Abstract
Cancer-induced bone pain (CIBP) is a frequent complication in patients suffering from bone metastases. Previous studies have demonstrated a pivotal role of reactive oxygen species (ROS) in inflammatory and neuropathic pain, and ROS scavengers exhibited potent antinociceptive effect. However, the role of spinal ROS remains unclear. In this study, we investigated the analgesic effect of two ROS scavengers in a well-established CIBP model. Our results found that intraperitoneal injection of N-tert-Butyl-α-phenylnitrone (PBN, 50 and 100 mg/kg) and 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol, 100 and 200 mg/kg) significantly suppressed the established mechanical allodynia in CIBP rats. Moreover, repeated injection of PBN and Tempol showed cumulative analgesic effect without tolerance. However, early treatment with PBN and Tempol failed to prevent the development of CIBP. Naive rats received repetitive injection of PBN and Tempol showed no significant change regarding the nociceptive responses. Finally, PBN and Tempol treatment notably suppressed the activation of spinal microglia in CIBP rats. In conclusion, ROS scavengers attenuated established CIBP by suppressing the activation of microglia in the spinal cord.
Keywords: Cancer-induced bone pain, Reactive oxygen species, PBN, Tempol
Graphical abstract
Highlights
-
•
PBN and Tempol could suppress established mechanical allodynia in CIBP rats.
-
•
Repeated injection of PBN and Tempol showed cumulative analgesic effect.
-
•
PBN and Tempol failed to prevent the development of CIBP.
-
•
PBN and Tempol could suppress the microglia activation in CIBP rats.
1. Introduction
Cancer-induced bone pain (CIBP) is a frequent complication in patients suffering from bone metastases [1], [2]. Currently, the World Health Organization (WHO) analgesic ladder remains the golden standard for the management of CIBP in clinic [3], [4]. However, current therapeutic strategies often provided inadequate pain relief and are associated with numerous unavoidable side effects which limit their prolonged application [5], [6]. Despite marked advance has been made regarding the mechanisms of CIBP, few effective drugs has been developed for the management of CIBP in the past decades. Therefore, further investigations are warranted to uncover the underlying mechanisms of CIBP.
Reactive oxygen species (ROS) are produced as a natural byproduct of normal metabolism and play an important role in cell signaling and homeostasis [7]. There are many types of ROS including hydroxyl radicals, superoxide radicals, nitric oxides, hydrogen peroxides and peroxynitrites [8]. Under normal conditions, the production of ROS is tightly regulated by antioxidant defense systems [9]. However, excessive ROS levels due to increased ROS production and/or decreased antioxidant defense ability leads to lipid peroxidation, protein oxidation, and nucleic acid oxidation [10]. Recently, emerging evidence suggested that ROS scavengers showed potent analgesic effect in rodent models of inflammatory pain and neuropathic pain [11], [12], [13], [14]. However, the role of ROS in CIBP remains largely unknown. Therefore, the present study examined the antinociceptive effect of two ROS scavengers in a well-established CIBP model.
2. Material and methods
2.1. Animals
In the present study, we chose virgin female Sprague-Dawley rats (180–200 g, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, PR China) since female rats are more susceptible to Walker 256 mammary gland carcinoma cells. The animals were housed under controlled conditions (24 ± 0.5 °C, 12 h alternating light-dark cycle, with free access to water and food). All experimental protocols were approved by the Animal Care and Use Committee of Huazhong University of Science & Technology.
2.2. Establishment of CIBP models
Tumor cells were harvested from the ascitic fluid of rats that were inoculated with Walker 256 cells (4 × 107 cells/mL, 1 mL) into the abdominal cavity. Suspensions of tumor cells (4 × 107 cells/mL) in phosphate buffer saline (PBS) were prepared for injection using a hemocytometer. The model of CIBP was performed as described previously [15], [16]. In brief, the right leg was disinfected after anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneal, i.p.). Then, Walker 256 cells (4 × 107 cells/mL, 10 μL) were slowly injected into the tibial cavity using a 10 μL Hamilton syringe. 10 μL PBS was injected instead of tumor cells for the sham group. The injection site was sealed with bone wax once the syringe was removed. Finally, the wound was sealed with 3-0 silk thread.
2.3. Drug administration
N-tert-Butyl-α-phenylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 0.9% saline. The dose of drugs was chosen according to our preliminary results and previous reports [17], [18]. To determine whether ROS scavengers could alleviate CIBP in advanced phase, a single dose of PBN (10, 50, 100 mg/kg, i.p.) or Tempol (10, 100, 200 mg/kg, i.p.) was given on day 14 (14 days) after the establishment of CIBP models. To determine whether repetitive treatment with ROS scavengers had a cumulative analgesic effect on CIBP, PBN (100 mg/kg, i.p.) or Tempol (200 mg/kg, i.p.) was given once daily from 14 days to 18 days. To determine whether early treatment with ROS scavengers could suppress the development of CIBP, PBN (100 mg/kg, i.p.) or Tempol (200 mg/kg, i.p.) was given once daily from 1 days to 5 days. To determine whether ROS scavengers could affect the mechanical sensitivity of naive rats, PBN (10, 50, 100 mg/kg, i.p.) or Tempol (10, 100, 200 mg/kg, i.p.) was given once daily for 5 consecutive days.
2.4. Behavioral tests
Mechanical allodynia was evaluated by measuring ipsilateral hind paw withdrawal threshold (PWT) in response to von Frey filament stimuli as described previously [19], [20]. Briefly, animals were put in individual chambers on a metal mesh floor and allowed to habituate for 30 min. Von Frey filaments were applied to the mid-plantar of the right hind paw for 6 s per filament or until paw withdrawal in ascending order of forces (1, 1.4, 2, 4, 6, 8, 10, and 15 g), starting at 2 g von Frey filament. Positive responses were defined as abrupt paw withdrawal, lickings, and shaking. Once a positive response occurred, the paw was re-tested after a 5 min rest, starting with the next lower von Frey filament. Once no response was observed, the next higher von Frey filament was applied after a 5 min rest. The PWT was defined as the lowest force (in grams) required to elicit a positive response. The investigator who performed the behavioral tests were blinded to the experimental design.
