2010).
Despite these advantages, initiating XR-NTX treatment can be challenging. Unlike methadone and buprenorphine, XR-NTX requires 7 to 10 days of abstinence from all opioids prior to beginning the medication to prevent potentially serious precipitated withdrawal (Alkermes®, 2015). Most patients relapse quickly following inpatient (Smyth, Barry, Keenan, & Ducray, 2010) and during outpatient buprenorphine-assisted detoxification (Dunn, Sigmon, Strain, Heil, & Higgins, 2011), and would not be eligible for XR-NTX. The subset of patients who are able to abstain throughout a recent buprenorphine-assisted detoxification must continue to remain opioid-free following buprenorphine taper and termination. This post-detoxification period is particularly challenging and has been associated with heightened withdrawal and relapse (Dunn et al., 2011; Horspool, Seivewright, Armitage, & Mathers, 2008; Sullivan et al., 2017).
As an alternative to requiring 7 to 10 days of opioid abstinence, efforts to increase the number of patients who could receive XR-NTX thus far have focused on developing rapid opioid detoxification procedures and evaluating investigational non-opioid medications to ease withdrawal (memantine and dronabinol, Bisaga et al., 2014; 2015). These procedures last 8 days; can occur in both inpatient and outpatient settings; and include brief buprenorphine stabilization, a washout period, and gradually increasing doses of oral naltrexone with XR-NTX delivery on the final day. Precipitated withdrawal symptoms are treated with non-opioid medications such as clonidine, clonazepam, zolpidem, trazodone, and other adjuvant medications. A similar outpatient rapid detoxification procedure that combines increasing very low dose oral naltrexone with decreasing low doses of buprenorphine has also been evaluated (Mannelli, Wu, Peindl, Swartz, & Woody, 2014). The investigational medications memantine and dronabinol have had no effect on XR-NTX induction outcomes. The rapid detoxification procedures in the above studies produced XR-NTX induction rates between 56% and 70%, and a recent outpatient randomized clinical trial showed that rapid outpatient naltrexone-assisted detoxification was superior to buprenorphine-only detoxification in promoting transition to XR-NTX (Sullivan et al., 2017).
In addition to developing optimal opioid detoxification protocols to promote XR-NTX induction, researchers and clinicians could further benefit from identifying treatment and patient characteristics that are associated with successful XR-NTX induction. Such information could inform clinical decision making, incorporate more personalized care, and potentially improve treatment outcomes. Few studies have examined patient characteristics predicting successful naltrexone induction. Those that have showed that older patients who used less opioids (Mogali et al., 2014), who were less prone to risk-taking (Aklin et al., 2012), and who used prescription opioids (Sullivan et al., 2017) were more likely to complete naltrexone induction than younger patients who used more opioids, were more prone to risk-taking, and who used heroin. The purpose of the current study was to extend this line of research by exploring predictors of XR-NTX induction among unemployed heroin-dependent adults enrolled in a randomized clinical trial to treat heroin use.
2. Materials and Methods
2.1 Main trial overview
Data were collected as part of an ongoing randomized clinical trial to evaluate the separate and combined effects of employment-based opiate abstinence reinforcement and XR-NTX for heroin use. Participants who completed the induction (described below) were invited to a 24-week outpatient treatment in a therapeutic workplace (for a description of the therapeutic workplace, see Silverman, Holtyn, & Morrison, 2016) and were randomized to one of four treatment conditions, two of which involved treatment with XR-NTX. All data were collected between November 2012 and June 2016.
2.2 Participants
Participants were recruited from detoxification programs in Baltimore, MD, through street outreach, from programs that provide services to heroin-dependent adults, and by word of mouth. Inclusion criteria for the main trial were that participants: (1) met DSM-IV criteria for opioid dependence, (2) reported using heroin at least 21 of the last 30 days while living in the community, (3) reported and show visible signs (track marks) of injection drug use, (4) were unemployed, (5) were between 18 and 65 years old, (6) were medically approved for naltrexone treatment, and (7) lived in or near the study area. Exclusion criteria were that participants: (1) had current DSM-IV major Axis I disorders, (2) had current suicidal or homicidal ideation, (3) expressed interest in methadone or buprenorphine maintenance treatment, (4) used opioids for prescribed medical purposes, (5) earned over $200 in taxable income in the past 30 days while living in the community, (6) had physical limitations that prevented them from using a keyboard, (7) were pregnant or breastfeeding, (8) had serum aminotransferase levels over three times normal, (9) had known intolerance to naltrexone or XR-NTX components, and (10) were enrolled in another clinical study.
