Abstract
Purpose
Little is known about disparities in colorectal cancer (CRC) incidence and mortality by community-level factors such as metropolitan status.
Methods
This analysis utilized data from the Surveillance, Epidemiology, and End Results (SEER) program from Utah. We included patients diagnosed with CRC from 1991 to 2010. To determine whether associations existed between metropolitan/nonmetropolitan county of residence and CRC incidence, Poisson regression models were used. CRC mortality was assessed using multivariable Cox regression models.
Findings
CRC incidence rates did not differ between metropolitan and nonmetropolitan counties by gender (males: 46.2 per 100,000 vs 45.1 per 100,000, P = .87; females: 34.4 per 100,000 vs 36.1 per 100,000, P = .70). However, CRC incidence between the years of 2006 and 2010 in nonmetropolitan counties was significantly higher in females (metropolitan: 30.4 vs nonmetropolitan: 37.0 per 100,000, P = .002). As compared to metropolitan counties, the incidence of unstaged CRC in nonmetropolitan counties was significantly higher in both males (1.7 vs 2.8 per 100,000, P = .003) and females (1.4 vs 1.6 per 100,000, P = .002). Among patients who were diagnosed between 2006 and 2010, metropolitan counties were found to have significantly increased survival among males and females, but nonmetropolitan counties showed increased survival only for males.
Conclusions
While we observed a decreasing incidence of CRC among men and women in Utah, this effect was not seen in women in nonmetropolitan areas nor among those with unstaged disease. Further studies should evaluate factors that may account for these differences. This analysis can inform interventions with a focus on women in nonmetropolitan areas.
Keywords: access to care, colorectal cancer, epidemiology, health disparities, rural health
Colorectal cancer (CRC) is the third most common cancer in the United States (US) and ranks among the leading causes of cancer-related deaths for both men and women.1 National statistics show decreasing incidence and mortality from CRC for both men and women over the past several decades.1,2 Although debated, these improvements are likely to be largely due to the increased uptake of CRC screening.1 Although screening rates for CRC have improved from 52.1% in 2008 to 58.2% in 2013, these rates are still well below the Healthy People 2020 and National Colorectal Cancer Roundtable goal of 80% of the US population receiving CRC screening by 2018.3.4 Because CRC is a top cancer killer and is largely preventable with screening, improved CRC prevention efforts are a national priority.1
Studies have found that incidence of CRC differs by geographic area, with some suggesting a lower incidence in nonmetropolitan areas, but with other studies showing higher CRC rates in nonmetropolitan locations.5–9 Stage at diagnosis is the most significant prognostic factor for CRC survival and is associated with a variety of factors including age, race, and socioeconomic status.1 To date, most analyses of CRC incidence by geographic factors have focused on national samples or have been limited to specific states that tend to be geographically dense.6,10,11
Utah, part of the Intermountain West, is a largely rural and frontier area and has many distinct characteristics with important implications for CRC. The majority of the land mass in Utah is classified as rural/frontier.12 However, only 9.4% of Utah’s population lives in nonmetropolitan areas as most of the population resides in the Wasatch Front, which is a large, metropolitan area where the majority of physicians, including gastroenterologists, are located.12 Nonmetropolitan residents in Utah are less likely than their metropolitan counterparts to be up to date with CRC screening,13 but there has been limited research on the relationship of geography on CRC incidence and mortality.8,9 The objectives of this study were to examine the incidence and mortality of CRC in Utah between the years of 1991 and 2010 and to identify disparities for residents living in nonmetropolitan counties of the state. As gender and stage of cancer at diagnosis are important factors in CRC incidence and mortality, we investigated differences between metropolitan and nonmetropolitan counties stratified for these factors.
Methods
Data
Using the Surveillance, Epidemiology, and End Results (SEER) program, we identified CRC cases between the years of 1991 and 2010 in Utah using SEER 9. CRC was defined following the site coding rules ICD-0-3/WHO 2008.14 SEER is composed of high quality population-based (state or metropolitan area) central cancer registries, enabling the monitoring of health disparities related to cancer incidence, mortality, patient survival, and treatment. There were N=13,026 CRC diagnoses available for analysis.
