Abstract
In the setting of ST-segment elevation myocardial infarction (STEMI) complicated by cardiogenic shock, three primary treatment objectives include providing circulatory support, ventricular unloading, and restoring myocardial perfusion. In addition to primary percutaneous coronary intervention, each of these three objectives can be achieved with appropriate use of an acute mechanical circulatory support (AMCS) pump. Over the past decade, utilization of percutaneously-delivered AMCS devices including the Impella axial-flow catheter, TandemHeart left atrial-to-femoral artery bypass system, and veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has grown exponentially. In this review, we will discuss the hemodynamic impact of each AMCS device and clinical data surrounding their use in the setting of STEMI complicated by cardiogenic shock.
Keywords: Mechanical circulatory support, Acute myocardial infarction, Hemodynamics
1. Introduction
A well-established goal for therapeutic success in the setting of ST-segment elevation myocardial infarction (STEMI) is a Door to Balloon (DTB) time less than 90 min, which is defined as the time interval from the electrocardiogram obtained at first medical contact demonstrating ST-segment elevations to interventional reperfusion of a thrombotically occluded coronary artery. However, recent studies suggest that DTB times less than 90 min are not associated with improved in-hospital mortality, especially for patients with anterior myocardial infarction and cardiogenic shock.1, 2 Furthermore, despite timely reperfusion, nearly 10% of acute myocardial infarction (AMI) subjects die during their index hospitalization and 76% of patients who survive progress to develop chronic heart failure within the next 5 years.3, 4, 5, 6 These findings suggest that new approaches are needed to reduce the burden of myocardial injury in AMI.
Described best by Braunwald and Kloner in 1985, myocardial reperfusion is a “double edged sword” due to the fact that reperfusion of ischemic myocardium promotes cardiomyocyte death and microvascular damage through a process referred to as myocardial ischemia-reperfusion injury (IRI).1 As an estimate of how much residual damage exists after successful reperfusion therapy in an anterior STEMI, the Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP-AMI) trial demonstrated that nearly 40% of the left ventricle is injured as quantified by cardiac magnetic resonance imaging within 1 week of successful reperfusion therapy.3 The actual number of these patients that go on to develop systolic heart failure remains poorly defined.
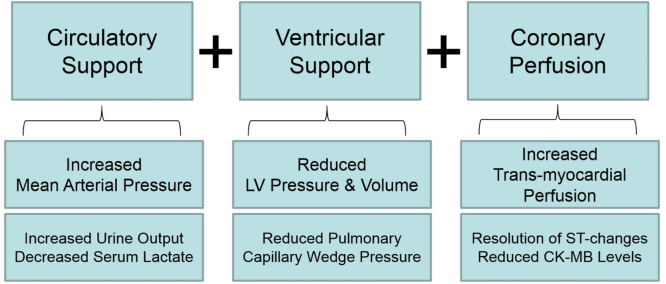
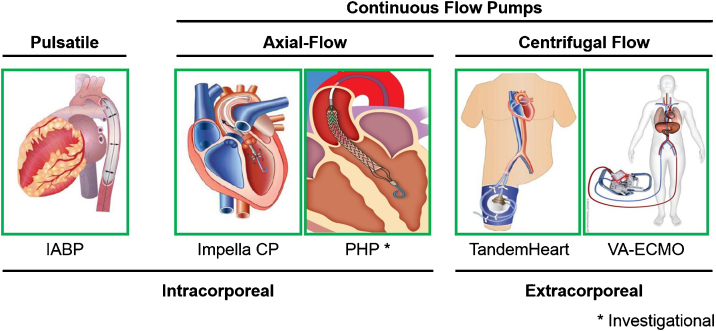
In the setting of STEMI and cardiogenic shock, there exist three primary treatment objectives. First, circulatory support must be achieved in order to preserve adequate mean arterial pressure and systemic organ perfusion. Second, ventricular unloading, which refers to a reduction in both left ventricular pressure and volume, is required to reduce myocardial oxygen demand given the limited myocardial oxygen supply during a STEMI. Third, augmenting myocardial perfusion can be achieved by re-opening an occluded coronary artery, sustaining coronary arterial pressure, and reducing left ventricular diastolic pressure. The net effect is a positive shift in the myocardial supply:demand ratio that further reduces the burden of myocardial ischemia and injury. In addition to balloon angioplasty, each of the three components of the hemodynamic support equation (Fig. 1) can be achieved with appropriate use of percutaneously inserted acute mechanical circulatory support (AMCS) pumps.6 These pumps can be utilized for multiple bridging strategies, including as a bridge-to-recovery, a bridge-to-durable left ventricular assist device, or as a bridge-to-decision in order to allow for further improvement in ventricular function prior to making an assignment. A contemporary analysis of the Nationwide Inpatient Sample from the Healthcare Cost and Utilization Project reported stable rates of intra-aortic balloon pump (IABP) implantation in the United States and increasing utilization of AMCS devices including the Impella axial-flow catheter, TandemHeart left atrial-to-femoral artery bypass system, and veno-arterial extracorporeal membrane oxygenation (VA-ECMO)7 (Fig. 2). In this review, we will discuss the hemodynamic impact of each AMCS device and clinical data surrounding their use in the setting of STEMI complicated by cardiogenic shock.
Fig. 1.