The requirement that participants be injection drug users was removed on January 2015 to broaden the target population and increase enrollment. For the present analyses, only eligible participants who formally enrolled in the main trial were included (i.e., those who signed a consent but never returned were excluded).
2.3 Intake assessments
At an intake session, participants completed a battery of self-report measures and interviews, behavioral tasks, and provided a urine sample that was tested for opiates, methadone, buprenorphine, oxycodone, cocaine, benzodiazepines, and THC.
2.3.1 Self-report measures and interviews
Self-report measures and interviews included the following: (1) Addiction Severity Index-Lite (ASI; McLellan et al., 1985), a semi-structured interview that assesses functioning in multiple dimensions (i.e., substance use, medical, legal, education, employment, and family histories); (2) Composite International Diagnostic Interview (CIDI; heroin and cocaine sections only; Compton, Cottler, Dorsey, Spitznagel, & Magera, 1996), a valid DSM-IV diagnostic tool for psychiatric disorders; (3) Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) a 21-item measure of depression severity; and (4) Prior treatment form, a questionnaire to gather information on participant’s most recent opioid detoxification. Participants were categorized as coming from shorter-term (< 21 days) or longer-term (≥ 21 days) detoxification programs. Three participants had recently been released from incarceration (≥ 21 days) and were categorized as longer-term. All longer-term detoxifications were inpatient programs, whereas short-term detoxifications were a mix of outpatient and inpatient programs. Specific details on detoxification protocols and medications were not collected.
2.3.2 Behavioral tasks
Behavioral tasks included the Balloon Analogue Risk Task (BART; Lejuez et al., 2002), a delay discounting task, and the Wisconsin Card Sorting Task (WCST; Heaton, Chelune, Talley, Kay, & Curtiss, 1993). The BART is a computer-based task that measures risk-taking propensity. The task displays a simulated balloon, a balloon pump button, an earnings collection button, a current earnings display, and a total earnings display. Participants were instructed to pump the simulated balloon to earn as much money as possible but to keep in mind that the balloon could pop at any time. Each click on the pump button increases the size of the simulated balloon and adds money ($0.05) to a current earnings display. Balloons that are pumped beyond their individual explosion points pop, and money accrued in the current earnings display is lost. Explosion points ranged from 1 to 128 pumps and were randomly determined for each balloon. At any time during the task, participants could collect their current earnings by pressing the earnings collection button. After a balloon was popped or earnings were collected, a new trial began with a new balloon, ending on the 20th trial. The primary measure of risk-taking propensity was the average number of pumps per trial, excluding those in which a balloon exploded.
Delay discounting is one behavioral index of impulsive choice and was measured using a computer-based task in which participants made hypothetical choices between receiving a smaller amount of money immediately or a larger amount of money after a delay. The larger amount was held constant at $1000 and the smaller amount varied across choice trials. The levels at which the subjective value of the smaller sooner and larger later rewards were equal (i.e., indifference points) were determined across seven delays: 1 week, 1 month, 6 months, 1 year, 2 years, 4 years, and 8 years. The primary measure of delay discounting was the area under the curve (Myerson, Green & Warusawithrana, 2001) of the seven indifference points. Nonsystematic data were screened and removed from analyses using an established algorithm (Johnson & Bickel, 2008). Area under the curve values range from 0 to 1, with lower values representing more impulsive choice.
The WCST is a computer-based neuropsychological test that measures executive functioning and impairment. Participants were asked to match 128 sample electronic cards that varied across three categories (number [1, 2, 3, 4], color [red, green, blue, yellow], and shape [circle, star, square, plus sign]), to one of four primary cards that also varied across these categories. Participants were not told the matching rule (i.e., based on number, color, or shape) but were given feedback (“Right” or “Wrong”) after sorting each sample card. After ten consecutive correct matches, the matching rule changed to a different category. The test concluded after six categories were completed or all 128 cards had been sorted. The primary outcomes were age- and education-adjusted percentiles for categories completed and perseverative errors made (i.e., making an error after receiving feedback that the match was wrong).