Census demographic data by county were pulled from county level attributes within SEER*Stat. The county level attributes that were included in the analysis were educational attainment (percent with less than high school education), age (percent under 18 years old and percent over 65 years old), median income, percent of families that live below the poverty level, and employment status (percent of county unemployed). Most of the county level demographics were provided with SEER*Stat in percentages and income as dollars in tens.
Our primary independent factor of interest, metropolitan or nonmetropolitan county of residence, was based on where the patient lived at diagnosis and was defined using the Rural-Urban Commuting Area (RUCA) Code Definitions that were developed by the United States Department of Agriculture (USDA). The RUCA codes examine population density, urbanization and daily commuting based on US Census tracts.15 Using SEER’s classification, RUCA codes were assigned at the county level.16 Counties were classified as metropolitan for RUCA codes 1–3 and the remaining codes, which included micropolitan areas, were considered nonmetropolitan (RUCA codes 4–9).16 Patient-level factors were also obtained from SEER*Stat including gender, stage of diagnosis, and year of diagnosis.
Statistical Methods
Demographic characteristics were compared between metropolitan and nonmetropolitan counties using median, interquartile range, minimum and maximum. We then generated incidence rates as cases per 100,000 persons with 95% confidence intervals. All rates were age-adjusted to the 2000 US standard population. P values for the rates comparison were calculated by Poisson regression and were adjusted by age. Poisson regression models were used to assess if there were statistically significant associations between metropolitan status and cancer incidence by diagnosis year and cancer stage within gender. The LOESS method to generate nonparametric local regression smoothing was used to produce smoothing incidence and 95% confidence interval scatterplots for CRC incidence rate trend from 1991 to 2010 by metropolitan status within gender.
Multivariate Cox proportional hazards models stratified by metropolitan status and gender, with adjustment for age at diagnosis and cancer stage, were used to calculate adjusted hazard ratios (HRs) and their 95% confidence intervals for the outcome of CRC mortality. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, North Carolina). All P values were 2-sided and were conducted at a significance level of .05.
Results
Ten counties were metropolitan and 19 were nonmetropolitan (Table 1). Census level demographic information showed that Utah metropolitan counties have a lower percentage of people with less than a high school education (median: 11.0%) as compared to Utah nonmetropolitan counties (median: 14.3%). Utah metropolitan counties also had fewer families living below poverty than nonmetropolitan (median: 6.3% vs 9.4%) and lower levels of unemployment (median: 4.7% vs 6.4%). Utah metropolitan counties had higher median household income (median: $45,800) as compared to nonmetropolitan counties (median: $34,250).