Solving the hemodynamic support equation in cardiogenic shock.73 Illustration of the three primary clinical objectives in the setting of acute myocardial infarction complicated by cardiogenic shock. Circulatory support is defined by an increase in mean arterial pressure. Ventricular support is defined by a reduction in left ventricular (LV) pressure and volume, thereby reducing myocardial wall stress and oxygen demand. Coronary perfusion is defined by an increase in the trans-myocardial gradient, which is determined by the difference between coronary arterial and LV end-diastolic pressure. The net effect of optimal hemodynamic support is increased urine output, reduced serum lactate, reduced pulmonary capillary wedge pressure, resolution of ischemic electrocardiographic changes, and reduced levels of myocardial injury biomarkers such as CK-MB.
Fig. 2.
Acute mechanical circulatory support devices for the left ventricle.73gr2
2. Intra-aortic balloon counterpulsation
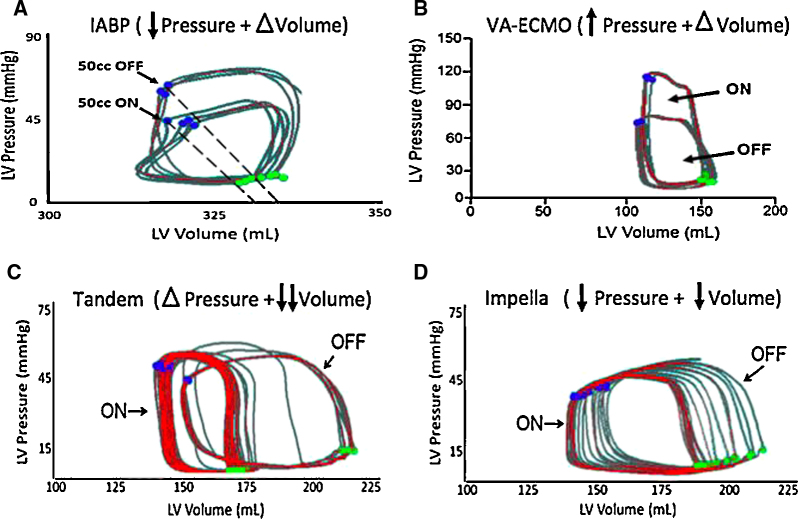
The IABP percutaneous support device is a catheter-mounted balloon that inflates during diastole, thereby displacing blood in the descending aorta and increasing mean aortic pressure, which in turn may augment coronary perfusion. The IABP deflates during systole and creates a pressure sink, into which blood is ejected from the left ventricle. As a result, the net hemodynamic effect of an IABP includes a reduction in ventricular afterload, increased mean arterial pressure, and an augmented left ventricular stroke volume (Fig. 3A). Multiple studies have identified that the hemodynamics of balloon counterpulsation are predominantly regulated by four factors: 1) the scale of diastolic pressure augmentation; 2) the scale of reduction in systolic pressure; 3) the degree of blood volume displacement; and4 the timing of balloon inflation and deflation. There is also evidence that IABPs improve microcirculatory support within the myocardium, especially during periods of active ischemia when coronary autoregulation is uncoupled, which may account for its beneficial effects in patients with active angina.8, 9, 10, 11, 12 More recently, larger capacity IABPs, known as the Mega Series (Maquet Inc), have been introduced into clinical practice. Advantages of IABPs include: ease of insertion, global familiarity with the technology, and relative cost.13, 14, 15, 16, 17, 18, 19, 20
Fig. 3.
Hemodynamic effects of acute mechanical circulatory support devices on the left ventricle. Pressure–volume loops for each device. (A) IABP reduces LV afterload but does not unload the ventricle. (B) VA-ECMO increases the wall stress and afterload of the LV and does not unload without an LV vent.74 (C) The LA-FA bypass, or TandemHeart device, unloads the left atrium, thereby decreasing LV end-diastolic volumes but does not decrease end-diastolic pressure.75 (D) The Impella device unloads the LV by decreasing end-diastolic volume and pressure.75gr3
Registry data have historically supported the use of IABPs.21, 22, 23, 24 In the thrombolysis era, the Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) trials assessed the use of IABP in patients after thrombolysis for STEMI. Out of 810 patients, 85 received an IABP and were found to have less re-occlusion of culprit vessel (7% vs 13%) at one week follow up. In this non-randomized study, there was, however, higher mortality among IABP recipients, which may reflect a selection bias toward sicker patients receiving IABP therapy. Despite this initial signal of potential benefit for IABP therapy in the setting of thrombolysis, data from primary percutaneous coronary intervention (PCI) cohorts has been less clear. Van’t Hof and colleagues randomized 238 patients with high risk MI post PCI to receive either IABP or usual care and found no difference in mortality, non-fatal MI, stroke and ejection fraction. Similar findings were observed in the Primary Angioplasty in Myocardial Infarction-II (PAMI-II) trial, which is an international multicenter randomized trial in which patients presenting with STEMI were randomized to primary PCI and IABP versus IABP alone. No differences in death, re-infarction, infarct related artery re-occlusion, stroke, new heart failure or ventricular arrhythmias were observed between groups.25, 26
Observational and randomized data also do not support routine use of IABP therapy among patients with AMI complicated by cardiogenic shock. Both the Should we emergently revascularize Occluded Coronary arteries for cardiogenic shock (SHOCK) trial as well as the National Registry of Myocardial Infarction-2 (NRMI-2) trial showed no clear benefit for IABP use in the setting of AMI and shock. Consistent with prior data, NRMI-2 showed lower in-hospital mortality among patients who received thrombolysis for STEMI and had an IABP placed compared to those receiving thrombolysis alone. In the NRMI-2 trial however, patients receiving PCI had no mortality benefit from IABP.27, 28 More recently, the IABP-SHOCK II study29 was a large randomized trial of 600 patients that effectively showed that not all patients presenting with an acute coronary syndrome (ACS) and clinical evidence of hypoperfusion should receive an IABP. In this study, trends toward benefit with IABP use were observed in younger patients with anterior myocardial infarction, no hypertension, and no prior infarction. The importance of this trial is that it confirms what most catheterization laboratories already practice, namely, to avoid non-discretionary use of an IABP in ACS.
