Abstract
Objectives
We evaluated the impact of implementation of the TN-STEMI programme on various characteristics of the pharmacoinvasive group by comparing clinical as well as angiographic outcomes between the pre- and post-implementation groups.
Methods
The TN-STEMI programme involved 2420 patients of which 423 patients had undergone a pharmacoinvasive strategy of reperfusion. Of these, 407 patients had a comprehensive blinded core-lab evaluation of their angiograms post-lysis and clinical evaluation of various parameters including time-delays and adverse cardio- and cerebro-vascular events at 1 year. Streptokinase was used as the thrombolytic agent in 94.6% of the patients.
Results
In the post-implementation phase, there was a significant improvement in ‘First medical contact (FMC)-to-ECG’ (11 vs. 5 min, p < 0.001) and ‘Lysis-to-angiogram’ (98.3 vs. 18.2 h, p < 0.001) times. There was also a significant improvement in the number of coronary angiograms performed within 24 h (20.7% vs. 69.3%, p < 0.001). The ‘Time-to-FMC’ (160 vs. 135 min, p = 0.07) and ‘Total ischemic time’ (210 vs. 176 min, p = 0.22) also showed a decreasing trend. IRA patency rate (70.2% vs. 86%, p < 0.001) and thrombus burden (TIMI grade 0: 49.1% vs. 73.4%, p < 0.001) were superior in this group. The MACCE rates were similar except for fewer readmissions (29.8% vs. 12.6%, p = 0.0002) and target revascularizations at 1 year (4.8% vs. none, p = 0.002) in the post-implementation group.
Conclusion
The implementation of a system-of-care (hub-and-spoke model) in the pharmacoinvasive group of the TN-STEMI programme demonstrated shorter lysis-to-angiogram times, better TIMI flow patterns and lower thrombus burden in the post-implementation phase.
Keywords: STEMI, System-of-care, Pharmacoinvasive, Thrombolysis, Streptokinase
1. Introduction
The primary aim of ST segment elevation myocardial infarction (STEMI) treatment is timely restoration of the patency of the infarct related artery (IRA). Though primary percutaneous coronary intervention (PCI) is considered the standard-of-care, it is accessible only for a fraction of patients in low-and-middle income countries (LMIC) like India where stand-alone thrombolysis still remains the commonest form of reperfusion therapy.1, 2, 3, 4 ‘Pharmacoinvasive (PI) strategy’ was developed to combine the advantages of early reperfusion with thrombolysis and improved IRA patency with PCI.
“Pharmacoinvasive strategy” has been endorsed by both the European Society5 as well as American Heart Association STEMI guidelines.6 In this strategy, where delay-to-primary PCI is more than 120 min or the door-to-balloon minus door-to-needle time is more than 60 min, the early presenters undergo thrombolysis and are then routinely transferred to a PCI capable centre for angiography within 3–24 h. The available evidence from the recent PI trials do not report any differences in the clinical outcomes (including mortality, reinfarction, stroke) between the primary PCI and PI group and these studies have shown shorter time-to-reperfusion and higher culprit vessel patency in the PI arm.7, 8, 9 In a prospective, observational, multicentre study comparing PI with tenecteplase versus primary PCI in Indian patients with STEMI (STEPP-AMI),10, 11 the mortality rates between the PI and primacy PCI arms were comparable. Though PI strategy may be an effective strategy in LMIC, there are no major studies in the Indian scenario that have evaluated this strategy using streptokinase. The current study is designed to assess the angiographic flow profiles and clinical outcomes in patients who underwent PI management strategy with streptokinase as the predominant thrombolytic agent in the TN-STEMI programme conducted in the state of Tamilnadu.
2. Methodology
The ‘Tamilnadu − ST segment elevation myocardial infarction (TN-STEMI) programme’ was designed with the aim of delivering timely reperfusion therapy for patients presenting with STEMI in the Indian set-up. The rationale and detailed design of the TN-STEMI network has been described previously.12 The programme introduced a ‘hub-and-spoke’ model to address the deficiencies in the infrastructure and patient transport in the existing STEMI-care, by forming strategic partnerships with the government and ambulance systems and utilising the latest developments in data collection. The programme systematically collected data of the existing practice of STEMI management in the first phase (pre-implementation) and then introduced a system-of-care for STEMI management in the second phase (post-implementation). The 1 year data has been published earlier.13 In the programme, 2420 consecutive patients were enrolled between 2012 and 2014 in ‘4 hub and 40 spoke centres’ in the state of Tamilnadu, India. There were 2167 patients in the study who received one of the three treatment strategies: stand-alone fibrinolysis (929 patients), fibrinolysis followed by angiography- PI approach (423 patients) and primary PCI (815 patients). The study observed a decrease in various time-delays and higher adoption of either PI or primary PCI as a reperfusion strategy.13 There was also a 3.4% absolute mortality reduction at the end of one year. Though we had shown a significant reduction in stand-alone thrombolysis and a significant increase in pharmacoinvasive strategy, the mechanisms which provided the improvements in the 1 year mortality remained unclear. We therefore evaluated clinical events, time-delays and coronary angiograms among patients who underwent fibrinolysis followed by cardiac catheterization (423 patients).
