Abstract
Objectives/Hypothesis
To identify functional network architecture differences in the brains of children with unilateral hearing loss (UHL) using resting state functional connectivity MRI (rs-fcMRI).
Study Design
Prospective observational study
Methods
Children (7 to 17 years) with severe to profound hearing loss in one ear, along with their normal hearing (NH) siblings, were recruited and imaged using rs-fcMRI. Eleven children had right UHL, 9 had left UHL, and 13 had normal hearing. Forty-one brain regions of interest culled from established brain networks such as the default mode (DMN), cingulo-opercular (CON), and frontoparietal networks (FPN), as well as regions for language, phonological and visual processing were analyzed using regionwise correlations and conjunction analysis to determine differences in functional connectivity between the UHL and normal hearing children.
Results
When compared to the NH group, children with UHL showed increased connectivity patterns between multiple networks, such as between the CON and visual processing centers. However, there were decreased, as well as aberrant connectivity patterns with the co-activation of the DMN and FPN, a relationship that is usually negatively correlated.
Conclusion
Children with UHL demonstrate multiple functional connectivity differences between brain networks involved with executive function, cognition, and language comprehension that may represent adaptive as well as maladaptive changes. These findings suggest possible interventions or habilitation, beyond amplification, might be able to affect some children's requirement for additional help at school.
Keywords: children, unilateral hearing loss, MRI
Introduction
Unilateral hearing loss (UHL) can affect the speech and language, cognitive, and behavioral development of a child. Studies have demonstrated that children with UHL have higher rates of academic difficulties, with up to 35% repeating a grade compared to 3.5% of their normal hearing (NH) peers.1-8 Nearly a third of children with UHL have reported behavioral problems, and ∼ 40% require individualized education plans (IEPs) 9 compared to 13% nationally10. Furthermore, similar to bilateral hearing loss in children, UHL can also lead to deficits in executive functions.11 Therefore, the goal of this study is to understand how UHL influences the development of neural systems responsible for language, cognition, and executive function in otherwise typically developing children.
We used resting state functional connectivity MRI (rs-fcMRI) to measure and characterize the development of the brain's functional network architecture in typically developing children with and without UHL. Rs-fcMRI relies on the pairwise temporal correlations of low frequency BOLD (blood oxygen level dependent) contrast signals measured during MRI (magnetic resonance imaging) from spatially disparate regions of the brain. When two regions of the brain have highly correlated time courses, they are thought to be activated together and therefore, functionally linked.12
This study adds a larger patient population to our prior pilot study, utilizing rs-fcMRI to explore additional interregional differences in the functional brain architecture of children with UHL compared to their NH siblings.13 Several key brain networks were analyzed such as the default mode network (DMN), which is less active during goal-directed behavior than during rest, and the frontoparietal network (FPN) and the cingulo-opercular network (CON), which are active during goal-directed tasks requiring attention, alertness, and error-monitoring.14-18 Additional regions involved in auditory and phonological processing, sensorimotor, language comprehension, and visual and auditory processing were also studied. We hypothesized that children with UHL have differences in functional brain architecture compared to their siblings with NH.
Methods
The Washington University Medical Center Human Research Protection Office granted institutional review board approval prior to the beginning of this study. All parents provided written informed consent, and all participants provided pediatric assent.
Participants
Children (7-17 years) with UHL were recruited from the Unilateral Hearing Loss in Children Study.6 Inclusion criteria required participants to have severe-to-profound sensorineural UHL, defined as a four-tone pure tone average (PTA) of ≥70 dB hearing level (HL) in the affected ear, and a three-tone PTA (500, 1000, and 2000 Hz) of ≤20 dB HL in the NH ear, with a threshold at 4000 Hz ≤30 dB. Participants were excluded if they were affected by any genetic or acquired intellectual and/or developmental disorder, temporary or conductive hearing loss, or had a contraindication to MRI scanning. NH siblings (7-17 years) of the children with UHL were recruited as controls. Exclusion criteria for the NH sibling controls were identical to those for the participants with UHL.
MRI Data Acquisition and Preprocessing
Scanning Protocol and Image Acquisition
The same Siemens TRIO 3.0 Tesla scanner (Erlangen, Germany) was used to acquire all images, which were obtained within a single scanning session. A high-resolution T1-weighted sagittal MPRAGE structural image (TE = 3.08 ms, TR (partition) =2.4 sec, TI = 1000 ms, flip angle = 8 degrees, 176 slices with 1×1×1 mm voxels) was obtained and used to compute the atlas transformation.
A BOLD contrast sensitive asymmetric spin-echo echo-planar sequence (volume TR=2.5 s, in-plane resolution 4×4 mm, T2* evolution time=27 ms, α=90°) was used for functional imaging. Thirty-two contiguous, 4 mm-thick axial slices parallel to the anterior commissure-posterior commissure plane were collected for whole brain coverage. For rs-fcMRI scanning data acquisition, participants maintained their gaze on a white fixation cross on a black background. Subjects had either three or four 3-minute runs or two to four 5-minute runs. At least 9 minutes of resting state data were collected for each participant.
fcMRI Data Preprocessing
First, the BOLD images produced in each run were combined into a 4D (x, y, z, time) time series. Sinc interpolation was used to compensate for timing offsets among slices within the same frame. Subsequently, differences in slice intensity due to contiguous interleaved slice acquisition were removed. Lastly, all frames were registered in a six-parameter rigid body alignment, which was used for standard motion correction in each subject.19 Three dimensional cubic spline interpolation was used for re-slicing. All image data were transformed to Talairach atlas space20 via a single common atlas21 derived from adult and pediatric brains22 by a warping mechanism. For each run, the first four image acquisitions (10 s) were removed to ensure steady-state magnetization. The mode voxel intensity value was normalized to 1000 for each fcMRI run.
