Abstract
Objective
Chronic symptomatic sinus node dysfunction (SND), the most common bradyarrhythmia, can be effectively managed by permanent cardiac pacing. Yet the care pathway and barriers to adoption of pacing therapy are not well understood – particularly in low volume implanting countries. The IMPROVE Brady study is a quality improvement initiative being conducted at centers in South Asia, Latin America, and Russia. We assessed the rates of SND diagnosis and pacemaker treatment for SND in the South Asia cohort.
Methods
The prospective study enrolled patients with heart rate of ≤50 beats per minute presenting with symptoms including syncope, dizziness, and/or dyspnea from ten centers in India and Bangladesh. Patients were followed to identify the proportion diagnosed with SND and subsequently treated with pacemaker therapy.
Results
A total of 508 patients meeting criteria were enrolled and followed on average for 8.3 ± 8.0 months. Patients were on average 58 years of age, 77% were male, and 91% had completed at least primary education. An SND diagnosis was made in 368 (72%) of patients, with the majority (80%) of diagnoses occurring within 1 month of enrollment. Of the patients with an SND diagnosis, 63 (17%) were treated with a pacemaker. Reasons for not receiving treatment were: subject refusal or deferred decision (45%), unaffordability (34%), physician determined – not-indicated (20%), and other (1%). Older age, female gender, history of hypertension, lower resting heart rate, and syncopal or pre-syncopal symptoms were associated with a higher probability of implant.
Conclusions
In a care pathway assessment for the diagnosis and treatment of symptomatic SND in South Asia only 1 in 6 patients received pacemaker indicated therapy, largely due to patient refusal and physician decision. Phase II of the study will be aimed to improve this treatment rate.
1. Introduction
Sinus node dysfunction (SND) is the most common bradyarrhythmia, with a prevalence estimated to be between 403–666 per million and increasing with age.1 SND has many causes but is commonly idiopathic. Symptoms range from asymptomatic to nonspecific and include syncope, pre-syncope, palpitations, dizziness, fatigue, and even sudden death. Associated arrhythmias include sinus bradycardia, sinoatrial block, sinus arrest, chronotropic incompetence, and tachycardia-bradycardia syndrome. Diagnosis of SND can be difficult due to its nonspecific symptoms and erratic course that can elude electrocardiographic monitoring. In the absence of reversible causes, the mainstay treatment for SND is placement of a permanent artificial pacemaker as supported by the guidelines from the American and European cardiology and heart rhythm societies.2, 3
Pacemaker therapy effectively manages symptoms associated with SND, although survival is unlikely to be impacted with treatment.4 Dual-chamber pacing or single-chamber atrial pacing is recommended over single-chamber ventricular pacing in patients with SND and intact atrioventricular conduction3 to mitigate risk of atrial fibrillation and stroke.5 In spite of the benefits of this well-tolerated therapy, adoption remains low in certain geographies around the world.6 The patient care pathway and reasons for low utilization in these geographies are unclear.
IMPROVE Brady is a prospective, multi-center, multi-phased study aimed at improving the diagnosis and treatment of SND in developing geographies around the world. Phase I examines the care pathway of patients presenting with symptoms associated with SND and their respective diagnosis and treatment rates. Phase II provides investigators with comprehensive resources that includes patient educational materials of their disease and therapy options and will measure the impact of this intervention on diagnosis and treatment rates compared to the baseline Phase I rates. The focus of this report is of the baseline diagnosis and treatment rates (Phase I) in the South Asia cohort.
2. Methods
2.1. Study design
The IMPROVE Brady Study is an ongoing, prospective, interventional, sequential, post-market quality improvement clinical study. The aim of the study is to use a practice-specific process improvement intervention consisting of education, diagnostic algorithms, and documentation tools that advocate and reinforce adherence to consensus treatment guidelines to improve the quality of care for patients with SND. The study is a sequential design and is being conducted in two phases.
During the Phase I period, physicians assessed and treated subjects per standard care practice at their center, recording the diagnostic assessments used to guide care and whether pacemaker therapy was prescribed. Phase I of the study was conducted at tertiary care centers in South Asia, Latin America, and Russia in accordance with the Declaration of Helsinki. All patients provided written informed consent to the study protocol that was reviewed and approved by the ethics committee of each participating institution. Phase I serves as a control period and the South Asia Phase I experience is the object of this report.
