Abstract
Magnetophoresis is a method of enhancement of drug permeation across the biological barriers by application of magnetic field. The present study investigated the mechanistic aspects of magnetophoretic transdermal drug delivery and also assessed the feasibility of designing a magnetophoretic transdermal patch system for the delivery of lidocaine. In vitro drug permeation studies were carried out across the porcine epidermis at different magnetic field strengths. The magnetophoretic drug permeation “flux enhancement factor” was found to increase with the applied magnetic field strength. The mechanistic studies revealed that the magnetic field applied in this study did not modulate permeability of the stratum corneum barrier. The predominant mechanism responsible for magnetically mediated drug permeation enhancement was found to be “magnetokinesis”. The octanol/water partition coefficient of drugs was also found to increase when exposed to the magnetic field. A reservoir type transdermal patch system with a magnetic backing was designed for in vivo studies. The dermal bioavailability (AUC0–6 h) from the magnetophoretic patch system in vivo, in rats was significantly higher than the similarly designed nonmagnetic control patch.
Keywords: Magnetophoresis, Transdermal, Lidocaine, Partition coefficient, Enhancer
1. Introduction
Transdermal permeation of polar and hydrophilic drugs is limited by the barrier property of stratum corneum. Therefore, several chemical and physical permeation enhancement techniques have been explored to improve the transdermal delivery of poorly permeable drugs [1–5]. In this direction, Murthy et al. investigated the plausibility of using magnetic field to enhance the transdermal permeation of drugs. The authors reported for the first time that the static magnetic field could facilitate the transdermal permeation of drugs such as benzoic acid, salbutamol sulfate and terbutaline sulfate [6–8]. The authors speculated that the enhanced transdermal drug permeation by the applied magnetic field could be a combination of multiple mechanisms such as magnetokinesis and alteration of barrier property of skin. A recent study by Krishnan et al. utilized an electromagnetic field to enhance the drug permeation across the skin. Krishnan et al. reported that the potential mechanism for enhanced transdermal drug permeation enhancement by the electromagnetic field of 5 mT was due to modulation of the permeability of stratum corneum [9]. Further, the present study was carried out with an objective to clearly resolve the predominant mechanisms responsible for enhanced transdermal drug permeation in the presence of the static gradient magnetic field similar to that applied by Murthy and co-workers [6–8]. More importantly, this study also assessed the feasibility of designing a magnetophoretic transdermal patch system. The base and salt form of a local anesthetic, lidocaine which is a diamagnetic molecule was selected as the candidate for the present study.
There are a number of reports regarding the use of magnetite incorporated particulate systems in drug delivery and imaging. An externally applied magnetic field has been shown to direct the systemically administered magnetoliposomes or nanoparticles to the target tissue [10,11]. The mechanism associated with this approach is attraction of the ferromagnetic substance, magnetite, towards the magnetic field. The present project involved delivery of a drug across the skin by application of magnetic field, where drug molecules which are diamagnetic in nature are repelled away from the magnetic field.
2. Materials and methods
2.1. Materials
Lidocaine hydrochloride (LH) was purchased from Spectrum Chemicals (New Brunswick, NJ), lidocaine base (LB), d-mannitol and sodium chloride were procured from Sigma-Aldrich Inc. (St. Louis, MO). [3H] Water and d-[1-14C] Mannitol were procured from American Radiolabeled Chemicals, Inc. (St. Louis, MO) and all other chemicals were obtained from Fischer Scientific (Fairway, NJ). Neodymium magnets were purchased from United Nuclear Scientific (Laingsburg, MI) and K&J Magnetics, Inc. (Jamison, PA).
2.2. Porcine epidermis
Porcine belly skin was obtained from a local abattoir. Pieces of skin wrapped in aluminum foil were heated to 60 °C for 2 min and the epidermis was gently peeled off the skin. The fresh epidermis was placed on glass microscopic slides and kept dry at 4 °C and was used within three days. Prior to use, the epidermis was hydrated with normal saline for an hour.
2.3. General in vitro experimental setup
A vertical Franz-type diffusion apparatus (PermeGear, Inc. Hellertown, PA) was used for all resistance measurements and drug permeation studies across the porcine epidermis. A piece of porcine epidermis was placed between the two compartments of the diffusion apparatus, one serving as the donor and other as the receiver compartment. The active diffusion area of the epidermis was 0.2 cm2. The receiver compartment (5 mL) and donor compartment (0.2 mL)were filled with isotonic saline solution. Ag/AgCl electrode wires of 0.5 mm diameter (Alfa Aesar, Ward Hill, MA) made in the form of concentric rings were placed 2 mm away from the porcine epidermis in both the donor and the receiver compartments. Before carrying out permeation studies, the electrodes were connected to the electrical set up for resistance measurement. The electrical resistance of the epidermis was measured by placing a load resistor RL (100 kΩ) in series with the epidermis. The voltage drop across the whole circuit (VO) and across the epidermis (VS) was measured using an electrical set up consisting of a wave form generator and a digital multimeter (Agilent Technologies, Santa Clara, CA). To measure resistance, a voltage of 100 mV was applied at 10 Hz and the skin resistance in kΩ was approximated. Only pieces of epidermis, which had resistances greater than 20 kΩ cm2 were used for the experiments. During the in vitro drug permeation studies the temperature of receiver compartment was maintained at 37 ± 1 °C by water circulation.
2.4. Passive drug permeation studies
After the electrical resistance measurement, the isotonic saline in the donor compartment was replaced with 0.2 mL of LH solution (10 mg/mL) or LB solution (1.66 mg/mL) prepared in isotonic saline and the permeation studies were carried out for 8 h. During the course of permeation studies, at each sampling time point (2, 3, 4, 5, 6, 7 and 8 h) 0.2 mL of the receiver compartment solution was sampled out, and was subsequently replaced with fresh isotonic saline solution. The amount of drug in the samples was analyzed by HPLC.