Functional Connectivity Preprocessing
As previously described, preprocessing for the functional connectivity analyses was carried out for optimization of the time-series data and to reduce artifacts and non-neurological variance.14,23 The steps include removing the linear trend, filtering temporal band-pass (0.009 Hz < f < 0.08 Hz) and spatial smoothing at 6 mm full width at half maximum, as well as performing regression of 24 motion parameters24, signals from whole brain, ventricle, and white matter, and the time-based derivatives of each.
Since motion artifact can be a source of spurious correlation, a motion censoring procedure was performed.23 Frames of MR data were removed if they had a frame-by-frame-displacement (FD) larger than 0.2 mm. If fewer than 6 contiguous frames remained between removed frames, these frames were removed as well. If fewer than 30 good frames remained in a bold run, the whole run was removed from the analysis.
Region of Interest (ROI) Definition
Hearing-related, cognitive control, default, visual, and sensorimotor ROIs were chosen based on previous literature.13,14,15,22,23,25,26,27,28 Coordinates were converted to the Talairach atlas as indicated. Spheres 10mm in diameter centered on the coordinates were used. Figure 1 depicts seed ROIs used in this study. Table 1 lists their coordinates and includes the regions that were identified from the conjunction analysis.
Figure 1.
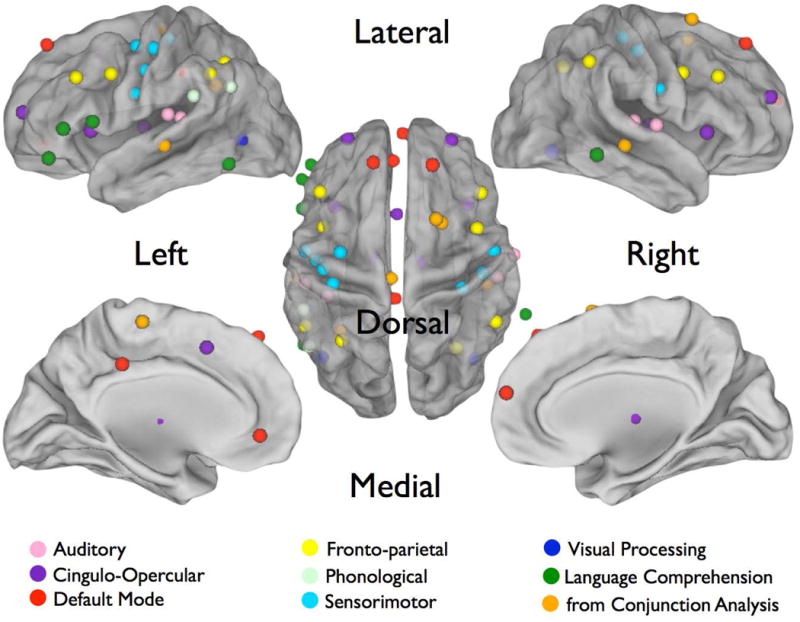
Seed regions from Table 1 projected onto a brain surface. Auditory regions are shown in pink, CON in purple, DMN in red, FPN in yellow, phonological in light blue, sensorimotor in blue, visual processing in deep blue, language network in green, and conjunction analysis in oranage. Representative dots are larger than the actual 10mm spheres used in analysis for ease of visualization.
Table 1. Brain system, regions and coordinates examined in this study.
| Coordinates | |||||
|---|---|---|---|---|---|
| System | Brain Region | Source | X | Y | Z |
| Auditory | L Heschl | Tibbetts et al | -39 | -35 | 12 |
| L BA41 | WOROI | -51 | -28 | 14 | |
| R BA41 | WOROI | 52 | -23 | 10 | |
| R Heschl | Jancke et al | 61 | -11 | 8 | |
| Cingulo-Opercular | dACC/msFC | Dosenbach et al | -1 | 10 | 46 |
| L aPFC | Dosenbach et al | -28 | 51 | 15 | |
| R aPFC | Dosenbach et al | 27 | 50 | 23 | |
| L anterior thalamus | Dosenbach et al | -12 | -15 | 7 | |
| R anterior thalamus | Dosenbach et al | 10 | -15 | 8 | |
| L aI/FO | Dosenbach et al | -35 | 14 | 5 | |
| R aI/FO | Dosenbachet al | 36 | 16 | 4 | |
| Default Mode | amPFC | Fox et al | 1 | 54 | 21 |
| vmPFC | Fox et al | -3 | 39 | -2 | |
| L superior frontal | Fox et al | -14 | 38 | 52 | |
| R superior frontal | Fox et al | 17 | 37 | 52 | |
| PCC | Fox et al | -2 | -36 | 37 | |
| Fronto-Parietal | L frontal | Dosenbach | -41 | 3 | 36 |
| R frontal | Dosenbach | 41 | 3 | 36 | |
| L IPL | Dosenbach | -51 | -51 | 36 | |
| R IPL | Dosenbach | 51 | -47 | 42 | |
| L IPS | Dosenbach | -31 | -59 | 42 | |
| R IPS | Dosenbach | 30 | -61 | 39 | |
| L dlPFC | Dosenbach | -43 | 22 | 34 | |
| R dlPFC | Dosenbach | 43 | 22 | 34 | |
| R SMA/pre-SMA | conjunction analysis | 19 | 8 | 65 | |
| R SMA/pre-SMA | conjunction analysis | 22 | 6 | 54 | |