2.4 XR-NTX induction procedure
All participants in the main trial had to complete an outpatient XR-NTX induction procedure prior to randomization. All procedures took place in the therapeutic workplace, where participants were invited to work up to four hours each weekday on computer-based jobs skills training programs. Participants earned $8 per hour in base pay and up to an additional $2 per hour in performance pay based on their progress in the various training programs. Mandatory urine samples were collected and tested every Monday, Wednesday, and Friday. Two induction procedures, delayed induction and immediate induction, were implemented based on participants’ opioid use determined at intake (Table 1).
Table 1.
XR-NTX induction procedures for participants who reported opioid use in the past 10 days or provided an opioid-positive urine sample at intake (delayed induction) or for participants who reported no opioid use in the past 10 days and provided an opioid-negative urine sample at intake (immediate induction).
| Phase |
Delayed induction
|
|
|---|---|---|
| Urine drug screening Monday, Wednesday, Friday | Employment-based reinforcement | |
| Washout (Days 1–7) | X | – |
| Opioid Abstinence (Days 8–17) | X | X for opioid-negative urines |
| Oral Naltrexone (Days 18–31) | X | X for taking scheduled doses of oral naltrexone |
|
| ||
| Phase |
Immediate induction
|
|
| Urine drug screening Monday, Wednesday, Friday | Employment-based reinforcement | |
| Oral Naltrexone (Days 1–14) | X | X for taking scheduled doses of oral naltrexone |
2.4.1 Delayed induction
Participants who reported opioid use in the past 10 days or who provided a urine sample that tested positive for opioids were exposed to the delayed induction procedure that consisted of three consecutive phases: washout, opioid abstinence, and oral naltrexone. The washout phase lasted 7 days and allowed time for the elimination of opioids. Participants could access the workplace and earn maximum wages independent of their urine test results during washout. The opioid abstinence phase generally lasted 10 days, during which employment-based abstinence reinforcement was implemented: Participants could access the workplace to earn wages only if their urine samples were negative for all opioids. Participants who tested positive for opioids were not allowed to work that day and their base pay was reset to $1. These participants were allowed up to 30 days to provide an opioid-negative urine sample and to resume work. Following a reset, base pay increased by $1 for each day the participant met the opioid abstinence criterion. After participants achieved 10 days of opioid abstinence, they were inducted onto oral naltrexone. The oral naltrexone phase lasted approximately two weeks, and access to the workplace and maximum wages was contingent on taking scheduled doses of oral naltrexone (i.e., employment-based reinforcement of oral naltrexone adherence). Dosing decisions were based on clinical judgment but participants received ascending doses of oral naltrexone (usually 25mg on day 1, 50mg on day 2, and 100 mg on day 3). All participants achieved a maintenance dose of 100mg on Monday, 100mg on Wednesday, and 150mg on Friday before randomization.
2.4.2 Immediate induction
Participants who reported no opioid use in the past 10 days and who provided a urine sample that tested negative for all opioids were placed on immediate induction, which included the oral naltrexone phase only.
2.4.3 Urine sample collection and testing
Urine samples were collected every Monday, Wednesday and Friday throughout the induction period. All samples were tested for opiates and cocaine. Samples collected during the washout and opioid abstinence phases were also tested for methadone, buprenorphine, and oxycodone.
2.5 Outcomes and data analysis
The primary outcome was successful completion of the XR-NTX induction, defined as taking all maintenance doses of oral naltrexone and being randomized into the main trial. A secondary outcome was whether participants began taking scheduled doses of oral naltrexone. We conducted bivariate logistic regressions to examine simple associations between baseline variables at intake and success during the XR-NTX induction. For this analysis, we selected a priori to examine only a subset of variables based on the literature and our experiences implementing similar induction procedures in previous trials (e.g., DeFulio et al., 2012). Baseline variables that were associated with XR-NTX induction success (p < .10) were entered simultaneously in a multiple logistic regression model. Statistically significant independent predictors of XR-NTX were analyzed descriptively among the subgroup of participants who reported opioid use in the past 10 days or who provided a urine sample that tested positive for opioids at intake.
3. Results
3.1 Participant characteristics
The participant characteristics that were selected for inclusion in this analysis are shown in Table 2. Participants were predominantly male, middle-aged, and Black. Most participants reported long histories of heroin use, injecting as their primary route of heroin administration, had recently used cocaine, and had recently completed a longer-term opioid detoxification.
Table 2.