2.5. Magnetophoretic drug permeation studies
The experimental set up was similar to passive drug permeation studies, and the magnetic field was applied by placing two neodymium magnets (1.2 cm length × 0.6 cm width × 0.6 cm thickness) on either side of the donor compartment and at a distance of 2 mm above the porcine epidermis (Fig. 1). Permeation studies were carried out for 8 h at different magnetic field strengths of 30, 150 and 300 mT. The amount of drug permeated across porcine epidermis into the receiver compartment solution was determined by analyzing the samples withdrawn at different time points as discussed in Section 2.4.
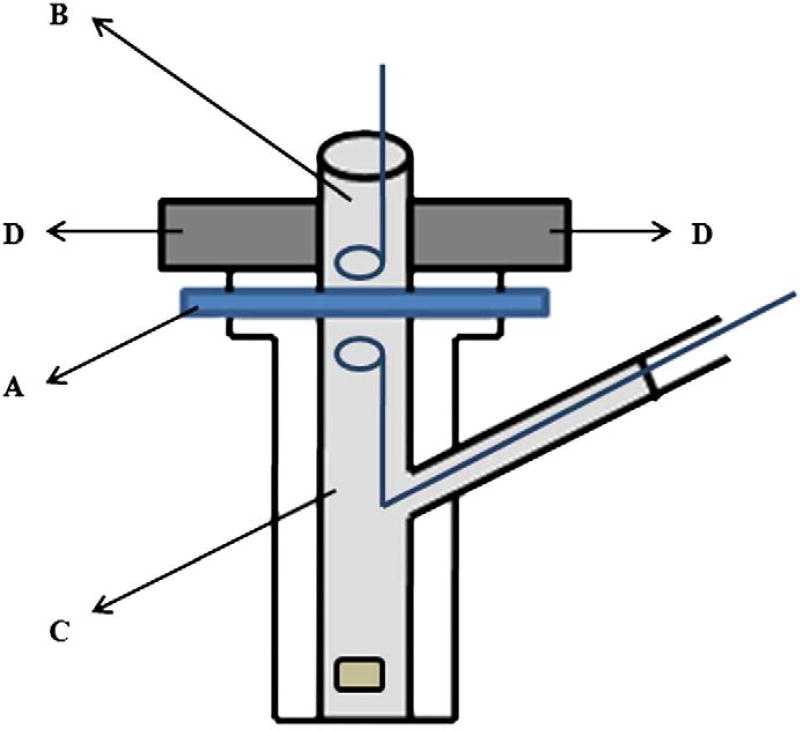
Fig. 1.
Experimental set up for permeation studies where the porcine epidermis ‘A’ was sandwiched between the donor chamber ‘B’ filled with drug solution and receiver chamber ‘C’ filled with isotonic saline. Two neodymium magnets ‘D’ were placed on either side of the donor compartment. The donor and receiver compartments were fitted with Ag/AgCl electrode wires for measurement of electrical resistance.
2.6. Drug permeation across the dialysis membrane
The drug permeation studies were carried out across Spectra/Por® dialysis membrane (1000 Da molecular weight cut-off) in the absence and presence of magnetic field (300 mT) following exactly the same procedure described in the passive and magnetophoretic drug permeation studies (Sections 2.4 and 2.5).
2.7. Drug permeation across the pretreated epidermis (epidermis exposed to magnetic field)
The porcine epidermis was pretreated (for 24 h) by exposure to the magnetic field by using a similar set up as shown in Fig. 1, without placing any medium in the donor compartment. After the pretreatment, the donor chamber was filled with drug solution and the passive drug permeation studies were carried out across the pretreated epidermis similar to that discussed in Section 2.4.
2.8. [3H] water and d-[1-14C] mannitol transport studies
The experimental set up was similar to that used for the passive and magnetophoretic drug permeation studies (Sections 2.4 and 2.5). The donor compartment was filled with 0.2 mL of [3H] water (20 μCi/ml) in isotonic saline for water transport studies. Mannitol (1 mg/ml) and [1-14C] mannitol (5 μCi/ml) in isotonic saline was placed in the donor compartment for mannitol transport studies. The receiver compartment was filled with isotonic saline in both the studies. In the case of the magnetophoretic permeation studies, a magnetic field of 300 mT was applied. After the passive and magnetophoretic permeation studies, tritiated water and mannitol transport across porcine epidermis was measured by withdrawing samples at regular intervals for a period of 8 h. The amount of [3H] water and [14C] mannitol was determined by scintillation counting (Scientisafe Econo 2 cocktail, Fischer Scientific, Fairway, NJ; liquid scintillation analyzer, Tri-Carb 1600 TR, Packard Bioscience, Meriden, CT).
2.9. Transepidermal Water Loss (TEWL) studies
The TEWL was measured using a Vapometer (Delfin Technologies, Kuopio, Finland). Porcine epidermis was mounted between the receiver and donor compartments of the diffusion cell (Fig. 1). Isotonic saline solution was placed only in the receiver compartment and the donor compartment was kept empty. The whole set up was placed in a temperature (37 °C) and humidity (RH 55%) controlled chamber and the TEWL was measured by fixing the Vapometer directly on the donor compartment. TEWL was measured prior to the start of the experiment. The test set was exposed to magnetic field (300 mT). The TEWL was measured at different time points up to 8 h.