| Phonological | Angular gyrus | Church et al | -49 | -62 | 29 |
| L Angular gyrus | conjunction analysis | -32 | -54 | 30 | |
| Supramarginal gyrus | Church et al | -52 | -42 | 24 | |
| Sensorimotor | L motor hand a | Tibbetts et al | -32 | -11 | 51 |
| L motor hand b | Tibbetts et al | -36 | -28 | 55 | |
| L sensory cortex | Tibbetts et al | -41 | -20 | 50 | |
| L motor mouth a | Tibbetts et al | -45 | -15 | 38 | |
| L motor mouth b | Tibbetts et al | -51 | -10 | 25 | |
| R sensory cortex | Tibbetts et al | 33 | -29 | 55 | |
| R motor hand | Tibbetts et al | 44 | -21 | 48 | |
| R motor mouth | Tibbetts et al | 50 | -9 | 27 | |
| L paracentral lobule | conjunction analysis | -4 | -25 | 60 | |
| Language Comprehension | L IFG | Turken et al | -52 | -61 | -13 |
| R MTG | Turken et al | 67 | -43 | -8 | |
| LIFG | Turken et al | -48 | 37 | -10 | |
| L IFG | Turken et al | -53 | 29 | 6 | |
| L IFG | Turken et al | -53 | 13 | 10 | |
| L MTG | conjunction analysis | -56 | -26 | -3 | |
| R MTG | conjunction analysis | 47 | -28 | -3 | |
| Visual Processing | L Occipito-temporal, ITG | Dubois et al | -42 | -68 | 0 |
| R Occipito-temporal, MOG | Dubois et al | 40 | -67 | -6 | |
All coordinates given are in Talairach 1988 space. Abbreviations: BA41, Brodmann's Area 41; dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; PFC, prefrontal cortex; aPFC, anterior PFC; aI/FO, anterior insula/frontal operculum; amPFC, anterior medial PFC; vmPFC, ventromedial PFC; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; IPL, inferior parietal lobule; IPS, intraparietal sulcus; dlPFC, dorsolateral PFC; FFG, fusiform gyrus; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; MOG, middle occipital gyrus; SMA, supplementary motor area.
Statistical Analysis
Computation of Mean Regionwise Correlations
A resting state BOLD time series was calculated for each of the 41 seed regions for each subject. A correlation of this time series with the time series in each voxel of the brain was calculated to produce a correlation seed map for each seed region in each subject. Fisher Z-transformed seed maps from each region were then created to perform t-tests to compare brain correlation patterns of the NH participants to those with UHL. Monte Carlo simulation was implemented to guard against false positives, using a z-score of greater ≥ 3 (P < 0.001 uncorrected) with a cluster size greater than 459 cubic mm (seventeen 3×3×3 mm voxels) to correct for multiple comparisons. For example, the t-test result for a seed placed in the ventromedial prefrontal region of the default mode network is illustrated in Figure 2.
Figure 2.
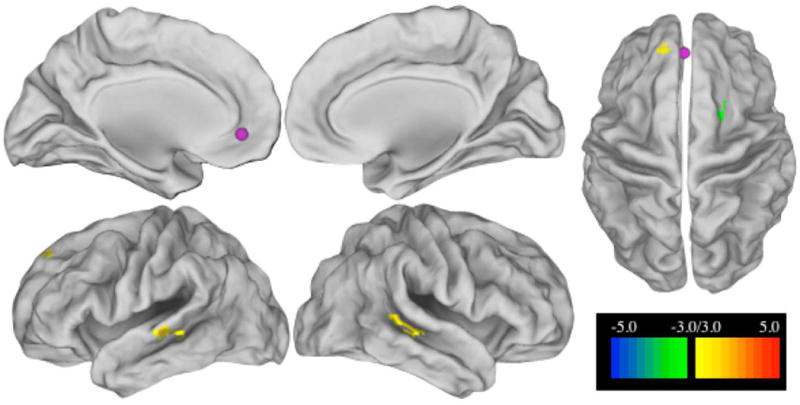
T-test on seed maps of L ventromedial prefrontal cortex (vmPFC) seed (-3, 39, -2; default system) showing differences between controls and all UHL subjects. The seed is shown in purple, the scale is the z-score from the t-test between control and UHL seedmaps, monte carlo corrected for multiple comparisons with z > 3 with a cluster size of 459 cubic mm or more. Positive z values, in warm colors, show regions where control subjects have higher connectivity to the seed than UHL subjects. Cool colors show negative z values where UHL subjects have higher connectivity than controls.