2.1. Objectives, data collection, definitions
The primary objective of the study was to assess the impact of implementation of a system-of-care for STEMI, on the IRA patency and thrombus load in the patients who underwent PI treatment. The secondary objective was to assess the effect of the programme on 1) time-delays, 2) MACCE, 3) Major and minor bleeds.
The PI cohort underwent onsite fibrinolysis in spoke centres within 12 h of onset of symptoms followed by transfer to one of the four PCI capable hospitals as a part of the PI strategy. Patients with failed lysis or those in cardiogenic shock were transferred for PCI as a part of the rescue PCI strategy.
Data were collected from the 4 clusters during both the phases of the programme with the help of electronic data capture application for the demographic characteristics, clinical findings, medications and cardiac catheterisation details. All the angiograms were collected from the hubs and analysed in an independent core-laboratory (Indian Cardiology Research Foundation Core laboratory, Chennai, India). The investigator overseeing the angiographic data of the total cohort was blinded to the distribution of the patients between the two groups. Angiographic determination of artery patency was based on TIMI flows described by the Thrombolysis In Myocardial Infarction group14 (Appendix A). Infarct related artery (IRA) patency rates were derived as a sum of TIMI 2 and 3 flows. Grading of thrombus burden was based on the definitions given by the TIMI group15 (Appendix B).
The clinical events reported in this study include all-cause mortality, reinfarction, ischemic stroke, haemorrhagic stroke, major bleed, minor bleed, angina or any cardiac symptoms requiring hospitalization and repeat revascularizations. The Major Cardio and Cerebro-Vascular Event (MACCE) rate has been reported as a composite of death, re-infarction and stroke. The reported follow-up of the patients are at three stages: in-hospital, 30 day and 1 year. The definitions (Appendix C, Appendix D) of stroke, reinfarction, major and minor bleeding is in accordance to the clinical data standards.16, 17
2.2. Statistical analysis
Continuous variables presented in the tables are summarised using descriptive statistics and the categorical data are presented as numbers with percentages. The time-delays are presented as medians with 25th and 75th percentiles. Comparison of variables between the two groups has been done with appropriate tests- chi-square/Fischer exact test for categorical variables, student T test for means of continuous variables, Mann-Whitney U test for the various time-delays. The various variables capable of predicting TIMI flows ≥2 have been studied using a binary logistic regression model where age, diabetes, hypertension, smoking status, heart rate, systolic blood pressure, various time-delays and pre-post group status were incorporated into a forward regression model. The likelihood of prediction of TIMI flows ≥2 is presented as odds ratio with 95% confidence intervals. The fitness of the model was tested using Hosmer and Lemeshow test. All calculated p values are two-tailed and are set at statistical significance of 0.05. All confidence intervals are reported at 95% level. The study conforms to ethical principles in the Declaration of Helsinki and the study has been approved by the local institutional ethics committee.
3. Results
Amongst the 423 patients in the PI cohort, 16 were excluded where either angiographic data was not available or patients had rescue PCI for failed lysis or directly transferred for PCI in the setting of cardiogenic shock. The study included 407 patients, 114 patients (28%) in the ‘Pre-implementation’ group and 293 (72%) in the ‘Post-implementation’ group.
The baseline characteristics of the pre- and post-implementation groups are summarised in Table 1 and the salient features are mentioned below. There were no significant differences in the baseline variables between the two groups except for heart rate, systolic blood pressure and distribution of hypertension. Streptokinase was the predominant thrombolytic agent (94.6%) used. Overall, the proportion of patients who received adhoc PCI, CABG and medical therapy were 50.9%, 14.5% and 34.1% respectively. Radial vascular access was used in 78.4% of the patients.
Table 1.
Comparison of baseline characteristics between Pre- and Post-implementation groups.