Table 1.
Census Level Demographics for Metro vs Nonmetropolitan Counties in Utah
| Metropolitan Counties (N=10)a | Nonmetropolitan Counties (N=19)a | |||
|---|---|---|---|---|
|
| ||||
| Median (IQR)% | Min/Max% | Median (IQR)% | Min/Max% | |
| Education | ||||
| <High school | 11.0 (7.6, 14.4) | 7.4/17.1 | 14.3 (12.2, 17.5) | 8.5/30.4 |
| Age | ||||
| <18 | 32.7 (31.0, 35.1) | 29.8/38.6 | 33.5 (30.7, 35.4) | 23.2/39.3 |
| 65+ | 7.72 (7.2, 9.8) | 4.9/17.0 | 12.5 (9.9, 14.1) | 8.4/17.1 |
| Income | ||||
| Median Household income ($) | 45,800 (39,730, 50,270) | 37,210/64,960 | 34,250 (32,000, 36,180) | 28,140/49,610 |
| % families below poverty | 6.3 (4.0, 7.7) | 3.1/8.0 | 9.4 (6.1, 12.0) | 4.2/26.9 |
| Employment | ||||
| Unemployed | 4.7 (3.9, 5.5) | 2.8/6.0 | 6.4 (5.3, 7.8) | 2.2/15.1 |
| Race/Ethnicity | ||||
| Non-white | 3.4 (1.8, 4.5) | 0.4/6.7 | 2.3 (1.6, 4.1) | 0.5/56.5 |
| Black | 0.5 (0.4, 1.4) | 0.2/1.7 | 0.2 (0.2, 0.4) | 0.1/1.2 |
| American Indian/Alaska Native | 0.9 (0.6, 1.3) | 0.1/2.0 | 1.3 (0.8, 2.5) | 0.0/56.1 |
| Asian/Pacific Islander | 1.4 (0.9, 2.3) | 0.1/4.3 | 0.4 (0.2, 0.7) | 0.0/1.2 |
| Hispanic | 6.7 (5.3, 10.4) | 1.4/12.8 | 4.5 (2.8, 5.4) | 1.8/10.3 |
| Other minority | 9.7 (8.0, 14.2) | 1.7/18.1 | 7.3 (5.3, 9.4) | 2.3/59.5 |
Metropolitan counties represent 88.3% of the total state population; Nonmetropolitan counties represent 22.7% of the total state population
CRC Incidence
We evaluated CRC incidence by diagnosis year and cancer stage within gender for metropolitan and nonmetropolitan counties (Table 2). Overall, the incidence rates by gender did not differ between metropolitan and nonmetropolitan counties, respectively (males: 46.2 per 100,000 vs 45.1 per 100,000, P = .87; females: 34.4 per 100,000 vs 36.1 per 100,000, P = .07). However, as compared to metropolitan counties, the incidence of unstaged CRC in nonmetropolitan counties was significantly higher in both males (1.7 vs 2.8 per 100,000, P = .003) and females (1.4 vs 2.2 per 100,000, P = .002). Among females, the incidence of CRC diagnosed in the years 2006 to 2010 in nonmetropolitan counties was significantly higher than in metropolitan counties (37.0 per 100,000 vs 30.4 per 100,000, P = .002). For the same years, there was no statistically significant difference in the rate for males.
Table 2.
Colorectal Cancer Incidence in Utah by Gender and Metropolitan Status, SEER 1991–2010 Data
| Gender | Cancer | Metropolitan Counties | Nonmetropolitan Counties | P valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Ratea | CI | Count | Pop | Rate* | CI | Count | Pop | |||