At the completion of Phase I, the investigators will complete an educational workshop and will be given access to the IMPROVE Brady toolkit. The impact of this process improvement intervention will be assessed in Phase II, which is currently ongoing.
2.2. Study patients and follow-up procedures
The study recruited subjects presenting with symptomatic bradycardia. The key patient inclusion criteria were: a sinus rate ≤50 beats per minute (BPM) or a junctional escape rhythm no faster than 50 BPM or documented primarily by ECG and other available subject medical records; symptoms of general fatigue, shortness of breath/dyspnea, shortness of breath with exertion, syncope, light headed dizziness, palpitations, lethargy, or malaise within 30 days of enrollment that are not related to other discovered causes (such as untreated hypothyroidism or anemia). Patients with type II 2nd degree AV block, high degree AV block (2:1, 3:1, 4:1 etc.), 3rd degree AV block, a recent history of blood loss, an acute medical illness associated with bradycardia, or a history of chronic atrial fibrillation were excluded.
Enrolled patients presented to a study investigator, generally an interventional cardiologist or electrophysiologist, for a baseline assessment. The study protocol did not dictate when the diagnostic assessment visit occurred. At the diagnostic assessment visit, which could take place on the same day as the baseline assessment, information about utilization of diagnostics tests, results of these tests, and cardiovascular medication use were collected. The final diagnosis and treatment plan recommended by the physician were recorded. For patients who were recommended for, but did not receive a pacemaker implant, the reason for not receiving treatment was recorded. There were several insurance/cost related responses that were grouped together for this analysis using the category ‘unaffordable’, where unaffordable was defined as either (1) the patient had insurance, but the device/procedure was not covered, (2) the patient had insurance and the device/procedure were covered, but the patient could not afford out of pocket expenses (e.g. device/procedure copayments), or (3) the patient did not have insurance and could not afford the device/procedure. For patients undergoing implant, the implant was performed according to the hospital’s standard implant practice.
Patients were followed until a treatment decision was made, after which patients were exited from the study. For patients without a treatment decision, follow-up continued until either the subject met study exit criteria or until 6 months after the last Phase I enrollment. Study exit criteria included, but were not limited to, the following: the patient did not meet an indication for pacing, the patient met an indication for pacing but not an SND indication for pacing, the patient was implanted with a market-released pacemaker, the patient had an SND indication for therapy but did not receive pacing therapy, patient lost to follow-up, patient withdrawal from the study, failure to meet inclusion/exclusion criteria, and death.
2.3. Statistical analysis
An exact 95% confidence interval of a binomial proportion was used to calculate the margin of error for the proportion of patients with SND diagnoses and pacemaker implantation in the study. Logistic regression models were then fit to determine the factors that may be associated with odds of diagnosis for all eligible enrolled patients and implant for all patients diagnosed with SND. Responses at the baseline visit for gender, age, resting heart rate, previous history of hypertension, education level and financial contribution to the household, along with presentation of syncope/presyncope in the 30 days prior to enrollment into the study, were included in the models. Cumulative incidence plots separate the timeline into the following categories: time 0 is day of enrollment or day of SND diagnosis, time 1 is >0 and ≤1 month, time 3 is >1 month and ≤3 months. Each subsequent category is grouped by additional 3 month intervals up to the maximal follow-up time period. Based upon the physician provided diagnoses, patients were classified by the Sponsor according to ACC/AHA/HRS guidelines.7
The study was powered to be able to measure the percentage of SND diagnosis and the percentage of SND patients treated with IPG therapy with sufficient precision. It was determined that 500 subjects were needed in Phase I to achieve this precision. The power calculations conservatively assumed that the SND diagnosis would be 20% within six months of follow-up. The margin of error will be ±4% in a sample of 500 subjects, based on a 95% confidence interval. Assuming that 20% of subjects are diagnosed with SND, this suggests that there will be 100 SND subjects in Phase I. Further assuming that IPG therapy will be adopted by 10% of SND subjects at three months after diagnosis, this means that the margin of error will be ±8.0% in a sample of 100, based on a 95% confidence interval.