Conjunction Analysis
A conjunction analysis was performed across all 41 seed regions to identify brain locations in which subjects with both right and left UHL differed from controls. Masks were first created from the z-transformed t-test image for each seed region by assigning each voxel with a statistical value of z ≥1.96 a mask value of 1, and all other voxels a value of 0. Then, the masks were summed across seeds to create conjunction images in which voxel values were now equal to the number of the seed ROIs that showed differences between UHL and control participants at that voxel. Conjunction images were created comparing 1) controls to all UHL participants, 2) controls to left UHL (LHL) participants, and 3) controls to right UHL (RHL). Each comparison group had both positive (controls more correlated than the UHL group) and negative (the UHL group more correlated than controls) t-test scores, thus creating six total conjunction images. Next, these positive and negative masks were summed to reveal overlap regions. Lastly, an automated algorithm was used to determine the center of mass of the overlapping regions that contained at least 10 contributing seeds.22 This in-house automatic peak selection algorithm smoothed the summed conjunction image with a 4mm at half-max Gaussian spatial filter, dropped 10mm diameter spheres on peak coordinates with 10 or more contributing seeds, and combined peaks within 10mm of each other to give us brain regions with differences between control and UHL participants.
Results
Demographics
Sixty-four children were recruited and participated, 41 with UHL and 23 with NH. A total of six children were excluded due to having braces (n=1), BAHA (n=2), failing to meet UHL criteria (n=1), difficulty staying awake during scanning (n=1), and being too anxious to complete scanning (n=1). After pre-processing, 33 participants had usable data: RHL (n=11), LHL (n=9), NH (n=13). We investigated whether children with UHL compared to NH controls were more likely to move in the scanner and lose usable data, but there was no significant difference (γ2 = 0.3535, P = 0.5521).
The demographic, socioeconomic, educational and medical histories of the study participants are shown in Table 2. The control group had more males. Significantly more children with UHL required IEPs relative to their NH siblings.
Table 2.
Demographic, socioeconomic, educational, and medical information about the participants.
| Characteristic | NH (n=13) |
All UHL (n=20) |
All participants (n=33) |
P value |
|---|---|---|---|---|
| Male sex, n (%) | 9 (69) | 6 (30) | 15 (46) | 0.04 |
| Age in years, mean, (SD) | 14.1 (2.8) | 13.3 (2.7) | 13.6 (2.7) | 0.43 |
| Race/ethnicity, n (%) | 0.32 | |||
| African American | 3 (23) | 3 (15) | 6 (18) | |
| White | 10 (77) | 14 (70) | 24 (73) | |
| Asian | 0 | 3 (15) | 3 (9) | |
| Hispanic/Latino | 0 | 0 | 0 | |
| Number of siblings, n (%) | 0.80 | |||
| 0 | 0 | 1 (5) | 1 (5) | |
| 1 | 2 (15) | 2 (10) | 4 (12) | |
| 2 | 5 (39) | 9 (45) | 14 (42) | |
| 3 or more | 6 (46) | 8 (40) | 14 (42) | |
| Type of insurance, n (%) | 0.56 | |||
| Medicaid | 2 (15) | 4 (20) | 6 (18) | |
| Private | 11 (85) | 16 (80) | 27 (82) | |
| Premature, n (%) | 3 (23) | 3 (15) | 6 (18) | 0.62 |
| Speech/language evaluation, n (%) | 2 (15) | 5 (25) | 7 (21) | 0.68 |
| Speech problems, n (%) | 3 (23) | 5 (25) | 8 (24) | 0.62 |
| Speech therapy, n (%) | 1 (8) | 4 (20) | 5 (15) | 0.63 |
| Repeat grade, n (%) | 1 (8) | 4 (20) | 5 (15) | 0.63 |
| Individualized education plan, n (%) | 0 | 7 (35) | 7 (21) | 0.03 |
| Asthma, n (%) | 0 | 0 | 0 | |
| Recurrent otitis media, n (%) | 0 | 3 (15) | 3 (9) | 0.26 |
| Chronic condition, n (%) | 3 (23) | 3 (15) | 6 (18) | 0.66 |
| Tympanostomy tubes, n (%) | 2 (15) | 9 (45) | 11 (33) | 0.13 |
| Other ear surgery, n (%) | 1 (8) | 7 (35) | 8 (24) | 0.11 |
| Dominant hand, n (%) | 0.54 | |||
| Left | 3 (23) | 2 (10) | 5 (15) | |
| Right | 9 (69) | 17 (85) | 26 (79) | |
| Both | 1 (8) | 1 (5) | 2 (6) | |
| Wear glasses, n (%) | 4 (31) | 12 (60) | 16 (49) | 0.16 |
NH, children with normal-hearing; UHL, children with unilateral hearing loss; SD, standard deviation
fcMRI Data Analysis
The findings of our seed-based correlations analysis are summarized in Table 3. To indicate greater or lesser resting-state functional connectivity (RSFC) between brain regions in UHL compared to NH controls, the terms “increased” and “decreased” RSFC are used, respectively.
Table 3. Locations of difference in connectivity between controls and unilateral hearing loss groups for seed regions showing a difference.