| Male | Overall | 46.2 | 45–47.4 | 5,876 | 20,073,090 | 45.1 | 42.2–48.0 | 975 | 2,662,224 | .87 |
| Diagnosis Year | ||||||||||
| 1991–1995 | 52.1 | 49.2–55.2 | 1,258 | 4,156,254 | 49.8 | 43.3–57.0 | 217 | 571,897 | .91 | |
| 1996–2000 | 49.3 | 46.7–52.0 | 1,407 | 4,762,969 | 48.5 | 42.4–55.2 | 235 | 639,867 | .79 | |
| 2001–2005 | 47.0 | 44.6–49.4 | 1,579 | 5,247,733 | 46.7 | 41.1–52.8 | 262 | 684,841 | .98 | |
| 2006–2010 | 39.6 | 37.7–41.7 | 1,632 | 5,906,134 | 37.6 | 33.1–42.5 | 261 | 765,619 | .92 | |
| Stage | ||||||||||
| Localized | 21.9 | 21.0–23.0 | 2,774 | 20,073,090 | 19.6 | 17.8–21.6 | 424 | 2,662,224 | .08 | |
| Regional | 14.4 | 13.7–15.0 | 1,857 | 20,073,090 | 15.4 | 13.8–17.2 | 336 | 2,662,224 | .15 | |
| Distant | 8.2 | 7.7–9.0 | 1,053 | 20,073,090 | 7.2 | 6.2–8.5 | 162 | 2,662,224 | .39 | |
| Unstaged | 1.7 | 1.5–2.0 | 192 | 20,073,090 | 2.8 | 2.1–3.7 | 53 | 2,662,224 | .003 | |
|
| ||||||||||
| Female | Overall | 34.4 | 33.4–35.3 | 5,291 | 20,032,423 | 36.1 | 33.8–38.6 | 884 | 2,635,666 | .07 |
| Diagnosis Year | ||||||||||
| 1991–1995 | 35.9 | 33.8–38.1 | 1,117 | 4,188,588 | 32.9 | 28.2–38.3 | 170 | 572,867 | .41 | |
| 1996–2000 | 37.6 | 35.6–39.6 | 1,339 | 4,761,176 | 38.5 | 33.6–43.9 | 224 | 637,692 | .58 | |
| 2001–2005 | 34.7 | 32.9–36.6 | 1,400 | 5,219,247 | 35.3 | 30.8–40.2 | 226 | 676,145 | .64 | |
| 2006–2010 | 30.4 | 28.8–32.0 | 1,435 | 5,863,412 | 37.0 | 32.6–41.8 | 264 | 748,962 | .002 | |
| Stage | ||||||||||
| Localized | 15.3 | 14.7–15.9 | 2,357 | 20,032,423 | 14.7 | 13.2–16.3 | 360 | 2,635,666 | .68 | |
| Regional | 11.5 | 11.0–12.1 | 1,772 | 20,032,423 | 12.5 | 11.2–14.0 | 306 | 2,635,666 | .11 | |
| Distant | 6.1 | 5.8–6.5 | 948 | 20,032,423 | 6.7 | 5.7–7.8 | 163 | 2,635,666 | .25 | |
| Unstaged | 1.4 | 1.2–1.6 | 214 | 20,032,423 | 2.2 | 1.6–2.8 | 55 | 2,635,666 | .002 | |
Rates are per 100,000 and age-adjusted to the 2000 U.S. Std. Population (19 age groups - Census P25–1130) standard; Confidence Intervals are 95% for rates.
P value was from Poisson regression and compares rates for each row.
Abbreviations: CI=Confidence Interval
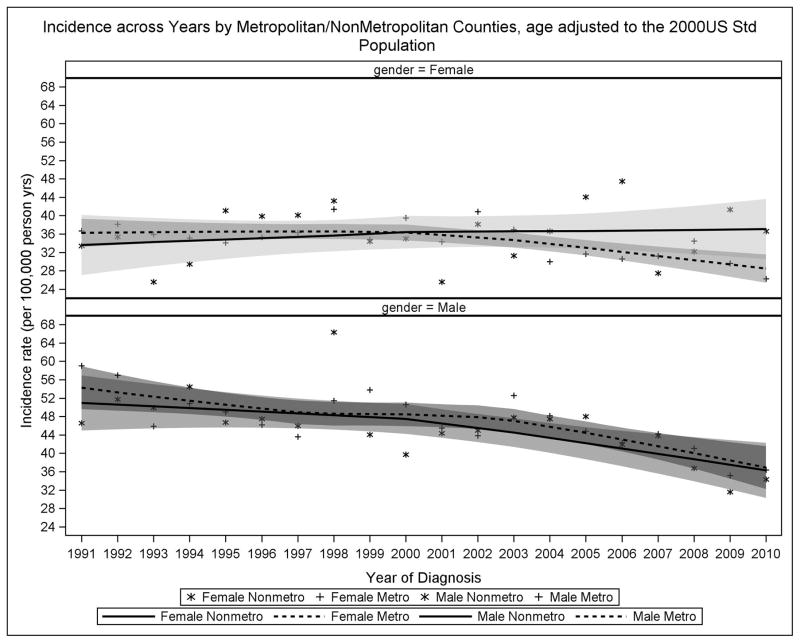
As depicted in Figure 1, the incidence of CRC in males has decreased in both metropolitan and nonmetropolitan counties, with a particular drop in the early 2000s when colonoscopies began to be routinely recommended as part of cancer prevention screening.17,18 For females in metropolitan counties, the decrease is only apparent after 2000, whereas for nonmetropolitan females the incidence stayed stable through 2010.
Figure 1. Year-Specific Colorectal Cancer Incidence by Metropolitan/ Nonmetropolitan Counties Within Gender, SEER 1991–2010.
These scatterplots demonstrate decreases in CRC incidence in both metropolitan and nonmetropolitan counties for males by year of diagnosis. This decrease is not observed for females in nonmetropolitan counties.