3. Results
3.1. Patients
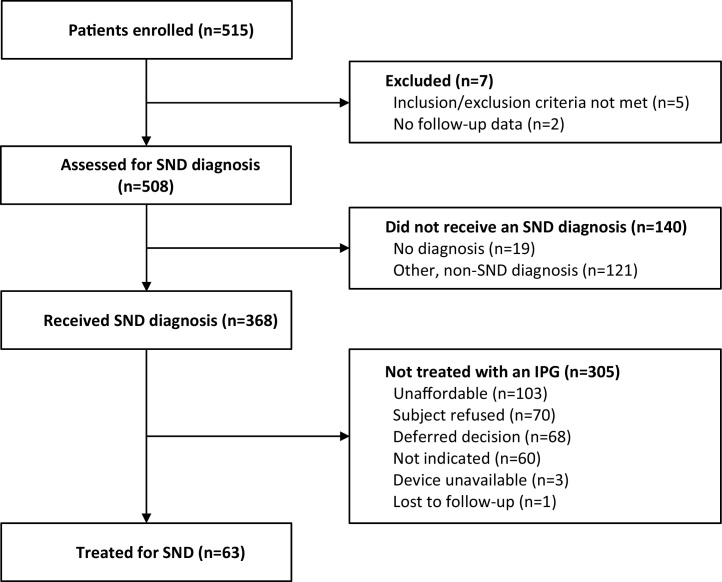
In the study, 515 patients were enrolled at 10 centers in India and Bangladesh between July 2012 and June 2014. Seven patients were excluded from analysis, 5 patients because a post-enrollment determination was made that they failed to satisfy inclusion/exclusion criteria and 2 patients were missing follow-up data (see Fig. 1). At their baseline assessments, subjects in these 10 centers were primarily seen by interventional cardiologists (302/500 = 60%) or electrophysiologists (197/500 = 39%).
Fig. 1.
Study Flow Diagram.
Baseline patient characteristics of the remaining 508 patients are summarized in Table 1. The overall mean age was 58 ± 15 years, 78% were male, 63% had a medical history of sinus bradycardia or SND prior to enrollment into the present study, and 40% reported symptoms of syncope/presyncope/dizziness within 30 days of enrollment into the study. Patients were followed for an average of 8.3 ± 8.0 months, where follow-up was calculated as time from enrollment.
Table 1.
Baseline Characteristics.
| Patient Characteristics | Total (n = 508) |
Diagnosed with SND (n = 368) |
Diagnosed with SND and Treated (n = 63) |
Diagnosed with SND vs. Treated p-value |
|---|---|---|---|---|
| Male Gender (N, %) | 394 (77.6%) | 285 (77.4%) | 38 (60.3%) | <0.001 |
| Age (years) Mean ± Standard Deviation | 57.6 (14.8) | 57.3 (15.5) | 63.9 (13.3) | <0.001 |
| BMI (kg/m2) Mean ± Standard Deviation | 24.1 (4.6) | 23.7 (4.5) | 23.5 (4.7) | 0.71 |
| Heart rate (intrinsic beats/min) Mean ± Standard Deviation | 55.7 (14.0) | 51.3 (11.3) | 48.5 (11.3) | 0.031 |
| History of sinus node dysfunction (N, %) | ||||
| Any history of SND | 320 (63.0%) | 283 (76.9%) | 47 (74.6%) | 0.11 |
| Bradycardia-tachycardia syndrome | 14 (2.8%) | 13 (3.5%) | 4 (6.3%) | 0.11 |
| Chronotropic incompetence | 4 (0.8%) | 4 (1.1%) | 2 (3.2%) | 0.12 |
| Sinus arrest/pause/exit block | 33 (6.5%) | 23 (6.3%) | 7 (11.1%) | 0.05 |
| Sinus bradycardia | 269 (53.0%) | 246 (66.8%) | 39 (61.9%) | 0.08 |
| Sinus tachycardia | 4 (0.8%) | 2 (0.5%) | 0 (0.0%) | 0.69 |
| Other SND | 3 (0.6%) | 2 (0.5%) | 0 (0.0%) | 0.69 |
| Symptoms (N, %)* | ||||
| Chest pain | 508 (100.0%) | 368 (100.0%) | 63 (100.0%) | |
| Dizziness/lightheadedness/presyncope | 180 (35.4%) | 123 (33.4%) | 14 (22.2%) | 0.013 |
