Abstract
Background
Interval timing, the ability to judge the duration of short events, has been shown to be compromised in Autism Spectrum Disorders (ASD). Timing abilities are ubiquitous and underlie behaviours as varied as sensory integration, motor coordination or communication. It has been suggested that atypical temporal processing in ASD could contribute to some of the disorder's symptoms, in particular motor clumsiness and difficulties in social interaction and communication. Recent behavioural investigations have suggested that interval timing in ASD is characterised by intact sensitivity but reduced precision in duration judgements.
Methods
In this study we investigated the processing of duration as compared to pitch in a group of high-functioning individuals with ASD using magnetoencephalography (MEG). 18 adolescents and adults with ASD and 18 age- and IQ-matched typically-developing control (TDC) individuals compared two consecutive tones according to their duration or pitch in separate experimental blocks. The analysis was carried out exclusively on physically identical stimuli (500 Hz tones lasting 600 ms), which served, according to instruction, as standard or probe in a Duration or Pitch task respectively.
Results
Our results suggest that compared to TDC individuals, individuals with ASD are less able to predict the duration of the standard tone accurately, affecting the sensitivity of the comparison process. In addition, contrary to TDC individuals who allocate resources at different times depending on the nature of the task (pitch or duration discrimination), individuals with ASD seem to engage less resources for the Duration task than for the Pitch task regardless of the context. Although individuals with ASD showed top-down adaptation to the context of the task, this neuronal strategy reflects a bias in the readiness to perform different types of tasks, and in particular a diminished allocation of resources to duration processing which could have cascading effect on learning and development of other cognitive functions.
Abbreviations: ASD, Autism Spectrum Disorder; TDC, typically developing controls; MEG, magnetoencephalography; EEG, electroencephalography
Keywords: Autism Spectrum Disorders, Interval timing, Duration perception, Pitch perception, Magnetoencephalography
Highlights
-
•
We investigated MEG response associated with duration or pitch comparison in ASD.
-
•
We found lower sensitivity for duration discrimination behaviourally in ASD.
-
•
ASD adults are less able to predict the offset of a standard tone.
-
•
ASD adults engage less neural resources in duration than pitch discrimination task.
1. Introduction
Autism Spectrum Disorder (ASD) is a developmental disorder characterised by impaired social interaction and communication, as well as stereotyped, repetitive behaviour (APA, 2000). Critical aspects of the diagnosis involve several factors pointing to a general decrease in the quality of social interactions including atypical turn taking, unusual verbal intonations and abnormal joint attention. Interestingly, these factors implicitly include timing factors: whether to judge the appropriate amount of time to leave between the examiner's last sentence and their reply, to produce speech at the right pace, or to pick up a visual cue at the right time, individuals have to be able to process short (i.e. sub-second) durations accurately. In fact time is fairly ubiquitous in cognitive processes such as sensory integration, motor control, language, and planning, all of which have been found to be areas of difficulties for individuals on the autism spectrum (Boucher, 2012, Eigsti et al., 2011, Hill, 2004, Marco et al., 2011). Importantly, the earliest signs of autism during the first couple of years of life consist in atypical motor behaviour (hypo- or hypertonia), reduced joint attention, atypical play patterns and general difficulties in producing and integrating multimodal behaviour (gaze, vocalisation, gesture), which are characterised by finely tuned temporal dynamics (Charman and Baird, 2002, Lord et al., 2008). Failure to develop these crucial skills in early life would certainly affect learning and development. For instance, the cues used by the carers to engage children's attention and orient them towards relevant aspects of the environment (such as eye gaze, pointing, speech) can become meaningless if they are not temporally integrated with the target event or behaviour (e.g. object, colour, movement).
Time processing includes interval timing, synchrony judgements and temporal order judgements (Eagleman, 2008, Wittmann and van Wassenhove, 2009) and importantly, these processes do not necessarily rely on the same cognitive resources and neural mechanisms (van Wassenhove, 2009). In particular, perturbations in one type of temporal judgement do not necessarily or automatically translate into perturbations in the other types of temporal processing. In this particular study we focused on interval timing i.e., the ability to judge the duration of short events (Buhusi and Meck, 2005).
Despite several mentions of possible time processing deficits in ASD (Boucher, 2001, Wimpory et al., 1995, Wimpory et al., 2002), timing and time perception have received little attention in the field of autism research until recently. To date, behavioural studies have found mixed evidence of time processing deficits. For instance, Szelag et al. (2004) found a dramatic impairment of time perception in children with ASD using temporal reproduction tasks in which children were asked to reproduce the duration of an auditory or a visual stimulus. Specifically, whereas typically developing control (TDC) children reproduced standard durations accurately, ASD children reproduced durations varying from 1 to 5.5 s as intervals around 3 s. Later research found more moderate differences between TDC and ASD performance on time perception tasks: using a temporal reproduction task with auditory durations ranging from 0.5 to 4.1 s, Martin et al. (2010) found that adults with ASD showed reduced sensitivity (temporal estimations further away from the veridical value) and precision (estimations are more variable) as compared to the TDC group - i.e. their reproduced durations were further away from the standards and judgements showed greater variability. Similar results were found using a bisection task (Allman et al., 2011) in which participants had to decide whether probe durations were closer to a short (1 or 2 s) or to a long (4 or 8 s) anchor duration shown at the beginning of the experiment. The authors found that children with ASD perceived a smaller bisection point (duration supporting the perception that the probe is equal to the standard), and showed lower precision compared to TDC children. Exploring shorter durations (125-800 ms) with a temporal reproduction and a temporal generalisation tasks in lower-functioning children with ASD, Brodeur et al. (2014) also found reduced sensitivity and higher variability in temporal judgements in children with autism.
In contrast with the described studies, Mostofsky et al. (2000) and Jones et al. (2009) found no differences between children and adolescents with ASD and their controls when using an auditory duration comparison task (below 1000 ms). Gil et al. (2012) found no group difference between ASD and TDC children in a time bisection task using durations between 0.5 and 16.62 s. Wallace and Happe (2008) even suggested that children with ASD can show superior performance to TDC children in a reproduction task using longer durations (2 to 45 s), and found no difference between groups in estimation and production tasks using the same durations. The latter results should be taken with caution as they were obtained with only 2 trials per duration and using a manual timing procedure.
In a previous study, we attempted to clarify previous inconsistencies in the literature by testing temporal processing of a range of intervals and unimodal auditory, unimodal visual and cross-modal interval comparisons using a temporal generalisation task in a sample of high-functioning individuals with a diagnosis of ASD and well-matched TDC controls (Falter et al., 2012). We chose the temporal generalisation paradigm as it allows a distinction between the sensitivity of interval discrimination and other aspects of timing, such as the scalar property. The scalar property of variance is a fundamental characteristic of human interval timing manifesting itself in constant timing sensitivity as the absolute intervals to be timed vary (e.g., Wearden and Lejeune, 2008). We found decreased duration discrimination sensitivity in the ASD group across the two interval ranges and modality conditions, but in particular driven by the auditory modality. At the same time, participants with ASD showed coherence to the scalar property of timing and even more strongly than the TDC group. Hence, some functional aspects of timing behaviour seem to be intact in ASD, while discrimination of time intervals was found to be impaired (Falter et al., 2012). Recent literature on time processing in autism (Allman, 2011, Allman et al., 2011) went as far as to propose that time processing deficit could underlie some of the core (e.g. atypical social interaction, repetitive behaviour) and secondary (e.g. language deficit, difficulties with planning) symptoms of ASD. In support of this theory, Maister and Plaisted-Grant (2011), using a dual-task paradigm in which participants performed a time reproduction task in concurrence with a verbal repetition task, provide correlational evidence that short duration deficits are associated with attention abilities.
Altogether, the discussed previous findings suggest subtle differences in time perception in individuals with ASD as compared to the performance observed in TDC individuals. These differences manifest themselves most reliably as diminished precision in timing tasks for durations in the range of a few seconds. These group differences are generally small in size and do not reflect a clear impairment in the ASD group. This performance pattern suggests that, to perform temporal judgements, individuals with ASD allocate fewer resources to the task or use different strategies (and different neural substrates) compared to TDC individuals. Timing differences could affect behaviour and cognitive functions relying on the perception and production of fine temporal signals such as social timing or motor coordination. Unfortunately, such subtle impairments in low-level neuronal functions (e.g. in the perception of the timing of events) could have cascading effects on the development of higher-level functions that are dependent on all neuronal functions downstream, for instance functions that rely directly on a fine temporal tuning (e.g. turn-taking in conversations) or functions that develop through time-dependent learning (e.g. social learning, motor learning).