| Variables | Total group (N = 407) | Pre-implementation (N = 114) | Post-implementation (N = 293) | p value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (years)a | 52.38 ± 10.7 | 51 ± 9.8 | 52.7 ± 10.9 | 0.24 |
| Male | 357 (87.7) | 97 (85.1) | 260 (88.7) | 0.32 |
| Diabetes | 90 (22.1) | 18 (15.8) | 72 (26.9) | 0.06 |
| Hypertension | 85 (20.9) | 15 (13.2) | 70 (23.9) | 0.02 |
| Active smoking | 188 (46.2) | 50 (43.9) | 138 (47.1) | 0.58 |
| Prior PCI | 6 (1.5) | 2 (1.8) | 4 (1.4) | 0.67 |
| Prior CAG | 2 (0.5) | 0 | 2 (0.7) | 0.9 |
| HR (beats/min)a | 76 ± 27 | 84 ± 17 | 72.9 ± 30 | <0.001 |
| Systolic BP (mm Hg)a | 121 ± 41 | 130 ± 27.3 | 117 ± 46 | 0.007 |
| DAPT treatment after thrombolysis | 388 (95.3) | 111 (97.4) | 276 (94.2) | 0.21 |
| Thrombolytic Agents | ||||
| Streptokinase | 385 (94.6) | 102 (89.5) | 283 (96.6) | 0.01 |
| Tenecteplase | 19 (4.7) | 11 (9.6) | 8 (2.7) | |
| Reteplase | 3 (0.7) | 1 (0.9) | 2 (0.7) | |
| Management strategy after thrombolysis | ||||
| Adhoc PCI | 207 (50.9) | 52 (45.6) | 155 (52.9) | 0.11 |
| Medical management | 124 (30.5) | 45 (39.5) | 79 (27) | |
| Elective PCI suggestedb | 15 (3.6) | 3 (2.6) | 12 (4.1) | |
| CABG | 59 (14.5) | 14 (12.3) | 45 (15.4) | |
CAG- coronary angiogram; HR- heart rate; BP- blood pressure; DAPT- dual anti-platelet treatment; CABG- coronary artery bypass graft surgery.
Continuous variables presented as mean ± SD.
Patients did not undergo PCI at the time of angiogram due to logistic reasons; PCI- percutaneous coronary intervention.
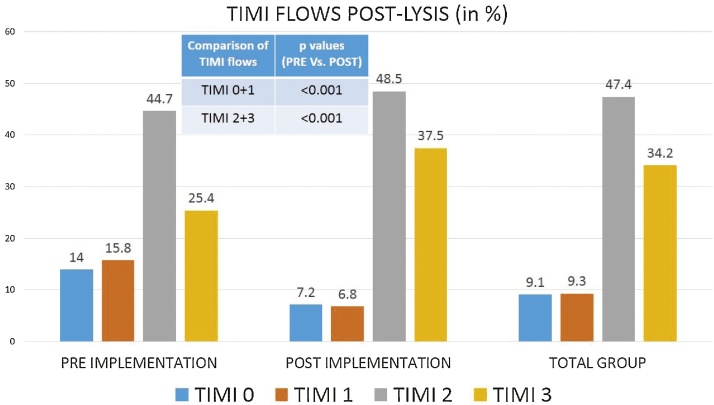
The time delays, TIMI flows (Fig. 1) and thrombus burden are detailed in Table 2. In the post-implementation phase, there was a significant improvement in ‘First medical contact (FMC)-to-ECG’ (11 vs. 5 min, p < 0.001) and ‘Lysis-to-angiogram’ (98.3 vs. 18.25 h, p < 0.001) times and number of coronary angiograms performed within 24 h (20.7% vs. 69.3%, p < 0.001). The ‘Time-to-FMC’ (160 vs. 135 min, p = 0.07) and ‘Total ischemic time’ (210 vs. 176 min, p = 0.22) showed a decreasing trend in the post-implementation period. IRA patency (after fibrinolysis) was noted in 86% of the patients in the post-implementation group compared to only 70.2% in the pre-implementation group (p < 0.001). TIMI 3 flows were noted in 37.5% and 25.4% in the post and pre-implementation groups respectively (p = 0.02). Similarly there was a significant reduction in the thrombus burden in the post-implementation group (49.1% vs. 73.4%, p < 0.001). TIMI 3 flows were achieved in 96.1% patients who underwent a PCI (96.1% in the pre- and 96.8% in the post-implementation groups respectively).
Fig. 1.
Comparison of angiographic timi flows after thrombolysis.
Table 2.
Comparison of time delays, TIMI flows and thrombus burden between Pre- and Post-implementation groups.
| Time Delaysa | Total group | Pre-implementation | Post-implementation | p valueb |
|---|---|---|---|---|
| Time to FMC | 140 (85,240) | 160 (97,260) | 135 (70,240) | 0.07 |
| FMC to ECG | 5 (5,11) | 11 (10,15) | 5 (5,10) | <0.001 |
| ECG to Lysis | 25 (14,42) | 15 (10,35) | 25 (15,43) | <0.001 |
| Door to needle | 30 (20, 50) | 30 (15, 45) | 30 (25, 58.5) | 0.004 |
| Total ischemic time | 185 (120,315) | 210 (125,289) | 176 (110,315) | 0.22 |
| Lysis to Angiogram | 1281 (580,4100) | 5900 (1860,9660) | 1095 (489,1659) | <0.001 |
| Lysis to PCI | 1064 (490,1792) | 2580 (480,7380) | 1030 (490,1327) | 0.001 |
| Thrombolysis to angio <24 h | 217 (53.3%) | 23 (20.7%) | 194 (69.03%) | <0.001 |
| TIMI-flows after lysis | Total group | Pre-implementation | Post-implementation | p valuec |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| 0 | 37 (9.1) | 16 (14) | 21 (7.2) | 0.04 |
| 1 | 38 (9.3) | 18 (15.8) | 20 (6.8) | 0.01 |
| 2 | 193 (47.4) | 51 (44.7) | 142 (48.5) | 0.51 |
| 3 | 139 (34.2) | 29 (25.4) | 110 (37.5) | 0.03 |
| TIMI flow 0 + 1 | 75 (18.4) | 34 (29.8) | 41 (14) | <0.001 |
| IRA patency (2 + 3) | 332 (81.6) | 80 (70.2) | 252 (86) | <0.001 |
| TIMI-thrombus grade after lysis | Total group | Pre-implementation | Post-implementation | p valuec |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Grade-0 | 271 (66.6) | 56 (49.1) | 215 (73.4) | <0.001 |
| Grade-1 | 54 (13.3) | 25 (21.9) | 29 (9.9) | 0.001 |
| Grade-2 | 32 (7.9) | 11 (9.6) | 21 (7.2) | 0.30 |
| Grade-3 | 15 (3.7) | 5 (4.4) | 10 (3.4) | 0.55 |
| Grade-4 | 5 (1.2) | 5 (4.4) | 0 | 0.002 |
| Grade-5 | 30 (7.4) | 12 (10.5) | 18 (6.1) | 0.15 |
FMC- first medical contact, ECG- electrocardiogram, PCI- percutaneous coronary intervention, TIMI- thrombolysis in myocardial infarction, IRA- infarct related artery.