Participant characteristics at intake
| Characteristic | Successful XR-NTX Induction (n = 84) % (n) or M (SD) |
Unsuccessful XR-NTX Induction (n = 60) % (n) or M (SD) |
p-value |
|---|---|---|---|
| Sex (male) | 71.4 (60) | 71.7 (43) | .975 |
| Age (years) | 42.7 (10.5) | 42.8 (10.1) | .951 |
| Race (black) | 81.0 (68) | 61.7 (37) | .011 |
| Currently on parole or probation | 22.6 (19) | 36.7 (22) | .068 |
| Opioid detoxification was longer-term | 84.5 (71) | 43.3 (26) | <.001 |
| Heroin | |||
| Lifetime use (years) | 16.4 (10.2) | 17.5 (10.9) | .569 |
| Primary route of administration intravenous | 81.0 (68) | 78.3 (47) | .699 |
| Cocaine | |||
| Past 30-day use (days) | 13.9 (13.5) | 11.8 (12.7) | .338 |
| Primary route of administration intravenous | 45.7 (32) | 40.8 (20) | .596 |
| Cannabis | |||
| Past 30-day use (days) | 4.6 (8.9) | 3.6 (7.8) | .485 |
| Beck Depression Inventory-II total score | 11.0 (8.7) | 14.7 (11.5) | .032 |
| Balloon Analogue Risk Task (total pumps) | 32.1 (12.6) | 30.3 (12.1) | .410 |
| Wisconsin Card Sorting Task | |||
| Categories completed (percentile) | .866 | ||
| ≤ 1 | 14.3 (12) | 10.2 (5) | |
| 2–5 | 16.7 (14) | 22.4 (11) | |
| 6–10 | 8.3 (7) | 6.1 (3) | |
| 11–16 | 8.3 (7) | 10.2 (5) | |
| > 16 | 52.4 (44) | 51.0 (49) | |
| Perseverative errors (percentile) | 38.8 (30.2) | 41.0 (31.7) | .690 |
| Delay discounting (area under the curve) | .257 (.218) | .226 (.207) | .469 |
3.2 Naltrexone initiation and XR-NTX induction success
Of the 144 participants who enrolled, 98 of 144 (68.1%) initiated oral naltrexone and 84 of 144 (58.3%) successfully completed the oral naltrexone phase and were eligible to receive XR-NTX. Nearly all participants (46/48; 95.8%) randomized to conditions that offered XR-NTX accepted their first injection.
Fifty-six (38.9%) participants reported opioid use in the past 10 days or provided a urine sample that tested positive for opioids and began the delayed induction procedure. Fifteen of the 56 (26.8%) who began the delayed induction procedure started taking scheduled doses of oral naltrexone, and 14 (25.0%) completed the XR-NTX induction and were randomized in the main trial. Among the 29 participants who tested positive for opiates at intake (i.e., recently used heroin), just 2 (6.9%) completed the XR-NTX induction. For participants who had no evidence of recent opioid use and were placed on immediate induction, rates of starting oral naltrexone (83 of 88; 94.3%) and completing the XR-NTX induction (70 of 88; 79.5%) were considerably higher.
3.3 Predictors of successful XR-NTX induction
Bivariate analyses revealed that four of the fifteen intake variables were associated (p < .10) with successful XR-NTX induction (Table 2). Participants who were black (vs. not black), not currently on parole or probation, recently completed a longer-term opioid detoxification (vs. shorter-term), and had lower levels of depression were more likely to complete the outpatient induction to be eligible to receive XR-NTX. Sex, age, lifetime heroin use, injection heroin use, injection cocaine use, and recent cocaine and cannabis use were not significantly associated with induction success. Outcomes on behavioral tasks to measure risk-taking propensity, impulsive choice, and executive functioning and impairment were also not related to completing the induction.