Thus, the current study aimed to explore the neural basis of reduced discriminatory sensitivity of time intervals, while timing functions did not seem to be completely disrupted in ASD. Considering the inconsistencies in behavioural findings, and the fact that differences between ASD and TDC groups might be the consequence of differential cognitive strategies as opposed to neurological impairments, investigating time processing in ASD using functional neuroimaging methods has previously proven fruitful (Falter et al., 2013). Consequently, here we aimed at characterising the neural correlates of duration perception in high-functioning individuals with ASD using magnetoencephalography (MEG). Specifically, we contrasted cortical responses to the presentation of the same auditory stimuli but while participants performed two possible tasks: a duration discrimination task or a pitch discrimination task. Indeed, and in contrast to time perception, the perception of auditory pitch has been systematically reported to be intact and sometimes superior in individuals with ASD. Behaviourally, children with ASD perform equally well or better than their TDC counterparts on pitch discrimination and categorisation tasks (Bonnel et al., 2003, Heaton et al., 2008, Heaton, 2005, Järvinen-Pasley and Heaton, 2007, Järvinen-Pasley et al., 2008a, Järvinen-Pasley et al., 2008b, Mottron et al., 2000, but see Bhatara et al., 2013). Superior pitch discrimination has also been observed in adolescents and adults with ASD, although only in those individuals also presenting some language delays or difficulties (Bonnel et al., 2010, Eigsti and Fein, 2013, Heaton et al., 2008, Jones et al., 2009). Other individuals on the autism spectrum did not perform differently from matched TDC controls (for review see Haesen et al., 2011, O'Connor, 2012).
Studies using electroencephalography (EEG) investigating the neural correlates of pitch perception in ASD reported a diminished auditory P1 (Buchwald et al., 1992, Ceponiene et al., 2003, Lepistö et al., 2005) and sometimes a diminished N2 and N4 (Lepistö et al., 2005). The Mismatch Negativity (MMN), which characterises the automatic detection of a deviant stimulus within a sequence of standard stimuli, has been found to be either intact (Ceponiene et al., 2003), enhanced (Ferri et al., 2003, Lepistö et al., 2005, Lepistö et al., 2006, Lepistö et al., 2008) or occurring with an earlier latency (Gomot et al., 2002, Kujala et al., 2007) in ASD in response to simple, complex and speech-like pitch changes. Yet, one MEG study found a delayed MMN in response to a change in pitch in children with ASD compared to TDC children (Oram Cardy et al., 2005). Very few studies have been conducted on the neural correlates of time perception in ASD and available evidence is so far quite inconsistent. Using an auditory oddball with duration deviants (104 vs 190 ms) in speech and non-speech stimuli, Lepistö et al., 2005, Lepistö et al., 2006 reported a diminished MMN for duration changes in non-speech stimuli in ASD and a similar trend (although non-significant) for speech stimuli. In line with these results, a diminished MMN in response to consonant change (/wa/ vs. /ba/) in young children with ASD was described Kuhl et al. (2005); interestingly in this study (Kuhl et al., 2005) the stimuli were acoustically identical except for the duration of the initial formant transitions. In contrast, using a multi-feature oddball paradigm with non-speech stimuli, Kujala et al. (2007) found a larger fronto-central MMN in adults with ASD in response to the duration deviant (65 vs. 100 ms). Overall, available data suggest atypical neural processing of time in ASD. Behaviourally, we thus predicted that ASD individuals would show comparable sensitivity to TDC individuals in the pitch comparison task, but a reduced sensitivity in the duration comparison task. In terms of neural correlates, and based on the observation that the auditory EEG response P1 is reduced in ASD (Buchwald et al., 1992, Ceponiene et al., 2003, Lepistö et al., 2005), we expected a slightly reduced MEG M100 in the ASD group compared to the TDC group in both tasks but an otherwise similar activity pattern in both groups. To the contrary, we expected a differential pattern reflecting atypical processing of time in ASD on the basis of behaviourally different performance patterns in similar tasks (e.g., Falter et al., 2012).
2. Methods
2.1. Participants
Nineteen ASD participants (16–38 years old, 1 female) were recruited through a database at the Department of Psychiatry, University of Oxford. ASD participants were only included in the study if they were free from any co-morbid psychiatric disorders, did not take any medications, were right-handed and had a full scale IQ > 85 measured with the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999). Four ASD participants had a diagnosis of high-functioning autism and fifteen ASD participants had a diagnosis of Asperger syndrome according to DSM-IV-TR (APA, 2000). Diagnoses of ASD were confirmed with the Autism Diagnostic Interview – Revised, ADI-R (Lord et al., 1994) and the Autism Diagnostic Observation Schedule – Generic, ADOS-G (Lord et al., 2000). Two participants did not meet the age of onset criterion of the ADI and three other participants scored one point below threshold on one ADI algorithm domain. Overall, all these participants scored above the ASD cut-off on other ADI domains and were included in the final analysis.
Nineteen TDC participants (15–38 years old, 5 females) were recruited through local advertisements and through their previous participation in studies at the University of Oxford. The same inclusion criteria applied to the TDC group as it did for the ASD group and in addition they were free of any psychiatric diagnosis.
Data of one ASD and one TDC participant were excluded from final analysis due to noisy MEG recordings. Thus, 18 participants with ASD and 18 TDC participants were included in the final MEG analysis (see Table 1 for demographic data). IQ data was not obtained from one female TDC participant. The remaining ASD and TDC participants were matched on age, verbal IQ, and performance IQ (largest t = 1.067). All individuals taking part in the study had normal or corrected-to-normal vision. Ethics approval for the study was formally obtained from the National Research Ethics Service UK and written informed consent was obtained from all participants prior to any testing in accordance with the Declaration of Helsinki (2008). The individuals included in the final analysis had also participated on the same day in an MEG study on perceptual simultaneity processing (Falter et al., 2013) and Gestalt perception (unpublished data).
Table 1.
Means, standard deviations (SD), and ranges of age (years:months), verbal IQ (VIQ), performance IQ (PIQ), and full IQ (FIQ) of participants with an Autism Spectrum Disorder (ASD) and typically-developing (TDC) participants. ADI-R Social Interaction Domain (ADI-A), ADI-R Communication Domain (ADI-B), ADI-R Repetitive Behaviours Domain (ADI-C), ADOS-G Communication Domain (ADOS-A), ADOS-G Reciprocal Social Interaction Domain (ADOS-B), and ADOS-G Stereotyped Behaviours and Restricted Interests Domain (ADOS-C) of participants with ASD.
| ASD (N = 18; 1 female) |
TDC (N = 18; 5 females) |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age | 25:3 | 8:1 | 16:9–42:11 | 26:4 | 6:4 | 15:9–38:6 |
| VIQ | 110 | 14 | 70–127 | 113 | 11 | 99–139 |
| PIQ | 112 | 15 | 75–136 | 116 | 8 | 104–125 |
| FIQ | 112 | 13 | 88–131 | 116 | 8 | 101–133 |
| ADI-A | 18 | 6 | 9–28 | |||
| ADI-B | 15 | 4 | 9–21 | |||
| ADI-C | 6 | 3 | 2–12 | |||
| ADOS-A | 4 | 2 | 1–7 | |||
| ADOS-B | 7 | 3 | 1–12 | |||
| ADOS-C | 1 | 1 | 0–4 | |||
2.2. Design and procedure
Stimuli were generated and presented using Stim2 software (Neuroscan, 2003) and back-projected onto a translucent screen in a dimly lit scanning room. The task followed the design of a repeated standard temporal generalisation task (McCormack et al., 2005), which allowed calculation of discrimination sensitivity (d prime). In this task, participants were presented on each trial with a standard stimulus followed by a probe stimulus. The probe stimulus was either identical to or different from the standard stimulus. In the current experiment, participants decided whether the duration (Duration task) or the pitch (Pitch task) of the probe auditory stimulus was the same as or different from the duration or the pitch of the standard stimulus, respectively.
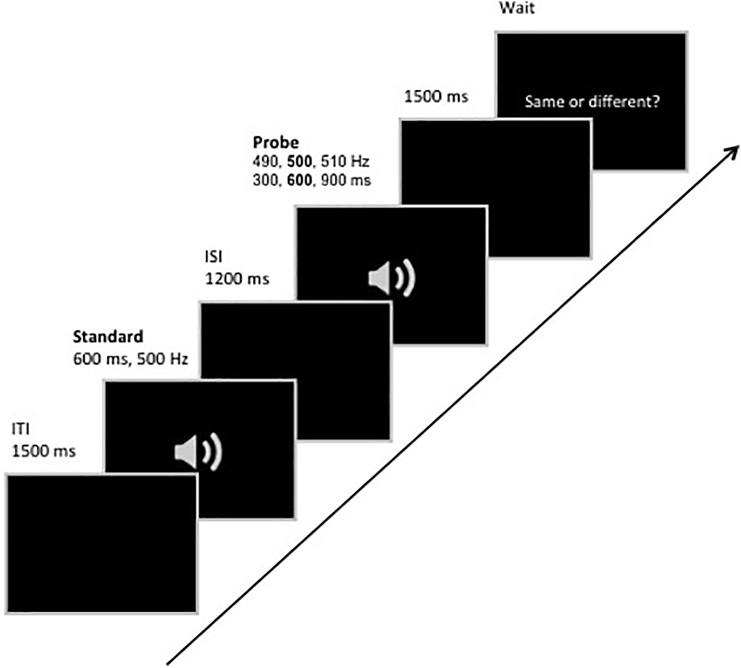
Duration and Pitch tasks were presented in separate blocks. The order of blocks was randomised across individuals. At the beginning of each block, an instruction screen indicated the type of comparison to be performed (i.e. ‘Duration’ or ‘Pitch’) followed by 12 trials of the specified condition. Each trial started with a 1500 ms period of black screen. A 500 Hz standard tone of 600 ms duration was then presented binaurally (standard tone). After a fixed Inter-Stimulus Interval (ISI) of 1200 ms, a probe tone of variable duration (Duration task) or frequency (Pitch task) was presented followed by a second ISI period of 1500 ms. After the presentation of the probe, participants were presented with a response cue, prompting them to register their answer by pressing one of two buttons (‘same’ vs. ‘different’). In the Duration task, three probes were tested in randomised order: 300 ms, 600 ms, or 900 ms and all probes were 500 Hz. In the Pitch task, three probes were presented in randomised order with a pitch of 490 Hz, 500 Hz, or 510 Hz; all probes lasted 600 ms (Fig. 1).