In summary, existing clinical data does not support routine IABP use in the setting of STEMI. Among STEMI patients with cardiogenic shock, recent data has not demonstrated clear benefit of IABP therapy. Whether future clinical trials employing large capacity 50 cc IABPs demonstrate better clinical outcomes than the 40 cc IABP remains unknown.
3. Impella in STEMI
The Impella pumps are catheter-mounted axial-flow devices that are deployed into the left ventricle across the aortic valve. The pump transfers kinetic energy from a circulating impeller to the blood in the left ventricle, resulting in continuous flow across the aortic valve (Fig. 2). The Impella 2.5 LP and CP devices, as well as the right-sided Impella RP, can be deployed percutaneously, while the Impella 5.0 device warrants surgical vascular access. In contrast to IABP therapy, the Impella pump reduces both native LV pressure and volume, thereby leading to a greater reduction in LV stroke work and myocardial oxygen demand (Fig. 3D).30, 31 While Impella pumps are primarily used to provide univentricular support to either the left or right ventricle, several cases have been reported using the pump for biventricular support, including configurations for the Impella 5.0/TandemHeart RVAD, Impella 5.0/Impella RP, and the Impella CP/Impella RP.32, 33, 34 More recently, preclinical data for another axial-flow catheter design, the HeartMate Percutaneous Heart Pump (PHP; St. Jude Inc) was reported.35 The PHP is approved for use in Europe after completion of the SHIELD I study and is currently undergoing clinical evaluation in the United States as part of the SHIELD II trial (Fig. 2C).
No studies have specifically examined the clinical utility of Impella unloading in the setting of STEMI. In 2008, the ISAR-SHOCK study (Impella LP 2.5 versus IABP in Cardiogenic SHOCK) randomly assigned 25 patients with AMI and cardiogenic shock to the Impella 2.5 LP device or an IABP. Patients receiving the Impella had a greater rise in cardiac index after 30 min of support 0.49 ± 0.46 L/min per m2 vs −.11 ± 0.31 L/min per m2. However, there was no difference in mortality, bleeding or distal limb ischemia between the two groups.36 Since then the EUROSHOCK registry evaluated the safety and efficacy of the Impella 2.5 LP in 120 patients from multiple centers.37, 38 This patient population had severe shock and had Impella placed as a last resort after failure of conventional therapy. Cardiopulmonary resuscitation prior to Impella implantation occurred in 41% of patients. The investigators found that within 24 h of support, there was a significant decrease in plasma lactate levels suggesting improved organ perfusion. The mortality rate in this population was 64% at 30 days, reflecting a sick patient population with poor baseline hemodynamics and high risk of impending death. The recently published IMPella versus IABP Reduces mortality in STEMI patients treated with primary PCI in SEVERE and deep cardiogenic SHOCK (IMPRESS) trial studied outcomes in patients with late-stage cardiogenic shock and identified no difference in outcome between the IABP and Impella devices. Mortality in this trial was above 50% in both arms and reflects the end-stage nature of cardiogenic shock among patients enrolled.39
4. The TandemHeart device in STEMI
The TandemHeart device is an extra-corporeal centrifugal flow pump that bypasses oxygenated blood from the left atrium (LA) to the descending aorta via two cannulas: a 21Fr trans-septal inflow cannula in the LA and an arterial outflow cannula in the femoral artery (FA) (Fig. 2). The TandemHeart delivers flows of 3.0 to 5.0 liters/minute contingent on the size of the arterial outflow cannula, which range between 15Fr to 19Fr in clinical practice.40 Previous studies have shown that the position of the arterial outflow cannula effects the magnitude of LV unloading. In particular, LA-to-ascending aortic bypass increases LVSW, whereas LA-to-descending aortic bypass decreases LVSW. By delivering blood from the LA to the arterial system, the TandemHeart pressurizes the aorta (Fig. 3C).41 In the ascending aorta, the rise in afterload restricts the degree of LV unloading.42 In the descending aorta, the increased afterload is alleviated by retrograde perfusion of the mesenteric and renal arteries, as well as the great vessels of the aortic arch, which decreases LVSW.43 Additionally, we previously reported that in a bovine model of AMI, activating the TandemHeart at 5500 RPM with a 17Fr arterial cannula produces 3.1 LPM of flow and decreases LVSW by 38%, while maximum speeds at 7500 RPM produce 4.4 LPM of flow and decrease LVSW by 67%.44 In comparison to the Impella CP, the main effect of the TandemHeart was a decrease in native LV stroke volume and thereby a reduction in LVSW.