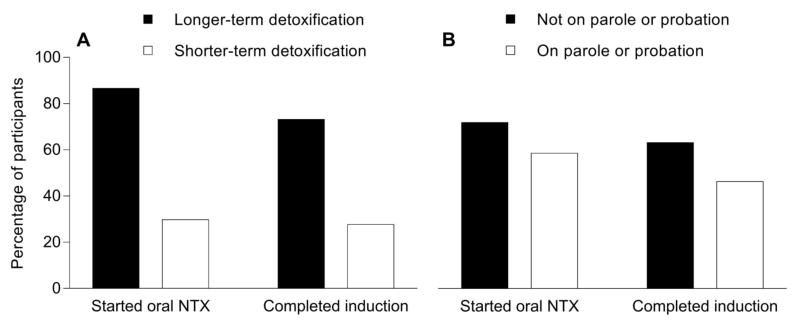
In the multivariate analyses, race, legal status, recent detoxification type, and depression were entered simultaneously to determine whether and to what extent each uniquely predicted XR-NTX induction success. Controlling for other variables in the model, only recent detoxification type and legal status were significant predictors of XR-NTX induction (Table 3; Figure 1). Compared to participants who were on parole or probation, those who were not were more likely (adjusted odds ratio of 2.5) to complete XR-NTX induction. The relationship between opioid detoxification type and XR-NTX induction was stronger – participants who were recently discharged from a longer-term opioid detoxification were more likely (adjusted odds ratio of 7.0) to complete the induction than those recently discharged from a shorter-term detoxification program. Within the subsample of participants who at intake reported opioid use in the past 10 days or who provided a urine sample that tested positive for opioids (n=56), similar findings were seen for legal status (on parole or probation = 13.3% success; not on parole or probation = 29.3%) and opioid detoxification type (shorter-term opioid detoxification = 17.5%; longer-term opioid detoxification = 43.8%), although the number of participants in these analyses were too small to conduct formal statistical analyses.
Table 3.
Multiple logistic regression results predicting successful XR-NTX induction
| Variable | β (SE) | Wald | Adjusted odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Intercept | −1.61 (0.65) | 6.11 | .013 | |
| Race | 0.27 (0.46) | 0.34 | 1.31 (0.53–3.20) | .558 |
| Currently on parole or probation | 0.91 (0.43) | 4.48 | 2.48 (1.07–5.74) | .034 |
| Recent opioid detoxification was longer-term | 1.95 (0.44) | 19.86 | 7.04 (2.98–16.61) | <.001 |
| Beck Depression Inventory-II total score | −0.01 (0.02) | 0.48 | 0.99 (0.95–1.03) | .489 |
R2 = .28 (Nagelkerke). χ2 (4) = 33.65, p < .001
Figure 1.
The relationships between opioid detoxification type (A), legal status (B), and successful initiation of oral naltrexone and completion of the XR-NTX induction
4. Discussion
Overall, 58.3% of participants enrolled and completed the outpatient XR-NTX induction. More participants who were eligible to begin oral naltrexone immediately completed the outpatient XR-NTX induction (79.5%) than those required to complete the washout and opioid abstinence phases before beginning oral naltrexone treatment (25.0%). Among participants who started taking oral naltrexone (68.1%), the vast majority completed the XR-NTX induction successfully (85.7%). This finding is consistent with prior research showing high rates of adherence to oral naltrexone when employment-based reinforcement for adherence is in place (Dunn et al., 2013). In contrast, for individuals who used heroin recently and tested positive for opiates immediately prior to entering the study, employment-based reinforcement to promote opiate abstinence and oral naltrexone adherence was largely unsuccessful.
To identify whether particular subsets of participants were better candidates for being eligible to receive XR-NTX, we examined several individual and treatment characteristics measured prior to induction procedures – demographics, legal status, recent opioid detoxification treatment, drug use, depression, risk-taking propensity, impulsive choice, and cognitive impairment. Of these characteristics, two were independently associated with XR-NTX induction. Participants who were not on parole or probation during the induction period and those who had recently completed a longer-term opioid detoxification were significantly more likely to be inducted onto XR-NTX than participants who were on parole or probation and who had recently completed a shorter-term opioid detoxification, respectively. Similar relationships were also observed in the subsample that could not begin oral naltrexone immediately due to recent opioid use.
One study that investigated predictors specific to XR-NTX induction did not find statistically significant associations of legal status and drug treatment history with induction success (Mogali et al, 2014). Differences in the induction procedures and how participant characteristics were measured may account for these disparate findings. Rather than current parole or probation status, Mogali et al. categorized legal status as whether a participant had a “legal history.” There was a non-significant trend suggesting higher rates of XR-NTX induction among those who had no legal history. Regarding drug treatment history, because all participants underwent the same opioid detoxification as part of the study procedures, treatment history was categorized as whether or not participants had received a specific type of treatment (e.g., detoxification, opioid agonist maintenance) in their lifetime. Duration was not noted.