Fig. 1.
Illustration of a typical experimental trial.
The full experiment consisted of 20 blocks, resulting in 120 trials per probe in the Duration task and 120 trials in the Pitch task. Participants were provided with short breaks in between blocks. The experiment was preceded by a training session in which participants performed one practice block on each condition and it was ascertained that the task instructions were understood. No feedback on performance was given during the experiment proper.
2.3. Data acquisition
MEG data were acquired at the Oxford University MEG unit using a Neuromag-306 VectorView™ system (Elekta-NeuromagOy; Helsinki, Finland), which consists of a helmet-shaped array of 102 pairs of orthogonal, first-order planar gradiometers (thereafter, “grad1” and “grad2” for the derivative along the latitude and the longitude, respectively) and 102 magnetometers (thereafter, “mags”). The data were sampled at 1000 Hz (0.03 to 330 Hz anti-alias filter). Individual head position was measured before each experimental run.
2.4. Data pre-processing
As typical for the Neuromag system using MaxShield, magnetic interferences originating outside of the MEG helmet were suppressed by the use of a Signal Space Separation method (Taulu and Simola, 2006) using MaxFilter (Elekta-NeuromagOy; Helsinki, Finland). The median position of each participant over the experimental runs was used as reference for the other runs based on the participant's head position recorded before each run. In addition to maxfiltering, PCA was performed to remove components explaining the ECG and EOG variance by using Graph (Elekta-NeuromagOy; Helsinki, Finland). The average cardiac and blink artefacts were performed using the ECG and EOG recordings. Components were checked manually for each sensor type (grad1, grad2, and mags) and saved as separate matrices (for detailed procedure, see: http://www.unicog.org/old_pm/pmwiki.php/MEG/CheckingDataWithNeuromagTools). Additional data rejection affected < 2% of epochs.
2.5. Event-related fields (ERF) analysis
Single-trials were epoched from − 200 ms to 800 ms from stimulus onset (either standard or probe) separately for each Pitch and Duration task and on a per individual basis. Epochs were low-pass filtered at 40 Hz and baseline corrected using the first 200 ms of the epoch. Single trials were first averaged on a per task and per individual basis, then grand-averaged across individuals of the same experimental group (ASD, TDC). Importantly, the analysis was carried out exclusively on the standard and on the probe stimuli, which were physically identical. Hence, comparisons were effectively carried out on 500 Hz tones lasting 600 ms. Brain responses to the 500 Hz, 600 ms tones were subsequently contrasted as a function of their position in the trial (standard or probe) and as a function of tasks (Duration or Pitch).
Within- and between-group analyses of ERFs were performed using Matlab (R2012) and Fieldtrip routines (Oostenveld et al., 2011). Cluster permutation analyses in sensor space (Maris and Oostenveld, 2007) were performed using Fieldtrip separately for all three types of MEG sensors and on the full epoch length.2 The minimal size for a cluster was two sensors with an alpha set to 0.05; the Monte Carlo method used 1000 permutations. All analyses were performed in accordance with accepted MEG analysis guidelines (Gross et al., 2013).
3. Results and discussion
3.1. Behavioural performance
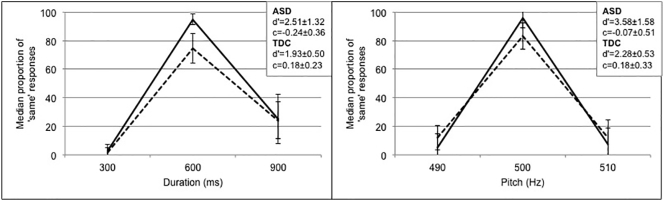
Mean sensitivity in the Duration and Pitch discrimination tasks for both groups are shown in Fig. 2. Using signal detection analyses, we calculated interval timing sensitivity (d′) independently of response bias (c) for each participant (Wearden, 2008) taking the hit rate and the false alarm rates into account.
Fig. 2.
Median proportion with quartile deviations of same responses plotted against the probe stimuli in Duration (left) and in Pitch (right) for the TDC group (solid line) and the ASD group (dashed line). The inlay shows the d’ and c scores for both groups.
The interval timing sensitivity indexed in d′ reflects participants' ability to distinguish between the standard and the probe intervals. The response bias indexed by c reflects the bias of responding ‘same’ across all intervals. In this respect, a general tendency of being cautious about responding ‘same’ would be seen as a rather conservative response bias. The hit rate reflects the proportion of ‘same’ responses in trials in which the probe duration was the same as the standard duration whereas the false alarm rate reflects the proportion of ‘same’ responses in trials in which the probe duration was different from the standard duration.
The d’ and c scores in the Pitch and Duration tasks were analysed separately. Due to the observed non-normality of d’ scores in both the TDC group (K-S test; D(18) = 0.226, p = 0.015) and in the ASD group (D(18) = 0.241, p = 0.007) in Duration, as well as in the ASD group in Pitch (D(18) = 0.212, p = 0.031), Mann-Whitney U tests were performed on the between-participants factor of Group (ASD, TDC). The ASD group had significantly lower d′ scores (Median = 1.93, QD = 0.50) than the TDC group (Median = 2.51, QD = 1.32) in Duration, U(36) = 88.5, p = 0.020. There were trends for lower d’ scores in the ASD (Median = 2.28, QD = 0.53) as compared to the TDC group (Median = 3.58, QD = 1.58) in Pitch, U(36) = 100.5, p = 0.052, and for higher c scores in the ASD (Median = 0.18, QD = 0.23) as compared to the TDC group (Median = − 0.24, QD = 0.36) in Duration, U(36) = 104.0, p = 0.066. There was no significant group differences or trends for c scores in Pitch, U(36) = 117.5, p = 0.159.
3.2. Auditory event-related fields
In order to examine contrasts in cognitive, task-specific processes rather than low-level sensory processes, the MEG analysis focused exclusively on standard and probe stimuli which were acoustically identical tones (500 Hz, 600 ms). Therefore, the sole difference between standard and probe stimuli was their temporal order in the course of a trial, and the sole major difference between Pitch and Duration probes was the task instruction.
3.2.1. Group differences for pitch and duration discrimination tasks
First, we performed a between-group comparison to evaluate differences in brain responses to the same auditory stimuli between the TDC and ASD participants, separately for Pitch and for Duration. It should be noted that despite some trends illustrated in the figures, few clusters contrasting ASD and TDC evoked responses with a between-group design reached significance after correction for multiple comparison, indicating the selectivity of our findings.
3.2.1.1. Pitch discrimination task
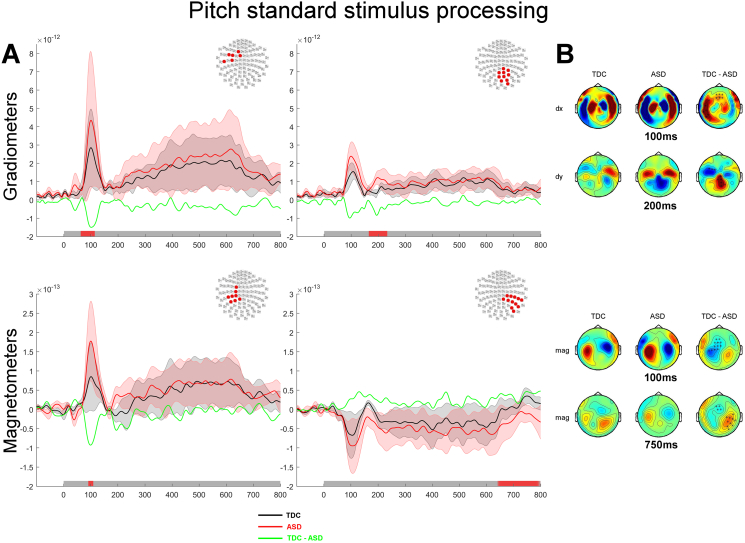
Between-group comparisons for the Pitch discrimination task revealed significant differences solely for the processing of the standard stimulus (and not the probe): larger auditory evoked M100 and M200 responses were found in the ASD group as compared to the TDC group over the left hemisphere (M100; mag, grad1: all p < 0.05; M200; grad2: p < 0.05) compared to the TDC group (Fig. 3). The ASD group also showed a significantly larger amplitude of their late auditory evoked responses (640 and 800 ms; mag: p < 0.05) over the right hemisphere as compared to TDC group (Fig. 3A, bottom right panel). The observed group differences in brain responses during the processing of the standard sound during the Pitch task are compatible with the idea of enhanced pitch processing in ASD early during the analysis of the auditory stimulus. The lack of group differences during the presentation of the probe suggests, however, that there were no major differences in how the Pitch discrimination task was performed in both groups.