CRC Mortality
In Table 3, for metropolitan counties, we found significantly better cause-specific survival for males diagnosed during the most recent time period, 2006–2010 (HR = 0.69, 95% CI = 0.59–0.79, P < .0001), and for 2001–2005 (HR = 0.81, 95% CI = 0.71–0.93, P = .003), as compared to those diagnosed 1991–1995. Similarly, females living in metropolitan counties diagnosed during the most recent period (2006–2010) also had better cause-specific survival (HR = 0.76, 95% CI = 0.65–0.88, P = .0003) compared to females diagnosed 1991–1995. For nonmetropolitan counties, improvements in cause-specific survival were seen in males who were diagnosed 1996–2000 (HR = 0.71; 95% CI = 0.51–0.99, P = .04) and 2006–2010 (HR = 0.70; 95% CI = 0.49–0.98, P = .04), as compared to those diagnosed between 1991 and 1995. We found no improvements in mortality among females from nonmetropolitan counties.
Table 3.
Cox Proportional Hazards Model for Colorectal Cancer Mortality in Utah by Gender and Metropolitan Status, SEER 1991–2010 Data
| Gender | Diagnosis year | Metropolitan | Nonmetropolitan | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HRa | CI | P value | HRa | CI | P value | ||
| Male | |||||||
| 1991–1995 | Reference | Reference | |||||
| 1996–2000 | 0.90 | 0.79–1.03 | .13 | 0.71 | 0.51–0.99 | .04 | |
| 2001–2005 | 0.81 | 0.71–0.93 | .003 | 0.82 | 0.59–1.14 | .23 | |
| 2006–2010 | 0.69 | 0.59–0.79 | < .0001 | 0.70 | 0.49–0.98 | .04 | |
| Female | |||||||
| 1991–1995 | Reference | Reference | |||||
| 1996–2000 | 1.01 | 0.88–1.16 | .84 | 1.11 | 0.80–1.54 | .53 | |
| 2001–2005 | 0.90 | 0.78–1.04 | .16 | 0.81 | 0.58–1.15 | .25 | |
| 2006–2010 | 0.76 | 0.65–0.88 | .0003 | 0.75 | 0.54–1.04 | .08 | |
Bold indicates P < .05.
Abbreviations: HR = Hazard Ratio; CI = Confidence Interval
Estimates were based on models that adjusted for age at diagnosis and cancer stages.
Discussion
This study examined differences in CRC incidence and mortality by metropolitan vs nonmetropolitan counties in a largely rural and frontier state. We found a decrease in CRC incidence in Utah for both men and women living in metropolitan counties since the onset of colonoscopy recommendations in the early 2000s. However, in nonmetropolitan counties, while men experienced a decrease in incidence over the same timeframe, women did not. Relatedly, women in nonmetropolitan counties did not experience improvements in CRC mortality from 2006–2010 compared to 1991–1995 whereas metropolitan women did. To our knowledge, there have been no previous reports examining differences by gender on CRC incidence and mortality among metropolitan and nonmetropolitan residents in rural states such as Utah. These findings suggest that women in nonmetropolitan areas represent a group that has not benefited fully from improvements in screening and treatment which have likely led to a decrease in CRC incidence nationally over the past 2 decades.
In our analyses, both men and women in nonmetropolitan counties had significantly higher incidence of unstaged CRC, whereas we found no other statistically significant differences by stage. These findings differ from previous studies which have reported that nonmetropolitan residents are diagnosed more often with late stage CRC and no specific differences in unstaged cancer. The Utah Cancer Registry has a very low rate of unstaged cancer; thus, this may reflect the lack of appropriate disease staging in certain jurisdictions.
Nonmetropolitan residents have been found to be less likely than their metropolitan counterparts to receive preventive services including CRC screening.19–21 These disparities may be in some part attributable to the generally lower income and education level of nonmetropolitan compared to metropolitan populations,19–21 but they are likely also affected by the lack of specialty physicians to provide screening in rural areas.22 We found a similar county level pattern in Utah, with nonmetropolitan counties having lower levels of education and higher levels of poverty and unemployment. While we were unable to investigate access to care directly, an earlier study of travel time to colonoscopy providers in Utah found that lower travel time was associated with increased screening compliance,14 although this association disappeared in adjusted analyses.