In contrast to Mogali et al. (2014), we did not observe significant associations between age and XR-NTX induction. Although we did not measure the amount of daily heroin use (e.g., bags per day), we did measure years of heroin use and did not observe a significant association with XR-NTX induction. Recent evidence (Sullivan et al., 2017) suggest that the type of opioid used (prescription opioids vs. heroin) may be a more robust predictor of XR-NTX induction than amount or route of administration, the latter of which was not associated with induction in past studies or the present study. Previous work by our group (Aklin et al., 2012) showed that participants with higher risk-taking propensity were less likely to complete outpatient oral naltrexone induction. We did not replicate this finding from the BART in the present study. Other variables explored based on prior studies were cocaine use (DeFulio et al., 2012), cannabis use (Bisaga et al., 2015), depression (Marsch et al., 2005), delay discounting (Stevens, Verdejo-Garcia, Roeyers, Goudriaan, & Vanderplasschen, 2015), and cognitive impairment (Adinoff et al., 2015), all of which were not associated with XR-NTX induction in the final model. While these have been previously shown to be related to various treatment outcomes and drug use, they may play little role in predicting XR-NTX induction. Future research examining these factors in different populations and with different induction procedures is needed.
Our finding that individuals currently on parole or probation have more difficulty initiating XR-NTX is relevant given the growing interest and research in using XR-NTX among individuals involved in the criminal justice system (Crits-Cristoph et al., 2016). This population faces a number of challenges including poor social supports, financial problems, difficulty reintegrating into the community, and residing in areas of high drug trafficking and use (Binswanger et al., 2012). Individuals involved in the criminal justice system being released from a controlled environment are also at high risk for relapse and fatal overdose (Binswanger, Blatchford, Mueller, & Stern, 2013), which can be significantly reduced with XR-NTX (Lee et al., 2016). Further, because of concerns about abuse and diversion associated with methadone and buprenorphine, XR-NTX may be ideal for adults in the criminal justice system, especially when initiated prior to release when patients have been detoxified for extended periods of time (Gordon et al., 2016). Despite the need and advantages of delivering XR-NTX to adults in the criminal justice system, our results suggest that they may require additional supports or modified procedures to increase their chances of successful XR-NTX induction (see National Institute on Drug Abuse, 2014 for discussion of treatment guidelines for this population).
The best predictor of XR-NTX induction was the type of opioid detoxification participants had received. Participants coming from longer-term detoxification programs (mean duration = 35.1 days, SD = 27.5) were substantially more likely to be inducted on XR-NTX compared to participants coming from shorter-term detoxification programs (mean duration = 7.0 days, SD = 4.9). This finding is consistent with observational data collected in an XR-NTX implementation study in Los Angeles, CA that found patients receiving XR-NTX were significantly more likely to have been referred from residential treatment (47.5% vs. 16.5%) and less likely to have been referred from outpatient treatment (32.5% vs. 67.5%) than patients who did not receive XR-NTX (Cousins, et al., 2016).
In the present study, the outpatient XR-NTX induction procedures and recent opioid detoxification type received were highly related. We chose to include detoxification type in the analyses rather than the induction procedure because of its broader application. Most participants (83.5%) coming from longer-term opioid detoxification programs were eligible to start oral naltrexone and began an immediate induction, whereas most participants (85.1%) coming from shorter-term opioid detoxification programs were not and began a delayed induction. The greater success in being eligible to receive XR-NTX for the longer-term opioid detoxification participants was due to the fact that most did start taking oral naltrexone, whereas most shorter-term detoxification participants did not begin taking oral naltrexone.
However, long-term residential treatment facilities in the United States, similar to those that were associated with high rates of XR-NTX induction success in the present study, make up less than 10% of all facilities that offer opioid treatment programs (SAMHSA, 2014). This number is not likely to change because of high treatment costs and lack of insurance coverage for such treatments. Nevertheless, the present study suggests that patients completing these types of programs are the most likely to successfully start XR-NTX. Efforts to expand XR-NTX to individuals being discharged from long-term treatments may be most successful, at least initially.