2.5. Immunohistochemistry
Briefly, rats were deeply anesthetized with pentobarbital sodium (60 mg/kg, i.p.) and then intracardially perfused with PBS followed by 4% ice-cold paraformaldehyde (PFA). The L4-L5 segments of spinal cord were removed and post-fixed using the same fixative. The embedded samples were sectioned 20 µm thick in a cryostat (CM1900, Leica, Germany). The sections were penetrated with 0.3% Triton X-100 for 15 min and blocked with 10% donkey serum for 1 h at room temperature (RT). Then, the sections were incubated with goat anti-ionized calcium-binding adapter molecule 1 (Iba1) antibody (microglial marker; 1:300; ab5076; Abcam) overnight at 4 °C. After been washed three times in PBS, the sections were incubated with Alexa Fluor 594-conjugated donkey anti-goat secondary antibody (1:500; 705-585-003; Jackson ImmunoResearch) for 2 h at RT. The sections were then captured using a fluorescence microscope (DM2500, Leica, Germany). The Iba1-immunolabeled surface areas were measured in laminae I-IV of the spinal cord dorsal horn using Image Pro Plus software as described previously [15]. Quantification of the immunoreactivity was accomplished by calculating the percentages of immunostaining ([positive immunofluorescent surface area]/[total measured picture area] × 100). Four rats of each group were used for statistical analysis. The investigator performed the image analyses was blinded to the experimental design.
2.6. Statistical analysis
Data are expressed as mean ± SEM and analyzed using the GraphPad Prism version 5.01 for Windows (Graph Pad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test was used for immunochemistry data. Two-way ANOVA with repeated measures, followed by Bonferroni post hoc test was used for PWT. p < 0.05 was considered statistically significant.
3. Results
3.1. Mechanical allodynia induced by intratibial injection of Walker 256 cells
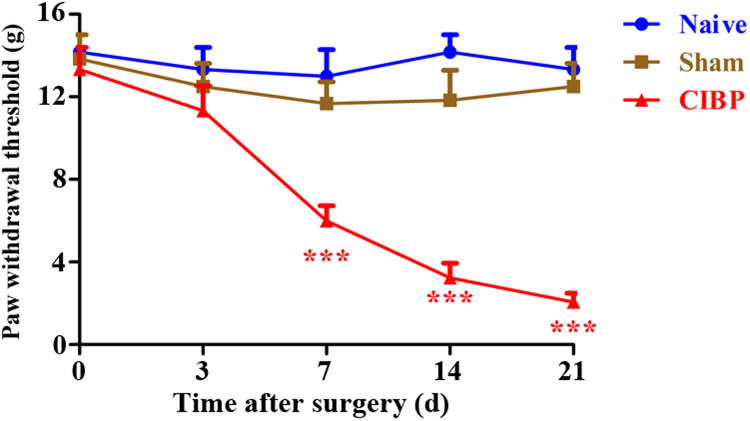
In this study, we used a well-established rat model of CIBP by intratibial injection of Walker 256 cells. To observe the development of mechanical allodynia, ipsilateral PWTs were evaluated at baseline and 3 days, 7 days, 14 days and 21 days. Similar baseline PWTs were observed among all groups. As shown in Fig. 1, the PWTs of ipsilateral hind paw were significantly decreased from 7 days to 21 days in CIBP rats. In contrast, naive and sham rats showed no significant change in PWTs during the 21-day observation period. These results indicate that mechanical allodynia is developed after intratibial injection of Walker 256 cells.
Fig. 1.
The time course of paw withdrawal threshold (PWT) in naive, sham and cancer-induced bone pain (CIBP) rats. The ipsilateral PWTs were significantly decreased from day 7 to day 21 after surgery in CIBP rats (***p < 0.001 compared with the naive group, n = 6 in each group). In contrast, naive and sham rats showed no significant change in PWTs during the 21-day observation period.
3.2. Analgesic effect of a single dose of PBN on established CIBP
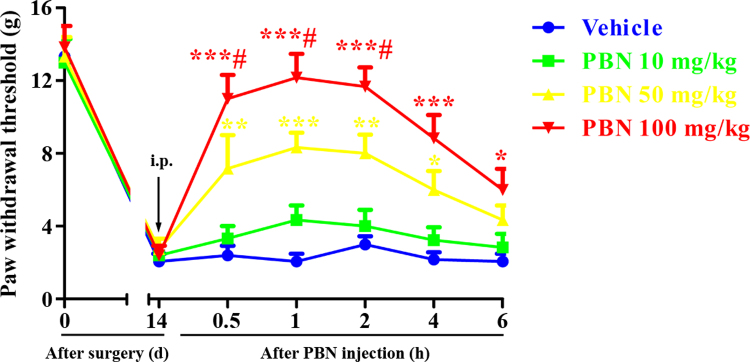
To determine whether PBN could alleviate CIBP in advanced phase, a single dose of PBN (10, 50, 100 mg/kg, i.p.) was given on 14 days. The behavioral tests were conducted at 0, 0.5, 1, 2, 4 and 6 h after PBN injection. As shown in Fig. 2, i.p. injection of PBN at the dose of 10 mg/kg had no significant effect on the PWTs compared with that of the vehicle group. However, i.p. injection of PBN at the dose of 50 and 100 mg/kg markedly increased the PWTs in CIBP rats, indicating alleviation of CIBP. The upregulation of PWTs began at 0.5 h, peaked at 1 h and lasted for at least 6 h. Moreover, significant differences were found between the groups (50 mg/kg vs 100 mg/kg), indicating that the antinociceptive effects of PBN are dose-dependent. These results suggest that a single injection of PBN could attenuate established CIBP in a dose-dependent manner.
Fig. 2.