2.10. Fourier Transform Infrared (FT-IR) spectroscopic studies
The porcine epidermis was subjected to FT-IR spectroscopic studies in absence and presence of magnetic field. Infrared spectra were obtained in the transmission mode using a Nicolet 6700 FT-IR spectrometer in the frequency range 4000–1000 cm−1. Sixty four scans were collected at 4 cm−1 resolution. The same piece of porcine epidermis was used for recording the spectra in absence and presence of magnets. Two magnets were placed on the surface of porcine epidermis at a distance of 6 mm with unlike poles facing each other (magnetic field of 300 mT) for recording the spectra. The magnets used, distance between magnets and polarity were same as those used for in vitro permeation studies.
2.11. Effect of magnetic field on octanol–water and stratum corneum (SC)–aqueous vehicle partitioning of drug
To determine the octanol–water partition coefficient, deionized water equilibrated with an equal volume of octanol was used to prepare the drug solution. 0.2 mL of drug solution (concentrations of 50, 100, 250 and 500 µg/mL) was placed in contact with 0.2 mL of octanol in a glass vial. The two phases were mixed thoroughly by vigorous shaking for 6 h and allowed to equilibrate for next 18 h. After equilibration, 20 µL of aqueous and octanol phases were withdrawn for measurement and calculation of “initial partition coefficient”. The vials from the test group were placed in presence of magnetic field (300 mT) and the control set were not. Only the aqueous phase was placed in presence of magnetic field. After 24 h, the “final partition coefficient” of the drug in the control and test set of vials was determined. The average difference in the partition coefficient (final partition coefficient value – initial partition coefficient value) was compared between control and the test sets.
To determine SC–vehicle partition coefficient, preweighed samples of SC (isolated by trypsin digestion method as shown by Essa et al.) were placed in 0.2 mL of drug solution in isotonic saline (100 µg/mL) in a glass vial for 48 h [12]. A magnetic field of 300 mT was applied on the bulk of the vehicle in the vial, only to the test group. At the end of 48 h, the amount of drug present in the vehicle and SC were measured to estimate the partition coefficient of drug.
2.12. Fabrication of magnetophoretic transdermal patches
Magnetophoretic transdermal patches were designed by affixing a series of neodymium magnets (10 mm length × 1.5 mm width × 1.5 mm thickness) on a nonmagnetic backing membrane which is placed over an adhesive 3M™ foam tape (3M Drug Delivery Systems, St. Paul, MN). About 200 mg of LH and LB gel (2%w/w) prepared in HPMC base (4%w/v) was spread over the magnets (1.5 cm2) as a thin layer, which acts as drug reservoir. The magnetic field strength at the skin surface of the magnetophoretic patch system was 450 mT. In the case of passive transdermal patches, the neodymium magnets were replaced with similar nonmagnetic metal pieces.
LH gel was prepared using HPMC in deionized water. Whereas, LB gel was prepared using HPMC in hydroalcoholic solution (water: alcohol, 70:30) due to the limited solubility of LB in aqueous medium.
2.13. In vivo dermal bioavailability studies of magnetophoretic patch system
The animal studies were approved by the Institutional Animal Care and Use committee (IACUC) at the University of Mississippi (Protocol # 09-031). The in vivo studies were performed on 12 male Sprague–Dawley rats (200–250 g) under ketamine (80 mg/kg) and xylazine (10 mg/kg) anesthesia administered intraperitoneally. The rats were procured from Harlan (Indianapolis, IN).
The in vivo studies in rats were carried out at 37 ± 1 °C by placing the animals on temperature control, water circulating pads. Cutaneous microdialysis was carried out in rats by inserting a 20G needle intradermally through a distance of 2 cm and a linear microdialysis probe of 5 mm membrane length and 30 kDa cut-off molecular weight (BASi, West Lafayette, IN) was inserted through this needle and the needle was withdrawn leaving the probe implanted in the dermal tissue [13,14]. The inlet tube was connected to an injection pump (BASi, West Lafayette) and isotonic saline solution was perfused at a flow rate of 2 µL/min. After equilibration of the probe for 30 min, a transdermal patch system was placed above the region where microdialysis probe was inserted and studies were carried out for a period of 6 h. For both the salt (LH) and base (LB) forms of the drug, animal studies were performed using passive and magnetophoretic patch systems respectively. The probe recovery was determined in vivo by retrodialysis method.
2.14. Analytical method
The amount of lidocaine hydrochloride and lidocaine were analyzed by high performance liquid chromatography. The HPLC system (Waters, MA) consisted of a chromatographic pump (Waters 1525), autosampler (Waters 717 plus) and an UV absorbance detector (Waters 2487). Symmetry® C18 column (4.6× 150 mm) was used and the mobile phase consisted of a mixture (14/86 v/v) of acetonitrile and potassium dihydrogen phosphate 0.05 M (pH adjusted to 4.0) 1.3 mL/min at 216 nm [15].
2.15. Statistical analysis
Statistical analysis was carried out using GraphPad InStat 3 software. Unpaired t-test was performed and P<0.05 was considered as the level of significance.
3. Results and discussion
Porcine epidermis has been accepted as one of the most appropriate models for transdermal drug delivery studies due to its similarities in structure and lipid composition of stratum corneum with the human [16–18]. Therefore, porcine epidermis was employed in all the in vitro experiments in the present study. The magnetic field of different strengths (30–300 mT) was generated by varying the distance between the poles of bar magnets or by using magnets of different strengths. The magnetic field strengths were measured at the surface of the epidermis using a hand-held Gauss/Tesla meter (F.W. Bell, Model # 4048).
