Abstract
Because adolescence is a period of heightened exploration of new behaviors, there is a natural increase in risk taking including initial use of alcohol and marijuana. In order to better understand potential differences in neurocognitive functioning among adolescents who use drugs, the current study aimed to identify the neural substrates of risky decision making that differ among adolescents who primary users of alcohol or marijuana, primary users of both alcohol and marijuana, and controls who report primary use of neither drug. Participants completed the Balloon Analogue Risk Task (BART) while undergoing functional magnetic resonance imaging. Comparison of brain activation during risky decisions versus non-risky decisions across all subjects revealed greater response to risky decisions in dorsal anterior cinguate cortex (dACC), anterior insula, ventral striatum, and lateral prefrontal cortex. Group comparisons across non-using controls, primary marijuana, primary alcohol, and alcohol and marijuana users revealed several notable differences in the recruitment of brain regions. Adolescents who use both alcohol and marijauna show decreased response during risky decision making compared to controls in insula, striatum, and thalamus, and reduced differentiation of increasing risk in dACC, insula, striatum, and superior parietal lobe compared to controls. These results provide evidence of differential engagement of risky decision making circuits among adolescents with varying levels of alcohol and marijuana use, and may provide useful targets for longitudinal studies that explicitly address causality of these differences.
Keywords: Risk taking, marijuana, alcohol, adolescence
Introduction
Initial exploration of substance use often occurs during adolescence, with 75.6% of all teens trying alcohol and 48.6% trying marijuana by the age of 18, with 46.8% and 27.7%, respectively, using each on a regular basis by the end of high school (Kann et al. 2014). While some argue for the normative nature of this exploration (Shedler and Block, 1990), others suggest that substance use during adolescence is associated with numerous harmful sequelae (Lisdahl et al. 2013; Tucker et al. 2006). Data from cross-sectional and longitudinal studies suggest that alcohol and marijuana use during adolescence is associated with changes in neural development and neurocognitive functioning (Gruber et al. 2013; Jacobus et al. 2015; Meier et al. 2012; Squeglia et al. 2014; White et al. 2010).
Differential rates of development of neural systems may be one reason for increased exploration of drugs during adolescence. While recruitment of frontal-based cognitive control systems increases linearly throughout development, reward based systems follow a quadratic increase with the peak occuring during adolescence (Somerville and Casey, 2010), resulting in an imbalance between the two systems during the critical age period when drug use increases. Studies have demonstrated reduced lateral frontal engagement during response inhibition among high-risk adolescents (i.e. those with a positive family history positive for substance use disorder; Heitzeg et al. 2010) and among current alcohol/marijuana users compared to controls (Norman et al. 2011; Schweinsburg et al. 2004; Wetherill et al. 2013), as well as differential response in ventral striatal regions in current alcohol/marijuana users compared to non-using controls (Schneider et al. 2012). While the above studies have examined the neural differences in cognitive and emotional functioning, they have not addressed potential competition between regions implicated in cognitive/emotional functioning. Examination of potential competition between cognitive and emotional functioning may be key for understanding drug use during adolescence. Decision making tasks are an ideal context in which to probe these interactions given that these tasks often involve trading off risks and rewards, requiring regions implicated in both cognitive and emotional processing.
While myriad measures of decision making exist, the Balloon Analogue Risk Task (BART) is one of the few laboratory tasks that predicts real-world risk-taking (Aklin et al. 2005; Lejuez et al. 2002) and substance using adolescents (Crowley et al. 2006; Hanson et al. 2014). In the BART, participants pump simulated air into a balloon, which increases the probability/risk of bursting. Participants can decide to “cash-out” at any time to collect accrued points, or continue pumping, which eventually causes the balloon to explode. The decision on each trial represents a conflict between reward accrual and potential negative consequences, making this task ideal for probing decision conflict. Neuroimaging studies of the BART have revealed increased responses during the risky decision phase in dorsal anterior cingulate cortex (dACC), insula, dorsolateral prefrontal cortex (DLPFC) and striatum (Bogg et al. 2012; Claus and Hutchison, 2012; Schonberg et al. 2012), regions implicated in choice conflict, prediction of rewarding outcomes, and cognitive control. In the one study to date that examined the neural correlates of risky decision making on the BART in substance abusing adolescents, adolescents with substance use showed reduced engagement of middle and medial frontal gyrus, ACC, and insula during risk-taking, suggesting a potential aberrant mechanism in avoiding risk (Crowley et al. 2010).
Although prior studies have investigated risk taking and other cognitive functions in adolescent substance users, the relative differences compared to controls attributable to alcohol or marijuana use or the combination of alcohol and marijuana has been largely neglected. Thus, the current study aimed to examine differences between adolescents who reported minimal/no use of alcohol or marijuana, adolescents reporting frequent use of either alcohol or marijuana, and adolescents reporting frequent use of both substances. Behaviorally, we expected that adolescents reporting alcohol and/or marijuana use would engage in more risk on the BART compared to non-users. Next, we hypothesized that frequent alcohol use and/or marijuana use would be associated with reduced response in dACC and anterior insula during risky decision making. Finally, frequent use of alcohol and marijuana was hypothesized to be associated with even greater reductions in BOLD response within regions implicated in decision making than individuals reporting use of a single substance.
Methods
Participants
One hundred ninety-eight adolescents were recruited through an alternative to incarceration program intended to reintegrate youth into school settings. Participants were recruited to take part in a study that was investigating an alcohol-related sexual risk reduction intervention; all data reported here were collected at the baseline session, prior to any intervention. Both informed assent (written) and informed parental/guardian consent (audiorecorded) were obtained prior to participation (see Magnan et al. 2013 for details). Participants were 14–18 years old, able to read and speak English, and were not currently taking psychotropic medications. Individuals were excluded from MRI procedures if they reported any history of brain injury or a neurological condition, or any contraindications for MRI. Participants were instructed to abstain from all substance use for a minimum of 24 hours prior to the MRI scan, which was verified by self-report prior to the scan session. Youth were screened for indicators of acute intoxication; no individuals were non-compliant, and no youth needed to be rescheduled. Participants received $20 for completing baseline assessments and the MRI session. All components of the study were completed with University Institutional Review Board Approval and a Federal Certificate of Confidentiality.
For the current study, participants were assigned to groups according to responses on the Risky Behavior questionnaire and Time Line Follow-Back (TLFB), both described below. To be classified as a non/infrequent user of alcohol or marijuana, participants had to endorse “Never” or “Occassionally” on the Risky Behavior questionnaire for the drug of interest and also report using alcohol or marijuana less than or equal to one time in the past month on the TLFB. Participants who were classified as primary alcohol or marijuana users reported using the respective drug more than one time in the past month on the TLFB. Two participants did not have data for classification into the relevant groups, so were dropped from the analysis. For the final sample included in the fMRI analyses, 37 were non/infrequent users of both substances, 39 primarily used marijuana, 23 primarily used alcohol, and 90 used both alcohol and marijuana.