Analgesic effect of intraperitoneal (i.p.) injection of a single dose of N-tert-Butyl-α-phenylnitrone (PBN) on established cancer-induced bone pain (CIBP). A single dose of PBN (10, 50, 100 mg/kg, i.p.) was given on day 14 after surgery. The behavioral tests were conducted at 0, 0.5, 1, 2, 4 and 6 h after PBN injection. PBN (i.p., 50 and 100 mg/kg) markedly increased the PWTs in CIBP rats, beginning at 0.5 h, peaking at 1 h and lasing for at least 6 h (*p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle group, #p < 0.05 compared with the group treated with PBN 50 mg/kg, n = 6 in each group). However, i.p. injection of PBN at the dose of 10 mg/kg had no significant effect on the PWTs compared with that of the vehicle group (p > 0.05).
3.3. Analgesic effect of repeated administration of PBN on established CIBP
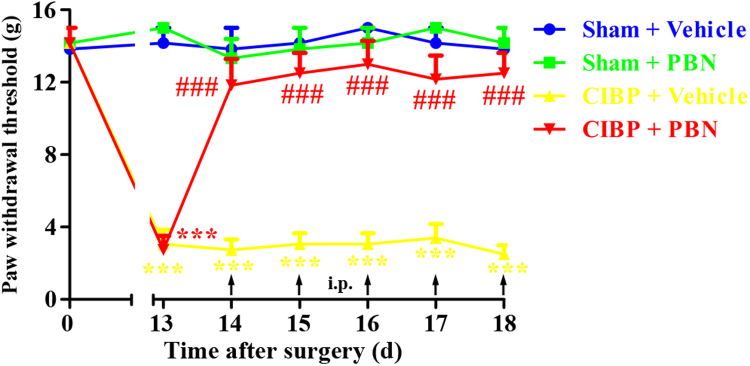
To determine whether repetitive treatment with PBN had a cumulative analgesic effect on CIBP, PBN (100 mg/kg, i.p.) was given once daily from 14 days to 18 days. The behavioral tests were conducted at 13 days and 1 h after PBN injection from 14 days to 18 days. As shown in Fig. 3, repeated injection of PBN (100 mg/kg, i.p.) notably reversed the mechanical allodynia in CIBP rats without signs of tolerance since the analgesic effect of PBN was similar during the treatment period. In contrast, CIBP rats treated with vehicle showed no significant change in PWTs. These results suggest that repetitive administration of PBN (100 mg/kg, i.p.) produce cumulative analgesic effect on CIBP without tolerance.
Fig. 3.
Analgesic effect of repeated intraperitoneal administration of N-tert-Butyl-α-phenylnitrone (PBN) on established cancer-induced bone pain (CIBP). PBN (100 mg/kg, i.p.) was given once daily from day 14 to day 18 after surgery. The behavioral tests were conducted at 13d and 1 h after PBN injection from day 14 to day 18. Repeated injection of PBN (100 mg/kg, i.p.) notably reversed the mechanical allodynia in CIBP rats without signs of tolerance (###p < 0.001 compared with the CIBP + vehicle group). In contrast, CIBP rats treated with vehicle showed no significant change in PWTs (***p < 0.001 compared with the sham + vehicle group).
3.4. Preventive effect of early treatment with PBN on the development of CIBP
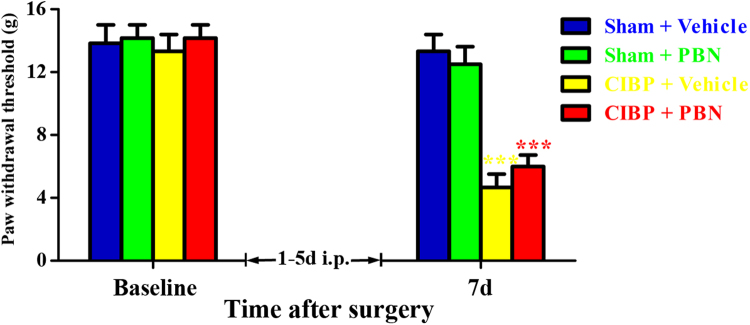
To determine whether early treatment with PBN could suppress the development of CIBP, PBN (100 mg/kg, i.p.) was given once daily from 1 days to 5 days. The behavioral tests were conducted at baseline and 7 days. As shown in Fig. 4, CIBP rats treated with PBN and vehicle showed significantly decreased PWTs at 7 days. Moreover, no significant difference was observed between the groups (CIBP + Vehicle vs CIBP + PBN). These results suggest that early treatment with PBN (100 mg/kg, i.p.) from 1 days to 5 days fail to prevent the development of mechanical allodynia in CIBP rats.
Fig. 4.
Preventive effect of early treatment with N-tert-Butyl-α-phenylnitrone (PBN) on the development of cancer-induced bone pain (CIBP). PBN (100 mg/kg, i.p.) was given once daily from day 1 to day 5. The behavioral tests were conducted at baseline and 7 days. CIBP rats treated with PBN and vehicle showed significantly decreased PWTs at 7d. Moreover, no significant difference was observed between the groups (CIBP + Vehicle vs CIBP + PBN, p > 0.05).
