Abstract
The nose-brain pathway is a potential route for drug delivery as it bypasses the brain barriers. The main objective of this study was to investigate the efficacy of peppermint oil in enhancing the bioavailability of intranasally administered neurotrophins like nerve growth factor (NGF). The effect of different concentrations of peppermint oil (PO) on the delivery of NGF across bovine olfactory epithelium was studied in vitro using Franz diffusion cells. Trans-olfactory epithelial electrical resistance (TEER) was measured to assess the permeability status of the bovine olfactory epithelium. The bioavailability of intranasally administered formulations in rat hippocampus was studied by carrying out brain microdialysis in male Sprague-Dawley rats. Peppermint oil at concentrations of 0.05, 0.1 and 0.5% v/v enhanced the in vitro transport of NGF by 5, 7 and 8 fold, respectively. In vivo studies employing brain microdialysis in rats demonstrated that intranasal administration of NGF formulation with 0.5% PO enhanced the bioavailability by ~8 fold compared to rats administered with NGF alone. The bioavailability of NGF in the brain could be enhanced by intranasal administration of peppermint oil.
1. Introduction
Neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and stroke are caused by degeneration of neurons in the brain (Dawbarn and Allen 2003). Endogenous levels of neurotrophins (NTs) become depleted during the neurodegenerative disorders. Therefore, restoration of the depleted endogenous NTs is one of the approaches to treat neurodegenerative disorders. The treatment with NTs has been a challenging task due to the lack of suitable delivery techniques which can bypass the blood-brain and blood-cerebrospinal fluid barriers. Currently practised methods of administration of NTs are invasive (intraparenchymal or intracerebroventricular or intrathecal administration) (Kordower et al. 1999). The nose to brain pathway has been explored as a potential route for targeting drugs to the brain, as it provides direct access, bypassing the blood brain barrier (Vaka et al. 2009; Baker and Spencer 1986; Frey et al. 1997; Hanson and Frey 2007; Shilpley 1985). This route allows frequent administration of drugs and is certainly more patient compliant than the currently followed invasive routes. Intranasally administered therapeutic agents are known to reach the brain via the olfactory pathway. However, this pathway is associated with a major limiting factor, the olfactory epithelial barrier. A natural barrier modulating agent, peppermint oil, has been found to improve the delivery of therapeutic agents by safe and transient permeabilization of the olfactory epithelium. The sensory and supporting cells of the olfactory epithelium form tight junctions with one another, regulating the movement of molecules across the olfactory pathway. It is most likely that peppermint oil opens the tight junction to enhance the transport of therapeutic agents.
Nerve growth factor (NGF), a member of the neurotrophin family synthesized primarily in the hippocampus and neocortex of the brain, plays a significant role in promoting growth, differentiation and function of both peripheral ganglion cells and central cholinergic neurons of the basal forebrain (Martinez et al. 1985; Krewson et al. 1995; Frey et al. 1997). NGF is a hydrophilic, dimeric protein of ~30kDa and is not bioavailable to the brain on systemic administration due to the brain barriers (Thorne and Frey 2001; Chen et al. 1998). The main objective of this study was to investigate the effect of peppermint oil (PO) as a permeation enhancer on brain uptake of intranasally administered NGF.
2. Investigations and results
2.1. In vitro permeation of NGF
PO at concentrations of 0.05, 0.1 and 0.5% v/v enhanced the in vitro permeation of NGF across the olfactory epithelium by 5,7 and 8 fold respectively over the control (1.21 ± 0.19ng/cm2) (Fig. 1). The transepithelial electrical resistance (TEER) dropped significantly by ~ 20.12 ± 2.13 % after 1–2h indicating the ability of PO to permeabilize the olfactory epithelial membrane.
Fig. 1.