The clinical effects of the TandemHeart have been studied in 18 patients with cardiogenic shock after myocardial infarction. Thiele and colleagues found that the device provided adequate support that resulted in improved CVP, MAP, cardiac index and pulmonary capillary wedge pressure.45 Gregoric and colleagues also demonstrated an overall clinical improvement and improvement in end-organ function in patients with refractory shock after the use of the TandemHeart.46 In 2006, the TandemHeart Investigators Group compared 42 patients presenting within 24 h of developing cardiogenic shock who were randomized to an IABP or TandemHeart device. AMI was the primary cause of shock in 70% of these patients. The TandemHeart generated a higher cardiac index, mean arterial pressure, and lower pulmonary capillary wedge pressure compared to IABP therapy. Despite this hemodynamic improvement, no difference in mortality at 30 days was observed in this small study.47
Among 117 patients with cardiogenic shock refractory to IABP and/or high dose vasopressors, Kar et al. also reported improved hemodynamic parameters. Systolic blood pressure increased from 75 to 100 mmHg, cardiac index increased from 0.52 to 3 l/min/m2, pulmonary capillary wedge pressure (PCWP) decreased from 31 to 17 mmHg and mixed venous oxygen saturation improved from 49 to 69.3%. Clinical parameters also improved: urine output increased from 70 to 1200 ml/day, lactic acid level from 11 to 1.5 mg/dl, and creatinine from 1.5 to 1.2 mg/dl. The mortality rates in this patient population were 40.2% and 45.3% at 30 days and 6 months respectively.40
5. Veno-arterial extracorporeal membrane oxygenation
VA-ECMO removes deoxygenated venous blood, circulates it through an oxygenator and extra-corporeal centrifugal flow pump, and returns oxygenated blood to the arterial circulation. The inflow cannula is often placed in the right atrium or across the superior and inferior vena cava (Fig. 2). The outflow cannula can be placed in the femoral or subclavian arteries. Since no venous reservoir is employed, VA-ECMO cannot be described as cardio-pulmonary bypass. The primary effect of VA-ECMO is to displace blood volume from the venous to the arterial circulation. As a result, reduced right and left ventricular volumes can be observed with a concomitant increase in mean arterial pressure. Depending on native LV function and the presence of aortic valve disease, VA-ECMO may be associated with increased LV pressures (Fig. 3B).48 The increase in LV pressures frequently leads to the use of a concomitant strategy to ‘vent’ the LV or reduce LV pressures. Common venting strategies include: inotropes or concomitant placement of either an IABP, LV Impella, or LA cannula.
The utility of VA-ECMO in AMI remains unknown. Several limitations preclude the use of VA-ECMO in AMI including: 1) the possibility for LV distention and increased LV stroke work, 2) a potentially higher risk for bleeding complications due to the need for large bore cannulas in the setting of aggressive antithrombotic and antiplatelet therapy, and 3) the risk of other complications including vascular injury, limb ischemia and insufficient upper body oxygenation in cases of relatively preserved LV systolic function. A recent, single-center experience reported a 67% survival to discharge rate among 18 patients with acute coronary syndromes complicated by cardiogenic shock. Bleeding complications were observed in 94% (17 out of 18) patients in this study.49
6. Right ventricular myocardial infarction
Several studies have examined the clinical importance of right ventricular (RV) failure in the setting of an AMI. RV dysfunction as defined by echocardiography can be identified in up to 50% of patients presenting with an acute IWMI.50 Of these patients, 15–25% will exhibit hemodynamic instability suggestive of RV involvement, yet histologic infarction of the RV free wall occurs in only 3–5% of patients with an acute IWMI. We recently identified the pulmonary artery pulsatility index (PAPi) as a hemodynamic indicator of right ventricular failure in patients presenting with an inferior MI51 as well as in patients post-LVAD.52 In a sub-study of the SHOCK trial, RV-dominant cardiogenic shock was associated with similar in-hospital mortality rates as LV-dominant cardiogenic shock (53.1% vs 60.8%, p = 0.3) despite a younger age, lower rate of anterior MI, and higher likelihood of single-vessel disease among RV-dominant shock patients.50 Furthermore, a meta-analysis of several studies, showed significantly higher in-hospital mortality and higher incidence of shock, ventricular arrhythmias, and advanced atrio-ventricular block if AMI involved the RV.53
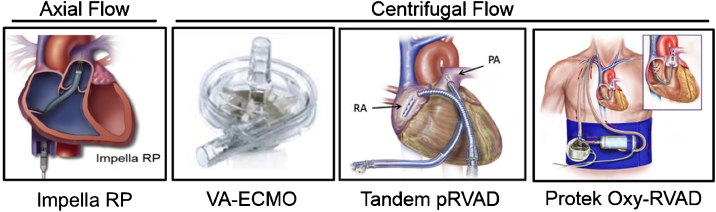
Current management of RV failure includes addressing the primary cause, which may necessitate volume resuscitation, inotropic therapy, or pulmonary vasodilation to maintain RV preload, promote RV contractility, and decrease RV afterload, respectively. In RV failure that is refractory to medical management, treatment options include surgical RV assist devices (RVAD), extracorporeal membrane oxygenation (ECMO), atrial septostomy, and cardiac transplantation. Percutaneously delivered circulatory support for RV failure is an emerging field with several device options available including the IABP, the TandemHeart percutaneous RVAD, the TandemHeart Protek Duo cannula, the axial-flow Impella RP catheter, and VA-ECMO (Fig. 4).
Fig. 4.
Acute mechanical circulatory support devices for the right ventricle.
At present, minimal data exploring the clinical utility of percutaneous RV support devices in RVMI exist. Several studies have shown the potential benefits of centrifugal flow pumps in RV failure using surgical and hybridized surgical-percutaneous deployment with the Centrimag (Thoratec Inc),54 Rotaflow (Maquet Inc),55 and TandemHeart pumps.56, 57 Most recently the Recover Right trial evaluated the Impella RP device in the setting of RVMI or post-cardiotomy RV failure.58 As experience with percutaneous RV support devices grows, their role in the armamentarium of the mechanical therapies for RMVI will depend less on the technical ability to place the device, but rather on improved algorithms for patient selection, patient and device monitoring, and weaning protocols.