Time delays are presented as median times (25th–75th percentile).
Comparison of distributions by independent samples Mann-Whitney U test.
by Fisher exact test.
Table 3 compares clinical events between the groups. The overall cumulative 1 year MACCE and all-cause mortality rates were 9.6% and 8.5% respectively. There was no significant difference in the overall MACCE or individual event rates between the groups except for lower readmissions (29.8% vs. 12.6%, p = 0.0002) and repeat revascularization at 1 year (4.8% vs. none, p = 0.002) in the post-implementation group.
Table 3.
Comparison of clinical outcomes between Pre- and Post-implementation groups.
| Variables | Total group | Pre-implementation | Post-implementation | p value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| In-hospital adverse events | ||||
| MACCE | 8 (1.96) | 2 (1.8) | 6 (2.0) | 0.9 |
| Death | 7 (1.7) | 2 (1.8) | 5 (1.7) | 0.9 |
| Stroke | 1 (0.2) | 0 | 1 (0.3) | 0.9 |
| Major bleed | 0 | 0 | 0 | 1 |
| Minor bleeding | 1 (0.2) | 0 | 1 (0.3) | 0.9 |
| Angina | 6 (1.5) | 2 (1.8) | 4 (1.4) | 0.67 |
| Others | 19 (4.7) | 5 (4.4) | 14 (4.8) | 0.9 |
| Clinical events after discharge upto 1 month | ||||
| MACCE | 8 (2) | 1 | 7 | 0.45 |
| Death | 7 (1.8) | 1 (0.9) | 6 (2.2) | 0.68 |
| Reinfarction | 1 (0.2) | 0 | 1 (0.003) | 0.9 |
| Stroke | 0 | 0 | 0 | 1 |
| Angina | 4 (1.05) | 0 | 4 (1.5) | 0.33 |
| Re-admission | 31 (8.2) | 12 (10.8) | 19 (7.1) | 0.22 |
| Re-intervention CABG | 7 (1.8) | 1 (0.9) | 6 (2.2) | 0.67 |
| Re-intervention PCI | 4 (1.05) | 4 (3.6) | 0 | 0.01 |
| Follow-up rate | 379 (93.1) | 111 (97.4) | 268 (91.5) | 0.05 |
| Clinical events after 1 month upto 1 year | ||||
| MACCE | 23 (5.85) | 5 (4.8) | 18 (7.1) | 0.49 |
| Death | 21 (5.8) | 5 (4.8) | 16 (6.3) | 0.81 |
| Reinfarction | 2 (0.56) | 0 | 2 (0.8) | 0.9 |
| Stroke | 0 | 0 | 0 | 1 |
| Angina | 22 (6.1) | 5 (4.8) | 17 (6.7) | 0.63 |
| Re Admission | 94 (23) | 31 (29.8) | 32 (12.6) | <0.001 |
| Re-intervention CABG | 14 (3.4) | 1 (0.96) | 6 (2.3) | 0.68 |
| Re-intervention PCI | 9 (2.2) | 5 (4.8) | 0 | 0.002 |
| Follow-up rate | 357 (87.7) | 104 (91.3) | 253 (86.3) | 0.24 |
| Cumulative events at 1 year | ||||
| MACCE | 39 (9.58) | 8 (7) | 31 (10.5) | 0.35 |
| Death | 35 (8.5) | 8 (7) | 27 (9.2) | 0.56 |
| Reinfarction | 3 (0.7) | 0 | 3 (1.02) | 0.56 |
| Stroke | 1 (0.2) | 0 | 1 (0.3) | 1 |
| Angina | 32 (7.8) | 7 (6.1) | 21 (7.16) | 0.83 |
| Re-admission | 94 (23) | 43 (37.7) | 51 (17.4) | <0.001 |
| Re-intervention CABG | 14 (3.4) | 2 (1.7) | 12 (4) | 0.37 |
| Re-intervention PCI | 9 (2.2) | 9 (7.8) | 0 | <0.001 |
MACCE- major adverse cardio- and cerebrovascular events (death, reinfarction or stroke); CABG- coronary artery bypass graft surgery; PCI- percutaneous coronary intervention
A binary logistic regression analysis to predict better TIMI flows (2 and 3) in the total cohort was performed with 14 variables (Table 4). It revealed that the ‘Implementation of the TN-STEMI programme’ was the only significant variable amongst the others in the regression model which was contributing substantially to better TIMI flows. In the model, the implementation of the TN-STEMI programme had 3.57 times more likelihood (Odds ratio = 1/0.28 = 3.57) of TIMI flows ≥2 compared to the pre-implementation group.