Assessments
Conner’s-Wells Self Report Scale-Short Version (CASS-S)
Given the high prevalence of Attention Deficit/Hyperactivity Disorder (AD/HD) among high risk youth, AD/HD symptoms were assessed with the Conner’s-Wells Self-Report Scale – Short version (CASS-S) (Conners et al. 1997). Participants indicated how much they thought each of 27 statements applied using a 4-point scale (0=not at all true to 3=very much true). Example statements include “I have too much energy to sit still for long” and “I bend the rules whenever I can.” Scores were calculated as the sum of these items where higher scores indicated greater AD/HD symptomology, α = .91.
Child Behavior Checklist-Youth Self Report (CBCL)
Due to the relationship between externalizing and adolescent risk behavior, the CBCL was used to access youth externalizing behaviors (Achenbach and Edelbrock, 1991). The CBCL-YSR externalizing scale includes 32 items, with higher sum scores indicating greater externalizing behavior, α = .94. This measure has been shown to be equally reliable across racial/ethnic groups of young people in our prior work (Feldstein Ewing et al. 2011).
Children's Depression Inventory (CDI)
The CDI (Kovacs, 2004) was used to assess potential group differences in depression, given the high rates of comorbidity between substance use and depression (Fergusson et al. 2002; Schuler et al. 2015). For each of 27 items, participants rate the degree to which statements about negative mood, interpersonal problems, ineffectiveness, anhedonia, and self-esteem are true (0=Not at all true to 2=Very true). A total score was calculated by summing across all items, with higher scores representing higher levels of depression, α = .84.
Revised Children's Manifest Anxiety Scale (RCMAS)
The RCMAS (Reynolds and Richmond, 1985) is a measure of anxiety, an important potential confound in adolescents with a history of substance use (Roberts et al. 2007). The RCMAS is a 37-item questionnaire that requires participants to respond “Yes” or “No” to statements about physiological anxiety, worry, and social concerns. A total score was computed by summing the total number of “Yes” responses on the 28 items that were not part of the “Lie” subscale; higher scores were indicative of higher anxiety, α = .87.
Impulsive Sensation Seeking (ImpSS)
To control for levels of sensation seeking across individuals, we used the ImpSS (Zuckerman et al. 1993), a well-validated measure of impulsive sensation seeking. For each of 19 items, “True” or “False” was selected to indicate whether the statement accurately described the participant. After reverse scoring two of the items, the total score was computed by summing the total number of “True” responses. Higher scores on the ImpSS indicate greater levels of impulsive sensation seeking, α = .77.
Risky Behavior
In order to improve the validity of reporting of high-risk behaviors, youth completed self-report measures via an audio-computer assisted self-interview assessment system. Among other assessments, participants were asked whether they had ever used alcohol and/or marijuana, and if they responded “yes” they were asked to indicate how often they consumed each drug over the past three months using a response scale with the following options: “Never”, “Occasionally”, “Once a month”, “2–3 times a month”, “4–5 times a month”, “Once a week”, “2–3 times a week”, “4–5 times a week”, “Every day”. Participants were also asked whether they had used a number of other substances (past 3 months and lifetime use), which included cocaine, methamphetamine, prescription drugs (e.g. Adderall, Oxycontin), ecstasy, mushrooms, LSD, GHB, heroin and ketamine. The total number of drugs used was summed to form a composite measure of hard drug use (past 3 months and lifetime; Robbins and Bryan, 2004). Participants were also asked about frequency of sexual intercourse and condom use (see Magnan et al, 2013 for details).
Time Line Follow-Back
All participants completed a TLFB (Sobell et al. 1992) interview to assess quantity and frequency of alcohol and frequency of marijuana use over the prior 30 days (see Table 1).
Table 1.
Demographics by group. Controls reported infrequent/no use of marijuana and alcohol. MJ only reported frequent marijuana use, but infrequent/no alcohol use. Alc only reported frequent alcohol use, but infrequent/no use of marijuana. Finally, the MJ + Alc group reported frequent use of both alcohol+marijuana.
| Control | MJ only | Alc only | MJ + Alc | |
|---|---|---|---|---|
| N | 37 | 39 | 23 | 90 |
| Proportion male | .54d | .72d | .61d | .88a,b,c |
| Age | 16.05 (1.18) | 15.97 (1.06) | 16.35 (1.11) | 16.31 (1.09) |
| Adjusted Pumps | 5.56 (.60) | 5.49 (.60) | 5.39 (.53) | 5.68 (.60) |
| Proportion Explosions | .20 (.10) | .18 (.11) | .18 (.08) | .20 (.10) |
| Total drinking days (out of 30; from TLFB) | .11 (.31)c,d | .23 (.43)c,d | 3.22 (3.63)a,b | 4.98 (5.17)a,b |
| Average drinks per drinking day (TLFB) | 0.62 (2.85)c,d | 1.08 (2.39)c,d | 4.66 (4.03)a,b | 6.99 (5.06)a,b |
| Total MJ days (out of 30; from TLFB) | 0.05 (.23)b,d | 14.64 (12.49)a,c | .09 (.29)b,d | 16.74 (11.95)a,c |
| Alcohol use frequency (Never=0 to Every day=8) | .32 (.48) c,d | .49 (.51) c,d | 3.39 (1.85) a,b | 3.92 (2.35) a,b |
| MJ use frequency (Never=0 to Every day=8) | .21 (.42)b,d | 4.69 (3.46)a,c | .13 (.34)b,d | 5.76 (3.00)a,c |
| Hard drug use (last 3 months); possible range 0 to 8 | .24 (.76)d | .56 (.99)d | .52 (.95)d | 1.27 (1.47)a,b,c |
| Hard drug use (ever); possible range 0 to 9 | 1.16 (1.85)d | 1.74 (1.71)d | 2.22 (2.11) | 2.83 (2.02)a,b |
| Proportion smoked cigarette in last month | .41d | .62 | .57 | .69a |
| IMPSS | 9.46 (3.53)d | 9.92 (4.30)d | 11.09 (4.21) | 11.87 (3.47)a,b |
| CASS-S | 22.59 (12.04)d | 26.18 (16.03)d | 27.91 (9.56) | 32.66 (14.97)a,b |
| CBCL | 15.00 (9.79)b,d | 23.46 (16.35)a | 19.70 (12.04) | 24.54 (11.99)a |
| CDI | 2.59 (3.29) | 3.59 (3.75) | 3.17 (3.41) | 2.83 (2.76) |
| RCMAS | 48.38 (12.95) | 49.26 (13.23) | 49.04 (10.50) | 49.26 (12.18) |
Superscripts show significant group differences of the respective cell and other groups that differ (where a = Control; b = MJ only; c = Alc only; d = MJ + Alc).