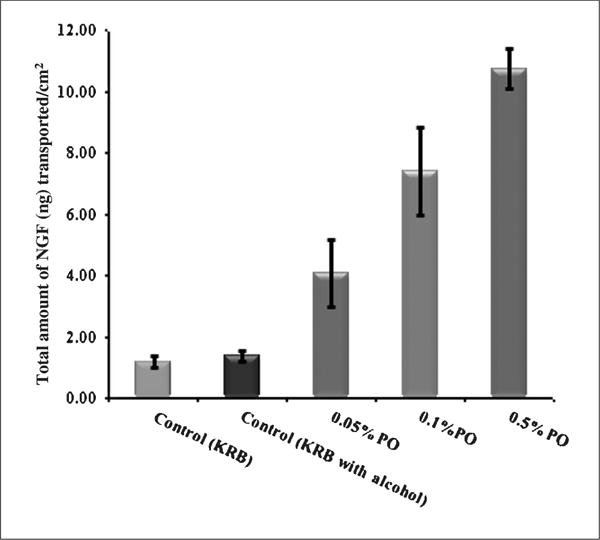
Effect of different concentrations of peppermint oil on in vitro permeability of NGF across the bovine olfactory epithelium. The data points provided are an average of four trails and error bars represent SEM
2.2. In vivo brain microdialysis
In vitro recovery of the microdialysis probe was 4.2 ± 0.3%. The basal value of unbound NGF in rat hippocampus (group 1) in our study was found to be 163.31 ± 3.36 pg/ml. The Cmax in the case of group 2 was 97.38 ± 10.66 pg/ml (baseline adjusted value) and the AUC0–6h was 209.79 ± 14.06 pg h/ml. In group 3, in which the formulation containing NGF + 0.5% PO was administered intranasally, the NGF levels were significantly higher than in groups 1 and 2. The Cmax was 446.39 ± 87.89 pg/ml (baseline adjusted value) and the AUC0–6 was 1787.85 ± 243.78 pg h/ml.
3. Discussion
The in vitro permeation of NGF was significantly enhanced due to a significant drop in TEER with the use of PO. However, there was no significant difference in the fall in TEER with different concentrations of PO. Interestingly, when the PO solution was replaced with KRB in donor, the TEER recovered completely within 2h. The recovery of TEER indicates the mechanism by which PO permeabilizes the epithelium without damaging the tissue. Similar observations were made with chitosan in the case of other biological membranes, where the authors (Smith et al. 2004; Dodane et al. 1999; Narai et al. 1997) inferred that such a phenomenon occurred due to the opening and closing of tight junctions during the permeabilization and recovery phases respectively. Based on these observations, one could speculate that PO could be acting as a drug permeation enhancer across the olfactory epithelium by opening the tight junctions.
Microdialysis is a method generally used to sample drugs from the perineural fluid of the brain (Chang et al. 2006; Bradberry 2000). By carrying out brain microdialysis in the rat hippocampus, the basal values of unbound NGF representing the endogenous levels in the perineural fluid were determined. The basal values observed for group 1 were 1/8th of the total NGF reported by other research groups, as they referred to bound NGF from the whole brain tissue homogenate rather than unbound NGF (Chen et al. 1998; Lapchak et al. 1993; Fawcett et al. 1999; Honer et al. 1996).
In case of group 2, in which the NGF formulation was administered via the intranasal route, NGF levels in the dialysate were raised significantly above the basal values, (Hanson and Frey 2007; Chen et al. 1998). The AUC0-t which is indicative of the bioavailability of the drug to the brain, was ~8 fold higher in the case of group 3 than in group 2 (Fig. 2). The bioavailability of NGF following i.v administration (group 4) was insignificant, indicating the ability of the brain barriers to prevent brain uptake of NGF. This observation is in agreement with Chen et al. who also reported that no detectable levels of rhNGF were found in rat hippocampus following i.v administration.
Fig. 2.
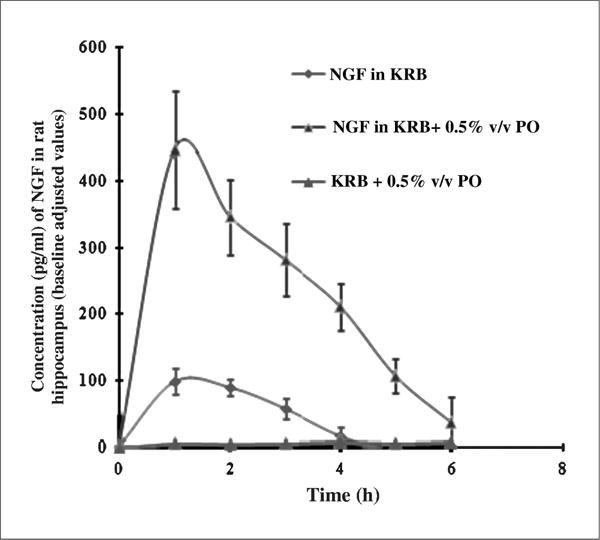
Concentration time profile of NGF in rat hippocampus following intranasal administration. The data points represent baseline adjusted values and are averages of three animals with SEM as error bars
These results provide evidence for our hypothesis that the bioavailability of NGF in the brain could be enhanced by intranasal administration of peppermint oil. The plausible mechanism of action of peppermint oil involves the opening of tight junctions. However, further studies need to be carried to demonstrate the safety and tolerability of intranasal administration of peppermint oil.