| System or region to which there is a difference in connectivity | ||||||
|---|---|---|---|---|---|---|
| System | Seed Coordinates, Location | Controls>UHL | Controls>RHL | UHL>Controls | RHL>Control | LHL>Control |
| Cingulo-Opercular | (-1, 10, 46), dACC/msFC | R MTG (50, -31, -4) | ||||
| (-28, 51, 15), L aPFC | R SLF (29, 33, 17); R anterior FP (24, 5, 45) | |||||
| (-12, -15, 7), L anterior thalamus | L FFG (-49, -48, -2) | |||||
| (10, -15, 8), R anterior thalamus | L FEF (-45, 13, 37) R MTG (50, -41, 3) | |||||
| (-35, 14, 5), L al/FO | Bilateral sensorimotor cortex [PreCG] (-48, -13, 37). and (60, -9, 23) | |||||
| Default Mode | (-3, 39, -2), vmPFC | Bilateral MTG (-56, -31, -4) and (48, -31, -2) | R FP (23, 5, 51) | |||
| Fronto-Parietal | (-41, 3, 36), L frontal | L aI/FO (-42, 19, 24) | R anterior Salience (20, 48, 19) | |||
| (-51, -51, 36), L IPL | R aI/FO (45, -15, 16) | |||||
| (30, -61, 39), R IPS | R FP (34, 15, 50) | Bilateral OG BA18 (-31, -87, 1) and (34, -85, 1) | ||||
| (43, 22, 34), R dlPFC | L superior dorsal motor cortex [PCL] (-8, -24, 66) | Bilateral OG BA18 (-11, -79, -11) and (16, -76, -14) | ||||
| Sensorimotor | (-45, -15, 38), L motor mouth a | Multiple, bilateral regions within aI/FO (51, 6, 5), (-38, 15, 1), (-59, -1, 14); L SMA (-2, -4, 58 | ||||
| Language Comprehension | (-48, 37, -10), L IFG | Multiple, bilateral regions within FP (-45, 28, 25), (47, 34, 12), (-40, 36, 11) | ||||
| (-53, 13, 10), L IFG | L FP (-49, 26, 18) L STG (-60, -37, 17) | |||||
UHL, children with unilateral hearing loss; RHL, children with right-sided unilateral hearing loss; LHL, children with left-sided unilateral hearing loss; dACC/msFC, dorsal anterior cingulate cortex/medial superior frontal cortex; PFC, prefrontal cortex; aPFC, anterior PFC; SLF, superior longitudinal fasciculus; FP, fronto-parietal; al/FO, anterior insula/frontal operculum; PreCG, precentral gyrus; vmPFC, ventromedial PFC; IPL, inferior parietal lobule ; PoCG, postcentral gyrus; IPS, intraparietal sulcus; OG, occipital gyrus; dlPFC, dorsolateral PFC; PCL, paracentral lobule; STG, superior temporal gyrus; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; FFG, fusiform gyrus; FEF, frontal eye field.
Cingulo-opercular seed regions showed increased RSFC to all regions with a significant difference between control subjects and those with UHL. Both dACC/msFC and right anterior thalamus showed increased RSFC to the right posterior middle temporal gyrus (MTG, language comprehension near auditory cortex), but only for the LHL group. The right anterior thalamus also showed increased connectivity to the left frontal eye field (FEF) for this group. The RHL group showed greater connectivity between the left anterior insula/frontal operculum and bilateral primary sensorimotor cortex. The combined UHL group had increased RSFC between the left aPFC and the right SMA, as well as between the left anterior thalamus and the left fusiform gyrus.
The vmPFC seed of the DMN had decreased RSFC to bilateral MTG, but increased RSFC to the right frontal region in the combined UHL group (Fig 2).
In the FPN, the combined UHL group showed increased RSFC between the left frontal and right aPFC, the left IPL and right operculum, R IPS and bilateral occipital cortex, and the R dlPFC and bilateral occipital cortex. There was decreased RSFC between the R IPS and right FEF, and right dlPFC to the left superior dorsal motor cortex. The left frontal seed also showed decreased RSFC to the left dlPFC in the RHL group.
The mouth portion of left motor cortex showed increased RSFC in UHL to multiple regions corresponding to the left and right aI/FO and left premotor cortex.
Two language comprehension network seeds within the left inferior frontal gyrus (IFG) showed increased RSFC to regions within the FPN and left superior temporal gyrus in the combined UHL group.
Conjunction Analysis
The results of our conjunction analysis are shown in Figure 3. Interestingly, multiple regions showed both increased and decreased connectivity findings. Therefore, we summed the positive and negative conjunction images to further explore these overlapping regions. Analysis of these summed conjunction images revealed six new regions (Fig 3C) in the FPN, auditory, language and sensorimotor cortex. For each of these six new regions, 10mm spheres were placed on the center of mass coordinates and used as new seeds in another round of correlation analysis. The results are summarized in Figure 4 and Table 4.
Figure 3.
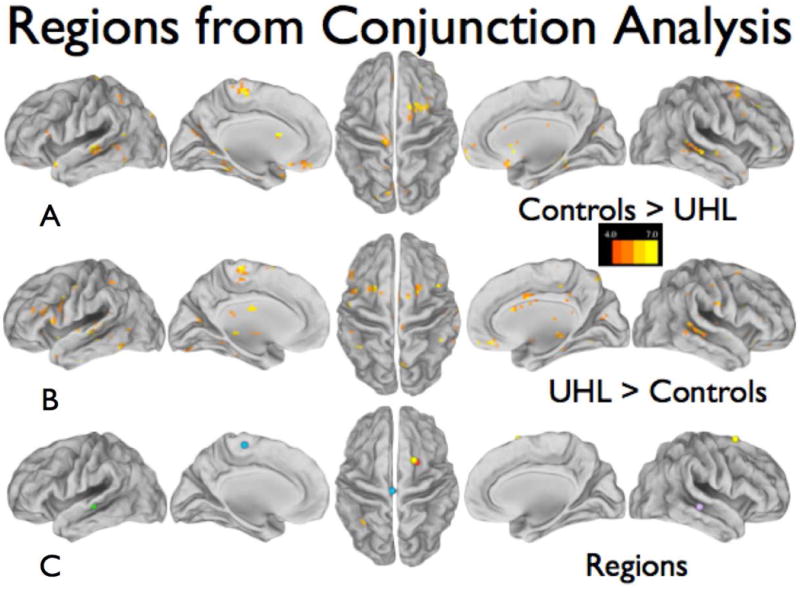
Conjunction analysis maps depicting regions of BOLD signal fluctuation correlations that differ between the control group and the UHL groups. At least four seed regions contributed to the differences shown; the scale shows how many seeds contributed to each voxel. (A) The control group had higher correlations with the seed regions than the UHL groups. (B) The UHL groups had higher correlations with the seed regions than the control groups. (C) Six new regions discovered from the sum of A and B, requiring at least 10 contributing seeds; the centers of mass were determined by an automated algorithm.