Modified Balloon Analogue Risk Task (BART)
The variant of the BART used in the current study was identical to the task used in a previous study (Claus & Hutchison, 2012) and utilized three balloons of different colors: blue, pink, and white. Blue balloons exploded after 5 pumps on average, pink balloons exploded after 8 pumps, and white balloons did not explode and were used as the control condition. Each balloon sequence began with a balloon on the screen. Participants were instructed to either pump or cashout by pressing a button with their index or middle finger, respectively. For blue and pink balloons, participants were instructed to pump until an explosion occurred or they decided to cash-out. Each decision to pump the balloon resulted in inflation over 200 milliseconds (msec), time during which participants could not make another pump response. During the pump, an inflation sound was played, the same sound used in the original BART (Lejuez et al, 2002). For each balloon, a predetermined number of pumps was selected from a Gaussian distribution with the average number of pumps as the mean and a standard deviation of one. The minimum number of pumps for each balloon was 2 for blue and 5 for pink, and the maximum number of pumps was 8 and 11. On explosion trials (i.e. those trials in which the number of pumps met the threshold for explosion), the balloon expanded for 50 msec, exploded on the screen with an accompaying explosion sound, and participants lost all points accumulated for the current balloon. At any time, participants were able to cashout instead of continuing to pump. Cashout responses resulted in an accumulation of the points earned on that balloon while the intact balloon remained on the screen; participants heard the sound of coins falling into a bank while the total number of points earned increased on the screen. Both explosion and cashout outcomes were displayed for 2 seconds. Between balloons, a blank screen appeared 2 to 16 seconds. The number of allowable pumps for the white balloons was chosen to approximately match the number of anticipated pumps for the blue and pink balloons, so had a similar range of potential pumps as that of the blue and pink balloons (i.e. 2 to 11 pumps). Because there was no outcome, per se, for white balloons, participants were instructed to pump until the balloon disappeared from the screen. The sequence of balloon bursting values was pseudorandomized within the run, and was presented in the same order for all participants. Participants did not receive any extra incentives for better task performance (i.e. higher total number of points).
MRI acquisition
MRI data was collected on a 3T Siemens Trio (Erlangen, Germany) whole body scanner. An echo-planar gradient-echo pulse sequence (TR=2000ms, TE=29, flip angle=75°, 33 axial slices, 64×64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap) was acquired with a 12-channel head coil, and images were acquired parallel to the ventral surface of a participant’s orbitofrontal cortex to reduce signal dropout and distortion in this region (Deichmann et al. 2003). Three hundred EPI volumes were acquired during the session. In addition, a high resolution T1-weighted MP-RAGE anatomical image was acquired (TR=2530ms, TE=1.64ms, flip angle=7°, 192 sagittal slices, 256×256 matrix, slice thickness = 1mm, no gap).
Image Analysis
The first 3 volumes of each functional run were discarded to allow the magnet to reach steady state. MCFLIRT (Motion Correction using FMRIB’s Linear Image Registration Tool) (Jenkinson et al. 2002) was used to motion correct images within a run; each volume was aligned to the first volume within the run. Mean relative framewise displacement was computed for each subject, and participants with a mean exceeding 0.5 mm were excluded from subsequent analyses. Images were deskulled using BET (Brain Extraction Tool) (Smith, 2002), spatially smoothed with a 5 mm full-width half-max Gaussian kernel, temporally filtered using a high-pass filter of 50 sec, and grand mean intensity normalized; all of these steps were performed using FMRIB’s Expert Analysis Tool (FEAT) (Smith et al. 2004).
BART Analysis
Each sequence of pumps for a given balloon was modeled as a block (mean pump), with the block duration equal to the total amount of time from the onset of the balloon to the last decision to pump. In addition, a parametric modulation regressor was included for each balloon to model the total number of pumps for a given balloon, which represented the increased risk of explosion with increased number of pumps (linear pump); the duration of a given event in the linear pump regressor was equal to the duration used for the corresponding event in the mean pump regressor (see Figure 1 in Claus and Hutchison, 2012 for details). Thus, the assumed amplitude for the mean pump regressor was one, whereas the linear regressor had varying amplitudes according to the number of pumps on a given balloon sequence. Linear pump regressors were orthogonalized with respect to the mean pump regressor. Note that while prior studies have used a constant duration for all pumps (e.g. Schonburg et al. 2012), this strategy was implemented in an event-related design, where each pump is modeled separately. In the current analysis approach, the entire pumping sequence for a given balloon is modeled as a block, and depending on how many pumps were chosen, the block length may differ from balloon to balloon. While we considered choosing a mean block length as the duration as in Schonburg et al (2012), we decided against this strategy because this duration would encompass both the pump decisions as well as the outcome on some proportion of the balloons, thus leading to an incorrect model of the events that occurred throughout the task. White balloons were modeled in the same manner as color balloons, with both a mean and linear regressor. For Explosions, the outcome was modeled separately from the decision phase despite the close temporal proximity of these events. For Cashout responses (Cashout), we included the decision to cashout as well as the outcome phase of the trial. Because many participants only had one or two explosions for a particular balloon type, we did not examine group level statistics for cashouts or explosions, as this was an insufficient number of trials for a valid fMRI analysis.
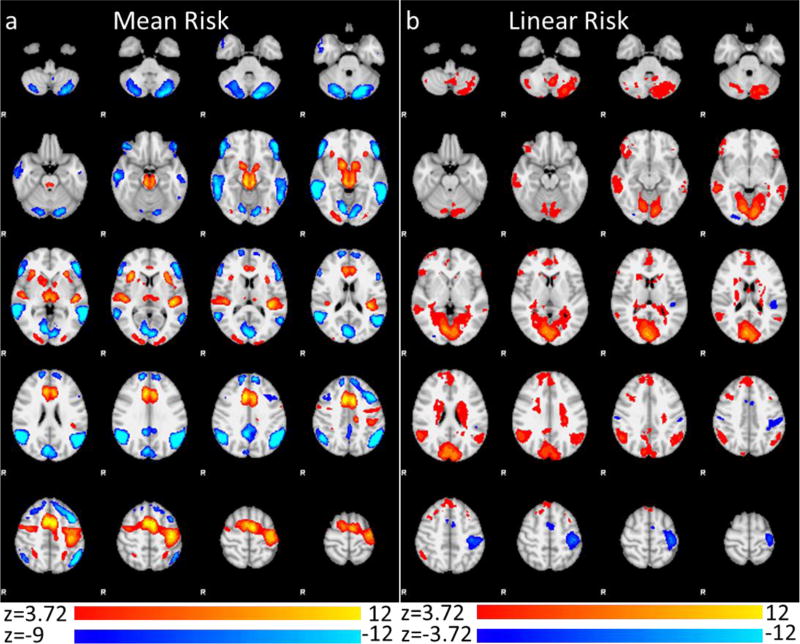
Figure 1.
fMRI results of main effect contrast of risky decision making across all participants in the (a) mean risk contrast (Mean Color > Mean White (orange) and Mean Color < Mean White (blue)) and (b) linear risk contrast (Linear Color > Linear White (orange) and Linear Color < Linear White (blue)). Images are cluster corrected using a voxel threshold of z > 3.72 and cluster p level of .05.