3.1. Magnetophoresis of LH across the porcine epidermis
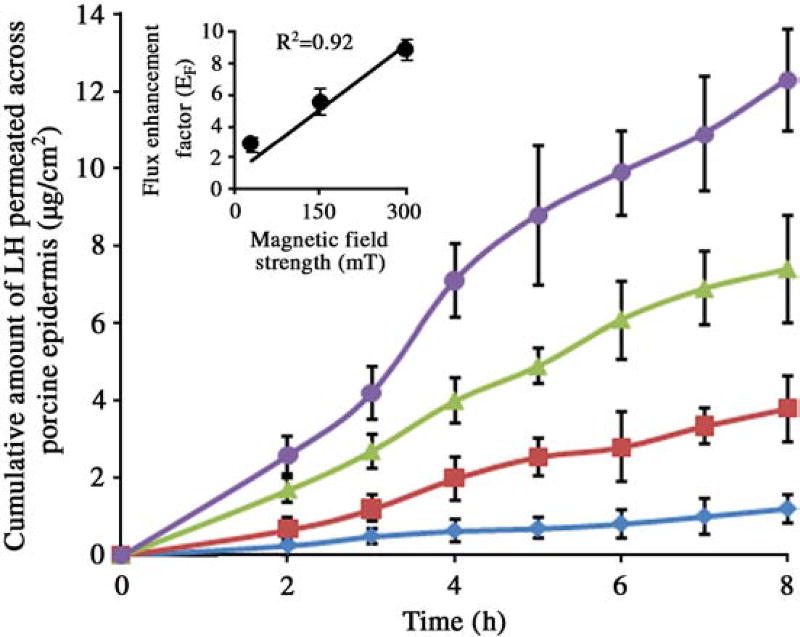
The data from Fig. 2 and Table 1 clearly demonstrate the ability of magnetic field to enhance the permeation of LH across the porcine epidermis. The permeation flux enhancement factor (EF) (permeation flux in presence of magnetic field/flux in absence of magnetic field) was found to increase linearly with increasing applied magnetic field strength as shown in the insert graph in Fig. 2. The relationship between the permeation flux enhancement factor for LH and magnetic field strength could be presented as EF = 0.027S + 1, (R2 = 0.92) where EF is the flux enhancement factor and S is the strength of magnetic field at the surface of the epidermis.
Fig. 2.
Cumulative permation of LH across porcine epidermis in case of passive (◆), magnetic field strength of 30 mT (■), 150 mT (▲) and 300 mT (●). Insert graph shows plot of magnetic field strength vs. flux enhancement factor. The data points represented are the average of n = 3 ± S.D.
Table 1.
Permeation flux and flux enhancement factor of LH and LB across porcine epidermis. The data points represented are the average of n = 3 ± S.D.
| Magnetic field (mT) |
LH | LB | ||
|---|---|---|---|---|
|
|
|
|||
| Permeation flux (µg/cm2/h) |
Flux enhancement factor (EF) |
Permeation flux (µg/cm2/h) |
Flux enhancement factor (EF) |
|
| 0 | 0.18 ± 0.06 | 1.0 | 0.57 ± 0.12 | 1.0 |
| 30 | 0.53 ± 0.09 | 2.9 | 0.75 ± 0.18 | 1.3 |
| 150 | 1.01 ± 0.17 | 5.6 | 1.14 ± 0.29 | 2.0 |
| 300 | 1.61 ± 0.12 | 8.9 | 2.23 ± 0.22 | 3.9 |
There was no significant change in the pH (~7.0) or temperature (~32 °C) of the donor compartment solution due to exposure to magnetic field during the permeation studies.
The concept of utilizing magnetic field for enhancement of transdermal drug delivery emanated from the hypothesis that diamagnetic substances, which are generally repelled away from the external magnetic field would be driven across the biological barrier along the direction of the magnetic field gradient [6]. However, the diamagnetic susceptibility is considered to be a very weak property and the driving force generated under mT range magnetic field is regarded as rather mild. Despite this, a significant transdermal permeation enhancement of drug was observed in the presence of magnetic field suggesting that the magnetophoresis phenomenon could involve multiple mechanisms.
Generally, transdermal physical permeation enhancement techniques are known to cause enhanced drug permeation across the biological barriers by mechanisms such as kinesis of drug molecules and/or by alteration of the biological barrier. Iontophoresis is one of the techniques which is known to enhance the transdermal drug permeation predominantly by driving the drug ions due to electrorepulsion. On the other hand, techniques such as electroporation, sonophoresis and thermoporation enhance the transdermal delivery of drugs by disruption of the stratum corneum barrier. To resolve the role of different mechanisms in case of magnetophoresis, systematic mechanistic studies were carried out as discussed further in this manuscript.
3.2. Permeation across the pretreated epidermis (epidermis exposed to magnetic field)
To assess the plausible effect of an applied magnetic field on the barrier property, the epidermis was pretreated by exposure to magnetic field (30, 150 and 300 mT) for 24 h. Passive permeation studies (in absence of magnetic field) were carried out across the pretreated epidermis. Permeation experiments across the untreated epidermis served as control. The permeation flux across the epidermis did not differ significantly between pretreated and untreated epidermis suggesting that prior exposure of the epidermis to magnetic field did not bring about any long lasting change in the epidermal permeability (Table 2). However, this experiment did not rule out the possibility of any potential real time microstructural changes in the epidermis during the magnetophoretic drug permeation studies.
Table 2.