| Dyspnea/shortness of breath | 145 (28.5%) | 122 (33.2%) | 32 (50.8%) | 0.001 |
| Edema | 267 (52.6%) | 177 (48.1%) | 28 (44.4%) | 0.09 |
| Exercise intolerance | 2 (0.4%) | 2 (0.5%) | 0 (0.0%) | 0.69 |
| Fatigue/weakness/lethargy | 141 (27.8%) | 100 (27.2%) | 19 (30.2%) | 0.1 |
| Malaise | 124 (24.4%) | 88 (23.9%) | 11 (17.5%) | 0.06 |
| Palpitations | 5 (1.0%) | 3 (0.8%) | 0 (0.0%) | 0.57 |
| Syncope | 73 (14.4%) | 54 (14.7%) | 9 (14.3%) | 0.16 |
| History of coronary artery disease (N, %) | 124 (24.4%) | 72 (19.6%) | 14 (22.2%) | 0.11 |
| History of hypertension (N, %) | 198 (39.0%) | 145 (39.4%) | 37 (58.7%) | <0.001 |
| History of myocardial infarction (N, %) | 19 (3.7%) | 7 (1.9%) | 1 (1.6%) | 0.39 |
| History of diabetes (N, %) | 81 (15.9%) | 53 (14.4%) | 14 (22.2%) | 0.025 |
| NYHA Class (N, %) | <0.001 | |||
| Subject does not have heart failure | 312 (61.4%) | 243 (66.0%) | 46 (73.0%) | |
| I | 23 (4.5%) | 16 (4.3%) | 4 (6.3%) | |
| II | 88 (17.3%) | 62 (16.8%) | 6 (9.5%) | |
| III | 35 (6.9%) | 13 (3.5%) | 3 (4.8%) | |
| IV | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) | |
| Not Available | 49 (9.6%) | 33 (9.0%) | 4 (6.3%) | |
| Education (N, %) | <0.001 | |||
| No formal education obtained | 46 (9.1%) | 42 (11.4%) | 6 (9.5%) | |
| Completed primary education | 152 (29.9%) | 107 (29.1%) | 17 (27.0%) | |
| Completed secondary (High School) education | 114 (22.4%) | 82 (22.3%) | 17 (27.0%) | |
| College-educated | 196 (38.6%) | 137 (37.2%) | 23 (36.5%) | |
| Contributes Financially to Their Household (N, %) | 0.012 | |||
| Yes | 259 (51.0%) | 196 (53.3%) | 26 (41.3%) | |
| No | 249 (49.0%) | 172 (46.7%) | 37 (58.7%) |
*Patients could have more than 1 symptom.
3.2. SND diagnosis
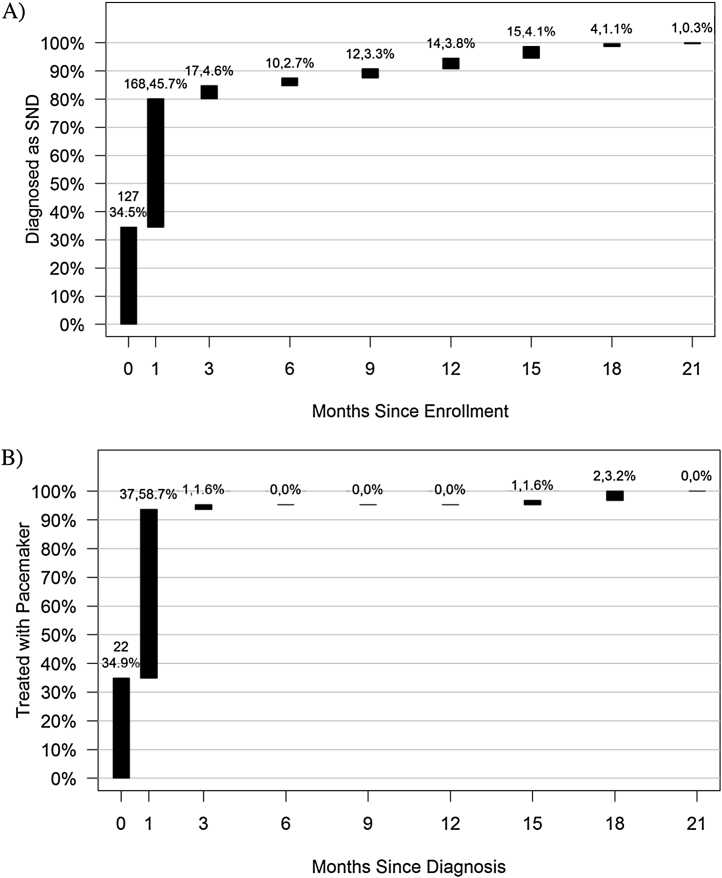
Of the 508 patients included in the analysis, 368 (75.3%) [95% CI: 71.2%–79.0%] were diagnosed with SND. Diagnostic tests used for the 368 patients diagnosed with SND were as follows: 353 (96%) patients had electrocardiographs, 257 (70%) had echocardiograms, 99 (27%) had event recorders, 55 (15%) had exercise tests, 11 (3%) had EP studies and 1 had a tilt table test. Fig. 2A shows the cumulative incidence from time of enrollment to time of SND diagnosis, which indicates that the majority (80.2%) of SND diagnoses were made within 1 month of being enrolled.