4. Experimental
4.1. Materials
NGF, 2.5 S, murine, and NGF Emax ImmunoAssay system were purchased from Promega Corporation (Madison, WI). The bovine olfactory epithelium was purchased from Pel-Freez Biologicals (Rogers, AR). Krebs Ringer bicarbonate buffer, peppermint oil and all other chemicals were procured from Sigma Chemicals (St. Louis,MO).
4.2. In vitro experimental set up
The in vitro permeation studies were carried out using bovine olfactory epithelium (Pel-Freez Biologicals, Rogers, AR) sandwiched between two compartments of a Franz diffusion cell (Logan Instruments, Somerset, NJ) such that the olfactory epithelium side was in contact with the upper donor compartment and the ventral side with the receiver compartment. Bovine olfactory epithelium has been reported to be a fairly close model for human olfactory mucosa due to the similarity in transepithelial electrical resistance (TEER) and metabolic enzyme profiles (Kandimalla et al. 2005; Schmidt et al. 2000). In order to determine the effect of peppermint oil (PO) on the permeability status of the olfactory epithelium, the trans-olfactory epithelial electrical resistance was monitored in the presence and absence of PO using an electrical set up consisting of a wave form generator and multimeter (Agilent Technologies, Santa Clara, CA).
4.3. Permeation studies across bovine olfactory epithelium, in vitro
The donor and receiver compartments were filled with NGF solution (100 μl of 100 μg/ml) and 5 ml of Krebs Ringer bicarbonate buffer (KRB) respectively. The active diffusion area was 0.64 cm2. Different concentrations of PO solutions were prepared by dissolving the required quantities of PO in 70% ethanol. The effect of different concentrations of PO (0.05, 0.1 and 0.5% v/v) on the permeation of NGF (Promega Corporation, Madison, WI) across bovine olfactory epithelium was studied by mixing these solutions with the NGF solution. A control set of experiments were run without incorporation of PO in the donor solution. Samples were collected from the receiver compartment after 2h and assayed by ELISA using an NGF Emax ImmunoAssay system (Promega Corporation, Madison, WI).
4.4. Brain microdialysis, in vivo
In vitro calibration of microdialysis probes (CMA 12) was carried out according to a published procedure (Vaka et al. 2009). The bioavailability of intranasally administered NGF in rat hippocampus was studied by carrying out brain microdialysis in male, Sprague-Dawley rats (250–300g; Harlan Company, Indianapolis, IN) under anesthesia [ketamine (80mg/kg) + xylazine (10mg/kg)] for a period of 6h. The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi (Protocol # 07–025). The rats were divided into 4 groups (n = 3). The formulations were administered directly into the posterior segment of the nose using a microsyringe connected to a soft polymer capillary. PO alone (0.5% v/v in KRB) was administered intranasally to the first group of rats (vehicle control group). NGF solution (100 μg/mL prepared in 100 μL of KRB) and NGF + 0.5% PO solution were administered intranasally to the 2nd and 3rd groups of rats, respectively. To the 4th group of rats, an NGF dose equivalent to that administered intranasally was given by the i.v route. The rats were secured on a stereotaxic frame (Harvard Instruments, Holliston, MA) and brain microdialysis was carried out by inserting the probe into the hippocampal region (anterior-posterior = 5.6 mm, medio-lateral = 5 mm, dorso-ventral = 7 mm, from bregma). The microdialysis probes were equilibrated by perfusing KRB at the rate of 2 μL/min using a microinjection pump for a period of 1h. Following intranasal administration of different formulations the dialysate samples were collected at hourly interval for a period of 6h and assayed by ELISA.