3.5. Analgesic effect of a single dose of Tempol on established CIBP
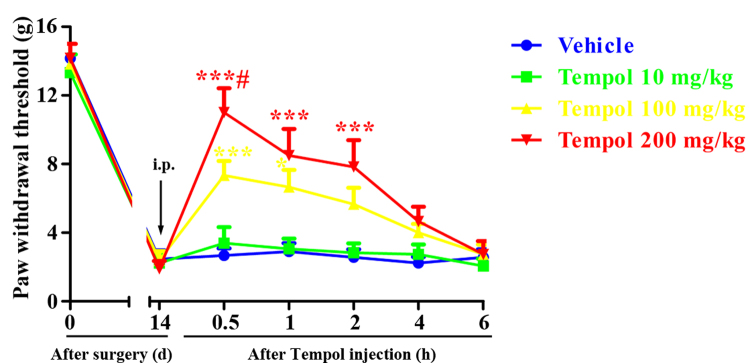
Given that the mechanical allodynia in CIBP rats could be substantially alleviated by a non-specific ROS scavenger, we next examined the analgesic effect of a superoxide selective scavenger Tempol. To determine whether Tempol could attenuate CIBP in advanced phase, a single dose of Tempol (10, 100, 200 mg/kg, i.p.) was given on 14 days. The behavioral tests were conducted at 0, 0.5, 1, 2, 4 and 6 h after Tempol injection. Compared with vehicle group, i.p. injection of Tempol at the dose of 10 mg/kg had no significant effect on the PWTs (Fig. 5). However, i.p. injection of Tempol at the dose of 100 and 200 mg/kg notably upregulated the PWTs in CIBP rats, beginning at 0.5 h, peaking at 1 h and lasting for at least 2 h. Moreover, significant differences were found between the groups (100 mg/kg vs 200 mg/kg), indicating that the analgesic effects of Tempol are dose-dependent. These results suggest that a single injection of Tempol could attenuate established CIBP in a dose-dependent manner.
Fig. 5.
Analgesic effect of intraperitoneal injection of a single dose of Tempol on established cancer-induced bone pain (CIBP). A single dose of Tempol (10, 100, 200 mg/kg, i.p.) was given on day 14 after surgery. The behavioral tests were conducted at 0, 0.5, 1, 2, 4 and 6 h after Tempol injection. i.p. injection of Tempol at the dose of 10 mg/kg had no significant effect on the PWTs (p > 0.05 compared with the vehicle group). However, i.p. injection of Tempol at the dose of 100 and 200 mg/kg notably upregulated the PWTs in CIBP rats, beginning at 0.5 h, peaking at 1 h and lasting for at least 2 h (*p < 0.05, ***p < 0.001 compared with the vehicle group, #p < 0.05 compared with the group treated with Tempol 100 mg/kg, n = 6 in each group).
3.6. Analgesic effect of repeated administration of Tempol on established CIBP
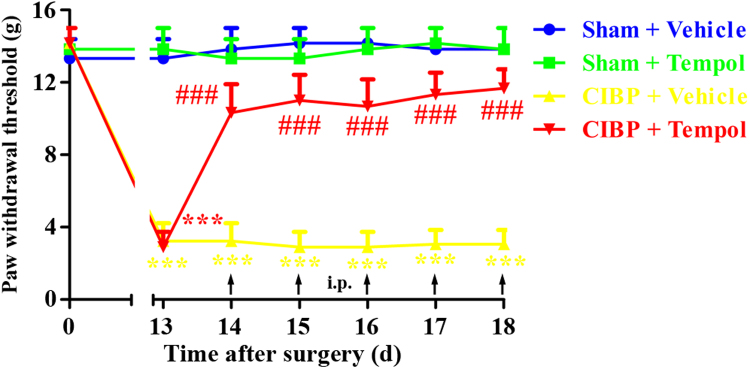
To determine whether repetitive treatment with Tempol had a cumulative analgesic effect on CIBP, Tempol (200 mg/kg, i.p.) was given once daily from 14 days to 18 days. The behavioral tests were conducted at 13 days and 0.5 h after Tempol injection from 14 days to 18 days. As shown in Fig. 6, repeated injection of Tempol (200 mg/kg, i.p.) obviously suppressed the mechanical allodynia in CIBP rats without signs of tolerance since the analgesic effect of Tempol was similar during the treatment period. In contrast, CIBP rats treated with vehicle showed no significant change in PWTs. These results suggest that repetitive administration of Tempol (200 mg/kg, i.p.) produce cumulative analgesic effect on CIBP without tolerance.
Fig. 6.
Analgesic effect of repeated intraperitoneal administration of Tempol on established cancer-induced bone pain (CIBP). Tempol (200 mg/kg, i.p.) was given once daily from day 14 to day 18 after surgery. The behavioral tests were conducted at 13 days and 0.5 h after Tempol injection from day 14 to day 18 after surgery. Repeated injection of Tempol (200 mg/kg, i.p.) obviously suppressed the mechanical allodynia in CIBP rats without signs of tolerance (###p < 0.001 compared with the CIBP + vehicle group). In contrast, CIBP rats treated with vehicle showed no significant change in PWTs (***p < 0.001 compared with the sham + vehicle group).
3.7. Preventive effect of early treatment with Tempol on the development of CIBP
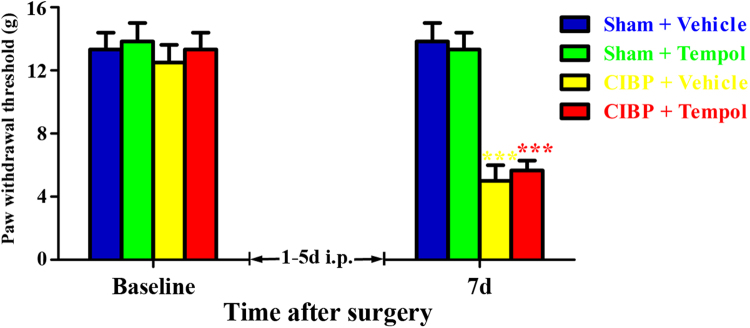
To determine whether early treatment with Tempol could suppress the development of CIBP, Tempol (200 mg/kg, i.p.) was given once daily from 1 days to 5 days. The behavioral tests were conducted at baseline and 7 days. As shown in Fig. 7, CIBP rats treated with Tempol and vehicle showed significantly decreased PWTs at 7 days. Moreover, no significant difference was observed between the groups (CIBP + Vehicle vs CIBP + Tempol). These results suggest that early treatment with Tempol (200 mg/kg, i.p.) from 1 days to 5 days fail to prevent the development of mechanical allodynia in CIBP rats.
Fig. 7.