Other factors such as difference in physician characteristics in metropolitan and nonmetropolitan areas may also play a role in individuals’ decisions to seek medical care and receipt of preventative services such as colonoscopies. These factors have been examined in relation to breast cancer screening, with studies demonstrating that physician recommendation is the most influential determinant of mammography among nonmetropolitan women and that the likelihood of physician recommendation may vary among nonmetropolitan and metropolitan physicians.23–25 A similar situation may be present with regard to recommendation for CRC screening among nonmetropolitan and metropolitan physicians and warrants future research.
Two recent initiatives in Utah have been implemented to improve CRC screening in our state. From 2009 to 2015, the Utah Department of Health had a grant from the Centers for Disease Control and Prevention to increase CRC screenings among low income and uninsured Utahns. While this program could have potentially affected CRC screening behaviors and awareness in Utah during the time of our study, the number of colonoscopies provided was limited and the program only overlapped with 2 years of our data. More recently, the 2016–2020 Utah Cancer Control Plan has key action steps to decrease structural barriers to CRC screening, such as encouraging in-home screening via fecal immunochemical tests, and these may help to address access to care disparities for nonmetropolitan residents.26
Culturally, Utah’s population is primarily of the Latter Day Saints (LDS) faith. LDS women often advocate for the health care of their families (which are often large and multi-generational) ahead of their own health. Since nonmetropolitan areas account for over 80% of the state of Utah and these areas are substantially higher in the population of LDS faith, one must factor in cultural considerations. We suggest that future studies to support CRC screening in Utah and other similar states that have large LDS populations, such as Idaho, incorporate potential cultural factors that may affect screening behaviors between men and women.12,27,28
The use of SEER data throughout the state of Utah, which is a large state with diversity in metropolitan and nonmetropolitan areas, is a key strength of this study. However, there are certain limitations that are important to consider in the interpretation of the results. As RUCA was developed for national use, there could be potential misclassification of rural areas or population growth in Utah that could dilute the ability to identify significant differences between classification areas. However, as RUCA is widely used and updated to reflect the growing US population, we believe it is an appropriate classification scheme for this analysis. Also, the use of county-level demographics rather than individual measures may limit inferences regarding sociodemographic factors and the outcome variables. Finally, we were not able to include additional measures (individual or ecologic) regarding health care access, health insurance penetration, or utilization in our study.
Conclusion
In summary, while we found a decreasing incidence of CRC among men and women in Utah, this effect was not seen in women in nonmetropolitan areas. Future studies need to focus on examining specific characteristics of individuals and their environment that may affect access to and utilization of CRC screening and care in nonmetropolitan areas and account for the discrepancy in CRC incidence noted in this study. Also, we found trends toward better survival over time, suggesting an important area for future research. The results of this study should be considered in the design and implementation of interventions with a focus on CRC prevention among women in nonmetropolitan areas.
Footnotes
Disclosures: The authors have no potential conflicts to disclose.
References
- 1.American Cancer Society. [Accessed April 26, 2016];Colorectal Cancer Facts and Figures 2014–2016. 2014 Available at: http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf.
- 2.Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D. Secular Trends in Colon and Rectal Cancer Relative Survival. J Natl Cancer Inst. 2013;105(23):1806–1813. doi: 10.1093/jnci/djt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healthy People 2020. [Accessed April 26, 2016];Cancer. 2016 Available at: https://www.healthypeople.gov/2020/data-search/Search-the-Data?nid=4054.