Permeation flux of LH and LB across pretreated porcine epidermis (epidermis exposed to magnetic field for 24 h). The epidermis was exposed to different magnetic field strengths and the drug permeation studies were carried out across the pretreated epidermis. The data points represented are the average of n = 3 ± S.D.
| Pretreatment magnetic field (mT) |
Permeation flux after pretreatment (µg/cm2/h) |
|
|---|---|---|
|
|
||
| LH | LB | |
| 0 | 0.17 ± 0.05 | 0.59 ± 0.09 |
| 30 | 0.18 ± 0.03 | 0.56 ± 0.13 |
| 150 | 0.16 ± 0.06 | 0.60 ± 0.19 |
| 300 | 0.17 ± 0.03 | 0.57 ± 0.16 |
TEWL and electrical resistance have been considered as two promising parameters used to assess the barrier integrity of the stratum corneum [19,20]. However, TEWL is known to be relatively less sensitive than electrical resistance as it can only reflect mechanical disruption of the barrier. On the other hand, electrical resistance is relatively more sensitive and could change significantly even due to any microstructural changes in the stratum corneum. TEWL and electrical resistance of the porcine epidermis were measured at different time points while the epidermis was being exposed to magnetic field. The initial TEWL and electrical resistance of the porcine epidermis was 6.15 ± 0.71 g/m2/h and 38.1 ± 1.2 kΩ cm2 respectively. The TEWL value across the intact epidermis that has been reported by other groups agrees well with that found in this case [21,22]. The TEWL and electrical resistance remained constant during study period of 8 h. Therefore, it is most likely that, at the applied magnetic field strengths in these experiments (30–300 mT), no significant real time transient structural alterations occurred in the epidermis. To further confirm the non-interaction of the magnetic field with the stratum corneum lipids, the epidermis were subjected to FT-IR studies. The FT-IR spectra of the stratum corneum side were recorded before and after exposure to the magnetic field. A custom designed skin holder was used to hold the magnets in place even while the spectra were recorded.
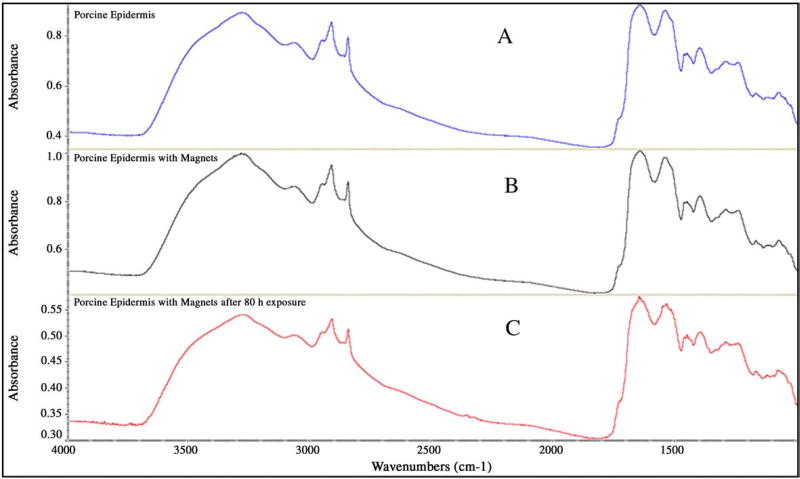
There was no significant shift in the peaks in the lipid region (2920 and 2850 cm−1) or in the amide regions (1650 and 1550 cm−1) as clearly seen in the representative spectra shown in Fig. 3, even after exposure to magnetic field for over 80 h. This data also supplements the hypothesis that the magnetic field strength used in this project has negligible or no effect on the skin structure and permeability.
Fig. 3.
Representative FT-IR spectra of porcine epidermis in absence of magnetic field (A), presence of magnetic field (B) and after exposure to magnetic field for 80 h (C).
3.3. Magnetokinesis of drug
Magnetokinesis is a phenomenon of forced propagation of drug molecules under the applied magnetic field. Magnetokinesis of molecules and particles under a gradient magnetic field has been reported in the past by several research groups [23–25]. There are at least two potential mechanisms that can contribute to magnetokinesis in the present experimental set up, magnetorepulsion and magnetohydrokinesis. Magnetorepulsion could be described as driving of the drug molecules (due to the repulsive force experienced by the induced diamagnet) in the presence of external magnetic field. Magnetohydrokinesis mediated drug transport is due to the movement of water across the membrane under the influence of an external magnetic field. These mechanisms are comparable to electrorepulsion and electroosmosis in the case of iontophoretic transdermal drug delivery. To reassess if magnetokinesis is one of the mechanisms leading to transdermal drug permeation enhancement, permeability studies were also carried out across the dialysis membrane with a cut-off size of 1000 Da. A nonbiological barrier like dialysis membrane was chosen to eliminate any effect of magnetic field on the barrier. Interestingly, application of magnetic field (300 mT) enhanced the permeation flux of LH across the dialysis membrane also by ~4-fold over passive permeation (passive: 10.79 ± 2.26 µg/cm2/h vs. magnet: 38.98 ± 6.21 µg/cm2/h). These results suggest that magnetokinesis is one of the predominant mechanisms responsible for drug permeation enhancement at these applied magnetic field strengths. However, these experiments did not differentiate the relative contribution due to magnetorepulsion and magnetohydrokinesis.
Magnetohydrokinesis across the epidermis under the influence of the applied magnetic field was investigated by studying the transport of tritiated water across the epidermis. There was a significant difference between the water transport flux across the epidermal membrane in the absence (32.63 ± 5.25 nL/cm2/h) and presence (57.21 ± 8.63 nL/cm2/h) of magnetic field (300 mT) indicating that hydrokinesis could be one of the factors contributing significantly to the overall drug transport due to magnetokinesis.
Enhanced transport of water would result in proportionately enhanced transport of dissolved substances as well. Therefore to add supportive evidence to the magnetohydrokinesis phenomenon, transport of 14C-mannitol across the epidermis was investigated. The application of magnetic field (300 mT) resulted in ~2.7-fold enhancement (5.18 ± 1.88 ng/cm2/h) in the transport flux of mannitol as compared to passive diffusion (1.89 ± 0.49 ng/cm2/h). The contribution of magnetohydrokinesis to the drug transport across biological membranes may become more apparent when higher magnetic field strengths are applied and may become remarkably significant when a more concentrated drug solution is placed in the donor chamber.