Preventive effect of early treatment with Tempol on the development of cancer-induced bone pain (CIBP). Tempol (200 mg/kg, i.p.) was given once daily from day 1 to day 5 after surgery. The behavioral tests were conducted at baseline and day 7. CIBP rats treated with Tempol and vehicle showed significantly decreased PWTs at day 7 after surgery. Moreover, no significant difference was observed between the groups (CIBP + Vehicle vs CIBP + Tempol, p > 0.05).
3.8. Effect of ROS scavengers on mechanical sensitivity in naive rats
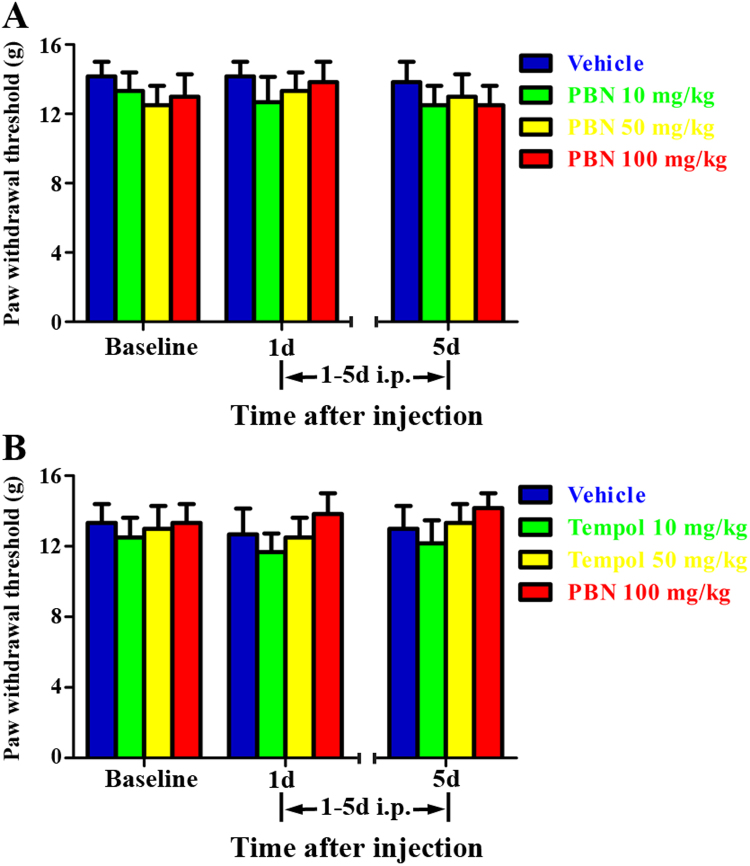
To determine whether ROS scavengers could affect the mechanical sensitivity of naive rats, PBN (10, 50, 100 mg/kg, i.p.) or Tempol (10, 100, 200 mg/kg, i.p.) was given once daily for 5 consecutive days. The behavioral tests were conducted at baseline and 30 min after drug administration at 1 days and 5 days. As shown in Fig. 8A, the PWTs in naive rats showed no significant change after consecutive administration of PBN (10, 50, 100 mg/kg, i.p.) for 5 days. A similar phenomenon was observed in naive rats treated with Tempol (10, 100, 200 mg/kg, i.p.). These results suggest that i.p. injection of ROS scavengers do not affect the mechanical sensitivity in naive rats.
Fig. 8.
Effect of intraperitoneal injection of N-tert-Butyl-α-phenylnitrone (PBN) or Tempol on paw withdrawal threshold in naïve rats. PBN (10, 50, 100 mg/kg, i.p.) or Tempol (10, 100, 200 mg/kg, i.p.) was given once daily for 5 consecutive days. The behavioral tests were conducted at baseline and 30 min after drug administration at day 1 and day 5. (A) The PWTs in naive rats showed no significant change after consecutive administration of PBN (10, 50, 100 mg/kg, i.p., p > 0.05 compared with the vehicle group) for 5 days. (B) A similar phenomenon was observed in naive rats treated with Tempol (10, 100, 200 mg/kg, i.p., p > 0.05 compared with the vehicle group).
3.9. Effect of ROS scavengers on spinal microglia activation in CIBP rats
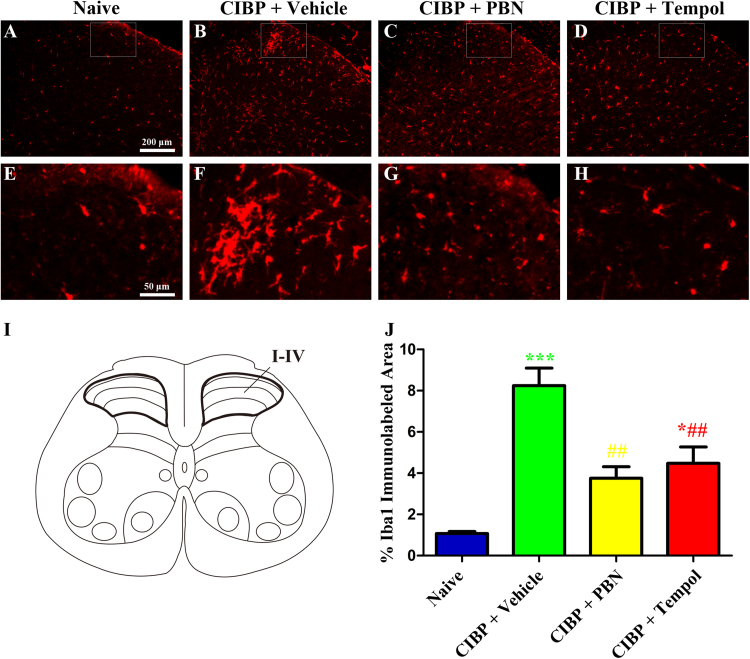
To determine whether ROS scavengers could suppress the activation of microglia in the spinal cord under CIBP condition, PBN (100 mg/kg, i.p.) or Tempol (200 mg/kg, i.p.) was given once daily from 14 days to 18 days. The rats were sacrificed 30 min after the last injection. Immunohistochemistry was used to evaluate the activation of microglia in the spinal cord. The Iba1-positive cells in CIBP rats treated with vehicle (Fig. 9B and F) showed obviously increased immunoreactivity and microgliosis compared with naive rats (Fig. 9A and E). Treatment with PBN (100 mg/kg, i.p., Fig. 9C and G) or Tempol (200 mg/kg, i.p., Fig. 9D and H) notably suppressed the activation of microglia in CIBP rats. These results suggest that ROS scavengers could suppress the activation of microglia in the spinal cord under CIBP condition.