7. Time for a paradigm shift: from primary reperfusion to primary unloading in STEMI
Current approaches to reduce myocardial injury without foregoing the benefit of reperfusion therapy in STEMI are limited.15 tone of the most promising approaches for cardioprotection in AMI is ischemic conditioning, which involves creating brief, intermittent periods of intentional coronary occlusion either before (preconditioning) or after (postconditioning) the initiation of a total coronary occlusion. Remote ischemic conditioning (RIC) involves the creation of intermittent periods of limb ischemia using a tourniquet during AMI. Theoretically, RIC leads to release of circulating cardioprotective chemokines such as stromal derived factor 1a (SDF1a) that reduces infarct size. RIC is actively under investigation.59, 60 Other modalities include pharmacologic treatments that are targeted to proteins involved in myocardial ischemia-reperfusion injury, such as cyclosporine.61, 62 Lastly, other methods, such as systemic hypothermia, have also been tested without any definitive evidence of benefit.
Critical barriers to contemporary cardioprotective strategies are2 the complex nature of reperfusion injury, which limits the impact of a single-target pharmacologic strategy5; the possibility of coronary dissection or perforation during ischemic conditioning; and6 the requirement for rapid coronary reperfusion within the 90 min DTB time and limited time for any cardioprotective strategy to beneficially impact myocardial injury zones. There is a clear need for improved approaches to restrict reperfusion injury that have a multifactorial benefit on the reperfusion injury cascade while also allowing time for drug penetration and effect.
Historically, surgical implementation of cardio-pulmonary bypass has been an effective method to reduce myocardial oxygen demand and has been associated with improved clinical outcomes and reduced infarct size in AMI complicated by cardiogenic shock.63, 64 Based on these early observations, activation of an intra-aortic balloon pump or catheter-mounted axial flow pump prior to coronary occlusion were found to reduce infarct size in preclinical models of AMI.65, 66, 67 We and others have reported that reducing LV wall stress using the TandemHeart left atrial-to-femoral artery bypass pump and delaying coronary reperfusion by 30 min significantly decreased infarct size in a preclinical model of AMI.68, 69 This preclinical study led to the TandemHeart to Reduce Infarct Size (TRIS) trial, which is currently underway in the United States.70 We recently reported that mechanically conditioning the myocardium using the Impella CP pump while delaying coronary reperfusion by 60 min reduces LV wall stress and activates a myocardial protection program that upregulates expression of the cytokine SDF-1a, increases cardioprotective signaling, reduces apoptosis, and limits myocardial damage in AMI.71
In contrast to multiple reports over the past 2 decades suggesting the potential benefit of mechanical unloading of the heart in acute MI, the novel aspects of this report included the concept that reducing left ventricular wall stress and delaying reperfusion led to small infarct sizes despite a higher ischemic burden, and the use of an AMCS device as a method to reduce left ventricular wall stress.2, 5, 17, 18 The clinical utility of primarily left ventricular unloading as opposed to primary reperfusion in STEMI with a left atrial-to-femoral artery bypass pump will be tested in the TRIS trial.
The concept of first unloading the heart with a circulatory support device and then providing reperfusion when it is safe to do so should not be so foreign to us. First, we know that myocardial perfusion is driven by a balance of several factors, including coronary perfusion pressure versus ventricular filling pressure and myocardial oxygen supply versus demand. The net effect of acute circulatory support may affect these factors in favor of optimal myocardial perfusion. Second, we can learn from our surgical colleagues, who often approach STEMI and shock management by first initiating cardiopulmonary bypass to unload both the right and left ventricles, followed by a period of time to harvest bypass conduits (during which time the culprit artery remains occluded), and ultimately reperfusion. Third, we know from a recent analysis of the USPELLA registry that implantation of an Impella device before PCI in STEMI and shock may improve survival.20 Based on this preclinical and clinical data, the Door to Unloading Trial was recently initiated in the United States to study the concept of first unloading the LV before reperfusion in STEMI.72
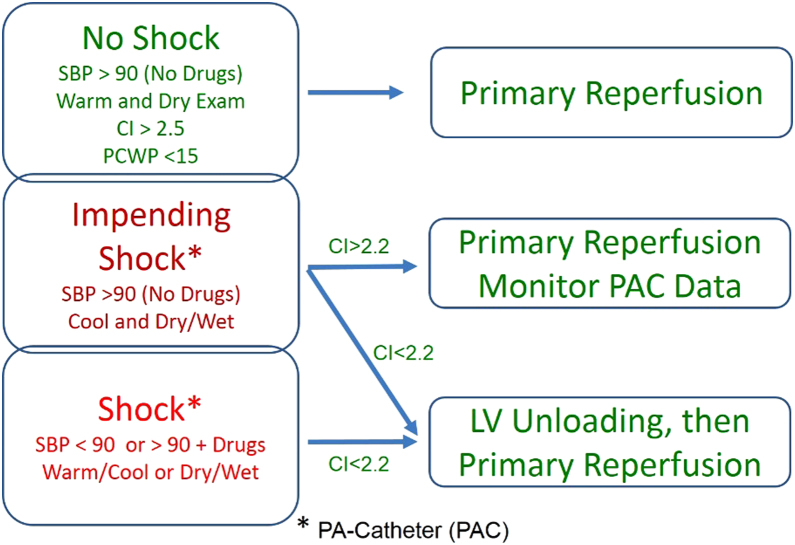
Over the next few years, the increase in utilization of acute mechanical circulatory support devices will hopefully translate into improved knowledge of advanced ventricular hemodynamics, coronary physiology, and the optimal management of cardiogenic shock in the setting of STEMI (Fig. 5). It remains to be decided whether a door-to-unload strategy will provide superior benefit in these patients compared to a DTB strategy, and further advances in this exciting field need to be achieved in order to bring this concept to clinical fruition.
Fig. 5.