Statistical analyses were performed using FMRIB’s Easy Analysis Tool (FEAT). Timing files were convolved with a double gamma hemodynamic response function and entered into a multiple regression along with first-order movement parameters. Analyses were conducted using FMRIB’s Improved Linear Model (FILM; Woolrich et al. 2004) with local autocorrelation estimation. Parameter estimates for each condition were combined to generate our primary contrasts: Mean Color vs. Mean White (mean risk) and Linear Color vs. Linear White (linear risk). The mean risk contrast modeled the mean level of response for risky versus riskless decisions across all balloons, whereas the linear risk contrast modeled the difference in partial correlations between BOLD response and number of pumps during risky and riskless choices. Contrast maps were registered to the participant’s high-resolution anatomical image and the MNI 152 brain template using FMRIB’s Linear Image Registration Tool (FLIRT) (Jenkinson et al. 2002).
Group analysis
Group analyses were conducted using FMRIB’s Local Analysis of Mixed Effects (FLAME) Stage 1. Analyses were corrected for multiple comparisons using cluster-based correction in FSL (Worsley et al. 1996; voxel z > 2.3, cluster p < .025); a cluster level significance of p < .025 was used to account for the fact that we tested two separate contrasts. A voxel level significance of z > 2.3 has been shown to sufficiently control false positives in event related designs when using FSL’s FLAME 1 for group level inference (Eklund et al., 2016). Group differences were assessed using a one-way ANOVA with group (Controls, Primary Marijuana, Primary Alcohol, Primary Alcohol and Marijauna) as the between subjects factor. Regions showing significance in the omnibus test were further interrogated using pairwise Tukey tests. In addition, we conducted one-way ANCOVAs that controlled for potential confounding factors such as hard drug use, gender, anxiety, depression, ADHD, impulsivity, and externalizing. Because these follow-up analyses were intended to rule out potential confounding variables, we did not require a cluster level significance that was more stringent than the level used in the primary analyses (i.e., cluster p < .025).
In addition to between group analyses on the main contrasts of interest, we also examined the correlation between each contrast of interest and the total number of adjusted average pumps to examine how neural response related to behavioral risk taking. We examined these correlations across all participants as well as in analysis that examined how the relationship between BOLD response and adjusted average pumps differed by group.
Results
Demographics/Behavior
All effects reported below include 189 partipants; seven participants were dropped because of in-scanner movement (mean framewise displacement > 0.5 mm).
Demographics and performance on the BART for each group from the final sample of 189 participants included in the analyses are presented in Table 1. Youth had been involved in the juvenile justice system for low-level status offenses primarily including assault or fighting (47.1%), burglary (23.5%), and possession of a controlled substance (22.1%). The groups differed in the number of other drugs used (lifetime: F(3, 185) = 7.44, p < .001; last 3 months: F(3, 185) = 7.97, p < .001), with the alcohol and marijuana group reporting greater use than the other groups. There were also significant differences in the proportion of males (χ2=19.94, p < .001) and proportion of smokers (χ2=8.97, p < .05) across the four groups, with the alcohol and marijuana group containing more males and smokers than the control group. On the BART, there were no effects of group on adjusted pumps (F(3, 185) = 1.97, p = 0.12), proportion of explosions (F(3, 185) = 0.80, p = 0.50), or mean balloon duration for color balloons (F(3, 185) = 0.95, p = 0.42) or white balloons (F(3, 185) = 0.38, p = 0.77). Significant group differences were also noted for the IMPSS, Connors, and CBCL (see Table 1).
fMRI Results: Main effects on the BART
In the mean risk contrast (i.e. mean color > mean white), risky choices were associated with greater activation than riskless choices in dACC/SMA, bilateral dorsal/ventral striatum (DS/VS) and anterior insula (for complete list see Fig 1, Table 2). In the contrast of linear risk (i.e. linear color > linear white), greater activation was found in pre/subgenual ACC/rostromedial MPFC, bilateral orbitofrontal cortex (OFC), as well as other regions throughout the temporal and parietal cortices (see Fig 1, Table 2 for complete list).
Table 2.
Main effects of risky decision making vs. non-risky decision making for mean pump actvity and linear pump activity.
| Contrast | Region | Voxels | Max Z | X | Y | Z |
|---|---|---|---|---|---|---|
| Mean Risk > Mean Control | DACC/RACC/DMPFC/Pre/Post-central gyrus | 9223 | 12.3 | −6 | 6 | 50 |
| Brainstem/Thalamus/Striatum/R Insula | 3242 | 11.5 | 8 | −26 | −12 | |
| L STG/ Posterior insula | 1246 | 10.7 | −38 | −32 | 12 | |
| R STG/ Posterior insula | 865 | 8.85 | 44 | −22 | 6 | |
| R Occipital pole | 658 | 6.07 | 14 | −100 | 6 | |
| R anterior insula | 500 | 9.37 | 32 | 22 | 6 | |
| L anterior insula | 471 | 8.93 | −30 | 24 | 4 | |
| L Occipital pole | 343 | 5.67 | −12 | −102 | 8 | |
| L SPL | 175 | 5.37 | −26 | −52 | 50 | |
| R SPL | 153 | 5.26 | 30 | −50 | 50 | |
| Mean Control > Mean Risk | Lingual Gyrus / Intracalcerine Cortex / PCC | 7728 | 13 | 12 | −76 | −2 |
| L IPL/MTG | 4943 | 14.4 | −48 | −64 | 40 | |
| R IPL/MTG | 4742 | 15.2 | 56 | −56 | 24 | |
| L MFG/SFG | 1921 | 12.7 | −42 | 14 | 46 | |
| L OFC/IFG | 1849 | 12.8 | −48 | 34 | −2 | |
| R OFC/IFG | 1492 | 12.5 | 50 | 38 | −8 | |
| R MFG/SFG | 1089 | 11.8 | 14 | 56 | 30 | |
| PCC | 867 | 12.4 | 6 | −46 | 34 | |
| Cerebellum | 15 | 9.98 | −6 | −54 | −46 | |
| L ITG | 4 | 9.22 | −52 | −8 | −32 | |
| SFG | 1 | 9.03 | 4 | 44 | 50 | |
| L MTG | 1 | 9.02 | −64 | −14 | −22 | |
| Linear Risk > Linear Control | Lingual Gyrus / Intracalcerine Cortex / Caudate / Thalamus | 16744 | 9.34 | 6 | −82 | 20 |
| RMPFC/DMPFC | 2176 | 5.49 | 6 | 44 | 52 | |
| R IPL | 1455 | 6.57 | 56 | −56 | 26 | |
| L IPL | 1040 | 5.68 | −52 | −62 | 32 | |
| R OFC | 860 | 5.53 | 50 | 36 | −12 | |
| R MTG | 856 | 6.72 | 60 | −36 | −8 | |
| L MTG | 279 | 4.74 | −54 | −36 | −8 | |
| Cerebellum | 133 | 4.68 | 44 | −62 | −42 | |
| L MFG | 117 | 4.35 | −32 | 20 | 50 | |
| L OFC | 114 | 4.54 | −46 | 32 | −8 | |
| Linear Control > Linear Risk | L Pre/Post Central | 1783 | 7.49 | −40 | −26 | 50 |
| DACC/SMA | 266 | 4.93 | −4 | 0 | 54 | |
| L Parietal Operculum | 191 | 5.29 | −44 | −34 | 16 | |
| R Occipital Fusiform | 132 | 4.59 | 28 | −88 | −6 | |
| R Post central gyrus | 77 | 4.52 | 58 | −20 | 38 |
DACC = dorsal anterior cingulate cortex; RACC = rostral anterior cingulate cortex; DMPFC = dorsomedial prefrontal cortex; SPL = superior parietal lobe; STG = superior temporal gyrus; IPL = inferior parietal lobe; OFC = orbitofrontal cortex; MFG = middle frontal gyrus.