Fig. 2.
Cumulative incidence curve from A) time of enrollment to time of SND diagnosis and B) time of SND diagnosis to pacemaker implant.
An exploratory multivariable logistic regression assessed the association between baseline characteristics and probability of receiving an SND diagnosis. Having syncope/presyncope within 30 days of the baseline visit was associated with a higher probability of being diagnosed with symptomatic SND (Table 2). In contrast, higher resting heart rate was associated with a lower probability of being diagnosed with SND. The diagnosis rate across the 10 centers varied considerably with a range from 18% to 100% (refer to Table 4).
Table 2.
Multivariable Analysis of Factors Affecting Probability of SND Diagnosis.
| Parameter | OR (95% CI) | p-value |
|---|---|---|
| Heart rate (per 5 beats per minute) | 0.63 (0.57, 0.69) | <0.00001 |
| Education (versus none) | ||
| Primary education | 0.12 (0.03, 0.42) | |
| Secondary education | 0.09 (0.02, 0.33) | 0.002 |
| College-educated | 0.08 (0.02, 0.3) | |
| Symptoms: Syncope/Presyncope | 1.68 (1, 2.83) | 0.05 |
| Contributes financially to household | 1.51 (0.86, 2.65) | 0.15 |
| Age (per year) | 0.99 (0.97, 1.01) | 0.37 |
| History of hypertension | 1.18 (0.71, 1.96) | 0.52 |
| Female gender | 1.14 (0.61, 2.14) | 0.69 |
Table 4.
Implant Rate of SND Diagnosed Patients by Study Center.
| Center | Total | SND Diagnosed | Treated |
|---|---|---|---|
| Overall | 508 | 368 (72%) | 63 (17%) |
| India center 1 | 110 | 90 (82%) | 26 (29%) |
| India center 2 | 109 | 46 (42%) | 5 (11%) |
| India center 3 | 72 | 49 (68%) | 2 (4%) |
| India center 4 | 55 | 51 (93%) | 1 (2%) |
| Bangladesh center 1 | 49 | 47 (96%) | 13 (28%) |
| India center 5 | 46 | 34 (74%) | 14 (41%) |
| India center 6 | 33 | 32 (97%) | 0 (0%) |
| India center 7 | 17 | 3 (18%) | 1 (33%) |
| Bangladesh center 2 | 13 | 12 (92%) | 0 (0%) |
| India center 8 | 4 | 4 (100%) | 1 (25%) |
3.3. SND treatment
Of the 368 patients diagnosed with symptomatic SND, 63 (17.1%) [95% CI: 13.7%–21.8%] were implanted with a pacemaker. Fig. 2B shows the cumulative incidence from time of diagnosis to time of pacemaker implant. The majority of patients (93.7%) were implanted with a pacemaker within 1 month of diagnosis. The primary reason for not receiving a pacemaker was the device/procedure was unaffordable for 103 patients (34%). Other reasons for not receiving a pacemaker were: patient refusal for the procedure in 70 patients (23%), deferred decision by patients who requested follow-up- 68(22%), Physician’s decision for treatment- 60 patients (20%), device availability issues for 3 patients (1%), and lost to follow up-1patient (<1%) (Fig. 1).
There were 360 class I, 4 class IIa, and 4 class IIb SND diagnoses indications for pacing [7]. None of the 4 class IIb indicated patients were implanted and 1 of the 4 class IIa patients was implanted. The remaining 62 implants were in class I indicated SND diagnosed patients, leaving 298 (82.8%) patients with a class I indication untreated.