Proposed algorithm for the use of acute mechanical circulatory support devices in STEMI complicated by cardiogenic shock.73gr5
Disclosure
Dr. Kapur: Research support from Heartware Inc, Abiomed Inc, Maquet Inc, and CardiacAssist Inc. Speaker Honoraria/Consultant: Abiomed Inc, CardiacAssist Inc, Thoratec Inc, Heartware Inc, and Maquet Inc. Dr. Esposito, Dr. Bader: None. Pedicini, Breton, Mullin: None.
Conflicts of interest
The authors have none to declare.
References
- 1.Braunwald E., Kloner R. Myocardial reperfusion: a double-edged sword. J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menees D.S., Peterson E.D., Wang Y. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. doi: 10.1056/NEJMoa1208200. [DOI] [PubMed] [Google Scholar]
- 3.Patel M., Smalling R., Thiele H. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock the CRISP AMI randomized trial. JAMA. 2011;306:1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 4.Rihal C., Naidu S., Givertz M. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care. J Am Coll Cardiol. 2015;65:e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz J.A., Kaul P., Bakal J.A. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Ezekowitz J.A., Armstrong P.W., Granger C.B. Predicting chronic left ventricular dysfunction 90 days after ST-segment elevation myocardial infarction: an assessment of pexelizumab in acute myocardial infarction (APEX-AMI) substudy. Am Heart J. 2010;160:272–278. doi: 10.1016/j.ahj.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Stretch R., Sauer C., Bonde P. National trends in the utilization of short-term mechanical circulatory support incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 8.Jung C., Lauten A., Roediger C. In vivo evaluation of tissue microflow under combined therapy with extracorporeal life support and intra-aortic balloon counterpulsation. Anaesth Intensive Care. 2009;37(5):833–835. doi: 10.1177/0310057X0903700517. [DOI] [PubMed] [Google Scholar]
- 9.Lauten A., Ferrari M., Goebel B. Microvascular tissue perfusion is impaired in acutely decompensated heart failure and improves following standard treatment. Eur J Heart Fail. 2011 Jul;13(7):711–717. doi: 10.1093/eurjhf/hfr043. [DOI] [PubMed] [Google Scholar]
- 10.Jung C., Lauten A., Ferrari M. Microcirculation in cardiogenic shock: from scientific bystander to therapy target. Crit Care. 2010;14(5):193. doi: 10.1186/cc9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Nunen L.X., Noc M., Kapur N.K., Patel M.R., Perera D., Pijls N.H. Usefulness of intra-aortic balloon pump counterpulsation. Am J Cardiol. 2016;117(3):469–476. doi: 10.1016/j.amjcard.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 12.De Silva K., Lumley M., Kailey B. Coronary and microvascular physiology during intra-aortic balloon counterpulsation. JACC Cardiovasc Interv. 2014;7(6):631–640. doi: 10.1016/j.jcin.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Kern M.J., Aguirre F., Bach R. Augmentation of coronary blood flow by intra-aortic balloon pumping in patients after coronary angioplasty. Circulation. 1993;87:500–511. doi: 10.1161/01.cir.87.2.500. [DOI] [PubMed] [Google Scholar]
- 14.Kern M.J., Aguirre F.V., Tatineni S. Enhanced coronary blood flow velocity during intraaortic balloon counterpulsation in critically ill patients. J Am Coll Cardiol. 1993;21:359–368. doi: 10.1016/0735-1097(93)90676-r. [DOI] [PubMed] [Google Scholar]
- 15.Schreuder J.J., Maisano F., Donelli A. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005;79:872–880. doi: 10.1016/j.athoracsur.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Schreuder J.J., Castiglioni A., Donelli A. Automatic intraaortic balloon pump timing using an intrabeat dicrotic notch prediction algorithm. Ann Thorac Surg. 2005;79:1017–1022. doi: 10.1016/j.athoracsur.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 17.Sarnoff S.J., Braunwald E., Welch G.H. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:148–156. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 18.Braunwald E., Sarnoff S.J., Case R.B. Hemodynamic determinants of coronary flow: effect of changes in aortic pressure and cardiac output on the relationship between myocardial oxygen consumption and coronary flow. Am J Physiol. 1958;192(1):157–163. doi: 10.1152/ajplegacy.1957.192.1.157. [DOI] [PubMed] [Google Scholar]
- 19.Weber K.T., Janicki J.S. Coronary collateral flow and intraaortic balloon counterpulsation. Trans Am Soc Artif Intern Organs. 1973;19:395–401. [PubMed] [Google Scholar]
- 20.Weber K.T., Janicki J.S. Intraaortic balloon counterpulsation. A review of physiological principles, clinical results, and device safety. Ann Thorac Surg. 1974;17:602–636. doi: 10.1016/s0003-4975(10)65706-2. [DOI] [PubMed] [Google Scholar]
- 21.Stone G.W., Ohman E.M., Miller M.F. Contemporary utilization and outcomes of intraaortic balloon counterpulsation in acute myocardial infarction: the benchmark registry. J Am Coll Cardiol. 2003;41:1940–1945. doi: 10.1016/s0735-1097(03)00400-5. [DOI] [PubMed] [Google Scholar]
- 22.Cohen M., Urban P., Christenson J.T. Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark Registry. Eur Heart J. 2003;24:1763–1770. doi: 10.1016/j.ehj.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Wahab M., Saad M., Kynast J. Comparison of hospital mortality with intraaortic balloon counterpulsation insertion before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2010;105:967–971. doi: 10.1016/j.amjcard.2009.11.021. [Epub 2010 Feb 13] [DOI] [PubMed] [Google Scholar]