Moderating Effect of Substance Use on BOLD Response and Task-Based Risk-taking
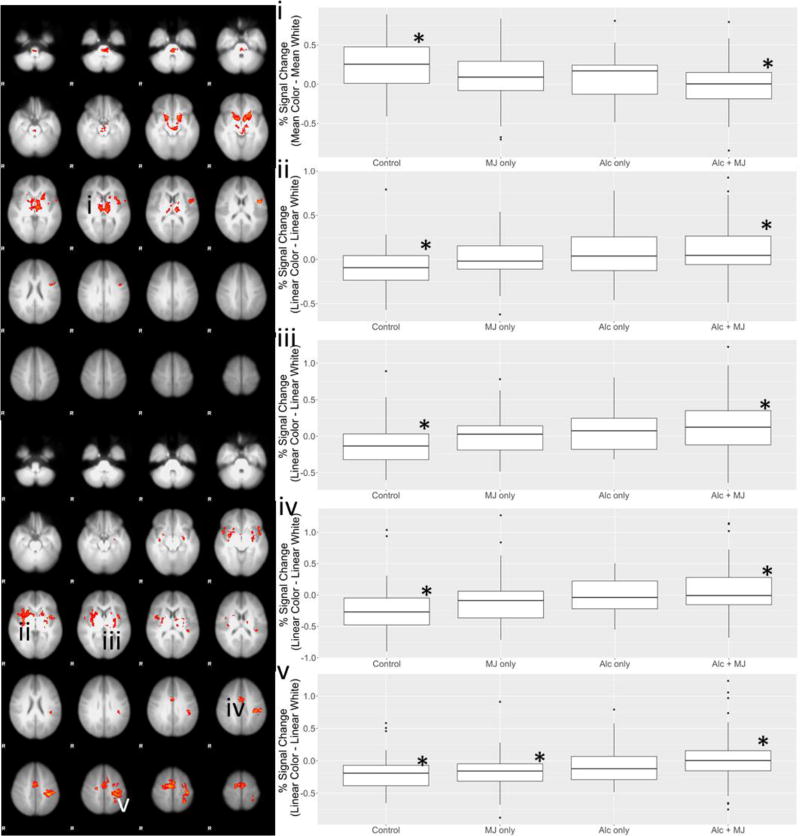
The omnibus F-test that examined whether there were any differences across the four groups demonstrated a significant effect for the mean risk contrast in a single cluster that included bilateral VS, thalamus/brainstem and left anterior insula/inferior frontal gyrus (IFG). Examination of percent signal change within this cluster by group revealed that the control group had greater response than the group using both alcohol and marijuana (p < .0001; Figure 2a).
Figure 2.
Regions that showed differences in the omnibus test across group for the mean risk contrast (a) and the linear risk contrast (b). Panels i-v display the signal change values by group within the regions in panels (a) and (b) showing group differences (significant differences are denoted by * for each ROI).
In the linear risk contrast, the omnibus F-test revealed group differences in supplementary motor area (SMA)/dorsal ACC, right putamen/insula, left insula/putamen/thalamus, and left postcentral gyrus (PoCG)/superior parietal lobe (SPL). Examination of percent signal change in these regions revealed that the group using alcohol and marijuana had greater response than the control group in supplementary motor area (SMA)/dorsal ACC, right putamen/insula, and left insula/putamen/thalamus (all p’s < .01), and greater response than the marijuana-only group and the control group in the left PoCG/SPL (p = .02 and p = .01, respectively) (see Figure 2b), but did not differ from the alcohol-only group.
Because the groups differed on hard drug use and gender, the above analyses were also conducted controlling for gender and hard drug use (past 3 months and ever), in three separate models (see Table 3). Controlling for these variables changed the results to some degree, with the most notable changes occurring when controlling for gender. When gender was controlled, the cluster showing signficance in the mean risk contrast was reduced in overall extent of activation, with group differences being restricted primarily to left lateralized NAc, putamen, and thalamus. In contrast, controlling for hard drug use resulted in very little change to overall cluster extent.
Table 3.
Group differences in the mean and linear risk contrasts. An omnibus test was used to test for group differences across all four groups, which was followed by individual comparisons (see Figure 2). In addition cluster sizes are reported for analyses that controlled for hard drug use (last 3 month use and lifetime use) and gender.
| Contrast | Region | Cluster size |
Max Z | x | y | z | Age | CBCL | CDI | CASS-S | Gender | IMPSS | RCMAS | Hard drugs (3 mo) |
Hard drugs (ever) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear risk > Linear control | L Pre/Post-central gyrus/SPL | 1495 | 4.27 | −36 | −30 | 50 | 1463 | 1107 | 1457 | 1073 | 968 | 4852 | 1471 | 1638 | 1858 |
| R Putamen/Caudate/Insula | 1338 | 3.43 | 48 | 12 | −4 | 970 | 507 | 1282 | 707 | 1021 | 3048 | 1222 | 1646 | 1531 | |
| DACC/SMA | 1234 | 4.09 | 10 | −8 | 60 | 1164 | 580 | 1229 | 713 | 816 | 1982 | 1222 | 1401 | 905 | |
| L Putamen/Thalamus/Insula | 812 | 3.53 | −46 | 20 | −6 | 37 | 32 | 826 | 44 | 77 | 4852* | 641 | 910 | 699 | |
| Mean risk > Mean control | Bilateral VS/Thalamus/Brainstem L Putamen, Insula/IFG | 3355 | 4.06 | −14 | 12 | −12 | 3221 | 3588 | 3301 | 2455 | 625 | 7702* | 3319 | 3799 | 5094 |
SPL = superior parietal lobe; DACC = dorsal anterior cingulate cortex; VS = ventral striatum; IFG = inferior frontal gyrus.