3.4. Effect of magnetic field on octanol–water and SC–aqueous vehicle partitioning of drug
One of the predominant factors known to determine the permeation of drugs across the skin is the partition coefficient. Generally, chemical permeation enhancers improve the drug permeation by increasing the partitioning of drug into the skin lipids. Sun et al. have shown that induction of magnetic field enhanced the extraction of acetone due to the increase in partition coefficient [26]. In the present work, the effect of exposure to magnetic field on the partition coefficient of drug between octanol and water phases was investigated. The partition coefficient of LH in the absence of magnetic field was 13.80 ± 4.79 and in the presence of magnetic field (300 mT)was found to be 28.94 ± 2.11. In the present set up for determination of partition coefficient, the aqueous phase was exposed to the magnetic field and the gradient exists along the aqueous→organic phase direction. It is likely that the drug molecules are driven to the interface along the field gradient which in turn modulates the thermodynamic equilibrium in a way that favors more partitioning of drug into the octanol phase. The other potential reason for enhanced octanol partitioning of the drug could be due to the changes in the solvent properties due to exposure to the magnetic field. The SC–aqueous vehicle partition coefficient of LH was also found to increase to 0.125 ± 0.02 in the presence of magnetic field compared to 0.037 ± 0.012 in the absence of magnetic field.
3.5. Magnetophoresis of lidocaine base
LB has a better skin permeation property than its salt (LH) as it is relatively more lipophilic than LH (log P of LB is 2.02 vs. 1.15 for LH under the present experimental conditions). Even in case of LB, an increasing trend in the enhancement factor was observed with increasing magnetic field strengths (Table 1) (EF=0.009S + 1). However, in general, at any given magnetic field strength, the enhancement factor was higher in case of LH than LB. This appears to be just because the passive permeation flux of LB was ~3-fold higher than that of LH (Table 1).
Similar to LH in the case of LB, there was no enhancement in permeation flux across the porcine epidermis pretreated by exposure to the magnetic field (Table 2). When permeation studies of LB were performed across the dialysis membrane, an enhancement of ~3-fold was observed in the presence of magnetic field (300 mT) (passive: 4.95 ± 1.99 µg/cm2/h vs. magnet: 14.47 ± 3.06 µg/cm2/h).
The octanol–water partition coefficient of LB was 103.61 ± 4.06 and it was increased to 128.55 ± 3.91 in the presence of magnetic field (300 mT). The SC–aqueous vehicle partition coefficient of in the presence of the magnetic field (0.171 ± 0.03) was increased when compared to that in the absence of magnetic field (0.092 ± 0.01). As compared to the ~110% increase in the partition coefficient of LH, the increase in the case of LB was significantly less (~24%). Comparable to this, the increase in partition coefficient between SC and vehicle was enhanced by ~237% in the case of LH as compared to ~85% in case of LB. This is likely due to the greater inherent ability of LB to partition into octanol or SC lipids as compared to LH. From all the above studies it is again evident that the predominant mechanism of drug permeation enhancement is likely to be magnetokinesis. Additionally, the enhanced drug partitioning into the lipid domain of the stratum corneum could be one of the potential mechanisms contributing, which is more apparent in the case of the hydrophilic molecule than the lipophilic molecule.
3.6. Magnetophoretic transdermal patch
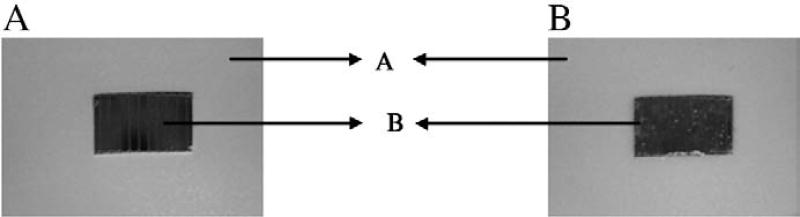
One of the major tasks that need to be addressed when any physical permeation enhancement technique is developed is its applicability in vivo. Techniques such as sonophoresis and electroporation have been developed as device based techniques for short duration treatment or pretreatment. The drug is delivered during the treatment or post treatment from a formulation. Approaches such as iontophoresis and microneedles could be conveniently incorporated into a transdermal patch system for prolonged drug delivery [27,28]. Magnetophoresis is a phenomenon which occurs only in presence of the magnetic field or it is rather an induced effect. Therefore it is more appropriate to incorporate this mechanism in the transdermal patch system intended for prolonged application. As a continuation of the transdermal magnetophoresis project, a simple transdermal patch system incorporated with magnetic field as the backing layer was designed for in vivo drug delivery applications. A patch system with better aesthetic appearance and workability could be made by improving the proposed system. LH and LB gels were prepared using HPMC (4% w/v) for use as a drug reservoir in the patch system. The backing membrane consists of a sequence of magnets arranged in parallel. The control patch was developed by using similar nonmagnetic metal pieces in the backing layer. The design of patch system is shown in Fig. 4.
Fig. 4.
Photographs of magnetophoretic patch system used for in vivo studies. In panel a, the magnetic backing could be clearly seen as it is not filled with the gel. In panel b, the gel is filled in the cavity ‘B’ (1.5 cm length, 1 cm width and 0.1 cm thickness) above the magnets. ‘A’ is the adhesive membrane to secure the patch onto the skin.