- 24.Curtis J.P., Rathore S.S., Wang Y. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high-risk percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes. 2012;5:21–30. doi: 10.1161/CIRCOUTCOMES.110.960385. [Epub 2011 Dec 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van’t Hof A., Liem A., de Boer M. A randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarction. Eur Heart J. 1999;20:659–665. doi: 10.1053/euhj.1998.1348. [DOI] [PubMed] [Google Scholar]
- 26.Stone G., Marsalese D., Brodie B. A Prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. J Am Coll Cardiol. 1997;29:1459–1467. doi: 10.1016/s0735-1097(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 27.Hochman J., Sleeper L., Webb J. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 28.Barron H.V., Every N.R., Parsons L.S. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001;141:933–939. doi: 10.1067/mhj.2001.115295. [DOI] [PubMed] [Google Scholar]
- 29.Thiele H., Zeymer U., Neumann F. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 30.Henriques J.P., Remmelink M., Baan J., Jr. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover Lp 2.5. Am J Cardiol. 2006;97:990–992. doi: 10.1016/j.amjcard.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Sjauw K.D., Remmelink M., Baan J., Jr. Left ventricular unloading in acute ST- segment elevation myocardial infarction patients is safe and feasible and provides acute and sustained left ventricular recovery. J Am Coll Cardiol. 2008;51:1044–1046. doi: 10.1016/j.jacc.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Nagy C.D., Jumean M.F., Pham D.T. Percutaneous circulatory support for biventricular failure. Circ Cardiovasc Interv. 2013;6:e12–e14. doi: 10.1161/CIRCINTERVENTIONS.112.000018. [DOI] [PubMed] [Google Scholar]
- 33.Aghili N., Bader Y., Vest A.R. Biventricular circulatory support using 2 axial flow catheters for cardiogenic shock without the need for surgical vascular access. Circ Cardiovasc Interv. 2016:9. doi: 10.1161/CIRCINTERVENTIONS.116.003636. pii: e003636. [DOI] [PubMed] [Google Scholar]
- 34.Kapur N.K., Jumean M., Ghuloom A. First successful use of 2 axial flow catheters for percutaneous biventricular circulatory support as a bridge to a durable left ventricular assist device. Circ Heart Fail. 2015;8:1006–1008. doi: 10.1161/CIRCHEARTFAILURE.115.002374. [DOI] [PubMed] [Google Scholar]
- 35.J. Granada , Thoractec Percutanous Heart Pump TCT Conference 2012; www.tctconference.com.
- 36.Seyfarth M., Sibbing D., Bauer I. A Randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 37.Lauten A., Engstrom A., Jung C. Percutaneous left-ventricular support with the Impella-2.5 – assist device in acute cardiogenic shock results of the Impella–EUROSHOCK-Registry. Circ Heart Fail. 2013;6:23–30. doi: 10.1161/CIRCHEARTFAILURE.112.967224. [DOI] [PubMed] [Google Scholar]
- 38.Acharya D., Loyaga-Rendon R.Y., Tallaj J.A. Circulatory support for shock complicating myocardial infarction. J Invasive Cardiol. 2014;26:E109–E114. [PubMed] [Google Scholar]
- 39.Ouweneel D.M., Eriksen E., Sjauw K.D. Impella CP versus intra-aortic balloon pump in acute myocardial infarction complicated by cardiogenic shock: The IMPRESS trial. J Am Coll Cardiol. 2016 pii: S0735-1097(16)36767-5. [Google Scholar]
- 40.Kar B., Gregoric I.D., Basra S.S. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011;57:688–696. doi: 10.1016/j.jacc.2010.08.613. [DOI] [PubMed] [Google Scholar]
- 41.Burkhoff D., Naidu S.S. The science behind percutaneous hemodynamic support: a review and comparison of support strategies. Catheter Cardiovasc Interv. 2012;80:816–829. doi: 10.1002/ccd.24421. [DOI] [PubMed] [Google Scholar]
- 42.Kono S., Nishimura K., Nishina T. Autosynchronized systolic unloading during left ventricular assist with a centrifugal pump. J Thorac Cardiovasc Surg. 2003;125:353–360. doi: 10.1067/mtc.2003.100. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein A.H., Pacella J.J., Clark R.E. Predictable reduction in left ventricular stroke work and oxygen utilization with an implantable centrifugal pump. Ann Thorac Surg. 1994;58:1018–1024. doi: 10.1016/0003-4975(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 44.Kapur N., Paruchuri V., Pham D. Hemodynamic effects of left atrial or left ventricular cannulation for acute circulatory support in a bovine model of left heart injury. ASAIO J. 2015;61:301–306. doi: 10.1097/MAT.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 45.Thiele H., Lauer B., Hambrecht R. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104:2917–2922. doi: 10.1161/hc4901.100361. [DOI] [PubMed] [Google Scholar]
- 46.Bruckner B.A., Jacob L.P., Gregoric I.D. Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Tex Heart Inst J. 2008;35:447–450. [PMC free article] [PubMed] [Google Scholar]
- 47.Burkhoff D., Cohen H., Brunckhorst C. TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(469):e1–e8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 48.Aghili N., Kang S., Kapur N.K. The fundamentals of extra-corporeal membrane oxygenation. Minerva Cardioangiol. 2015;63:75–85. [PubMed] [Google Scholar]
- 49.Esper S.A., Bermudez C., Dueweke E.J. Extracorporeal membrane oxygenation support in acute coronary syndromes complicated by cardiogenic shock. Cathet Cardiovasc Intervent. 2015;86(Suppl 1):S45–S50. doi: 10.1002/ccd.25871. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs A.K., Leopold J.A., Bates E. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol. 2003;41:1273–1279. doi: 10.1016/s0735-1097(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 51.Korabathina R., Heffernan K.S., Paruchuri V. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593–600. doi: 10.1002/ccd.23309. [DOI] [PubMed] [Google Scholar]