We also examined the group effects while controlling for the clinical variables of anxiety, depression, ADHD, impulsivity, and externalizing, and found that while cluster sizes were reduced to some degree, the group differences remained significant in all clusters except the left putamen/insula, which was no longer significant once controlling for age or externalizing. Notably, this effect was similar to that reported when controlling for gender. In all other cases, inclusion of the additional potential confounding variable resulted in changes in the cluster extent, but main group differences remained significant (see Table 3 for cluster sizes and Supplementary Figure 1 and 2 for group maps that included covariates of interest in the mean and linear risk contrasts, respectively). Finally, we examined our group differences after controlling for all variables in the context of a multiple regression. As seen in Supplementary Figure 3, the only cluster that was no longer significant was the dorsal ACC/SMA cluster, which was reduced to a cluster size of 346 voxels.
Relationships Between BOLD Response and Behavioral Risk Taking
Examination of the correlations between the mean and linear contrast and behavioral performance on the BART revealed several significant relationships. In the mean contrast, there was a negative correlation between adjusted average pumps and response throughout the brain including ventral ACC, bilateral orbitofrontal cortex/insula, bilateral IFG, and precentral gyrus (see supplemental Table for complete list). For the linear contrast, there was a positive relationship between adjusted average pumps and response in the right visual processing stream (occipital pole, lateral occipital cortex, and middle temporal gyrus). There were no significant group differences in the relationship between adjusted avearge pumps and BOLD response in either contrast.
Discussion
The goal of this study was to examine the neural mechanisms of risk taking that differ among adolescent alcohol and marijuana users compared to non-users/infrequent users. Although we observed no behavioral differences on the BART across the four groups, activation differences during risky decision making emerged in the striatum, thalamus, and anterior insula/IFG in the mean risk contrast, and in SMA/dACC, putamen/insula, and PoCG/SPL in the linear risk contrast. Interestingly, differences between groups were isolated to comparisons of controls to the alcohol+marijuana group and alcohol-only group, and the comparison of the marijuana-only group to the alcohol+marijuana group. While the group differences in the mean risk contrast may suggest differential valuation of rewarding outcomes, group differences in the linear risk contrast point to reduced differentiation of potential risk across balloons despite increased risk of explosion as pumps increased. Both of these possibilities are discussed below in the context of group differences.
Compared to controls, frequent use of alcohol+marijuana was associated with reduced response in VS and bilateral thalamus in the mean risk contrast. Of note, the observed reduction in VS response among frequent substance users replicates other work in substance using adolescents (Schneider et al. 2012) and adolescents with a family history of alcoholism (Heitzeg et al. 2010). The VS has been implicated in reward processes, including reward anticipation (Hsu et al. 2009; Knutson et al. 2001), reward receipt (Liu et al. 2007), and encoding of temporal difference errors (Rolls et al. 2008). In the BART, VS may play a significant role in anticipation of a positive outcome, leading to an approach-based motivational state and risk engagement. The valuation of each action may be greater for non-users, who may place greater value on each pump (i.e., reward), thereby resulting in greater VS response. Future studies that parametrically manipulate per-pump value (Bornovalova et al. 2009) and separate anticipatory and outcome related processes (Bogg et al. 2012) may allow clearer differentiation of the precise mechanism contributing to differential striatal engagement in adolescent polysubstance alcohol+marijuana user.
While multiple regions showed group differences in the linear contrast, the difference in SMA is most relevant to prior work using the BART to examine risky decision making (e.g. Bogg et al. 2012). The SMA/dACC plays a role in detecting conflict at the response level, and has been implicated in risk-taking, particularly in light of the opposing influences of potential positive and negative outcomes (Alexander and Brown, 2010; 2011); in the BART, reduced response in SMA/dACC as function of pump number is proposed to coincide with a “de-escalation of risk appraisal” (Bogg et al. 2012). In other words, more risk taking is expected when levels of SMA/dACC (and other regions) are reduced; we found evidence of this pattern of cognitive neural processing for the high-risk adolescents within this study.
We found that the relationship between pumps and dACC/SMA response was more negative in the control group as compared to youth using alcohol+marijuana, suggesting greater sensitivity of dACC/SMA to levels of risk as assessed by non-using youth. This finding is opposite to that of Bogg et al. (2012), who reported that heavier weekly alcohol use was associated with greater decreases the relationship between pump number and dACC/SMA response. While the disparate results between these two studies may be attributable to numerous factors including sample or design differences, the sample used here was significantly younger than the Bogg et al. study. Studies continue to reflect that adolescents engage portions of the dACC/SMA to a lesser degree than adults (Bjork et al. 2007), which could have driven observed outcomes with our adolescent sample. However, design differences may be a more salient difference; Bogg et al. (2012) used an event related modeling approach that enabled the separation of each pump decision, whereas this study modeled each sequence of pumps as a single block. In addition, the current study used a consistent reward (i.e. 1 point), whereas Bogg et al. used increasing rewards for each pump. Either task difference could potentially account for the difference in observed empirical outcomes between these two studies. Future studies that explcitly examine increasing versus constant rewards may clarify these results.
In addition to differences in the SMA, the adolescent alcohol+marijuana group showed a decreased relationship between pump number and response in the right insula/putamen compared to the non-using group, and in left PoCG/SPL compared to both the non-using and marijuana-only group. The anterior insula plays a critical role in anticipating negative outcomes in adults (Lovero et al. 2009; Paulus et al. 2003) and may serve as a stop signal to prevent risk-taking. The SPL is an area whose response typically increases with degree of perceived uncertainty (Vickery and Jiang, 2008).
The reduced parametric relationship between pump number and signal in these areas among the substance use groups thus may suggest less differentiation of varying levels of risk in the alcohol+marijuana group. If reduced differentiation of levels of risk is the underlying mechanism that differentiates the substance users from non-users, it could then be expected that with a greater range of available pumps, a behavioral difference might be observed in future examinations. Future studies that examine greater ranges of risk taking as well as those that specifically manipulate uncertainty processing (Van Leijenhorst et al. 2006) will provide a stronger test of this differentiability hypothesis. Interestingly, the difference in SPL/IPL is in the opposite direction to the results of a prior study of risky decision making in marijuana users (De Bellis et al. 2013). Whether this reflects task differences or the different developmental stage of the adolescents examined herein is unclear; yet, future studies that specifically address the task and sample differences will shed light on these disparate results.