Fig. 3.
Processing of the standard tone during the Pitch discrimination task contrasted between groups. A. Auditory evoked responses elicited by the presentation of the standard tone in the Pitch task. The top panel shows the normed evoked response from the paired gradiometers, the bottom panel shows the average evoked response measured by magnetometers. The topography at the top right corner of each panel illustrates the significant cluster of sensors across which the average evoked responses were obtained. Coloured traces depict the time course of brain responses evoked by the presentation of the standard stimuli in the Pitch task for the TDC group (black) and the ASD group (red). The difference in auditory evoked responses are plotted in green. The shadowed areas represent 2 standard deviations of the mean time course for each group. Temporal intervals showing a significant difference between groups for the cluster of sensors are denoted by a red bar along the x axis. B. Topographies of the auditory responses evoked by the presentation of the standard tone in clusters showing significant differences across experimental groups. The top panel illustrates the topographies for gradiometers (dx are derivative along the latitude; dy are derivative along the longitude), the bottom panel shows the topographies for magnetometers. The right column of sensors shows the difference in the evoked response between the two groups. Significantly clusters are denoted by x (p < 0.05) or * (p < 0.01).
3.2.1.2. Duration discrimination task
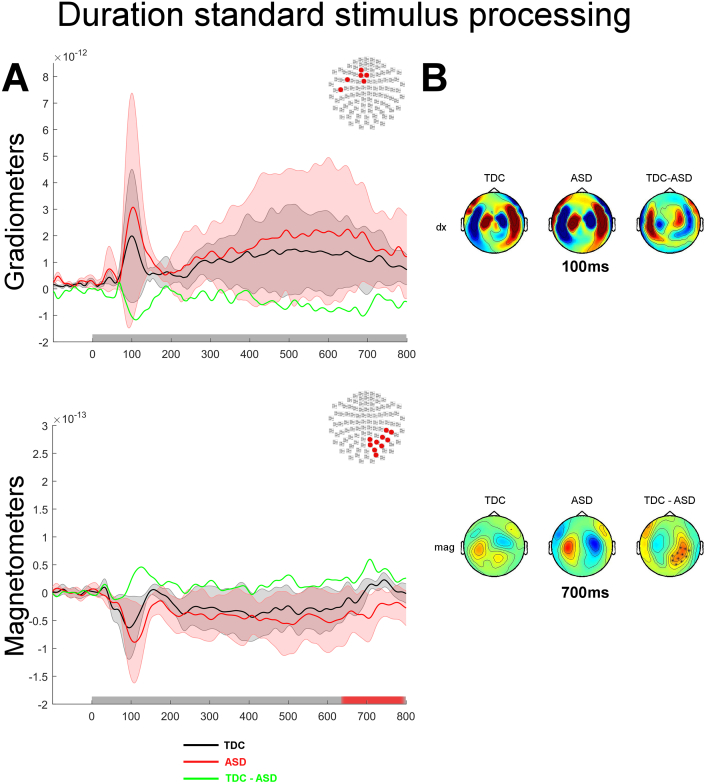
Similarly to the Pitch task, between-group comparisons for the Duration discrimination task revealed significant differences in the processing of the standard but not the probe stimulus. Although brain responses of the ASD group (Fig. 4; red traces) may suggest an enhanced activity throughout the course of the trial, this was not significant. Rather, the only significant difference between groups was that the auditory evoked responses persisted longer after the offset of the stimulus in the ASD group. This persistent activity in the ASD group resulted in a significantly larger brain evoked response between 630 and 800 ms as compared to the TDC group (mag: p < 0.05; Fig. 4, bottom panel). This group difference suggests that a possible neural correlate of the behaviourally observed diminished sensitivity in duration comparison may be due to the less clear demarcation of the offset response at the end of the standard stimulus.
Fig. 4.
Processing of the standard tone during the Duration discrimination task contrasted between groups. A. Auditory evoked responses elicited by the presentation of the standard tone in the Duration task. The top panel shows the normed evoked response from the paired gradiometers, the bottom panel shows the average evoked response measured by magnetometers. The topography at the top right corner of each panel illustrates the significant cluster of sensors across which the average evoked responses were obtained. Coloured traces depict the time course of brain responses evoked by the presentation of the standard stimuli in the Duration task for the TDC group (black) and the ASD group (red). The difference in auditory evoked responses are plotted in green. The shadowed areas represent 2 standard deviations of the mean time course for each group. Temporal intervals showing a significant difference between groups for the cluster of sensors are denoted by a red bar along the x axis. B. Topographies of the auditory responses evoked by the presentation of the standard tone in clusters showing significant differences across experimental groups. The top panel illustrates the topographies for gradiometers (dx are derivative along the latitude; dy are derivative along the longitude), the bottom panel shows the topographies for magnetometers. The right column of sensors shows the difference in the evoked response between the two groups. Significantly clusters are denoted by x (p < 0.05) or * (p < 0.01).
3.2.2. Task specificity: differential encoding of identical stimuli as a function of task instructions (Pitch vs. Duration contrast)
We then investigated whether the physically identical stimuli were encoded differently depending on task instructions within each experimental group. We tested whether and when, pitch and duration were differentially encoded by contrasting the standard and the probe stimuli in the Pitch and Duration tasks, separately for each group of participants.
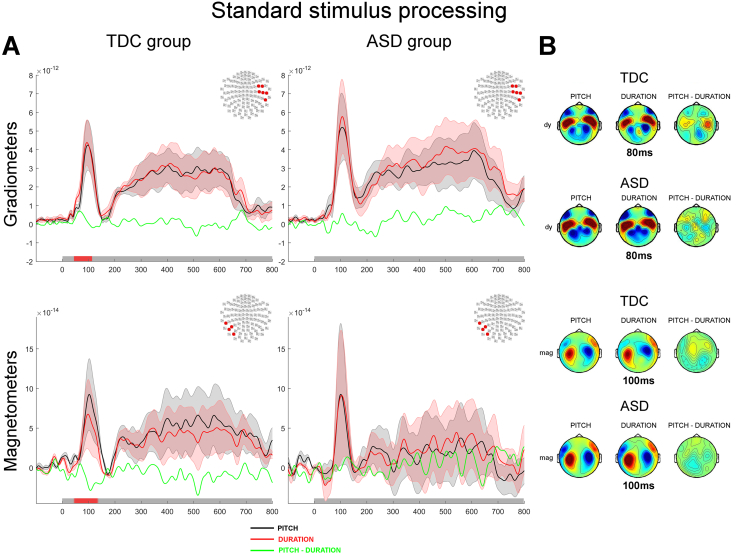
3.2.2.1. Standard stimulus processing
In the TDC group, the early M50 and M100 components of the auditory evoked responses elicited by the presentation of the standard tone differed significantly as a function of task requirements. First, auditory evoked responses during the Duration task were significantly enhanced compared to their homologues in the Pitch task over the right hemisphere (grad2: p < 0.05; Fig. 5A–B, top left panels). On the other hand a significantly larger M50 and M100 in the Pitch task was found in the left hemisphere as contrasted with the Duration task (mag: p < 0.05; Fig. 5A–B, bottom left panels). No other significant differences was found between the two tasks at these and later latencies.
Fig. 5.
Processing of the standard tone contrasted between Pitch and Duration discrimination tasks. A. Auditory evoked responses elicited by the presentation of the standard tone. The top panels show the normed evoked responses from the paired gradiometers, the bottom panels show the average evoked responses measured by magnetometers. The left panels show responses for the TDC group and the right panels show responses for the ASD group. The topography at the top right corner of each panel illustrates the significant cluster of sensors across which the average evoked responses were obtained. Coloured traces depict the time course of the brain responses evoked by the presentation of the standard tone in the Pitch task (black) and the Duration task (red). The difference in auditory evoked responses are plotted in green. The shadowed areas represent 2 standard deviations of the mean time course for each task. Temporal intervals showing a significant difference between groups for the cluster of sensors are denoted by a red bar along the x axis. B. Topographies of the auditory responses evoked by the presentation of the standard tone in clusters showing significant differences across experimental tasks. The top panel illustrates the topographies for gradiometers (dx are derivative along the latitude; dy are derivative along the longitude), the bottom panel shows the topographies for magnetometers. The right column of sensors shows the difference in the evoked response between the two tasks. Significantly clusters are denoted by x (p < 0.05) or * (p < 0.01).
In the ASD group, although the graphs suggest a similar trend in the right hemisphere, no significant differences were found when contrasting brain responses evoked by the presentation of the standard stimuli in the Pitch or in the Duration tasks (see Fig. 5).