Fig. 9.
Effect of ROS scavengers on spinal microglia activation in CIBP rats. (A-H) Representative photomicrographs showed that ROS scavengers suppressed the activation of microglia in the spinal cord dorsal horn in CIBP rats. PBN (100 mg/kg, i.p.) or Tempol (200 mg/kg, i.p.) was given once daily from day 14 to day 18. The rats were sacrificed 30 min after the last injection. (I) Schematic representation of the spinal cord. (J) Iba1-immunolabeled surface area were quantified from the spinal dorsal horn (laminae I-IV; as indicated in I) using Image Pro Plus software. Quantification of Iba1immunoreactivity was accomplished by calculating the percentages of immunostaining ([positive immunofluorescent surface area]/[total measured picture area] × 100). Four rats of each group were used for statistical analysis (*p < 0.05, ***p < 0.001 compared with naive group. ##p < 0.01 compared with the CIBP + vehicle group).
4. Discussion
In this study, we demonstrated that (1) i.p. injection of a non-specific ROS scavenger PBN reversed the established mechanical allodynia in CIBP rats, (2) i.p. injection of a superoxide selective scavenger Tempol suppressed the established mechanical allodynia in CIBP rats, (3) repeated injection of PBN and Tempol produced cumulative analgesic effect on CIBP without tolerance, (4) early treatment with PBN and Tempol failed to prevent the development of mechanical allodynia in CIBP rats, (5) treatment with PBN and Tempol suppressed the activation of microglia in the spinal cord. Taken together, these results provide the first direct evidence that ROS scavengers are effective in alleviating CIBP in rats.
The involvement of ROS in the pathogenesis of inflammatory pain [21], [22] and neuropathic pain [23], [24] has been repeatedly reported. Moreover, ROS scavengers, superoxide dismutase (SOD) mimetic compound and antioxidants have been demonstrated to exhibit potent analgesic effect in rodent pain models [25], [26], [27], [28]. However, the role of ROS in the development and maintenance of CIBP has not been reported. In the present study, we found that i.p. injection of PBN 100 mg/kg, a non-specific ROS scavenger, alleviated the established mechanical allodynia in CIBP rats for at least 6 h (Fig. 2). Moreover, repetitive administration of PBN (i.p., 100 mg/kg) for 5 days from 14 days to 18 days almost completely reversed established mechanical allodynia in CIBP rats without tolerance (Fig. 3). It is noteworthy to mention that Fidanboylu et al. reported that repeated PBN administration showed analgesic tolerance in paclitaxel-induced painful neuropathy [29]. The discrepancy may be due to different animal model, sex and experimental design. Given that the mechanical allodynia in CIBP rats could be substantially alleviated by a non-specific ROS scavenger, we also examined the analgesic effect of a superoxide selective scavenger Tempol. Similarly, i.p. injection of Tempol 200 mg/kg attenuated the established mechanical allodynia in CIBP rats for at least 2 h (Fig. 5). Likewise, repetitive administration of Tempol (i.p., 200 mg/kg) for 5 days from 14 days to 18 days produced cumulative analgesic effect on CIBP (Fig. 6). Our results also demonstrated that PBN exhibited stronger analgesic potency and longer duration time compared with that of Tempol. Therefore, we may conclude that the maintenance of CIBP are not the result of overproduction of superoxide anion alone.
Previously, several studies have reported that ROS also contributed to the development of neuropathic pain since early treatment with ROS scavengers almost completely blocked the onset of mechanical allodynia [17], [18], [29]. To determine the prophylactic effect of ROS scavengers on CIBP, PBN (100 mg/kg, i.p.) or Tempol (200 mg/kg, i.p.) was given once daily from 1 days to 5 days. Unexpectedly, both PBN and Tempol failed to prevent the development of CIBP (Fig. 4, Fig. 7). The reasons remain to be elucidated. Although CIBP share some common mechanisms with inflammatory and neuropathic pain, there are distinctive aspects underlying the mechanisms of CIBP. Previous studies have demonstrated that early activation of microglia in the spinal cord contributed to the development rather than the maintenance of neuropathic pain [30], [31]. However, our recent study and others demonstrated that the marked spinal activation of microglia was not observed at 7 days (early phase), but at 14 days (advanced phase) and 21 days (late phase) in CIBP rats [32], [33], [34]. Moreover, early repeated administration of minocycline, a selective microglia inhibitor, failed prevent the development of CIBP [33]. Additionally, upregulation of ROS mainly occurred in neuron and microglia [13]. Therefore, it is possible that ROS scavengers may alleviated established CIBP by inhibiting the activation of spinal microglia. To confirm this hypothesis, we treated CIBP rats with PBN (100 mg/kg, i.p.) or Tempol (200 mg/kg, i.p.) once daily for 5 days from 14 days to 18 days. As expected, our immunohistochemistry results showed that both PBN and Tempol notably suppressed the activation of microglia in the spinal cord (Fig. 9). Finally, to determine whether ROS scavengers could affect the mechanical sensitivity of naive rats, PBN (10, 50, 100 mg/kg, i.p.) or Tempol (10, 100, 200 mg/kg, i.p.) was given once daily for 5 consecutive days. Our behavioral study showed that i.p. injection of ROS scavengers do not affect the mechanical sensitivity in naive rats (Fig. 8).