Table 4.
Binary logistic regression analysis of variables predicting IRA patency.a
| Variables | Odds ratio (95% C.I) | p value |
|---|---|---|
| Baseline demographic variables | ||
| Age | 1.01 (0.97–1.03) | 0.72 |
| Gender | 1.39 (0.55–3.5) | 0.49 |
| Diabetes | 1.53 (0.75–3.15) | 0.24 |
| Hypertension | 0.84 (0.38–1.85) | 0.67 |
| Smoking status | 0.58 (0.31–1.08) | 0.09 |
| DAPT treatment | 1.25 (0.26–5.9) | 0.78 |
| Clinical variables | ||
| Heart rate | 1.01 (0.99–1.02) | 0.57 |
| Systolic blood pressure | 0.99 (0.99–1.009) | 0.79 |
| Time delays | ||
| Time to FMC | 1.0 (0.99–1.01) | 0.40 |
| FMC to ECG | 1.0 (0.99–1.01) | 0.83 |
| ECG to Lysis | 1.01 (0.99–1.03) | 0.34 |
| Door to needle | 0.99 (0.98–1.01) | 0.44 |
| Thrombolysis to Angio time | 1.0 (1–1) | 0.19 |
| Implementation of Programme | ||
| Pre- and post-programme | 0.29 (0.145–0.572) | <0.001 |
Infarct related artery (IRA) patency = TIMI flows ≥2; p value for Hosmer and Lemeshow test = 0.188 (Model is good fit); DAPT- dual antiplatelet treatment; FMC- first medical contact; ECG- electrocardiogram.
4. Discussion
The current study shows that implementation of a system-of-care for management of STEMI was associated with 1) shorter times to cardiac catheterisation, 2) better IRA patency rates, and 3) lower thrombus burden.
The TN-STEMI programme addresses the gap in research in acute cardiovascular diseases with a long term follow-up in India.18 In an earlier paper, we have shown the impact of the model on various health metrics during the post-implementation phase and observed a decrease in various time-delays, higher rates of coronary angiography (35.0% vs. 60.8%; p < 0.001) and PCI (29.5% vs. 46.5%; p < 0.001), and greater adoption of either PI or primary PCI as a reperfusion strategy (46.4% vs. 70.6%; p < 0.001). The TN-STEMI model-of-care resulted in 3.4% absolute mortality reduction at end of 1 year with ‘any’ form of reperfusion therapy in 2167 patients. The majority of this difference was in the spoke centers (3.3% mortality reduction compared to only 1.1% in the hub centers).13 In the pharmacoinvasive cohort, which has been analysed in the present paper, majority of the patients were from spoke centers (245 patients, 60.2%). This could have contributed to the higher mortality reduction observed in the spoke centers than in the hub centers in the total cohort of the earlier paper.
The impact of PI strategy with newer generation thrombolytic agents has been established.7, 8, 9 However with financial constraints in LMIC, streptokinase continues to be the commonly used thrombolytic agent.1, 2, 3, 4 There are no major studies which have evaluated streptokinase as a part of PI strategy in contemporary practice. In addition the impact of systems-of-care on angiographic outcomes with PI strategy utilising streptokinase is also not known. TN-STEMI programme provides a unique data on this subset of patients and should be of great interest in other LMIC, where a similar strategy can be used.
Timely restoration of IRA patency in STEMI is critical for myocardial salvage. Fibrinolytic agents and primary PCI are equally effective in the initial hours of STEMI.5, 6 Hence for thrombolytic to be effective reperfusion agents, shorter times-to-treatment is important, more so with agents like streptokinase. This underscores the need of a system-of care in order to deliver timely reperfusion. In this study there was an improvement in timelines- total ischemia time, lysis-to-angiogram and the percentage of angiograms within the mandated time of 24 h. The median total ischemic time was shorter (176 min) in the post-implementation phase compared to a longer time (210 min) in the pre-implementation phase. This is comparable to the 165 min in the KAMIR registry19 and 130 min observed in the FAST MI 2010 registry.20, 21 In our study, prior to implementation of the programme, the lysis-to-angiogram times were very high (nearly 4 days) and introducing a system-of-care has immensely helped in reducing the lysis-to-angiogram times to nearly 18 h. Our analysis also shows that 60.9% patients had an angiographic evaluation within 24 h of lysis compared to a mere 21.8% prior implementation of the programme. In addition, the programme has also impacted the lysis-to-PCI time, causing a significantly less time-to-PCI (17 h) in the post-implementation phase compared to the pre-implementation phase (40 h).