Our findings add to the limited literature on factors associated with XR-NTX induction; however, some limitations are worth noting. First, the outpatient induction procedure used a unique intervention (employment-based reinforcement) to promote opioid abstinence (when necessary) and oral naltrexone adherence. Future work will be necessary to determine whether legal status and recent detoxification type are associated with XR-NTX initiation using other, more common induction procedures. Second, the study sample was restricted to primary heroin users who were unemployed and recruited from one urban area. The relationship between participant characteristics measured in the present study and XR-NTX induction success may differ for other more general populations. Third, although all participants had to complete the XR-NTX induction procedures to be randomized into the main trial, not all were randomized to conditions that received XR-NTX. Of the 84 randomized participants, 48 were assigned to receive XR-NTX, and all but two participants received their first injection (one was withheld due to pregnancy and another because the participant was unable to leave a controlled environment). Fourth, the model significantly predicted whether participants were inducted onto XR-NTX but there is still much variance that is unexplained. Other factors not analyzed or measured may be more predictive and/or clinically relevant to XR-NTX induction. For example, withdrawal was not formally assessed following oral naltrexone initiation and maintenance and may have contributed to drop out for the subset that started but did not complete the oral naltrexone phase. Finally, our analyses were correlational and the measures we used did not collect detailed information about the nature of participants’ parole and probation or specific protocols about the opioid detoxification programs they attended prior to enrolling in the study. Experimental analyses of opioid detoxification length and improved measures are needed to evaluate more clearly the mechanisms through which opioid detoxification length and legal status may impact XR-NTX induction.
5. Conclusions
This study adds to the limited literature characterizing XR-NTX induction and factors associated with its success. Being on parole or probation and coming from a shorter-term opioid detoxification (vs. longer-term) were both independently associated with failing an outpatient XR-NTX induction protocol necessary to be eligible to receive an injection. These patients may need additional supports or alternative induction strategies to begin XR-NTX. Future research investigating these variables in other populations and induction procedures is needed.
Highlights.
Starting XR-NTX can be difficult because it requires extended opioid abstinence
Patient characteristics may predict XR-NTX induction and help inform treatment
Patients on parole or probation had less XR-NTX induction success
Patients from longer-term detoxification programs had more XR-NTX induction success
Acknowledgments
Role of Funding Source
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (R01DA019497 and T32DA07209). Alkermes, Inc. supplied the medication (Vivitrol®) at no cost for the main trial. Neither Alkermes, Inc. or the National Institutes of Health had any further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Carmody TJ, Walker R, Donovan DM, Brigham GS, Winhusen T. Decision-making processes as predictors of relapse and subsequent use in stimulant-dependent patients. The American Journal of Drug and Alcohol Abuse. 2015;42:88–97. doi: 10.3109/00952990.2015.1106550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aklin WM, Severtson G, Umbricht A, Fingerhood M, Bigelow GE, Lejuez CW, Silverman K. Risk-taking propensity as a predictor of induction onto naltrexone treatment for opioid dependence. Journal of Clinical Psychiatry. 2012;73:1056–1061. doi: 10.4088/JCP.09m05807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkermes, Inc. Vivitrol® (naltrexone for extended-release injectable suspension) 2015 Retrieved from https://www.vivitrol.com/Content/pdf/prescribing_info.pdf.
- Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bigelow GE, Preston KL, Schmittner J, Dong Q, Gastfriend DR. Opioid challenge evaluation of blockade by extended-release naltrexone in opioid-abusing adults: Dose-effects and time-course. Drug and Alcohol Dependence. 2012;123:57–65. doi: 10.1016/j.drugalcdep.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Nowels C, Corsi KF, Glanz J, Long J, Booth RE, Steiner JF. Return to drug use and overdose after release from prison: A qualitative study of risk and protective factors. Addiction Science & Clinical Practice. 2012;7 doi: 10.1186/1940-0640-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: Opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine. 2013;159:592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Carpenter KM, Mariani JJ, … Nunes EV. A placebo-controlled trial of memantine as an adjunct to injectable extended-release naltrexone for opioid dependence. Journal of Substance Abuse Treatment. 2014;46:546–552. doi: 10.1016/j.jsat.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Pavlicova M, Haney M, … Nunes EV. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug and Alcohol Dependence. 2015;154:38–45. doi: 10.1016/j.drugalcdep.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AC, Comer SD, Sullivan MA, Bisaga A, Carpenter KM, Raby WM, … Nunes EV. Long-acting injectable versus oral naltrexone maintenance therapy with psychosocial intervention for heroin dependence: A quasi-experimental experiment. Journal of Clinical Psychiatry. 2010;71:1371–1378. doi: 10.4088/JCP.09m05080ecr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Dorsey KB, Spitznagel EL, Mager DE. Comparing assessments of DSM-IV substance dependence disorders using CIDI-SAM and SCAN. Drug and Alcohol Dependence. 1996;41:179–187. doi: 10.1016/0376-8716(96)01249-5. [DOI] [PubMed] [Google Scholar]