Although emerging evidence demonstrating the critical role of ROS in pain facilitation, the exact primary source of ROS production under different pain models remains elusive. There are multiple sources of ROS production in the central nervous system such as mitochondria, NADPH oxidase, nitric oxide synthase, etc [35]. Previously, Park et al. reported that ROS was primarily produced in the mitochondria of dorsal horn neurons using a neuropathic pain model established by L5 spinal nerve ligation [36]. In another study, Schwartz et al. found that ROS accumulation was also observed primarily in the mitochondria of dorsal horn neurons after intradermal capsaicin injection in mice [37]. Their further study demonstrated that mitochondrial antioxidant superoxide dismutase (SOD-2) was a critical determinant for mitochondrial ROS accumulation in the neurons and subsequent capsaicin-induced secondary hyperalgesia [38]. Moreover, increased production of mitochondrial ROS by intrathecal injection of electron transport complex inhibitors induced pain behaviors in naive mice [39]. These studies indicated an important role of mitochondrial ROS in the neurons in nociception. In a rat model of neuropathic pain produced by T10 spinal cord injury, Gwak et al. found increased ROS production in neurons and microglia in the L4-L5 spinal cord dorsal horn [13]. Additionally, they demonstrated that ROS contributed to neuropathic pain and locomotor dysfunction via activation of neuronal calcium/calmodulin-dependent protein kinase II (CaMKII) by phosphorylation. A recent study performed by Kallenborn-Gerhardt et al. provided evidence that NADPH oxidase 4 (Nox4) was expressed in a subset of nonpeptidergic nociceptors and myelinated dorsal root ganglia (DRG) neurons, and mice lacking Nox4 showed attenuated injury-induced ROS production and neuropathic pain [40]. Interestingly, Kim et al. demonstrated that Nox2-derived ROS in spinal cord microglia was a significant driver of peripheral nerve injury-induced neuropathic pain [41]. In this study, we demonstrated that ROS scavengers could suppressed the activation of microglia in the spinal cord of CIBP rats (Fig. 9). However, whether microglia is the major source of ROS under CIBP condition and how ROS are involved in CIBP remain to be elucidated.
In summary, this study demonstrated that i.p. injection of PBN and Tempol significantly suppressed established mechanical allodynia in CIBP rat, and repetitive administration showed cumulative analgesic effect without tolerance. However, early treatment with PBN and Tempol failed to prevent the development to CIBP. The analgesic effect of PBN and Tempol on advanced CIBP might be due to suppression of the activation of microglia in the spinal cord. We, thus, conclude that ROS are involved in the maintenance rather than the initiation of CIBP, and ROS scavengers might be useful in attenuating established CIBP in clinic.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (Grant nos. 81400917, 81371250 and 81571053). Fei Chen and Da-Wei Ye contributed equally as senior authors.
Acknowledgments
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154(Suppl 1):S54–S62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y.Q., Gao H.Y., Guan X.H., Yuan X., Fang G.G., Chen Y., Ye D.W. Chemokines and their receptors: potential therapeutic targets for bone cancer pain. Curr. Pharm. Des. 2015;21(34):5029–5033. doi: 10.2174/1381612821666150831141931. [DOI] [PubMed] [Google Scholar]
- 3.Mantyh P.W. Bone cancer pain: from mechanism to therapy. Curr. Opin. Support. Palliat. Care. 2014;8(2):83–90. doi: 10.1097/SPC.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y.Q., Liu Z., Liu Z.H., Chen S.P., Li M., Shahveranov A., Ye D.W., Tian Y.K. Interleukin-6: an emerging regulator of pathological pain. J. Neuroinflamm. 2016;13(1):141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y.Q., Liu D.Q., Chen S.P., Sun J., Zhou X.R., Luo F., Tian Y.K., Ye D.W. Cellular and molecular mechanisms of calcium/calmodulin-dependent protein kinase II in chronic pain. J. Pharmacol. Exp. Ther. 2017;363(2):176–183. doi: 10.1124/jpet.117.243048. [DOI] [PubMed] [Google Scholar]
- 6.Song Z.P., Xiong B.R., Guan X.H., Cao F., Manyande A., Zhou Y.Q., Zheng H., Tian Y.K. Minocycline attenuates bone cancer pain in rats by inhibiting NF-kappaB in spinal astrocytes. Acta Pharmacol. Sin. 2016;37(6):753–762. doi: 10.1038/aps.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moris D., Spartalis M., Tzatzaki E., Spartalis E., Karachaliou G.S., Triantafyllis A.S., Karaolanis G.I., Tsilimigras D.I., Theocharis S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann. Transl. Med. 2017;5(16):324. doi: 10.21037/atm.2017.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhou T., Chuang C.C., Zuo L. Molecular characterization of reactive oxygen species in myocardial ischemia-reperfusion injury. BioMed. Res. Int. 2015;2015:864946. doi: 10.1155/2015/864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winyard P.G., Moody C.J., Jacob C. Oxidative activation of antioxidant defence. Trends Biochem. Sci. 2005;30(8):453–461. doi: 10.1016/j.tibs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Khattab M.M. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur. J. Pharmacol. 2006;548(1–3):167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.K., Park S.K., Zhou J.L., Taglialatela G., Chung K., Coggeshall R.E., Chung J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111(1–2):116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Gwak Y.S., Hassler S.E., Hulsebosch C.E. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 2013;154(9):1699–1708. doi: 10.1016/j.pain.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Lee D.Z., Chung J.M., Chung K., Kang M.G. Reactive oxygen species (ROS) modulate AMPA receptor phosphorylation and cell-surface localization in concert with pain-related behavior. Pain. 2012;153(9):1905–1915. doi: 10.1016/j.pain.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y.Q., Chen S.P., Liu D.Q., Manyande A., Zhang W., Yang S.B., Xiong B.R., Fu Q.C., Song Z.P., Rittner H., Ye D.W., Tian Y.K. The role of spinal GABAB receptors in cancer-induced bone pain in rats. J. Pain: Off. J. Am. Pain Soc. 2017;18(8):933–946. doi: 10.1016/j.jpain.2017.02.438. [DOI] [PubMed] [Google Scholar]