- 52.Morine K.J., Kiernan M.S., Pham D.T. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22:110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Mehta S.R., Elkelboom J.W., Natarajan M.K. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37:37–43. doi: 10.1016/s0735-1097(00)01089-5. [DOI] [PubMed] [Google Scholar]
- 54.Takayama H., Naka Y., Kodali S.K. A novel approach to percutaneous right-ventricular mechanical support. Eur J Cardiothorac Surg. 2012;41:423–426. doi: 10.1016/j.ejcts.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 55.Loor G., Khani-Hanjani A., Gonzalez-Stawinski G.V. Use of RotaFlow (MAQUET) for temporary right ventricular support during implantation of HeartMate II left ventricular assist device. ASAIO J. 2012;58:275–277. doi: 10.1097/MAT.0b013e318247088c. [DOI] [PubMed] [Google Scholar]
- 56.Kapur N.K., Paruchuri V., Korabathina R. Effects of a percutaneous mechanical circulatory support device for medically refractory right ventricular failure. J Heart Lung Transplant. 2011;30:1360–1367. doi: 10.1016/j.healun.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Kapur N.K., Paruchuri V., Jagannathan A. Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013;1:127–134. doi: 10.1016/j.jchf.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Anderson M.B., Goldstein J., Milano C. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015;34:1549–1560. doi: 10.1016/j.healun.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Hausenloy D.J., Kharbanda R., Rahbek Schmidt M. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2015;36(29):1846–1848. [PubMed] [Google Scholar]
- 60.Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13(4):193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 61.Cung T.T., Morel O., Cayla G. Cyclosporine before PCI in patient with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 62.Ottani F., Latini R., Staszewsky L. CYCLE Investigators. Cyclosporine A in reperfused myocardial infarction: the multicenter, controlled, open-label CYCLE Trial. J Am Coll Cardiol. 2016;67:365–374. doi: 10.1016/j.jacc.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 63.Kanter K.R., Schaff H.V., Gott V.L. Reduced oxygen consumption with effective left ventricular venting during postischemic reperfusion. Circulation. 1982;66:I50–I154. [PubMed] [Google Scholar]
- 64.Allen B.S., Buckberg G.D., Fontan F.M. Superiority of controlled surgical reperfusion versus percutaneous transluminal coronary angioplasty in acute coronary occlusion. J Thorac Cardiovasc Surg. 1993;105:864–879. [PubMed] [Google Scholar]
- 65.Smalling R.W., Cassidy D.B., Barrett R. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial-flow, transvalvular left ventricular assist device. A comparison with intra-aortic balloon counterpulsation and reperfusion alone in a canine infarction model. Circulation. 1992;85:1152–1159. doi: 10.1161/01.cir.85.3.1152. [DOI] [PubMed] [Google Scholar]
- 66.Achour H., Boccalandro F., Felli P. Mechanical left ventricular unloading prior to reperfusion reduces infarct size in a canine infarction model. Catheter Cardiovasc Interv. 2005;64:182–192. doi: 10.1002/ccd.20271. [DOI] [PubMed] [Google Scholar]
- 67.Meyns B., Stolinski J., Leunens V. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol. 2003;41:1087–1095. doi: 10.1016/s0735-1097(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 68.Kapur N.K., Paruchuri V., Urbano-Morales J.A. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation. 2013;128:328–336. doi: 10.1161/CIRCULATIONAHA.112.000029. [DOI] [PubMed] [Google Scholar]
- 69.Catinella F.P., Cunningham J.N., Jr., Glassman E. Left atrium-to-femoral artery bypass: effectiveness in reduction of acute experimental myocardial infarction. J Thorac Cardiovasc Surg. 1983;86:887–896. [PubMed] [Google Scholar]
- 70.Cardiac Assist Inc . 2015. TandemHeart to Reduce Infarct Size (TRIS Trial) (TRIS) Available from https://clinicaltrials.gov/ct2/show/NCT02164058?.term=NCT02164058&rank=1. [Google Scholar]
- 71.Kapur N.K., Paruchuri V., Qiao X. Acute left ventricular unloading and delayed coronary reperfusion promotes stromal cell derived factor-1 expression and cardioprotective signaling in acute myocardial infarction. (Abstract #432). Presented at the 2014 Annual Meeting of Transcatheter Cardiovascular Therapeutics; Washington, DC; 2014 September 13. [Google Scholar]
- 72.Abiomed Inc . 2016. Door To Unloading With IMPELLA CP System in Acute Myocardial Infarction Trial (DTU) Available from https://clinicaltrials.gov/ct2/show/NCT03000270. [Google Scholar]
- 73.Kapur N.K., Esposito M.L. Door to unload: a new paradigm for the management of cardiogenic shock. Curr Cardiovasc Risk Rep. 2016;10:41. [Google Scholar]
- 74.Esposito M.L., Shah N., Dow S. Distinct effects of left or right atrial cannulation on left ventricular hemodynamics in a swine model of acute myocardial injury. ASAIO J. 2016;62(6):671–676. doi: 10.1097/MAT.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 75.Kapur N.K., Paruchuri V., Pham D.T. Hemodynamic effects of left atrial or left ventricular cannulation for acute circulatory support in a bovine model of left heart injury. ASAIO J. 2015;61(3):301–306. doi: 10.1097/MAT.0000000000000195. [DOI] [PubMed] [Google Scholar]