Figure 4.
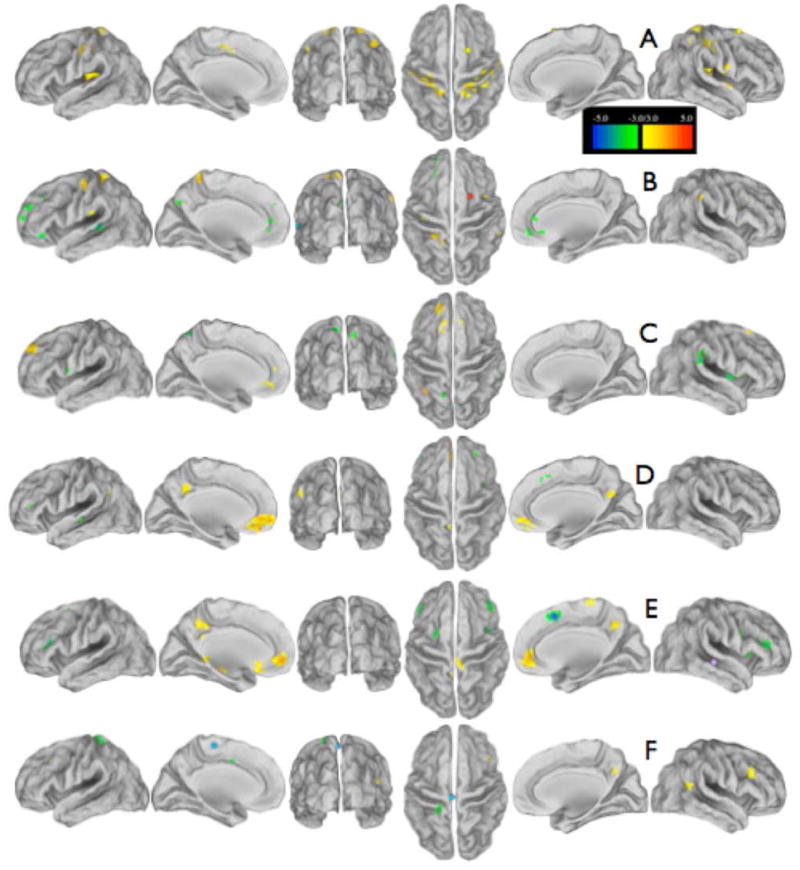
T-test maps between control and UHL subjects for the new regions discovered from conjunction analysis; the seeds are shown as spheres of the color listed. (A) R supplementary motor area seed (yellow) in fronto-parietal system (19, 8, 65). (B) R supplementary motor area seed (red) in fronto-parietal system (22, 6, 54). (C) L angular gyrus seed (orange) in fronto-parietal system (-32, -54, 30). (D) L middle temporal gyrus seed (green) in auditory cortex (-56, -26, -3). (E) R middle temporal gyrus seed (purple) in auditory cortex (47, -28, -3). (F) L paracentral lobular seed (blue) in sensorimotor cortex (-4, -25, 60). Most of these seeds are more connected to some brain regions for controls (hot colors) and more connected in UHL subjects to other regions (cool colors). The scale shows the z-score significance from the controls vs all UHL subject t-tests, monte carlo corrected for multiple comparisons.
Table 4.
Region-to-system differences in connectivity between controls and unilateral hearing loss groups in seeds discovered by conjunction analysis. Color of region refers to Figure 4.
| System or region to which there is a difference in connectivity | ||||
|---|---|---|---|---|
| System | (Seed Coordinates), Location | Color of Region | Controls > UHL | UHL > Controls |
| Fronto-Parietal | (19,8,65), R SMA | 3A – yellow | STG ; STS; dACC; PreCG, PoCG | |
| (22,6,54), R SMA | 3B – red | PreCG | MFG ; STG; vmPFC, PCUN | |
| Phonologic | (-32,-54,30), L ANG | 3C – orange | SFG | aI/FO ; SMG |
| Language Comprehension | (-56,-26,-3), L MTG | 3D – green | vmPFC ; PCC | STS ; dlPFC ; aI/FO; dACC |
| (47,-28,-3), R MTG | 3E – purple | vmPFC ; PCC ; PCL | dACC ; aI/FO | |
| Sensorimotor | (-4,-25,60), L PCL | 3F - blue | MFG; PCC; ANG | PoCG ;dACC |
Abbreviations: UHL, children with unilateral hearing loss; RHL, children with right-sided unilateral hearing loss; LHL, children with left-sided unilateral hearing loss; PreCG, precentral gyrus; PoCG, postcentral gyrus; FP, fronto-parietal; FG, frontal gyrus; MFG, middle FG; SFG, superior FG; MTG, middle temporal gyrus; ANG, angular gyrus; CO, cingulo-opercular; DA, dorsal attention; PCUN, precuneus; CG, cingulate gyri; PCL, paracentral lobule; STS, superior temporal sulcus; STG, superior temporal gyrus; SMG, supramarginal gyrus; dlPFC, dorsolateral PFC.