- 4.National Colorectal Cancer Roundtable. [Accessed May 16, 2016];Our Straregic Plan. 2016 Available at: http://nccrt.org/about/strategic-plan/
- 5.Coughlin SS, Thompson TD. Rural/nonrural differences in colorectal cancer incidence in the United States, 1998–2001. Cancer. 2006;107(5 Suppl):1181–1188. doi: 10.1002/cncr.22015. [DOI] [PubMed] [Google Scholar]
- 6.Yoo W, De S, Wilkins T, Smith SA, Blumenthal D. Age, Race and Regional Disparities in Colorectal Cancer Incidence Rates in Georgia between 2000 and 2012. Ann Public Health Res. 2016;3(2) pii: 1040 Epub 2016 Mar 11. [PMC free article] [PubMed] [Google Scholar]
- 7.Kinney AY, Harrell J, Slattery M, Martin C, Sandler RS. Rural-urban differences in colon cancer risk in blacks and whites: the North Carolina Colon Cancer Study. J Rural Health. 2006;22(2):124–130. doi: 10.1111/j.1748-0361.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 8.Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and Whites in Georgia. J Rural Health. 2012;28:296–305. doi: 10.1111/j.1748-0361.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 9.Wan N, Zhan FB, Lu Y, Tiefenbacher JP. Access to healthcare and disparities in colorectal cancer survival in Texas. Health Place. 2012;18(2):321–329. doi: 10.1016/j.healthplace.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol. 2011;2011:1–27. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zahand WE, Mueller GS, Fogleman AJ, Jenkins WD. Intrastate Variations in Rural Cancer Risk and Incidence: An Illinois Case Study. J Public Health Manag Pract. 2016;22(5):472–478. doi: 10.1097/PHH.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 12.United States Census Bureau. Utah: 2012. [Accessed April 26, 2016];2010 Census of Population and Housing. 2012 Available at: https://www.census.gov/prod/cen2010/cph-2-46.pdf.
- 13.Anderson AE, Henry KA, Samadder NJ, Merrill RM, Kinney AY. Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11(5):526–533. doi: 10.1016/j.cgh.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute Surveillance, Epidemiology, and End Results Program. [Accessed June 14, 2016];Site Recode. 2016 Available at: http://seer.cancer.gov/siterecode/
- 15.United States Department of Agriculture. [Accessed April 26, 2016];Rural-Urban Commuting Area Codes (RUCAs) 2014 Available at: http://depts.washington.edu/uwruca/ruca-uses.php.
- 16.National Cancer Institute Surveilance, Epidemiology, and End Results Program. [Accessed April 26, 2016];Rural-Urban Continuum Codes. 2014 Available at: https://seer.cancer.gov/seerstat/variables/countyattribs/ruralurban.html.
- 17.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 18.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 19.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: cross-sectional analysis of 1998–2005 data from the Centers for Disease Control’s Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coughlin SS, Richards TB, Thompson T, et al. Colorectal Cancer Screening Practices Among Men and Women in Rural and Nonrural Areas of the United States, 1999. J Rural Health. 2004;20(2):118–124. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Wimberly MC. Geographic Variations of Colorectal and Breast Cancer Late-Stage Diagnosis and the Effects of Neighborhood-Level Factors. [Accessed February 22, 2017];J Rural Health. 2016 doi: 10.1111/jrh.12179. Epub ahead of print 14 March 2016. Available at: http://onlinelibrary.wiley.com/doi/10.1111/jrh.12179/full. [DOI] [PubMed]
- 22.Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of Rural Cancer Care in the United States. Oncology. 2015;29(9):633–640. [PubMed] [Google Scholar]
- 23.Davis TC, Arnold CL, Rademaker A, et al. Differences in barriers ot mammography between rural and urban women. J Womens Health. 2012;21(7):748–755. doi: 10.1089/jwh.2011.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina Y, Thompson B, Ceballos RM. Physician and Family Recommendations to Obtain a Mammogram and Mammography Intentions: The Moderating Effects of Perceived Seriousness and Risk of Breast Cancer. J Womens Health Care. 2014;3(6):199. doi: 10.4172/2167-0420.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauscher GH, Hawley ST, Earp JA. Baseline predictors of initiation vs. maintenance of regular mammography use among rural women. Prev Med. 2005;40(6):822–830. doi: 10.1016/j.ypmed.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Utah Cancer Action Network. [Accessed June 3, 2016];2016–2020 Utah Comprehensive Cancer Prevention and Control Plan. 2016 Available at: http://www.ucan.cc/wp-content/uploads/2015/12/State-Cancer-Plan-Revision-2.pdf.
- 27.The Association of Religion Data Archives. [Accessed June 3, 2016];Religion, 2009 County (InfoGroup) - % of Total Reported Members Who Are Latter Day Saints. 2009 Available at: http://www.thearda.com/DemographicMap/displayIGMap.asp?ZipCode=utah.
- 28.Gallup. [Accessed April 26, 2016];Tracking Religious Affiliation State by State. 2004 Available at: http://www.gallup.com/poll/12091/tracking-religious-affiliation-state-state.aspx.