The reported TIMI 3 flows of 34.2% in the total cohort are comparable to the 33% of TIMI 3 flows observed in the GUSTO-1 trial with streptokinase.22 The most important observation of this study was the significant improvement in artery patency rates (86% vs. 70.2%; p < 0.001) which was driven by increase in the TIMI 3 flows (25.4% vs. 37.5%; p = 0.027) and reduction in TIMI 0 and 1 flows (18.4% vs. 29.8%; p < 0.001) in the post-implementation phase. There was also a concomitant significant reduction in the thrombus burden (49.1% vs. 73.4%; p < 0.001). The improvement in TIMI flow and reduction of thrombus load can be attributed to the reduced ischemia times and time-to-lysis, though not significant individually in this analysis. The system-of-care could have impacted all these factors and hence was responsible for this improvement.
In the present study, our observations in the PI cohort did not reveal any change in the primary clinical outcome of MACCE rate including mortality, stroke or reinfarction between both the groups. The observed 30 day and 1 year mortality rates of 1.7% and 9.2% respectively in the post-implementation phase is comparable to the 30 day and 6-to-12 month mortality rates of 3.3% and 4.8% respectively observed in a meta-analysis of studies with an early PCI strategy following thrombolysis with newer generation lytic agents.23 In the same meta-analysis, the observed stroke and 6-to-12 month reinfarction rates were 0.7% and 3.9% respectively comparable to 0.3% and 1.02% at 1 year respectively in our study.
The safety end points were not different and shows that though there was a significant reduction in time-to-catheterisation, safety was not an issue. There were no major bleeding events observed in the cohort. Though the observed median time to an invasive strategy (nearly 21 h) is within the current recommendation of 3–24 h, this was achieved only in 53.3% of the study patients. These longer times and in addition a predominantly radial vascular access might have contributed to lower bleeding events. Re-admission and re-interventions improved in the post-implementation phase possibly due to the impact of the system-of-care on multiple parameters.
The study has reported observations on a cohort who underwent angiography following fibrinolysis and thus has the inherent limitation of an observational quasi-experimental study design. The project was not designed to address the safety and efficacy of the PI strategy against primary PCI or stand-alone fibrinolysis, which has been proven in prior trials. However the study reflects the real-world scenario with the available systems-of-care in our region. The study documents important facts pertinent to the adoption of a PI management strategy with improved systems-of-care to improve the contemporary STEMI facilities in the region.
5. Conclusion
In this study, the implementation of a ‘system-of-care’ with PI management strategy in the form of the ‘Hub-and-Spoke’ model for the management of STEMI population, resulted in shorter lysis-to-angio times, better TIMI flow patterns and lower thrombus burden. If streptokinase is used as the lytic agent, as in this cohort, it is necessary to follow systems-of-care in order to achieve a favourable outcome for patients. Importantly, increased use of the PI strategy with streptokinase in the post-implementation phase, did not increase the bleeding complications. Methodical implementation of a system-of-care24, 25, 26, 27 will help in getting guideline-based treatments and help in achieving better clinical outcomes.
What is already known?
The TN-STEMI programme showed an improvement in the various process measures as well as clinical outcomes at 1 year in patients presenting with STEMI. Pharmacoinvasive strategy is a time tested reperfusion strategy for patients of STEMI and has shown to reduce inadvertent time-delays in establishing the infarct artery patency by primary PCI.
What this study adds?
The TN-STEMI model-of care in STEMI patients who were offered pharmacoinvasive treatment predominantly with streptokinase, has resulted in improvement in the angiographic outcomes in the form of reduction in thrombus burden, improvement in TIMI flows and artery patency rates in the post-implementation phase.
Source of funding
The research drew support and funding from the Indian Council of Medical Research (ICMR); Independent grant 5/4/1-14/11-ncd-ii
Funding/support
The Tamil Nadu ST-Segment Elevation Myocardial Infarction (TN-STEMI) programme was supported by independent grant 5/4/1-14/11-NCD-II from the Indian Council for Medical Research.
Role of the funding/sponsor
The TN-STEMI programme did not have a role in the writing of the manuscript or the decision to submit the manuscript for publication. STEMI India, a nonprofit organization dedicated to the improvement of care for patients with STEMI in India, played a role in the development of the study protocol.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Acknowledgments
The authors acknowledge the contributions of all participants of the study, efforts of each member of the TN-STEMI network and the Government of Tamilnadu who are working towards pursuing the goal of delivering timely reperfusion to patients of STEMI.