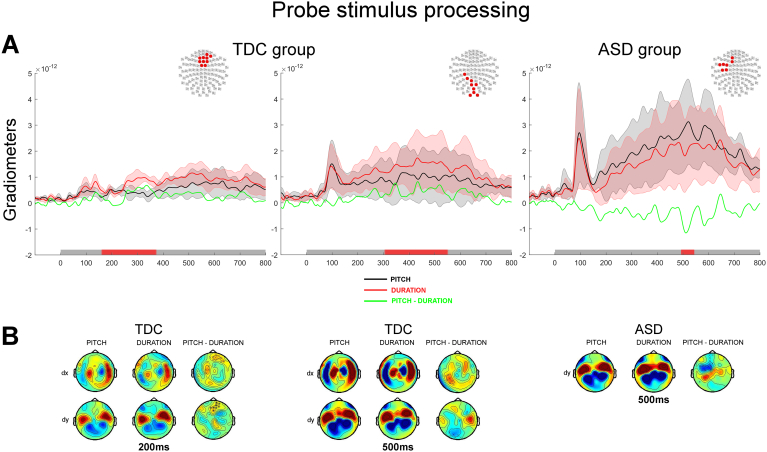
3.2.2.2. Probe stimulus processing
During the probe presentation, the TDC group showed clear enhanced evoked responses during Duration task as compared to Pitch task in several consecutive clusters starting around 200 ms, and lasting up to 400 ms post-stimulus onset (grad1: p < 0.05; grad 2: p < 0.001; Fig. 6, left panel), between 300 and 410 ms and again between 460 and 550 ms (grads: p < 0.01 and p < 0.05 respectively; Fig. 6, middle panel). Significant clusters involved different set of sensors over both hemispheres, suggesting the contribution of multiple sources to the enhanced response elicited by the Duration task.
Fig. 6.
Processing of the probe tone contrasted between Pitch and Duration discrimination tasks. A. Auditory evoked responses elicited by the presentation of the probe tone. The panels show the normed evoked responses from the paired gradiometers. The left and middle panels show responses for the TDC group and the right panel shows responses for the ASD group. The topography at the top right corner of each panel illustrates the significant cluster of sensors across which the average evoked responses were obtained. Coloured traces depict the time course of the brain responses evoked by the presentation of the standard tone in the Pitch task (black) and the Duration task (red). The difference in auditory evoked responses are plotted in green. The shadowed areas represent 2 standard deviations of the mean time course for each task. Temporal intervals showing a significant difference between groups for the cluster of sensors are denoted by a red bar along the x axis. B. Topographies of the auditory responses evoked by the presentation of the standard tone in clusters showing significant differences across experimental tasks from gradiometers (dx are derivative along the latitude; dy are derivative along the longitude). The right column of sensors shows the difference in the evoked response between the two tasks. Significantly clusters are denoted by x (p < 0.05) or * (p < 0.01).
In stark contrast, response profiles in the ASD group indicated a sustained enhanced evoked response in the Pitch task as compared to the Duration task starting at around 150 ms post-stimulus onset, and lasting throughout the presentation of the sound. This difference only reached statistical significance for a short interval between 495 and 540 ms over the left hemisphere (grad2: p < 0.05, Fig. 6, right panel).
Hence, both TDC and ASD individuals showed specific processing differences of the same physical stimuli between the two tasks (Pitch vs. Duration), suggesting that task instructions provided top-down guidance on bottom-up information processing. An important difference between the TDC and ASD groups was that in the TDC group, brain responses had greater amplitude in the Duration task as compared to the Pitch task after the early components of the evoked responses. To the contrary the ASD group showed a larger amplitude of the evoked response in the Pitch task as compared to the Duration task. One major difference between the two tasks is that the Pitch discrimination task can be resolved based on early sensory evidence provided by the frequency of the stimulus, whereas the Duration task can only be resolved later in the trial (specifically for the 600 ms stimuli, the task can be fully resolved from 600 ms after the offset of the signal). This seems to be reflected in the TDC group by a greater response to duration after 150 ms, but not in the ASD group in which the diminished amplitude in the Duration task (as compared to the Pitch task) might underlie the reduced sensitivity for duration discrimination in the behavioural results.
3.2.3. Comparison processes: standard vs. probe stimuli
Our last question concerned the neural correlates of the comparison process that led participants to make a decision on a given trial. For this, we investigated the differences in brain responses evoked by the presentation of the standard and the probe stimuli. Keeping in mind that we only considered trials in which the probe was identical to the standard stimulus, any differences could only reflect top-down and decision processes as opposed to bottom-up sensory analysis.
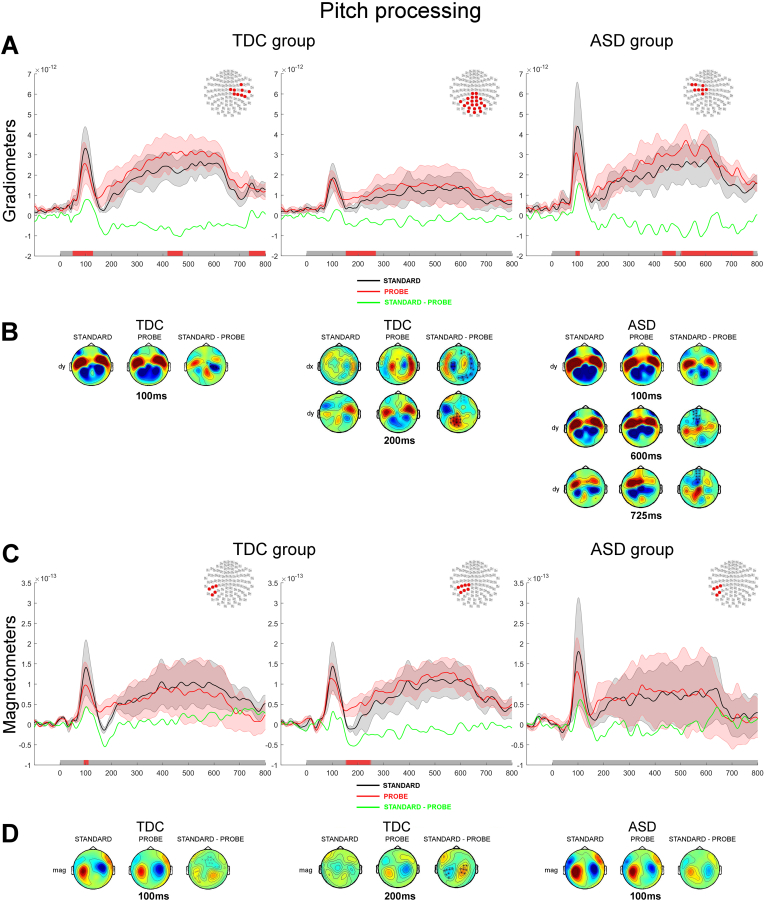
3.2.3.1. Pitch discrimination task
In the TDC group, the comparison of the standard and the probe during the Pitch task revealed a significant sustained difference of the auditory evoked response from 44 ms post-stimulus onset onwards, with the probe stimulus eliciting a smaller auditory M100 than the standard over both the right (grad2: p < 0.01; Fig. 7A–B, left panel) and the left sensors (mag: p < 0.05; Fig. 7C–D, left panel). This pattern suggests that the cortical processing of the standard and the probe stimuli were significantly different as early as the mid-latency responses. This difference was followed by an enhanced bilateral auditory M200 elicited by the presentation of the probe as compared to the standard (mag, grad2: p < 0.05; Fig. 7A–D, middle panels). A significantly larger response to the presentation of the probe as compared to the standard was also observed bilaterally between 420 and 475 ms (grad2: p < 0.05; Fig. 7A–B, left panel); late significant clusters were also found in the left hemisphere between 740 and 800 ms (grad1: p < 0.05).
Fig. 7.
Processing of auditory tone during the Pitch discrimination task contrasted between standard and probe tone presentations. A&C. Normed auditory evoked responses from the paired gradiometers (A) and from the magnetometers (C). The left and middle panels show responses for the TDC group and the right panels shows responses for the ASD group. The topography at the top right corner of each panel illustrates the significant cluster of sensors across which the average evoked responses were obtained. Coloured traces depict the time course of the brain responses evoked by the presentation of the standard tone (black) and the probe tone (red) in the Pitch task. The difference in auditory evoked responses are plotted in green. The shadowed areas represent 2 standard deviations of the mean time course for each type of tone. Temporal intervals showing a significant difference between groups for the cluster of sensors are denoted by a red bar along the x axis. B&D. Topographies of the auditory responses evoked by the presentation of the auditory tone in clusters showing significant differences across tone type from gradiometers (B; dx are derivative along the latitude; dy are derivative along the longitude) and magnetometers (D). The right column of sensors shows the difference in the evoked response between the two tasks. Significantly clusters are denoted by x (p < 0.05) or * (p < 0.01).
In the ASD group, the same analysis yielded largely similar patterns of significant effects. As in the TDC group, a diminished auditory M100 response was observed in response to the presentation of the probe as compared to the standard during the Pitch task over the left sensors (grads: 90–110 ms, p < 0.05; Fig. 7A–B, right panel). An enhanced auditory response to the presentation of the probe as compared to the standard was confined to the left sensors between 424 and 481 ms and again between 500 and 800 ms post-stimulus onset (grad2: p < 0.001; Fig. 7A-B, right panel).