3.7. Transdermal drug delivery in vivo in rats
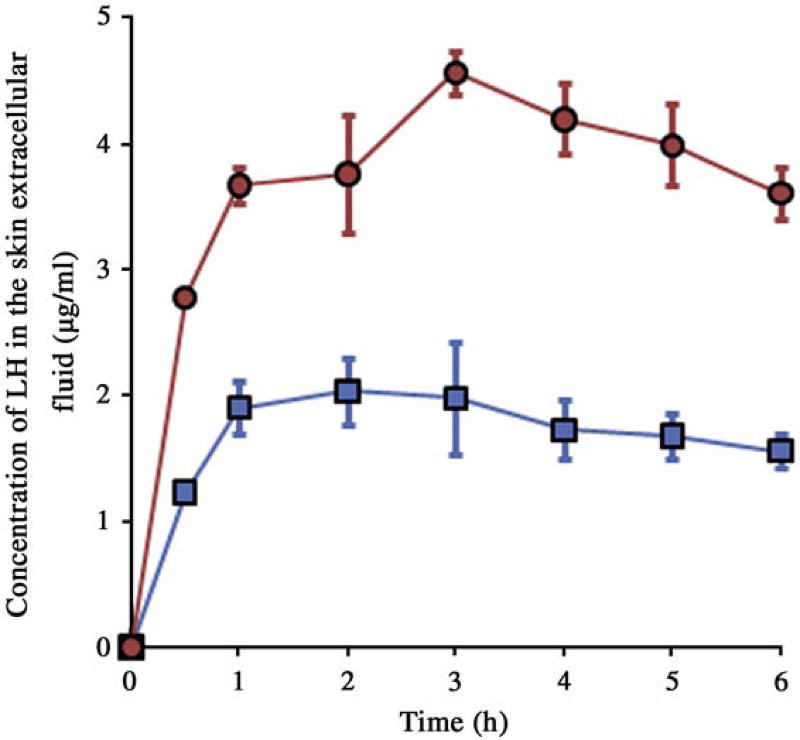
In vivo studies were carried out in rats whereas porcine epidermis was used for all the in vitro studies. Though in vitro and in vivo data cannot be correlated due to the use of different skin models, the in vivo data with rat model would supplement the findings from in vitro studies and thus demonstrate the workability of the technique. Cutaneous microdialysis was employed as a tool to investigate the time course of drug concentration in the skin. The in vivo microdialysis probe recovery was found to 28.3 ± 5.2 and 12.9 ± 3.8% for LH and LB respectively. The patch systems were applied on the abdomen region of rats. The time course of drug in the skin extracellular fluid upon application of control and magnetophoretic transdermal patch systems is shown in Figs. 5 and 6 for LH and LB respectively.
Fig. 5.
Time course of LH in the skin extracellular fluid following application of passive transdermal patch (■) and magnetophoretic transdermal patch (●) in rats. The data points represented are the average of n = 3 ± S.D.
Fig. 6.
Time course of LB in the skin extracellular fluid following application of passive transdermal patch (■) and magnetophoretic transdermal patch (●) in rats. The data points represented are the average of n = 3 ± S.D.
The area under the curve (AUC0–6 h) which represents the dermal bioavailability and the maximum concentration (Cmax) for both the salt and base forms of drug are given in Table 3. LB being more lipophilic tends to permeate in higher amounts into the skin compared to LH. Moreover, the use of ethanol for incorporating the lipophilic LB in the aqueous gel systems might have also lead to enhanced passive drug permeation. The AUC0–6 h and the drug retained in the skin was ~2–3-fold higher in case of magnetophoretic patch system as compared to nonmagnetic control patch system (Table 3).
Table 3.
AUC0–6 h, Cmax in the skin extracellular fluid and drug content of LH and LB in the skin following transdermal application of passive and magnetophoretic patch systems in vivo in rats. The data points represented are the average of n = 4 ± S.D.
| Parameter | LH | LB | ||
|---|---|---|---|---|
|
|
|
|||
| Passive patch | Magnetophoretic patch | Passive patch | Magnetophoretic patch | |
| AUC0–6 h (µg h/mL) | 10.57 ± 2.05 | 23.02 ± 2.96 | 25.21 ± 4.05 | 53.96 ± 2.63 |
| Cmax (µg/mL) | 2.03 ± 0.26 | 4.56 ± 0.16 | 4.87 ± 0.48 | 10.390 ± 0.72 |
| Skin drug content (µg/mg) | 0.63 ± 0.18 | 1.19 ± 0.44 | 0.89 ± 0.14 | 2.03 ± 0.83 |
4. Conclusions
Transdermal magnetophoresis is a phenomenon of the application of magnetic field to enhance the drug delivery across the skin. This study demonstrates that the magnetic field could be utilized to enhance the drug delivery across the skin. The magnetic field is believed to be a relatively safer technique for use on skin as it was found to have no effect on the skin structure at the field strengths utilized in this project. The in vivo studies demonstrated the feasibility of developing a magnetophoretic transdermal patch system. The magnetophoretic patch systems deliver drugs at a higher rate than the nonmagnetic patch systems.
Acknowledgments
The project described was partially supported by Grant Number 5P20RR021929 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors would like to thank Dr. Babu Tekwani (Principal Scientist, NCNPR, The University of Mississippi) for the radioactive lab facility and Dr. Franck E. Dayan (Plant physiologist/Biochemist, USDAARS, NCNPR, The University of Mississippi) for the liquid scintillation counter. The authors would also like to thank Mr. Thomas Jamerson (Laboratory Physicist, Department of Physics and Astronomy, The University of Mississippi) for helping with the magnetic field strength measurements.
The authors would also like to thank Dr. Ryan Gordon, 3M Drug Delivery Systems (St. Paul, MN) for providing gift samples of 3M™ 9773 Foam Tape.