An important question that arises from the current study is the specificity of the results to substance use per se as opposed to more general proclivity toward risk taking during this developmental phase, and whether the differences observed could potentially be a marker of future drug use or the sequelae of prior drug use. First, the studied population is known to engage in greater levels of risk than individuals not involved in the juvenile justice system, and in this sense are a fairly homogenous group with regard to general propensity for risk-taking. If it is true that there are patterns of activation that underlie risk-taking more generally, then of importance for interpretation, these patterns are, in fact, matched in our groups, and thus any observed differences could be viewed as specific to substance use (Weiland et al. 2015). Although our findings are likely specific to substance use, we are still unable with this design to determine whether the differences existed prior to the initiation of substance use or as a consequence of adolescent substance use. Future longitudinal studies will be well-positioned to answer this key question.
Finally, one question that often emerges following fMRI analyses of adolescent task-based behavior is the potential applicability of the results to treatment. The field has been moving towards integration of basic biological metrics, including neuroimaging (MRI/fMRI) as indices of mechanisms of treatment response for adolescents (Feldstein Ewing et al. 2016a), as well as increasing use of neuroimaging as a more sensitive metric to identify the nature of adolescent cognitive processing. While neuroimaging itself is unlikely to be a widely disseminated tool for day-to-day clinical settings, starting to understand how the adolescent brain processes risk information is critical to informing treatment development and tailoring of interventions so that they are more responsive and effective for this developmental phase. This is particularly critical at this time, when interventions for adolescent alcohol and cannabis use have much lower effect sizes for adolescents, as compared with adults (Feldstein Ewing et al. 2016b). Concretely, brain-based differences may play a critical role in helping interventionists determine which factors to address and enhance within the context of intervention efforts (Feldstein Ewing and Chung, 2013). For example, results from the current study may suggest that it may be fruitful to target cognitive processes such as reward valuation or risk assessment in individuals who use alcohol+marijuana. The next step would be to examine the degree to which interventions that incorporate reward valuation and risk assessment (e.g., contingency management; cognitive behavioral therapy) change response in these regions, and whether those modulations of brain response predict subsequent reductions in adolescent drug use and/or risk taking.
Limitations and Future Directions
While the current study has many strengths, some limitations must also be acknowledged. First, the groups differed in the use of hard drugs and proportion of males, both of which could have influenced group differences. However, when controlling for these variables, clusters showing group differences either increased in extent or decreased slightly, but remained significant suggesting that group differences were not driven solely by these variables. Next, abstinence from alcohol/marijuana at the time of scanning was confirmed using only self-report. Thus there is some possiblility that a portion of the group differences may have emerged from acute drug effects from past 24-hour use. Finally, the variant of the BART used in the current study had some limitations. First, we failed to observe any group differences in behavior on the BART, which may have been the result of the reduced range of pumps required to cause an explosion; this limited range may have placed artificial constraints on risk taking. In addition to a lack of group differences, the limited range of available pumps on color balloons caused participants to pump significantly less on color balloons than the control balloons, a difference that could contribute to group differences in BOLD response. However, examination of the percent signal change across regions that demonstrated group differences suggests that variation in BOLD reponse occurred primarily in the color balloon condition, suggesting our results are not merely the result of an imperfect control condition. Finally, we acknowledge that the variant of the BART used in the current study is limited in its ablity to parse specific cognitive functions. Although we have attempted to control for some of the more obvious motor related activity, the contrasts included in the study are unlikley to control for factors such as executive control, working memory, and attention. Given that we do not have any estimate of intelligence or school performance in our participants, the possibility of group differences in overall executive ablility could be driving some of the group differences noted in the task. Future studies that carefully assess risk taking in more controlled tasks alongside assessment of general cognitive ability will be key for further advancing our understanding of risk taking mechanisms among substance using groups.
While several group differences emerged between non-using and substance-using individuals at the neural level, the precise cognitive process contributing to these differences remains unclear. Futher studies that manipulate reward expectancy, choice conflict, and risk assessment may provide further clues about the underlying cognitive differences in substance using adolescents. In addition, future studies should examine how neurocognitive functioning changes throughout the course of interventions targeting reductions in substance use, and whether an increase in response over the course of treatment corresponds to a decrease in substance use outside the laboratory.
Supplementary Material
Supplemental Figure 1. Regions that showed differences in the omnibus test across control, marijuana only, alcohol only, and marijuana + alcohol groups for the mean risk contrast controlling for a) age; b) CBCL; c) CDI; d) CASS-S; e) Gender; f) Hard drug use, past 3 months; g) Hard drug use, ever; h) IMPSS; i) RCMAS.
Supplemental Figure 2. Regions that showed differences in the omnibus test across control, marijuana only, alcohol only, and marijuana + alcohol groups for the linear risk contrast controlling for a) age; b) CBCL; c) CDI; d) CASS-S; e) Gender; f) Hard drug use, past 3 months; g) Hard drug use, ever; h) IMPSS; i) RCMAS.
Supplemental Figure 3. Regions that showed differences in the omnibus test across control, marijuana only, alcohol only, and marijuana + alcohol groups for the mean risk (a) and linear risk (b) contrast controlling for age, gender, CBCL, CDI, CASS-S, Hard drug use past 3 months, Hard drug use ever, IMPSS, and RCMAS.
Acknowledgments
Funding: This study was funded by the National Institute on Alcohol Abuse and Alcoholism (AA017390).
Footnotes
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of Univesrity of New Mexico Human Research Protection Office and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: The authors declare no conflicts of interest.
Informed consent: Informed assent (written) and informed parental/guardian consent (audiorecorded) were obtained for all individual participants included in the study.