In summary, for both TDC and ASD participants in the Pitch comparison task, early auditory evoked responses to the probe showed smaller amplitudes as compared to the standard stimulus, possibly reflecting sensory habituation (diminished early sensory response to a repeated stimulus). In both groups, the probe also elicited larger auditory evoked responses from 400 ms onwards although this difference was bilateral in TDC group and mostly left-lateralized in the ASD group. Together with the behavioural findings, this suggests that both groups of individuals shared similar response in a Pitch comparison task which allows them to resolve pitch judgement successfully.
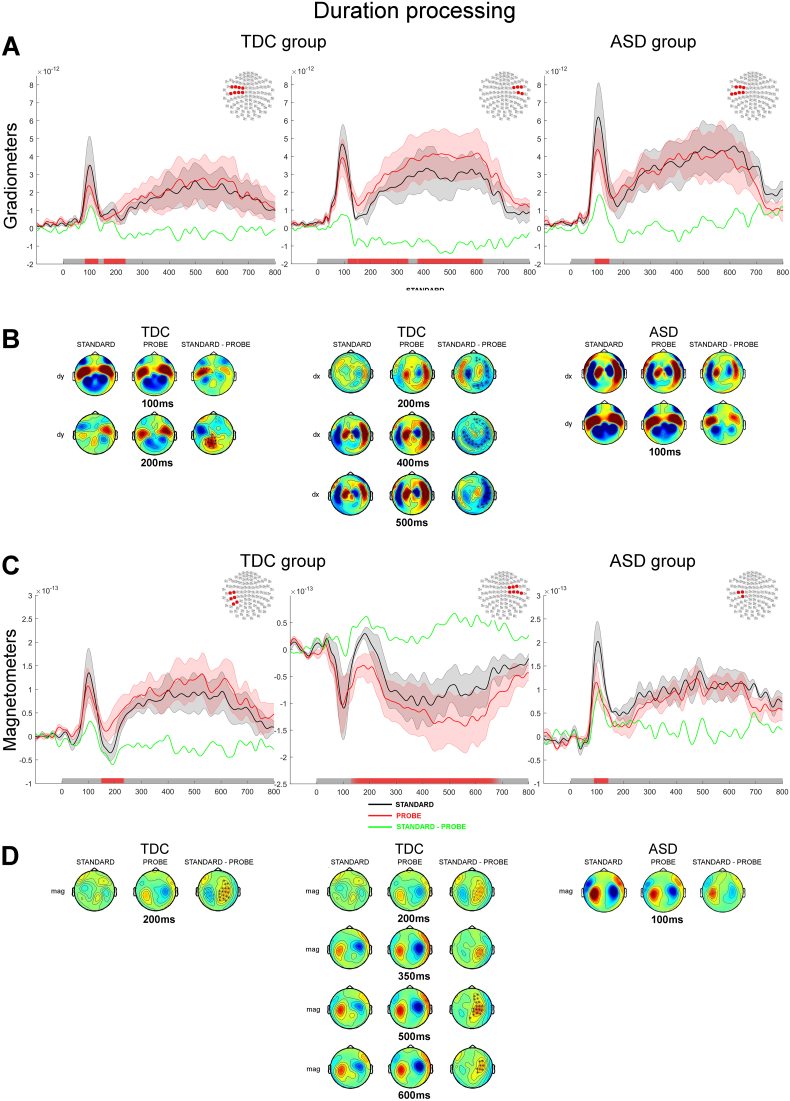
3.2.3.2. Duration discrimination task
In the duration discrimination task, and similar to the pitch discrimination task, TDC participants showed an early and sustained differential processing of the standard and the probe (Fig. 8A–D, left and middle panels). The probe elicited a significantly smaller auditory evoked responses than the standard: specifically, a smaller M100 response over the left sensors (grad2: p < 0.01) and a smaller M200 over the left hemisphere then sustained over both hemispheres until 340 ms (mag, grad2: p < 0.05; grad1: p < 0.001). The sustained response was followed by a greater response to the probe from 330 to 700 ms (mag, grad1: p < 0.01). The latter sustained and offset differences could be due to the prediction of possible probe durations which could take on three different values i.e. 300, 600 and 900 ms but which are not addressed here (van Wassenhove and Lecoutre, 2015).
Fig. 8.
Processing of auditory tone during the Duration discrimination task contrasted between standard and probe tone presentations (gradiometers). A&C. Normed auditory evoked responses from the paired gradiometers (A) and magnetometers (C). The left and middle panels show responses for the TDC group and the right panel shows responses for the ASD group. The topography at the top right corner of each panel illustrates the significant cluster of sensors across which the average evoked responses were obtained. Coloured traces depict the time course of the brain responses evoked by the presentation of the standard tone (black) and the probe tone (red) in the Duration task. The difference in auditory evoked responses are plotted in green. The shadowed areas represent 2 standard deviations of the mean time course for each type of tone. Temporal intervals showing a significant difference between groups for the cluster of sensors are denoted by a red bar along the x axis. B&D. Topographies of the auditory responses evoked by the presentation of the auditory tone in clusters showing significant differences across tone type from gradiometers (B; dx are derivative along the latitude; dy are derivative along the longitude) and magnetometers (D). The right column of sensors shows the difference in the evoked response between the two tasks. Significantly clusters are denoted by x (p < 0.05) or * (p < 0.01).
In the ASD group, comparing the responses elicited by the presentation of standard and probe revealed a bilaterally diminished auditory M50 and M100 for the probe (bilaterally, mag: p < 0.05; and left hemisphere, mag: p < 0.05; grad1: p < 0.05; grad2: p < 0.01; Fig. 8A–D, right panels). No other significant differences were found between standard and probe in the ASD group.
4. General discussion
The present study investigated the neural correlates of interval timing in ASD. Participants performed two auditory comparison tasks in which they had to decide whether two consecutive tones were the same duration (Duration task) or the same pitch (Pitch task) while being recorded with MEG. The first tone in the pair was always the same and served as a standard, whereas the second tone served as a probe and varied in duration or in pitch with respect to the standard according to the task. Data analysis was carried out exclusively on identical standard and probe stimuli (i.e., 500 Hz tones lasting 600 ms). This procedure allowed us to investigate the processing related to the encoding (standard) and to the comparison (probe) of duration and pitch, while keeping all physical stimulus aspects constant.
First, looking at direct group contrasts, we found an enhanced amplitude of the auditory evoked responses to the standard in the ASD group as compared to the TDC group. This was found in both the Pitch and in the Duration tasks. This enhanced amplitude was apparent just after the offset of the standard tone. The significantly prolonged neural response to the stimulus beyond its physical duration and the slower slope of the signal offset observed in ASD could reflect their diminished sensitivity to duration (d′) observed in the current study and in previous work (Falter et al., 2012). One possible interpretation is that ASD individuals encoded the offset of the standard with less precision than TDC individuals, in the sense of a seemingly delayed offset: considering that the standard tone was a reference to which the probe was subsequently compared to, this would de facto affect the comparison process when reaching the offset of the probe. Hence, even if the probe was encoded similarly between the ASD and the TDC groups, the comparison process in the ASD group would be hampered on the basis of the gradual (as opposed to sharp) offset response to the standard offset. The lengthened offset response may indicate that, despite the high predictability of the standard tones, ASD participants failed to automatically predict its precise offset, thereby misencoding its duration. Interestingly, and in line with this hypothesis, no group differences were found in the processing of the probe tone. Thus, our results suggest that differences in duration processing could be due to standard offsets not being automatically predicted in ASD and thereby leading to the difficulty of shunting the comparison process.
Secondly, looking at the comparison process itself, we contrasted the evoked brain responses elicited by the standard and the probe tones separately in each group. In the Pitch task, the ASD group, just like the TDC group, displayed a sustained response difference between standard and probe, supporting the behavioural finding of intact processing of pitch in ASD. In the Duration task, however, the ASD group showed an early reduced response amplitude (~ 100 ms) elicited by the probe as compared to the standard tone; to the contrary, the TDC group showed almost sustained differences throughout the response period ranging from 80 to 700 ms post-stimulus onset. Like in the ASD group, the early latency auditory responses elicited by the probe tone (around 50 to 100 ms post-stimulus onset) were smaller than the responses to the standard, but then from ~ 150 ms onwards, the responses evoked by the probe in the TDC group were enhanced compared to the standard. In the TDC group, these sustained differences strongly suggested distinct processes in the encoding and in the comparison of durations. Specifically, the enhanced amplitude of the early evoked response (~ 150 ms) to the probe tone may reflect additional neural resources allocated during the probe presentation in order to perform the comparison task. In the ASD group, the lack of differences after 140 ms suggests that individuals with ASD processed stimuli more similarly irrespective of position within a trial, in other words they may not allocate additional resources to the processing of the probe tone. This interpretation has to be taken with caution as it is based on a null difference in the ASD group, but the lack of significant difference after 140 ms in the ASD group is quite striking, and supports the idea of atypical processing of duration in autism.