In the UHL group, the right frontal cortex showed decreased RSFC between the left optic radiation and bilateral primary sensorimotor regions, and increased RSFC to the right primary visual cortex. The left angular gyrus had decreased RSFC to the SFG, but increased RSFC to regions associated with the CON and FPN. Similar to the seed-based correlations, left and right MTG had decreased RSFC to regions in the DMN, but increased RSFC to regions in the CON and FPN. Finally, the left paracentral lobule region had decreased RSFC to bilateral middle frontal gyrus, similar to our correlations analysis above, and had increased RSFC to bilateral STG, or secondary auditory regions.
Discussion
In this study, we identified differences in RSFC between networks known to be involved with executive control and cognition to regions involving visual processing, motor attention, and language comprehension in children with UHL compared to NH siblings. Consistent with previous results6-11, children with UHL had a higher utilization rate of IEPs in school. Combined, our results appear consistent with the idea that UHL in children is disadvantageous, and that differences in the functional architecture of the brain may reflect both adaptive and maladaptive changes.
The CON, involved with flexible control of goal-directed behavior16-18, had increased RSFC changes in children with UHL. For instance, the L APC, thought to implement complex task rules and strategies14, was more connected to the SMA, possibly to improve motor attention skills.16 On the other hand, the conjunction analysis showed decreased RSFC between the PCL and MFG. This is similar to a study by Sweet et al where decreased activation in the PCL occurred during a task that presented increasing degrees of phonological complexity, presumably to direct more cortical activity in other brain regions more involved with analyzing complex sounds.29
The thalamus, a subcortical hub of the CON16, had increased RSFC in UHL to the FEF, which mediates top-down control over visual areas during spatial orienting of attention30 to the fusiform gyrus, which plays a key role in the DAN and visual memory.31,32 Church et al showed activation of visual regions in verbal repeat tasks in healthy adults and children, suggesting mental visualization of spoken words.22 Similarly, children with UHL may be utilizing these visual regions to compensate for their hearing loss.
The FPN, which controls initiation of tasks and is responsible for executive top-down control16, showed increased RSFC to bilateral occipital cortex, the visual processing center. As in our pilot study13, the IPL, associated with short term memory and attention29, had increased RSFC with a region around the posterior operculum, associated with echoic memory and domain-general working memory33,34, possibly due to increased subvocal rehearsal.
Fortifying “within network” connections has been shown to be a part of normal development through adolescence, and decreased within network connectivity has been associated with lower IQ.35 Decreased connectivity between regions within the FPN was seen in the UHL participants, such as between the IPS and right frontal. This altered RSFC pattern in the executive function networks may correlate with the increased rate of difficulty with tasks involving executive function seen in children with UHL.11
The DMN, believed to be involved with implicit learning and introspection14,15, showed an unusual pattern of RSFC in UHL. While the FPN and DMN is considered to be negatively correlated networks, the vmPFC, a DMN region, had increased connectivity with the right frontal region of the FPN, and coactivation between multiple FPN and DMN regions were seen in our conjunction analysis as well (Table 4).12,14 Interestingly, lesion-based studies of the vmPFC show significant lapses in moral judgment and decision making36,37, and aberrant activity of the DMN was also found in a recent study comparing rs-fcMRI in children with UHL to normal hearing controls.38
One limitation of this study was the small sample size. Despite acquiring more subjects since the pilot study in 2010, six subjects had to be excluded and 25 subjects had unusable data due to movement, attesting to the difficulty of balancing the scan time required for data acquisition with the time a child is able to hold still. Although we found no significant correlations of the academic, cognitive and executive performance of our subjects with their connectivity profiles due to limited available data, another study examing the microstructural integrity of brain white matter in the same group of children using diffusion tensor imaging MRI found a correlation between the need for IEP with the degree of organization in Heschl's gyrus.39 Further study will be needed to determine the impact of the presumed adaptive and maladaptive brain network connectivity findings on the academic and executive function performance of children with UHL. Finally, since we only examined children with severe to profound UHL, additional research is required to determine if a gradient in functional brain connectivity exists with varying severity of UHL.
In conclusion, pediatric UHL is associated with cognitive, speech and language, and behavioral deficits. Our study found distinct differences in the functional brain architecture of children with UHL compared to their NH siblings. Some changes suggest compensatory co-activation of networks whereas other changes could be a result of aberrant development. These findings portend the impact of UHL on the functional neural development in children, and suggest that hearing rehabilitation for children with UHL should be investigated as a way to mitigate academic and behavioral difficulties.
Acknowledgments
Funding Source: Supported by the American Hearing Research Foundation; the McDonnell Center for Systems Neuroscience New Resource Proposal, Washington University School of Medicine; and the St. Louis Children's Hospital Foundation/Children's Surgical Sciences Institute. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University.
Footnotes
Presented at The Triological Society 119th Annual Meeting at the Combined Otolaryngology Spring Meetings, May 21, 2016, Chicago, Illinois.