References
- 1.Trommer H, Neubert RHH. Overcoming the stratum corneum: the modulation of skin penetration. Skin Pharmacol. Physiol. 2006;19:106–121. doi: 10.1159/000091978. [DOI] [PubMed] [Google Scholar]
- 2.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Natl Acad. Sci. USA. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chein YW, Banga AK. Iontophoretic (transdermal) delivery of drugs: overview of historical development. J. Pharm. Sci. 1989;78:353–354. doi: 10.1002/jps.2600780502. [DOI] [PubMed] [Google Scholar]
- 4.Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc. Natl Acad. Sci. USA. 1993;90:10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitragotri S, Blankschtein D, Langer R. Ultrasound mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 6.Murthy SN. Magnetophoresis: an approach to enhance transdermal drug diffusion. Pharmazie. 1999;54:377–379. [PubMed] [Google Scholar]
- 7.Murthy SN, Hiremath SR. Effect of magnetic field on the permeation of salbutamol sulphate. Indian Drugs. 1999;36:663–664. [Google Scholar]
- 8.Murthy SN, Hiremath SR. Physical and chemical permeation enhancers in transdermal delivery of terbutaline sulphate. AAPS PharmSciTech. 2000;2 doi: 10.1208/pt0201_tn1. E-TN1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan G, Edwards J, Chan Y, Benson HAE. Enhanced skin permeation of naltrexone by pulsed electromagnetic fields in human skin in vitro. J. Pharm. Sci. 2010;99:2724–2731. doi: 10.1002/jps.22024. [DOI] [PubMed] [Google Scholar]
- 10.Hafeli UO. Magnetically modulated therapeutic systems. Int. J. Pharm. 2004;277:19–24. doi: 10.1016/j.ijpharm.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Arruebo M, Fernandez-Pacheco R, Ibarra MR, Santamaria J. Magnetic nanoparticles for drug delivery. Nanotoday. 2007;2:22–32. [Google Scholar]
- 12.Essa EA, Bonner MC, Barry BW. Human skin sandwich for assessing shunt route penetration during passive and iontophoretic drug and liposome delivery. J. Pharm. Pharmacol. 2002;54:1481–1490. doi: 10.1211/002235702135. [DOI] [PubMed] [Google Scholar]
- 13.Sammeta SM, Vaka SRK, Murthy SN. Dermal levels of antibiotic (cephalexin) determined by electroporation and transcutaneous sampling (ETS) technique. J. Pharm. Sci. 2009;99:2677–2685. doi: 10.1002/jps.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sammeta SM, Vaka SRK, Murthy SN. Transcutaneous sampling of ciprofloxacin and 8-methoxypsoralen by electroporation (ETS) technique. Int. J. Pharm. 2009;369:24–29. doi: 10.1016/j.ijpharm.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padula C, Colombo G, Nicoli S, Catellani PL, Massimo G, Santi P. Bioadhesive film for the transdermal delivery of lidocaine in vitro and in vivo behavior. J. Control. Release. 2003;88:277–285. doi: 10.1016/s0168-3659(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 16.Pliquett U, Gallo S, Hui SW, Gusbeth Ch, Neumann E. Local and transient structural changes in stratum corneum at high electric fields: contribution of joule heating. Bioelectrochemistry. 2005;67:37–46. doi: 10.1016/j.bioelechem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Ferry LL, Argenteiri G, Lochner DH. The comparative histology of porcine and guinea pig skin with respect to iontophoretic drug delivery. Pharm. Acta Helv. 1995;70:43–56. doi: 10.1016/0031-6865(94)00050-6. [DOI] [PubMed] [Google Scholar]
- 18.Schurer NY, Elias PM. The biochemistry and function of stratum corneum lipids. Adv. Lipid Res. 1991;24:27–56. doi: 10.1016/b978-0-12-024924-4.50006-7. [DOI] [PubMed] [Google Scholar]
- 19.Netzlaff F, Kostka K-H, Lehr C-M, Schaefer UF. TEWL measurements as a routine method for evaluating the integrity of epidermis sheets in Franz type diffusion cells in vitro. Limitation shown by transport data testing. Eur. J. Pharm. Biopharm. 2006;63:44–50. doi: 10.1016/j.ejpb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Heylings JR, Clowes HM, Hughes L. Compaison of tissue sourcesfor the skin integrity function test (SIFT) Toxicol. In Vitro. 2001;15:597–600. doi: 10.1016/s0887-2333(01)00069-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhao K, Singh J. In vitro percutaneous absorption enhancement of propranolol hydrochloride through porcine epidermis by terpenes/ethanol. J. Control. Release. 1999;62:359–366. doi: 10.1016/s0168-3659(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 22.Levang AK, Zhao K, Singh J. Effect of ethanol/propylene glycol on the in vitro percutaneous absorption of aspirin, biophysical changes and macroscopic barrier properties of the skin. Int. J. Pharm. 1999;181:255–263. doi: 10.1016/s0378-5173(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaka M, Miyakoshi J, Ueno S. Magnetophoresis of diamagnetic cells and microorganisms in culture medium. IEEE Trans. Magn. 2001;37:2644–2646. [Google Scholar]
- 24.Hitoshi W, Masayori S, Yoshinori I. Magnetophoresis and electromagnetophoresis of microparticles in liquids. Anal. Bioanal. Chem. 2004;378:1693–1699. doi: 10.1007/s00216-003-2354-7. [DOI] [PubMed] [Google Scholar]
- 25.Kuznetsov OA, Schwuchow J, Sack FD, Hasenstein KH. Curvature induced by amyloplast magnetophoresis in protonemata of the moss ceratodon purpureus. Plant Physiol. 1999;119:645–650. doi: 10.1104/pp.119.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Liu Y, Wu S, Jia S. Effect of magnetic field on the extraction process of acetone–water–trichloromethane system. Chin. J. Chem. Eng. 2007;15:916–918. [Google Scholar]
- 27.Siegel SJ, O'Neill C, Dube LM, Kaldeway P, Morris R, Jackson D, Sebree T. A unique iontophoretic patch for optimal transdermal delivery of sumatriptan. Phar. Res. 2007;24:1919–1926. doi: 10.1007/s11095-007-9317-1. [DOI] [PubMed] [Google Scholar]
- 28.Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]