References
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist. Burlington: University of Vermont; 1991. [Google Scholar]
- Aklin W, Lejuez C, Zvolensky M, Kahler C, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behaviour Research and Therapy. 2005;43:215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Alexander G, Crutcher M. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosciences. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander G, Crutcher M, DeLong M. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander W, Brown J. Competition between learned reward and error outcome predictions in anterior cingulate cortex. Neuroimage. 2010;49:3210–3218. doi: 10.1016/j.neuroimage.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental Differences in Posterior Mesofrontal Cortex Recruitment by Risky Rewards. The Journal of Neuroscience. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Fukunaga R, Finn PR, Brown JW. Cognitive control links alcohol use, trait disinhibition, and reduced cognitive capacity: Evidence for medial prefrontal cortex dysregulation during reward-seeking behavior. Drug and Alcohol Dependence. 2012;122:112–118. doi: 10.1016/j.drugalcdep.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova M, Cashman-Rolls A, O'Donnell J, Ettinger K, Richards J, deWit H, et al. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacol Biochem Behav. 2009;93:258–262. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Claus ED, Hutchison KE. Neural mechanisms of risk taking and relationships with hazardous drinking. Alcohol Clin Exp Res. 2012;36:932–940. doi: 10.1111/j.1530-0277.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- Conners CK, Wells KC, Parker JDA, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for the assessment of adolescent psychopathology: factor structure, reliability, validity and diagnostic sensitivity. J Abnorm Child Psychol. 1997;25:487–497. doi: 10.1023/a:1022637815797. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, et al. Risky Decisions and Their Consequences: Neural Processing by Boys with Antisocial Substance Disorder. PLoS ONE. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Raymond KM, Mikulich-Gilbertson SK, Thompson LL, Lejuez CW. A Risk-Taking “Set” in a Novel Task Among Adolescents With Serious Conduct and Substance Problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:175–183. doi: 10.1097/01.chi.0000188893.60551.31. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, Huettel SA. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug and Alcohol Dependence. 2013;133:134–145. doi: 10.1016/j.drugalcdep.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried J, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. Psychology of Addictive Behaviors. 2013;27:329–335. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Apodaca TR, Gaume J. Ambivalence: Prerequisite for success in motivational interviewing with adolescents? Addiction. 2016a;111:1900–1907. doi: 10.1111/add.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Molina BSG, Tapert SF. Uniting adolescent neuroimaging and treatment research: Recommendations in pursuit of improved integration. Neuroscience & Biobehavioral Reviews. 2016b;62:109–114. doi: 10.1016/j.neubiorev.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Sakhardande A, Blakemore S-J. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage: Clinical. 2014;5:420–437. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Venner KL, Mead HK, Bryan AD. Exploring racial/ethnic differences in substance use: a preliminary theory-based investigation with juvenile justice-involved youth. BMC Pediatr. 2011;11:1–10. doi: 10.1186/1471-2431-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–35. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2013;231:1455–1465. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Thayer RE, Tapert SF. Adolescent marijuana users have elevated risk-taking on the balloon analog risk task. Journal of Psychopharmacology. 2014;28:1080–1087. doi: 10.1177/0269881114550352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zucker RA, Zubieta J-K. Striatal Dysfunction Marks Preexisting Risk and Medial Prefrontal Dysfunction Is Related to Problem Drinking in Children of Alcoholics. Biological Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer CF. Neural Response to Reward Anticipation under Risk Is Nonlinear in Probabilities. Journal of Neuroscience. 2009;29:2231–2237. doi: 10.1523/JNEUROSCI.5296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, et al. Developmental Cognitive Neuroscience. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, et al. Youth risk behavior surveillance--United States, 2013. MMWR Surveill Summ. 2014;63(Suppl 4):1–168. [PubMed] [Google Scholar]
- Knutson B, Adams C, Fong G, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:15. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children's depression inventory (CDI) Toronto: Multi-Health Systems Inc; 2004. [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell D, Wang H, Gold B, Corbly C, Joseph J. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. The Journal of Neuroscience. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovero K, Simmons A, Aron J, Paulus M. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45:976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan RE, Callahan TC, Ladd BO, Claus E, Hutchison K, Bryan AD. Evaluating an integrative theoretical framework for HIV sexual risk among juvenile justice involved adolescents. Journal of AIDS & Clinical Research. 2013;4:217. [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences. 2012;109:E2657–64. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. Revised Children's Manifest Anxiety Scale. RCMAS Manual. Los Angeles: Western Psychological Services; 1985. [Google Scholar]
- Robbins RN, Bryan A. Relationships Between Future Orientation, Impulsive Sensation Seeking, and Risk Behavior among Adjudicated Adolescents. J Adol Research. 2004;19:428–445. doi: 10.1177/0743558403258860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E, McCabe C, Redoute J. Expected Value, Reward Outcome, and Temporal Difference Error Representations in a Probabilistic Decision Task. Cereb Cortex. 2008;18:652–663. doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA. Decreasing Ventromedial Prefrontal Cortex Activity During Sequential Risk-Taking: An fMRI Investigation of the Balloon Analog Risk Task. Front Neurosci. 2012;6:1–11. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler MS, Vasilenko SA, Lanza ST. Age-varying associations between substance use behaviors and depressive symptoms during adolescence and young adulthood. Drug and Alcohol Dependence. 2015;157:75–82. doi: 10.1016/j.drugalcdep.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Shedler J, Block J. Adolescent drug use and psychological health. A longitudinal inquiry. American Psychologist. 1990;45:612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M, Litten R, Allen J. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: Psychosocial and biochemical methods. 1992:41–72. [Google Scholar]
- Somerville LH, Casey B. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–25. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Are Drug Experimenters Better Adjusted Than Abstainers and Users?: A Longitudinal Study of Adolescent Marijuana Use. Journal of Adolescent Health. 2006;39:488–494. doi: 10.1016/j.jadohealth.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Crone E, Bunge S. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Vickery TJ, Jiang YV. Inferior Parietal Lobule Supports Decision Making under Uncertainty in Humans. Cerebral Cortex. 2008;19:916–925. doi: 10.1093/cercor/bhn140. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. Journal of Neuroscience. 2015;35:1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY, Loeber R. Associations Between Heavy Drinking and Changes in Impulsive Behavior Among Adolescent Boys. Alcohol Clin Exp Res. 2010;35:295–303. doi: 10.1111/j.1530-0277.2010.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M, Behrens T, Beckmann C, Jenkinson M, Smith S. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman D, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. Journal of Personality and Social Psychology. 1993;65:757–768. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Regions that showed differences in the omnibus test across control, marijuana only, alcohol only, and marijuana + alcohol groups for the mean risk contrast controlling for a) age; b) CBCL; c) CDI; d) CASS-S; e) Gender; f) Hard drug use, past 3 months; g) Hard drug use, ever; h) IMPSS; i) RCMAS.
Supplemental Figure 2. Regions that showed differences in the omnibus test across control, marijuana only, alcohol only, and marijuana + alcohol groups for the linear risk contrast controlling for a) age; b) CBCL; c) CDI; d) CASS-S; e) Gender; f) Hard drug use, past 3 months; g) Hard drug use, ever; h) IMPSS; i) RCMAS.
Supplemental Figure 3. Regions that showed differences in the omnibus test across control, marijuana only, alcohol only, and marijuana + alcohol groups for the mean risk (a) and linear risk (b) contrast controlling for age, gender, CBCL, CDI, CASS-S, Hard drug use past 3 months, Hard drug use ever, IMPSS, and RCMAS.