- 16.Fu Q., Shi D., Zhou Y., Zheng H., Xiang H., Tian X., Gao F., Manyande A., Cao F., Tian Y., Ye D. MHC-I promotes apoptosis of GABAergic interneurons in the spinal dorsal horn and contributes to cancer induced bone pain. Exp. Neurol. 2016;286:12–20. doi: 10.1016/j.expneurol.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.K., Hwang S.H., Abdi S. Tempol ameliorates and prevents mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain. Front. Pharmacol. 2016;7:532. doi: 10.3389/fphar.2016.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.K., Zhang Y.P., Gwak Y.S., Abdi S. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology. 2010;112(2):432–439. doi: 10.1097/ALN.0b013e3181ca31bd. [DOI] [PubMed] [Google Scholar]
- 19.Bu H., Shu B., Gao F., Liu C., Guan X., Ke C., Cao F., Hinton A.O., Jr., Xiang H., Yang H., Tian X., Tian Y. Spinal IFN-gamma-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res. Treat. 2014;143(2):255–263. doi: 10.1007/s10549-013-2807-4. [DOI] [PubMed] [Google Scholar]
- 20.Guan X., Fu Q., Xiong B., Song Z., Shu B., Bu H., Xu B., Manyande A., Cao F., Tian Y. Activation of PI3Kgamma/Akt pathway mediates bone cancer pain in rats. J. Neurochem. 2015;134(3):590–600. doi: 10.1111/jnc.13139. [DOI] [PubMed] [Google Scholar]
- 21.Ibi M., Matsuno K., Shiba D., Katsuyama M., Iwata K., Kakehi T., Nakagawa T., Sango K., Shirai Y., Yokoyama T., Kaneko S., Saito N., Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J. Neurosci.: Off. J. Soc. Neurosci. 2008;28(38):9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z.Q., Porreca F., Cuzzocrea S., Galen K., Lightfoot R., Masini E., Muscoli C., Mollace V., Ndengele M., Ischiropoulos H., Salvemini D. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004;309(3):869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 23.Hassler S.N., Johnson K.M., Hulsebosch C.E. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J. Neurochem. 2014;131(4):413–417. doi: 10.1111/jnc.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yowtak J., Lee K.Y., Kim H.Y., Wang J., Kim H.K., Chung K., Chung J.M. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152(4):844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Cesare Mannelli L., Bani D., Bencini A., Brandi M.L., Calosi L., Cantore M., Carossino A.M., Ghelardini C., Valtancoli B., Failli P. Therapeutic effects of the superoxide dismutase mimetic compound MnIIMe2DO2A on experimental articular pain in rats. Mediat. Inflamm. 2013;2013:905360. doi: 10.1155/2013/905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X., Chen C., Ke Q., Tang H., Hou J., Fang W. EGb761 ameliorates neuropathic pain by scavenging reactive oxygen species. Pharmacology. 2015;95(5–6):293–299. doi: 10.1159/000430769. [DOI] [PubMed] [Google Scholar]
- 27.Mao Y.F., Yan N., Xu H., Sun J.H., Xiong Y.C., Deng X.M. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res. 2009;1248:68–75. doi: 10.1016/j.brainres.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Cochran V., Abdi S., Chung J.M., Chung K., Kim H.K. Phenyl N-t-butylnitrone, a reactive oxygen species scavenger, reduces zymosan-induced visceral pain in rats. Neurosci. Lett. 2008;439(2):216–219. doi: 10.1016/j.neulet.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Fidanboylu M., Griffiths L.A., Flatters S.J. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One. 2011;6(9):e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghavendra V., Tanga F., DeLeo J.A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 31.Ledeboer A., Sloane E.M., Milligan E.D., Frank M.G., Mahony J.H., Maier S.F., Watkins L.R. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Song Z., Xiong B., Zheng H., Manyande A., Guan X., Cao F., Ren L., Zhou Y., Ye D., Tian Y. STAT1 as a downstream mediator of ERK signaling contributes to bone cancer pain by regulating MHC II expression in spinal microglia. Brain Behav. Immun. 2017;60:161–173. doi: 10.1016/j.bbi.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y., Li H., Li T.T., Luo H., Gu X.Y., Lu N., Ji R.R., Zhang Y.Q. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J. Neurosci.: Off. J. Soc. Neurosci. 2015;35(20):7950–7963. doi: 10.1523/JNEUROSCI.5250-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y.Q., Liu Z., Liu H.Q., Liu D.Q., Chen S.P., Ye D.W., Tian Y.K. Targeting glia for bone cancer pain. Expert Opin. Ther. Targets. 2016;20(11):1365–1374. doi: 10.1080/14728222.2016.1214716. [DOI] [PubMed] [Google Scholar]
- 35.Kishida K.T., Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid. Redox Signal. 2007;9(2):233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park E.S., Gao X., Chung J.M., Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci. Lett. 2006;391(3):108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz E.S., Lee I., Chung K., Chung J.M. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138(3):514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz E.S., Kim H.Y., Wang J., Lee I., Klann E., Chung J.M., Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J. Neurosci.: Off. J. Soc. Neurosci. 2009;29(1):159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H.Y., Chung J.M., Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: the effect of mitochondrial electron transport complex inhibitors. Neurosci. Lett. 2008;447(1):87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallenborn-Gerhardt W., Schroder K., Del Turco D., Lu R., Kynast K., Kosowski J., Niederberger E., Shah A.M., Brandes R.P., Geisslinger G., Schmidtko A. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci.: Off. J. Soc. Neurosci. 2012;32(30):10136–10145. doi: 10.1523/JNEUROSCI.6227-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D., You B., Jo E.K., Han S.K., Simon M.I., Lee S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA. 2010;107(33):14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]