Appendix A.
| TIMI Flow Grade Classification14 | |
|---|---|
| Grade | Definition |
| 3 (complete reperfusion) | Antegrade flow in the terminal coronary artery segment through a stenosis is as prompt as antegrade flow into a comparable segment proximal to the stenosis. Contrast material clears as rapidly from the distal segment as from an uninvolved, more proximal segment. |
| 2 (partial reperfusion) | Contrast material flows through the stenosis to opacify the terminal artery segment. However, contrast enters the terminal segment perceptibly more slowly than more proximal segments. Alternatively, contrast material clears from a segment distal to a stenosis noticeably more slowly than from a comparable segment not preceded by a significant stenosis. |
| 1 (penetration with minimal perfusion) | A small amount of contrast flows through the stenosis but fails to fully opacify the artery beyond. |
| 0 (no perfusion) | No contrast flow through the stenosis |
Appendix B.
| TIMI Thrombus classification15 | |
|---|---|
| Grade 0 | no cineangiographic characteristics of thrombus are present. |
| Grade 1 | possible thrombus is present, with such angiography characteristics as reduced contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion suggestive but not diagnostic of thrombus; |
| Grade 2 | there is definite thrombus, with greatest dimensions ≤1/2 the vessel diameter. |
| Grade 3 | there is definite thrombus but with greatest linear dimension >1/2 but <2 vessel diameters. |
| Grade 4 | there is definite thrombus, with the largest dimension ≥2 vessel diameters. |
| Grade 5 | there is total occlusion. |
Appendix C.
| TIMI Bleeding Criteria16 |
|---|
| Non-CABG Related Bleeding: |
| 1. Major |
|
| 2. Minor: Clinically overt (including imaging), resulting in hemoglobin drop of 3–<5 g/dL or ≥10% decrease inhaematocrit |
|
| 3. Minimal |
| Any overt bleeding event that does not meet the criteria above or Any clinically overt sign of haemorrhage (including imaging) associated with a <3 g/dL decrease in haemoglobin concentration or <9% decrease in haematocrit |
| Bleeding in the Setting of CABG: |
|
| Chest tube output >2 L within a 24-h period |
Appendix D.
| Clinical events17 | |
|---|---|
| ST elevation | New ST elevation at the J point in two contiguous leads with the cut-points: ≥0.1 mV in all leads other than leads V2–V3 where the following cut-points apply: ≥0.2 mV in men ≥40 years; ≥0.25 mV in men |
| MACCE (Major Adverse Cardio- and Cerebro vascular event) | Composite of death, nonfatal myocardial infarction, stroke |
| Death | Patient died during hospitalization/documentation of death during follow-up |
| Myocardial Infarction | Detection of a rise and/or fall of cardiac biomarker values (preferably cardiac troponin) with at least one value above the 99th percentile URL and with at least one of the following: (i) symptoms of ischemia, or (ii) new or presumed new significant ST-segment–T wave (ST–T) changes or new left bundle branch block, or (iii) development of pathological Q waves in the electrocardiogram, or (iv) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, or (v) identification of an intracoronary thrombus by angiography or autopsy |
| Stroke | Loss of neurological function caused by an ischemic or hemorrhagic event with residual symptoms at least 24 h after onset or leading to death |
| Cardiogenic shock | Hypotension (a systolic blood pressure of less than 90 mmHg for at least 30 min or the need for supportive measures to maintain a systolic blood pressure of greater than or equal to 90 mmHg), end-organ hypoperfusion (cool extremities or a urine output of less than 30 mL/h, and a heart rate of greater than or equal to 60 beats per minute). |
References
- 1.Xavier D., Pais P., Devereaux P.J. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371(April (9622)):1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 2.Alexander T. STEMI in India. Eur Heart J. 2016;37(31):2449–2453. doi: 10.1093/eurheartj/ehw279. [DOI] [PubMed] [Google Scholar]
- 3.Alexander T., Mullasari A.S., Narula J. Developing a STEMI system of care for low- and middle-income countries: the STEMI-India model. Global Heart. 2014;9(December(4)):419–423. doi: 10.1016/j.gheart.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Alexander T., Mehta S., Mullasari A. Systems of care for ST-elevation myocardial infarction in India. Heart. 2012;98(Januray (1)):15–17. doi: 10.1136/heartjnl-2011-301009. [DOI] [PubMed] [Google Scholar]
- 5.Kolh P., Windecker S., Alfonso F. ESC/EACTS guidelines on myocardial revascularization the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur J Cardio-thorac Surg. 2014;46(4):517–592. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 6.O'Gara P.T., Kushner F.G., Ascheim D.D. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary. J Am Coll Cardiol. 2013;61(Januray (4)):485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong P.W., Gershlick A.H., Goldstein P. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction (STREAM investigative team) N Engl J Med. 2013;368(April (15)):1379–1387. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 8.Rashid M.K., Guron N., Bernick J. Safety and efficacy of a PI strategy in ST-segment elevation myocardial infarction: a patient population study comparing a PI strategy with a primary percutaneous coronary intervention strategy within a regional system. JACC Cardiovasc Interv. 2016;9(1October (19)):2014–2020. doi: 10.1016/j.jcin.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Larson D.M., Duval S., Sharkey S.W. Safety and efficacy of a pharmaco-invasive reperfusion strategy in rural ST-elevation myocardial infarction patients with expected delays due to long-distance transfers. Eur Heart J. 2012;33(May (10)):1232–1240. doi: 10.1093/eurheartj/ehr403. [DOI] [PubMed] [Google Scholar]