- Connery HS. Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry. 2015;23:63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Cousins SJ, Denering L, Crevecoeur-MacPhail D, Viernes J, Sugita W, Barger J, … Rawson RA. A demonstration project implementing extended-release naltrexone in Los Angeles County. Substance Abuse. 2016;37:54–62. doi: 10.1080/08897077.2015.1052868. [DOI] [PubMed] [Google Scholar]
- Crits-Cristoph P, Markell HM, Gibbons MBC, Gallop R, Lundy C, Stringer M, Gastfriend DR. A naturalistic evaluation of extended-release naltrexone in clinical practice in Missouri. Journal of Substance Abuse Treatment. 2016;70:50–57. doi: 10.1016/j.jsat.2016.07.014. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Everly JJ, Leoutsakos JM, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: A randomized controlled trial. Drug and Alcohol Dependence. 2012;120:48–54. doi: 10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, DeFulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, … Silverman K. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Experimental and Clinical Psychopharmacology. 2013;21:74–83. doi: 10.1037/a0030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: A review. Drug and Alcohol Dependence. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. Journal of the American Medical Association. 2015;314:1468–1478. doi: 10.10001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and expanded. Psychological Assessment Resources Inc; Odessa, FL: 1993. [Google Scholar]
- Horspool MJ, Seivewright N, Armitage C, Mathers N. Post-treatment outcomes of buprenorphine detoxification in community settings: A systematic review. European Addiction Research. 2008;14:179–185. doi: 10.1159/000141641. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Experimental and Clinical Psychopharmacology. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden M, Bohm MK. Vital signs: Demographic and substance use trends among heroin users – United States, 2002–2013. Morbitity and Mortality Weekly Report. 64:719–725. [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, … O’Brien CP. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. The New England Journal of Medicine. 2016;374:1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, … Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Wu L-T, Peindl KS, Swartz MS, Woody GE. Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: A very low dose naltrexone and buprenorphine open label trial. Drug and Alcohol Dependence. 2014;138:83–88. doi: 10.1016/j.drugalcdep.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Stephens M, Mudric T, Strain EC, Bigelow GE, Johnson RE. Predictors of outcomes in LAAM, buprenorphine, and methadone treatment for opioid dependence. Experimental and Clinical Psychopharmacology. 2005;13:293–302. doi: 10.1037/1064-1297.13.4.293. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mogali S, Khan NA, Drill ES, Pavlicova M, Sullivan MA, Nunes E, Bisaga A. Baseline characteristics of patients predicting suitability for rapid naltrexone induction. The American Journal on Addictions. 2014;24:258–264. doi: 10.1111/ajad.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–43. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Principles of drug abuse treatment for criminal justice populations: A research-based guide. 2014 (NIH Publication No. 11-5316). Retrieved from https://www.drugabuse.gov/publications/principles-drug-abuse-treatment-criminal-justice-populations/principles.
- Silverman K, Holtyn AF, Morrison R. The therapeutic utility of employment in drug addiction: Science to application. Translational Issues in Psychological Science. 2016;2:203–212. doi: 10.1037/tps0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Irish Medical Journal. 2010;103:176–179. [PubMed] [Google Scholar]
- Stevens L, Verdejo-Garcia A, Roeyers H, Goudriaan AE, Vanderplasschen W. Delay discounting, treatment motivation and treatment retention among substance-dependent individuals attending an inpatient detoxification program. Journal of Substance Abuse Treatment. 2015;49:58–64. doi: 10.1016/j.jsat.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Service Administration. National Survey on Substance Abuse Treatment Services (N-SSATS) 2013: Data on substance abuse treatment facilities. 2014 (HHS Publication No. 14-4890). Retrieved from http://wwwdasis.samhsa.gov/dasis2/nssats/2013_nssats_rpt.pdf.
- Substance Abuse and Mental Health Service Administration. Federal guidelines for opioid treatment programs. 2015 (HHS Publication No. PEP15-FEDGUIDEOTP). Retrieved from http://store.samhsa.gov/shin/content/PEP15-FEDGUIDEOTP/PEP15-FEDGUIDEOTP.pdf.
- Sullivan M, Bisaga A, Pavlicova M, Choi J, Mishlen K, Carpenter KM, … Nunes EV. Long-acting injectable naltrexone induction: A randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. The American Journal of Psychiatry. 2017 doi: 10.1176/appi.ajp.2016.16050548. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker LA, Bailey G, Herman D, Anderson B, Stein M. Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. Journal of Substance Abuse Treatment. 2016;66:48–53. doi: 10.1016/j.jsat.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]