An exploratory multivariable logistic regression assessed the association between baseline characteristics and probability of receiving a pacemaker implant after symptomatic SND diagnosis. Older age, female gender, history of hypertension, lower resting heart rate, and syncopal or pre-syncopal symptoms 30 days prior to baseline visit were associated with a higher probability of implant (Table 3). Implant rate varied considerably across the 10 centers with a range from 0% to 41% (Table 4).
Table 3.
Multivariable Analysis of Factors Affecting Probability of Pacemaker Implant for SND Diagnosed Patients.
| Parameter | OR (95% CI) | p-value |
|---|---|---|
| Symptoms: Syncope/Presyncope | 4.61 (2.37, 8.99) | <0.00001 |
| Female gender | 2.77 (1.36, 5.64) | 0.005 |
| History of hypertension | 2.26 (1.21, 4.21) | 0.01 |
| Heart rate (per 5 beats per minute) | 0.85 (0.73, 0.99) | 0.03 |
| Age (per year) | 1.03 (1, 1.05) | 0.03 |
| Contributes financially to household | 0.53 (0.26, 1.07) | 0.08 |
| Education (versus none) | ||
| Primary education | 0.89 (0.28, 2.78) | |
| Secondary education | 1.5 (0.46, 4.83) | 0.38 |
| College-educated | 1.81 (0.57, 5.77) | |
4. Discussion
In this observational study of the care pathway of 508 patients presenting with symptoms and low heart rate from 10 centers in India and Bangladesh, 72% were diagnosed with SND. Of these, 17% were treated – leaving 83% of patients left untreated. Many of the untreated patients had a class I indication according to American and European guidelines.1, 7, 3 Primary reasons for not receiving treatment were due to patient refusal or deferral of decision (45%), affordability issues with treatment (34%), and the physician determining that pacemaker was not-indicated (20%). Treatment tended to occur quickly with 94% of pacemaker implants occurring within a month of diagnosis. We also observed wide variability in diagnosis and treatment rates across the 10 centers.
To our knowledge, this is the first comprehensive report that characterizes the SND care pathway in South Asia. Permanent cardiac pacing therapy for symptomatic bradycardia has been available for more than half a century.
Affordability was a major barrier to being treated for a substantial proportion of patients as 34% of patients reported no or insufficient insurance coverage as a reason to decline therapy. A majority of patients (53%) diagnosed with SND contributed financially to their household. Of patients diagnosed with SND, only 26 of 63 (41%) of those that received a pacemaker contributed financially to their household, while 167 out of 298 (56%) of those that did not receive a pacemaker contributed financially, which suggests some type of socioeconomic sensitivity towards healthcare spending for the treatment of SND. Since a more direct measure of income or socioeconomic status was not collected, the relationship between therapy cost and treatment decision is not clear.
Affordability of therapy was not a primary barrier for 66% of patients who were left untreated. Reasons included the patient reporting an aversion to the procedure, not agreeing that their condition warranted a device, or deferring their decision and preferring continued follow-up. Also, in many cases the physician determined that the patient was not indicated in spite of the SND diagnosis and the guideline indications. Phase II of the IMPROVE Brady study is designed to address these conditions via patient and physician educational resource tools in effort to improve treatment rates.
Syncope or pre-syncopal symptoms within 30 days of the baseline visit strongly correlated with subsequent pacemaker implantation, with more than a 4-fold higher chance of receiving a pacemaker than a patient without such symptoms. There were also higher implant rates for women (Odds ratio = 2.77; 95% CI: [1.36, 5.64]), older patients (Odds ratio = 1.03; 95% CI: [1.00, 1.05]), and patients with a history of hypertension (Odds ratio = 2.26; 95% CI: [1.21, 4.21]). Not surprisingly, lower heart rate was associated with increased likelihood of pacemaker implant.