- 10.Victor S.M., Subban V., Alexander T. A prospective, observational, multicentre study comparing tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI. Open Heart. 2014;1(August (1)):e000133. doi: 10.1136/openhrt-2014-000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor S.M., Vijayakumar S., Alexander T. Two-year follow-up data from the STEPP-AMI study: a prospective, observational, multicenter study comparing tenecteplase-facilitated PCI versus primary PCI in Indian patients with STEMI. Indian Heart J. 2016;68(March–April (2)):169–173. doi: 10.1016/j.ihj.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander T., Victor S.M., Mullasari A.S. Protocol for a prospective, controlled study of assertive and timely reperfusion for patients with ST-segment elevation myocardial infarction in Tamil Nadu: the TN-STEMI programme. BMJ Open. 2013;3(December (12)):e003850. doi: 10.1136/bmjopen-2013-003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander T., Mullasari A.S., Joseph G. A system of care for patients with ST-Segment elevation myocardial infarction in India: the tamil nadu-ST-Segment elevation myocardial infarction program. JAMA Cardiol. 2017;(March 8) doi: 10.1001/jamacardio.2016.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312(April (14)):932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 15.Gibson C.M., de Lemos J.A., Murphy S.A. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001;103(21):2550–2554. doi: 10.1161/01.cir.103.21.2550. [DOI] [PubMed] [Google Scholar]
- 16.Bovill E.G., Terrin M.L., Stump D.C. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med. 1991;115(August (4)):256–265. doi: 10.7326/0003-4819-115-4-256. [DOI] [PubMed] [Google Scholar]
- 17.Cannon C.P., Battler A., Brindis R.G. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee A. Quality improvement in cardiac services in India quo vadis? JAMA Cardiol. 2017 doi: 10.1001/jamacardio.2016.6008. [Publishedonline March 08] [DOI] [PubMed] [Google Scholar]
- 19.Sim D.S., Jeong M.H., Ahn Y. Korea acute myocardial infarction registry (KAMIR) investigators. PI strategy versus primary percutaneous Coronary intervention in patients with ST-segment-elevation myocardial infarction: a propensity score-matched analysis. Circ Cardiovasc Interv. 2016;9(September (9)) doi: 10.1161/CIRCINTERVENTIONS.115.003508. [DOI] [PubMed] [Google Scholar]
- 20.Bailleul C., Puymirat E., Aissaoui N. Factors associated with infarct-related artery patency before primary percutaneous Coronary intervention for ST-elevation myocardial infarction (from the FAST-MI 2010 registry) Am J Cardiol. 2016;117(Januray (1)):17–21. doi: 10.1016/j.amjcard.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Hanssen M., Cottin Y., Khalife K. French registry on acute ST-elevation and non ST-elevation myocardial infarction 2010. FAST-MI 2010. Heart. 2012;98(May (9)):699–705. doi: 10.1136/heartjnl-2012-301700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Califf R.M., White H.D., Van de Werf F. One-year results from the global utilization of streptokinase and TPA for occluded Coronary arteries trial. GUSTO-I Investigators. Circulation. 1996;94(September (6)):1233–1238. doi: 10.1161/01.cir.94.6.1233. [DOI] [PubMed] [Google Scholar]
- 23.Borgia F., Goodman S.G., Halvorsen S. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: a meta- analysis. Eur Heart J. 2010;31:2156. doi: 10.1093/eurheartj/ehq204. [DOI] [PubMed] [Google Scholar]
- 24.Dalal J.J., Alexander T., Banerjee P.S. Cardiocare STEMI experts. 2013 consensus statement for early reperfusion and pharmaco-invasive approach in patients presenting with chest pain diagnosed as STEMI (ST elevation myocardial infarction) in an Indian setting. J Assoc Phys. India. 2014;62(June (6)):473–483. [PubMed] [Google Scholar]
- 25.Alexander T., Mullasari A.S., Kaifoszova Z. Framework for a national STEMI program: consensus document developed by STEMI INDIA, cardiological society of india and association physicians of India. Indian Heart J. 2015;67(September–October (5)):497–502. doi: 10.1016/j.ihj.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guha S., Sethi R., Ray S. Cardiological Society of India: position statement for the management of ST elevation myocardial infarction in India. Indian Heart J. 2017;69:S63–S97. doi: 10.1016/j.ihj.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra S., Ramkrishnan S., Babu A.S. Management algorithms for acute ST elevation myocardial infarction in less industrialized world. Indian Heart J. 2017;69:S98–S103. doi: 10.1016/j.ihj.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]