4.5. Data analysis
The statistical analysis was carried out using Graph pad Prism 5 software. The t-test was selected as the test of significance; p<0.05 was considered statistically significant.
Acknowledgments
The project was funded by Grant # 5P20RR021929 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We thank Dr. Karen E. Sabol and Dr. Premalatha Balachandran for technical help.
References
- Baker H, Spencer RF. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res. 1986;63:461–473. doi: 10.1007/BF00237470. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Applications of microdialysis methodology in nonhuman primates: practice and rationale. Crit Rev Neurobiol. 2000;14:143–163. [PubMed] [Google Scholar]
- Chang Q, Savage LM, Gold PE. Microdialysis measures of functional increases in Ach release in the hippocampus with and without inclusion of acetylcholinesterase inhibitors in the perfusate. J Neurochem. 2006;97:697–706. doi: 10.1111/j.1471-4159.2006.03765.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Fawett JR, Rahman Y, Ala TA, Frey WH. Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis. 1998;1:35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol. 2003;29:211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Dodane V, Khan MA, Merwin J. Effect of chitosan on Caco-2 on epithelial permeability and structure. Int J Pharm. 1999;182:21–32. doi: 10.1016/s0378-5173(99)00030-7. [DOI] [PubMed] [Google Scholar]
- Fawcett JR, Chen X, Rahaman Y, Frey WH. Previously reported nerve growth factor levels are underestimated due to an incomplete release from receptors and interaction with standard curve media. Brain Res. 1999;842:206–210. doi: 10.1016/s0006-8993(99)01817-x. [DOI] [PubMed] [Google Scholar]
- Frey WH, Liu L, Chen XQ, Thorne RG, Faweett JR, Ala TA, Rahman YE. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. [Google Scholar]
- Hanson LR, Frey WH., II Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune Pharmacol. 2007;2:81–86. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- Honer MC, Hewitt E, Conner JM, Costello JW, Varon S. Nerve growth factor content in adult rat brain tissue is several-fold higher than generally reported and is largely associated with sedimentable fractions. Brain Res. 1996;728:47–56. [PubMed] [Google Scholar]
- Kandimalla KK, Donovan MD. Carrier mediated transport of chlorpheniramine and chlor-cyclizine across bovine olfactory mucosa: implications on nose-to-brain transport. J Pharm Sci. 2005;3:613–624. doi: 10.1002/jps.20284. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Palfi S, Chen EY. Clinicopathological findings following intraventricular glialderived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Krewson CE, Klarman ML, Saltzman WM. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 1995;680:196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Hefti F. Emerging pharmacology of nerve growth factor. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:851–860. doi: 10.1016/0278-5846(91)90013-q. [DOI] [PubMed] [Google Scholar]
- Martinez HJ, Dreyfus CF, Jonakait GM, Black IB. Nerve growth factor promotes cholinergic development in brain striatal cultures. Neurobiology. 1985;82:7777–7781. doi: 10.1073/pnas.82.22.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narai A, Arai S, Shimizu M. Rapid decrease in transepithelial electrical resistance of human intestinal Caco-2 cell monolayers by cytotoxic membrane perturbents. Toxicol In Vitro. 1997;11:347–354. doi: 10.1016/s0887-2333(97)00026-x. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Simmen D, Hilbe M, Boderke P, Ditzinger G, Sandow J, Lang S, Rubas W, Merkle HP. Validation of excised bovine nasal mucosa as in in vitro model to study drug transport and metabolic pathways in nasal epithelium. J Pharm Sci. 2000;89:396–407. doi: 10.1002/(SICI)1520-6017(200003)89:3<396::AID-JPS10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Shilpley MT. Transport of molecules from nose to brain: Transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res Bull. 1985;15:129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm Res. 2004;21:43–49. doi: 10.1023/b:pham.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Frey WH. Delivery of neurotrophic factors to the central nervous system. pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- Vaka SRK, Sammeta SM, Day LB, Murthy SN. Delivery of nerve growth factor to brain via intranasal administration and enhancement of brain uptake. J Pharm Sci. 2009;98:3640–3646. doi: 10.1002/jps.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]