Finally, we investigated each group's ability to adjust neural processing to the task instructions by contrasting the responses evoked in the Duration and Pitch tasks. TDC individuals showed differential evoked responses to the standard tone showing that the same acoustic signal was processed differentially from 50 ms onwards as a function of the cognitive context or task instructions. This suggests feature-specific neural processing of otherwise physically identical stimuli. In contrast, in the ASD group, no difference was found in the responses evoked by the standard tone between the Pitch and the Duration task. When looking at the responses evoked by the probe tone, TDC individuals showed a sustained enhanced response when performing the Duration as compared to the Pitch task. The opposite pattern was found in the ASD group with enhanced evoked response to the probe in the Pitch task as compared to the Duration task, and the difference reached significance for a shorter interval starting around 500 ms. This provides evidence that both groups are processing the identical auditory signals in a feature-specific manner. In the TDC group, the significant differences in the amplitude of the evoked responses when comparing the Pitch and the Duration task likely reflected different demands for each task, the Duration one inducing larger brain responses. Because of the very features of pitch and duration, pitch discrimination could be resolved shortly after the onset of the tone, whereas duration comparison can only be resolved after the offset of the tone – duration, by definition, accumulates over time (Lambrechts et al., 2013, Martin et al., 2017, Shi et al., 2013): a first reduction of uncertainty may therefore be attained after 300 ms if the stimulus is not interrupted, and evidence is sufficient after 600 ms to inform a concluding decision (Kononowicz et al., 2015, van Wassenhove and Lecoutre, 2015). This rationale would explain why in the Duration task the probe elicited a larger response than in the Pitch task in the TDC group. In contrast, the response to pitch was enhanced throughout the probe tone presentation in the ASD group, which seems to indicate preferential processing of pitch or reduced processing of duration in autism, and could underlie the slightly diminished sensitivity in duration comparison in the ASD group. This is in line with reports of increased sensitivity to pitch in autism (Bonnel et al., 2003, Bonnel et al., 2010, Eigsti and Fein, 2013, Heaton et al., 2008, Heaton et al., 2008, Heaton, 2005, Järvinen-Pasley and Heaton, 2007, Järvinen-Pasley et al., 2008a, Järvinen-Pasley et al., 2008b, Jones et al., 2009, Mottron et al., 2000, but see Bhatara et al., 2013). These results also support the hypothesis that individuals with ASD allocate less resources to duration processing, potentially affecting the sensitivity of their temporal judgements. If this is the case early in development, imprecise timing could have cascading effects on learning and on the acquisition of important functions such as joint attention and communication.
The current results together with our previous behavioural data (Falter et al., 2012) indicate that interval timing abilities in individuals with ASD are not entirely disrupted, but rather hampered by a suboptimal engagement of task-specific resources. In particular, while individuals with ASD may have a crisp encoding of the onset of an interval, the predictive coding of the offset of the standard interval, which requires top-down processing (Kononowicz and Rijn, 2015, van Wassenhove and Lecoutre, 2015, Wiener et al., 2010), may be perturbed. This could mean that while TDC individuals can efficiently compare the ongoing duration to a reliable template of the standard interval, individuals with ASD are comparing the probe interval to a less well encoded standard and therefore have greater noise in their decisions. In addition, our results suggest that resources are not allocated optimally according to task demand in ASD: whereas pitch comparison can be solved earlier than duration comparison, the Pitch task claims a greater response throughout the probe tone compared to the Duration task.
A limitation of our result is the absence of control for the effect of time and fatigue on the neural response. TDC and ASD participants might be differentially susceptible to fatigue which would affect the comparison of their neural responses over time, an effect not limited to the current study. It is also possible that the Duration task is in fact more demanding for individuals on the spectrum, especially because it requires to stay engaged for longer than during a Pitch task trial (in which the decision can be reached quickly) and that when their attention fades away, so do the neural resources allocated. Because of a limited number of trials, it was not possible here to gain any more insight in the change in signal over time but this question could be addressed in future research.
5. Conclusions
In this study we investigated the processing of duration as compared to pitch in a group of adults with ASD using MEG. Our results suggest that individuals with ASD are less able to predict the duration of the standard tone accurately, hampering the comparison process. In addition, contrary to TDC individuals who adjust allocation of processing resources at different times during stimulus processing, depending on task instructions and context, individuals with ASD seem to allocate less resources overall to the processing of duration in comparison to pitch, which could lead to less accurate timing judgments. Overall, a diminished ability to judge short durations accurately could have cascading effects in the development of motor behaviour and cognitive functions such as joint attention and communication.
Acknowledgments
Acknowledgments
We thank all participants for their time and commitment and the Baily Thomas Charitable Trust (2216/1) for financial support. We also thank Anthony Bailey for his help and support of this project. We are grateful to Tadeusz W. Kononowicz for his useful feed-back on the manuscript, and to Hedderik van Rijn and two additional anonymous reviewers for their feedback on earlier versions of the MS. CMF was supported by a German Research Council (DFG) Fellowship. The collaboration between CMF, VvW and AL was supported by a European Cooperation in Science and Technology (COST) action on Time in Mental Activity (TIMELY; TD0904). VvW was supported by an ERC-YStG-263584 and an ANR10JCJC-1904.
Footnotes
Due to the high sensitivity of cluster permutations to the number of samples, cluster permutations analyses were first performed for predefined, overlapping time windows of interest around characteristic auditory event related fields (M50, M100, M200, 600 ms response to the stimulus offset). Significant clusters which were either found to overlap or be contiguous, and which shared a majority of sensors were subsequently identified as a single cluster. The analysis was thus rerun with an extended time window. If a cluster had a common boundary with the predefined time-window, the window was extended to capture the real onset and offset of the cluster. These steps are well known short-comings of the implemented cluster permutation analysis. This procedure insured a clear reporting of the effect while preserving no a priori regarding the selection of the appropriate time windows for the hypothesized effect. For these reasons, the final time windows in each condition differed slightly, but aimed to identify independent, robust clusters of significant differences in brain responses between two groups or tasks.
Contributor Information
Anna Lambrechts, Email: Anna.Lambrechts.2@city.ac.uk.
Christine M. Falter-Wagner, Email: Christine.Falter@cantab.net.
Virginie van Wassenhove, Email: Virginie.van.Wassenhove@gmail.com.
References
- Allman M.J. Deficits in temporal processing associated with autistic disorder. Front. Integr. Neurosci. 2011;5(March):2. doi: 10.3389/fnint.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman M.J., DeLeon I.G., Wearden J.H. Psychophysical assessment of timing in individuals with autism. Am. J. Intellect. Dev. Disabil. 2011;116(2):165–178. doi: 10.1352/1944-7558-116.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. [Google Scholar]
- Bhatara A., Babikian T., Laugeson E., Tachdjian R., Sininger Y.S. Impaired timing and frequency discrimination in high-functioning autism spectrum disorders. J. Autism Dev. Disord. 2013;43(10):2312–2328. doi: 10.1007/s10803-013-1778-y. [DOI] [PubMed] [Google Scholar]
- Bonnel A., Mottron L., Peretz I., Trudel M., Gallun E., Bonnel A.-M. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J. Cogn. Neurosci. 2003;15(2):226–235. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Bonnel A., McAdams S., Smith B., Berthiaume C., Bertone A., Ciocca V.…Mottron L. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48(9):2465–2475. doi: 10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Boucher J. Lost in a sea of time: Time parsing and autism. In: McCormack T., Hoerl C., editors. Time and Memory: Issues in Philosophy and Psychology (Oxford Uni) Oxford University Press; Oxford: 2001. [Google Scholar]
- Boucher J. Research review: Structural language in autistic spectrum disorder - Characteristics and causes. J. Child Psychol. Psychiatry Allied Discip. 2012;53(3):219–233. doi: 10.1111/j.1469-7610.2011.02508.x. [DOI] [PubMed] [Google Scholar]
- Brodeur D.a., Gordon Green C., Flores H., Burack J.a. Time estimation among low-functioning individuals with autism spectrum disorders: evidence of poor sensitivity to variability of short durations. Autism Res. 2014;7(2):237–244. doi: 10.1002/aur.1364. [DOI] [PubMed] [Google Scholar]
- Buchwald J.S., Erwin R., Van Lancker D., Guthrie D., Schwafel J., Tanguay P. Midlatency auditory evoked responses: P1 abnormalities in adult autistic subjects. Electroencephalogr. Clin. Neurophysiol. 1992;84(2):164–171. doi: 10.1016/0168-5597(92)90021-3. [DOI] [PubMed] [Google Scholar]
- Ceponiene R., Lepistö T., Shestakova, a, Vanhala, R., Alku, P., Näätänen, R., & Yaguchi, K. Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T., Baird G. Practitioner review: diagnosis of autism spectrum disorder in 2-and 3-year-old children. J. Child Psychol. Psychiatry. 2002;43(3):289–305. doi: 10.1111/1469-7610.00022. [DOI] [PubMed] [Google Scholar]