Investigators often indicated that the symptoms of SND were mild and did not merit pacemaker implantation. Guidelines for cardiac pacing consider symptomatic SND diagnoses as Class I or II indication for pacemaker implant.2, 3 Furthermore, delaying pacemaker therapy for individuals with Class I indications has been shown to have negative clinical outcomes and result in poorer quality of life.8, 9 A recent survey of Cardiac Implantable Electronic Devices (CIEDs) across several centers in India reported that 80% of pacemaker implants were for bradycardia due to AV block, while only 20% were for SND.10 This may highlight a preference for device implant to prevent death rather than improve quality of life in a relatively mild disease. Sinus node dysfunction has been specifically shown to represent the largest group of patients for whom a referral for pacing was not made at initial documentation.8, 11 Thus, better education of patients and physicians about guidelines and the course of disease progression of SND are warranted in designing process improvement strategies for the study in the region. Furthermore, local geographic society recommendations, such as Indian guidelines or expert consensus statement, may influence the cardiologist and patient in their acceptance of therapy. Local cost effective analysis or quality of life evidence may also help make a more informed decision when treating SND.
We observed wide variability across centers with respect to diagnosis and treatment of SND. Half of the centers had diagnosis rates >90%, but rates also dipped as low as 18%. There was no evidence of positive correlation between center diagnosis and treatment as 3 centers with high diagnosis rates had the lowest treatment rates. Although speculative, this variability across centers could be reflective of lack of physician agreement with Western guideline recommendations.
There are several important limitations of this study. These data were collected over a relatively brief period of time across 10 specialized centers in India and Bangladesh. The type of sites that were selected might have also led to data collection on a patient population that was better able to afford healthcare spending than the general population in this region. Inferences on the effect of therapy affordability were limited because patients were not asked direct questions about socioeconomic status and income.
Phase I of IMPROVE Brady demonstrates that there remains a significant gap in the use of Western guideline-based pacemaker therapy for patients diagnosed with SND in India and Bangladesh, particularly among younger patients and males. Although affordability of therapy is a primary barrier for one third of patients, the remaining barriers are non-economical. Phase II of the study will investigate whether specific educational tools, as designed, can be successful in improving these rates. Future work is needed that follows individuals earlier in the bradycardia care pathway to identify the rate of SND in the general population of this region and to explore ways to improve the initial diagnosis of SND.
Acknowledgements
The authors would like to thank Harrison Hudnall and Dedra Fagan of Medtronic for their editorial support of this manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ihj.2017.02.013.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Brignole M., Menozzi C., Lolli G., Oddone D., Gianfranchi L., Bertulla A. Pacing for carotid sinus syndrome and sick sinus syndrome. Pacing Clin Electrophysiol. 1990;13(12 Pt 2):2071–2075. doi: 10.1111/j.1540-8159.1990.tb06944.x. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M., Auricchio A., Baron-Esquivias G. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34(29):2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 3.Gillis A.M., Russo A.M., Ellenbogen K.A. HRS/ACCF expert consensus statement on pacemaker device and mode selection. J Am Coll Cardiol. 2012;60(7):682–703. doi: 10.1016/j.jacc.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Shaw D.B., Holman R.R., Gowers J.I. Survival in sinoatrial disorder (sick-sinus syndrome) Br Med J. 1980;280(6208):139–141. doi: 10.1136/bmj.280.6208.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healey J.S., Toff W.D., Lamas G.A. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114(1):11–17. doi: 10.1161/CIRCULATIONAHA.105.610303. [DOI] [PubMed] [Google Scholar]
- 6.Mond H.G., Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009 – a world society of arrhythmia's project. Pacing Clin Electrophysiol. 2011;34(8):1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein A.E., Dimarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5(6):e1–e62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Cunnington M.S., Plummer C.J., McComb J.M. Delays and adverse clinical outcomes associated with unrecognized pacing indications. QJM. 2009;102(7):485–490. doi: 10.1093/qjmed/hcp066. [DOI] [PubMed] [Google Scholar]
- 9.Risgaard B., Elming H., Jensen G.V., Johansen J.B., Toft J.C. Waiting for a pacemaker: is it dangerous? Europace. 2012;14(7):975–980. doi: 10.1093/europace/eus016. [DOI] [PubMed] [Google Scholar]
- 10.Shenthar J., Bohra S., Jetley V. A survey of cardiac implantable electronic device implantation in India: by indian society of electrocardiology and indian heart rhythm society. Indian Heart J. 2016;68(1):68–71. doi: 10.1016/j.ihj.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Eck J.W., van Hemel N.M., Kelder J.C. Poor health-related quality of life of patients with indication for chronic cardiac pacemaker therapy. Pacing Clin Electrophysiol. 2008;31(4):480–486. doi: 10.1111/j.1540-8159.2008.01018.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.