Financial Disclosures: None
Conflict of Interest: None
Level of Evidence: 3b
References
- 1.Bess FH, Tharpe AM. An introduction to unilateral sensorineural hearing loss in children. Ear Hear. 1986;7(1):3–13. doi: 10.1097/00003446-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Oyler Rf, Oyler AL, Matkin ND. Warning: a unilateral hearing loss may be detrimental to a child's academic career. Hear J. 1987;40:18–22. [Google Scholar]
- 4.English K, Church G. Unilateral hearing loss in children: an update for the 1990's. Lang Speech Hear serv Sch. 1999;30:26–31. doi: 10.1044/0161-1461.3001.26. [DOI] [PubMed] [Google Scholar]
- 5.Ruben RJ, Schwartz RG. Necessity versus sufficiency: auditory input in language acquisition. Int J Otolaryngol. 1999;47:137–140. doi: 10.1016/s0165-5876(98)00132-3. [DOI] [PubMed] [Google Scholar]
- 6.Lieu JE, Tye-Murray N, Karzon RK, Piccirillo JF. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 2010;125(6):e1348–e1355. doi: 10.1542/peds.2009-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieu JE. Unilateral hearing loss in children: speech-language and school performance. B-ENT. 2013;9(Suppl 21):107–115. [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer C, Lieu J. Unilateral hearing loss is associated with a negative effect on language scores in adolescents. Int J Pediatr Otorhinolaryngol. 2014;78:1611–1617. doi: 10.1016/j.ijporl.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieu J, Tye-Murray N, Qiang F. Longitudinal study of children with unilateral hearing loss. Laryngoscope. 2012;122:2088–2095. doi: 10.1002/lary.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ED Data express. U.S. Department of Education; Percent children with disabilities: 2013-2014. Web 14 May 2016 http://eddataexpress.ed.gov/data-element-explorer.cfm/tab/data/deid/5/ [Google Scholar]
- 11.Ead B, Hale S, DeAlwis D, Lieu J. Pilot study of cognition in children with unilateral hearing loss. Int J Pediatr Otorhinolaryngol. 2013;77:1856–1860. doi: 10.1016/j.ijporl.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Fox M, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 13.Tibbetts K, Ead B, Umansky A, Coalson R, Schlaggar BL, Firszt J, Lieu JE. Inter-regional brain interactions in children with unilateral hearing loss. Otolaryngol Head Neck Surg. 2011 Apr;144(4):602–611. doi: 10.1177/0194599810394954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason M, Norton M, Horn J, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;19:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power J, Petersen S. Control-related systems in the human brain. Curr Opi Neur. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power JD, Schlaggar BL, Petersen SE. Studying brain organization via spontaneous fMRI signal. Neuron. 2014 Nov 19;84(4):681–96. doi: 10.1016/j.neuron.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 Pt 1):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- 21.Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 22.Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex. 2008;18(9):2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power J, Cohen A, Nelson S, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston K, Holmes A, Price C, et al. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 25.Dubois C, Otzenberger H, Gounot D, Sock R, Metz-Lutz MN. Visemic processing in audiovisual discrimination of natural speech: A simultaneous fMRI-EEG study. Neuropsychologia. 2012;50:1316–1326. doi: 10.1016/j.neuropsychologia.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Jancke L, Buchanan TW, Lutz K, Shah NJ. Focused and nonfocused attention in verbal and emotional dichotic listening: an FMRI study. Brain Lang. 2001;78(3):349–363. doi: 10.1006/brln.2000.2476. [DOI] [PubMed] [Google Scholar]
- 27.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011 Feb 10;5:1. doi: 10.3389/fnsys.2011.00001. eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WOROI: 347 - Brodmann Area 41. The Human Brain Project - Denmark. [Accessed 5-5-2009] http://neuro.imm.dtu.dk/services/jerne/brede/WOROI_347.html.
- 29.Sweet L, Paskavitz J, Haley A, et al. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46(4):1114–1123. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Vossel S, Geng J, Fink G. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel A, Miezin F, Petersen S, Schlaggar B. The putative visual word form area is functionally connected to the dorsal attention network. Cerebral Cortex. 2012;22:537–549. doi: 10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai S, Chong T, Zhang Y, et al. Altered functional connectivity of fusiform gyrus in subjects with amnestic mild cognitive impairment: a resting-state fMRI study. Front Hum Neurosci. 2015;9(471):1–13. doi: 10.3389/fnhum.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravizza S, Delgado M, Chein J, et al. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 34.Buchsbaum B, Olsen R, Koch P, Berman K. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48(4):687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Sherman L, Rudie J, Pfeifer, et al. Development of the default mode and central executive networks across early adolescence: A longitudinal study. Develop Cogn Neurosc. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenigs M, Young L, Adolphs R, et al. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van den Bos W, Guroglu B. The role of the ventral medial prefrontal cortex in social decision making. J of Neur. 2009;29(24):7631–7632. doi: 10.1523/JNEUROSCI.1821-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmithorst V, Plante E, Holland S. Unilateral deafness in children affects development of multi-modal nodulation and default mode networks. Front Hum Neurosc. 2014;8(164):1–9. doi: 10.3389/fnhum.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rachakonda T, Shimony J, Coalson R, Lieu J. Diffusion tensor imaging in children with unilateral hearing loss: a pilot study. Front Syst Neurosci. 2014;8(87):1–12. doi: 10.3389/fnsys.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]