- Eagleman D.M. Human time perception and its illusions. Curr. Opin. Neurobiol. 2008;18(2):131–136. doi: 10.1016/j.conb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I.-M.M., Fein D.a. More is less: pitch discrimination and language delays in children with optimal outcomes from autism. Autism Res. 2013;6(6):605–613. doi: 10.1002/aur.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I.M., De Marchena A.B., Schuh J.M., Kelley E. Language acquisition in autism spectrum disorders: a developmental review. Res. Autism Spectr. Disord. 2011;5(2):681–691. [Google Scholar]
- Falter C.M., Noreika V., Wearden J.H., Bailey A.J. More consistent, yet less sensitive: Interval timing in autism spectrum disorders. Q. J. Exp. Psychol. 2012;65(11):2093–2107. doi: 10.1080/17470218.2012.690770. [DOI] [PubMed] [Google Scholar]
- Falter C.M., Braeutigam S., Nathan R., Carrington S., Bailey A.J. Enhanced access to early visual processing of perceptual simultaneity in autism spectrum disorders. J. Autism Dev. Disord. 2013;43(8):1857–1866. doi: 10.1007/s10803-012-1735-1. [DOI] [PubMed] [Google Scholar]
- Ferri R., Elia M., Agarwal N., Lanuzza B., Musumeci, S. a, & Pennisi, G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clin. Neurophysiol. 2003;114(9):1671–1680. doi: 10.1016/s1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- Gil S., Chambres P., Hyvert C., Fanget M., Droit-Volet S. Children with autism spectrum disorders have “The Working Raw Material” for time perception. PLoS One. 2012;7(11):1–10. doi: 10.1371/journal.pone.0049116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M., Giard M.-H., Adrien J.-L., Barthelemy C., Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39(5):577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gross J., Baillet S., Barnes G.R., Henson R.N., Hillebrand A., Jensen O.…Schoffelen J.-M. Good practice for conducting and reporting MEG research. NeuroImage. 2013;65:349–363. doi: 10.1016/j.neuroimage.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesen B., Boets B., Wagemans J. A review of behavioural and electrophysiological studies on auditory processing and speech perception in autism spectrum disorders. Res. Autism Spectrum Disord. 2011;5(2):701–714. [Google Scholar]
- Heaton P. Interval and contour processing in autism. J. Autism Dev. Disord. 2005;35(6):787–793. doi: 10.1007/s10803-005-0024-7. [DOI] [PubMed] [Google Scholar]
- Heaton P., Davis R.E., Happé F.G.E. Research note: exceptional absolute pitch perception for spoken words in an able adult with autism. Neuropsychologia. 2008;46(7):2095–2098. doi: 10.1016/j.neuropsychologia.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Hill E.L. Executive dysfunction in autism. Trends Cogn. Sci. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A., Heaton P. Evidence for reduced domain-specificity in auditory processing in autism. Dev. Sci. 2007;10(6):786–793. doi: 10.1111/j.1467-7687.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A., Wallace G.L., Ramus F., Happé F., Heaton P. Enhanced perceptual processing of speech in autism. Dev. Sci. 2008;11(1):109–121. doi: 10.1111/j.1467-7687.2007.00644.x. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A., Pasley J., Heaton P. Is the linguistic content of speech less salient than its perceptual features in autism? J. Autism Dev. Disord. 2008;38(2):239–248. doi: 10.1007/s10803-007-0386-0. [DOI] [PubMed] [Google Scholar]
- Jones C.R.G., Happé F., Baird G., Simonoff E., Anita J.S., Tregay J.…Charman T. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47(13):2850–2858. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Kononowicz T.W., Rijn H. Van. Single trial beta oscillations index time estimation. Neuropsychologia. 2015;75:381–389. doi: 10.1016/j.neuropsychologia.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Kononowicz T.W., Sander T., van Rijn H. Neuroelectromagnetic signatures of the reproduction of supra-second durations. Neuropsychologia. 2015;75:201–213. doi: 10.1016/j.neuropsychologia.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K., Kuhl P.K., Coffey-corina S., Coffey-corina S., Padden D., Padden D.…Dawson G. Links between social and linguistic processing of speech in children with autism: behavioural and electrophysiological measures. Dev. Sci. 2005;8(1):1–12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Kujala T., Aho E., Lepistö T., Jansson-Verkasalo E., Nieminen-von Wendt T., von Wendt L., Näätänen R. Atypical pattern of discriminating sound features in adults with Asperger syndrome as reflected by the mismatch negativity. Biol. Psychol. 2007;75(1):109–114. doi: 10.1016/j.biopsycho.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Lambrechts A., Walsh V., Van Wassenhove V. Evidence accumulation in the magnitude system. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepistö T., Kujala T., Vanhala R., Alku P., Huotilainen M., Näätänen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Res. 2005;1066(1–2):147–157. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lepistö T., Silokallio S., Nieminen-von Wendt T., Alku P., Näätänen R., Kujala T. Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clin. Neurophysiol. 2006;117(10):2161–2171. doi: 10.1016/j.clinph.2006.06.709. [DOI] [PubMed] [Google Scholar]
- Lepistö T., Kajander M., Vanhala R., Alku P., Huotilainen M., Näätänen R., Kujala T. The perception of invariant speech features in children with autism. Biol. Psychol. 2008;77(1):25–31. doi: 10.1016/j.biopsycho.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994 doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H.J., Leventhal B.L., DiLavore P.C.…Rutter M. The Autism Diagnostic Schedule – generic: A standard measures of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., Dilavore P.C., Risi S. Hogrefe; 2008. ADOS: Autism diagnostic Observation Schedule. [Google Scholar]
- Maister L., Plaisted-Grant K.C. Time perception and its relationship to memory Autism Spectrum Conditions. Dev. Sci. 2011;14(6):1311–1322. doi: 10.1111/j.1467-7687.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- Marco E.J., Hinkley L.B.N., Hill S.S., Nagarajan S. Sensory processing in autism: a review of neuropsychologic findings. Pediatr. Res. 2011;69(5):48–54. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martin J.S., Poirier M., Bowler D.M. Brief report: Impaired temporal reproduction performance in adults with autism spectrum disorder. J. Autism Dev. Disord. 2010;40(5):640–646. doi: 10.1007/s10803-009-0904-3. [DOI] [PubMed] [Google Scholar]
- Martin B., Wiener M., Van Wassenhove V. A Bayesian perspective on accumulation in the magnitude system. Sci Rep. 2017;7(1):630. doi: 10.1038/s41598-017-00680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T., Wearden J.H., Smith M.C., Brown G.D.A. Episodic temporal generalization: a developmental study. Q. J. Exp. Psychol. A Hum. Exp. Psychol. 2005;58(4):693–704. doi: 10.1080/02724980443000250. [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Goldberg M.C., Landa R.J., Denckla M.B. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. J. Int. Neuropsychol. Soc. 2000;6(7):752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Mottron L., Peretz I., Ménard E. Local and global processing of music in high-functioning persons with autism: beyond central coherence? J. Child Psychol. Psychiatry Allied Discip. 2000;41(8):1057–1065. [PubMed] [Google Scholar]
- O'Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci. Biobehav. Rev. 2012;36(2):836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. 2011. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram Cardy J.E., Flagg C.A.E.J., Roberts W., Brian J., Roberts T.P.L. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport. 2005;16(4):329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- Shi Z., Church R.M., Meck W.H. Bayesian optimization of time perception. Trends Cogn. Sci. 2013;17(11):556–564. doi: 10.1016/j.tics.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Szelag E., Kowalska J., Galkowski T., Pöppel E. Temporal processing deficits in high-functioning children with autism. Br. J. Psychol. 2004;95(Pt 3):269–282. doi: 10.1348/0007126041528167. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51(7):1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Wallace G., Happe F. Time perception in autism spectrum disorders. Res. Autism Spectr. Disord. 2008;2(3):447–455. [Google Scholar]
- van Wassenhove V. Minding time in an amodal representational space. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364(1525):1815–1830. doi: 10.1098/rstb.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wassenhove V., Lecoutre L. Duration estimation entails predicting when. NeuroImage. 2015;106(July):272–283. doi: 10.1016/j.neuroimage.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Wearden J.H. Detectability and criterion measures in temporal generalization. Behav. Process. 2008;78(3):374–379. doi: 10.1016/j.beproc.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Wearden J.H., Lejeune H. Scalar properties in human timing: conformity and violations. Q. J. Exp. Psychol. 2008;61(4):569–587. doi: 10.1080/17470210701282576. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Wiener M., Turkeltaub P., Coslett H.B. The image of time: a voxel-wise meta-analysis. NeuroImage. 2010;49(2):1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Wimpory D., Chadwick P., Nash S. Brief report: musical interaction therapy for children with autism: an evaluative case study with two-year follow-up. J. Autism Dev. Disord. 1995;25(5):541–552. doi: 10.1007/BF02178299. [DOI] [PubMed] [Google Scholar]
- Wimpory D., Nicholas B., Nash S. Social timing, clock genes and autism: a new hypothesis. J. Intellect. Disabil. Res. 2002;46(4):352–358. doi: 10.1046/j.1365-2788.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- Wittmann M., van Wassenhove V. The experience of time: neural mechanisms and the interplay of emotion, cognition and embodiment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364(1525):1809–1813. doi: 10.1098/rstb.2009.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]