Abstract
In HIV-infected individuals, impaired mitochondrial function may contribute to cardiometabolic disease as well as to fatigue and frailty. Aerobic exercise improves total body energy reserves; however, its impact at the cellular level is unknown. We assessed alterations in cellular bioenergetics in peripheral blood mononuclear cells (PBMC) before and after a 12-week aerobic exercise study in sedentary HIV-infected subjects on stable antiretroviral therapy who successfully completed a 12-week aerobic exercise program. In this prospective study, participants underwent supervised 20–40 min of light aerobic exercise (walking or jogging) performed three times per week for 12 weeks, gradually increasing to maintain an intensity of 50%–80% of heart rate reserve. Maximal aerobic capacity (VO2MAX) was assessed by a graded exercise test on a cycle ergometer before and after completion of the study. PBMC from compliant subjects (attended at least 70% of exercise sessions) were assessed for mitochondrial respiration using the Seahorse XF24 Bio-Analyzer. Seven of 24 enrolled subjects were compliant with the exercise regimen. In these individuals, a significant increase (p = .04) in VO2MAX over 12 weeks was found with a median increase of 14%. During the same interval, a 2.45-fold increase in PBMC mitochondrial respiratory capacity (p = .04), a 5.65-fold increase in spare respiratory capacity (p = .01), and a 3.15-fold (p = .04) increase in nonmitochondrial respiration was observed. Aerobic exercise improves respiration at the cellular level. The diagnostic and prognostic value of such improved cellular respiration in the setting of chronic HIV warrants further investigation.
Keywords: : aerobic, cardiovascular fitness, VO2MAX
Advances in antiretroviral therapy (ART) have led to longer life expectancies for HIV-infected individuals and markedly improved clinical outcomes. Fatigue and frailty, as well as non-AIDS morbidity and mortality related to cardiometabolic abnormalities are increasingly common. This has led to interest in exercise training as a strategy to maintain health, energy, and function.1,2 Aerobic exercise improves aerobic capacity and cognitive function, increases lean body mass, reduces body fat and abdominal girth, and has positive impacts on quality of life without detrimental changes in CD4 T cell count or in plasma HIV RNA.2 We assessed changes in fitness level and mitochondrial respiration induced by exercise in peripheral blood mononuclear cells (PBMC) after a 12-week group-based aerobic exercise intervention study among HIV-infected individuals on stable ART.
This was a prospective pilot study of 24 sedentary HIV-infected individuals. Inclusionary criteria specified documentation of HIV positivity, between 18 and 65 years, ART unchanged for at least 6 months, and no reported regular aerobic conditioning in the previous 3 months. Exclusionary criteria included active illicit substance abuse, self-reported neurological disease, pregnancy, and any absolute contraindications to exercise as outlined by the American College of Sports Medicine (ACSM).3 The study was approved by the Human Studies Program of the University of Hawaii and informed consent was obtained from all participants.
The exercise intervention consisted of a 12-week aerobic exercise program. Duration was initially set at 20 min up to a maximum of 40 min of exercise per session (in 2-min per week increments) with a target intensity of 50%–80% of maximum heart rate. Participants were considered compliant if they attended at least 70% of exercise sessions.
Following a 5-min warm-up on a cycle ergometer (Model 818E; Monark AB, Varberg, Sweden) at a self-selected pace, maximal oxygen consumption (VO2MAX) data were collected at 60 rpm with a starting intensity of 50 W (increased by 12.5 W/min). All participants were tested in the morning in a nonfasted, hydrated state. The VO2MAX tests were considered valid if the participants met the ACSM criteria.3 VO2MAX was reported as unscaled (liter/min), ratio-scaled (ml kg−0.67 min−1), and allometrically scaled (ml kg−0.67 min−1) values.
PBMCs were isolated from anticoagulated whole blood with a Ficoll-Paque gradient per AIDS Clinical Trial Group Guidelines.4 Cell viability was determined using AOPI (Acridine Orange/Propidium Iodide) and PBMCs were seeded at a density of 5.0e5 cells per well in duplicate on cell culture plates treated with poly-l-lysine. PBMC mitochondrial oxygen consumption rate (OCR) was assessed using the Mito Stress Test and Seahorse XF24 (Agilent Technologies, Santa Clara, CA), which uses high-throughput oximetry to simultaneously measure OCR and extracellular acidification rates. Basal respiration, ATP turnover, proton leak, maximal respiration, spare respiratory capacity, and nonmitochondrial respiration were then determined as previously described.5
Differences between pre- and posttest parameters were assessed by paired Wilcoxon Signed Rank tests and correlations between parameters were assessed by Spearman's rho. Statistical analyses were performed using SPSS (ver. 22, IBM, Armonk, New York) with an alpha level set at 0.05.
Of the 24 participants originally recruited into the exercise study, 12 completed the exercise protocol; three of these 12 were noncompliant with the exercise intervention (attended <70% of exercise sessions) and were excluded. Of the remaining nine, two had invalid posttreatment VO2MAX tests as per ACSM criteria,3 leaving a total of seven subjects who could be analyzed for exercise-induced changes in PBMC cellular respiration. In the noncompliant individuals, there were no differences at baseline in terms of VO2MAX and Seahorse metabolic data. Baseline characteristics among the seven compliant participants (all male) were as follows: age range of 36–58 years; Caucasian (n = 5), Hispanic (n = 1), Asian (n = 1); past use of ZDV or d4T, 28.6%; mean current CD4 count of 513 ± 247 cells/μl; HIV viral load (<40 copies/ml) was undetectable in 6 of the 7; and body mass index (BMI) of 24.6 ± 4.2 kg/m2. No significant pre- and postexercise changes in CD4 count or plasma HIV RNA values were observed; nor were there significant changes in BMI, fasting glucose, and lipid parameters.
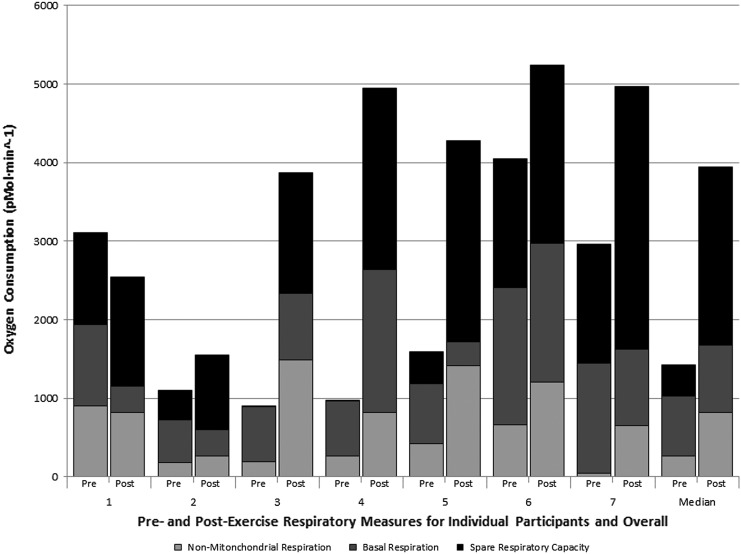
There was a significant median increase of 14% in unscaled VO2MAX (p = .04), a 12% increase in ratio-scaled VO2MAX (p = .04), and a trending significant increase of 13% in allometrically scaled VO2MAX (p = .06). PBMC mitochondrial respiratory capacity also substantially improved, resulting in a 2.45-fold increase in PBMC mitochondrial respiratory capacity (p = .04) and a 5.65-fold increase in spare respiratory capacity (p = .01), whereas basal respiration remained relatively stable. Interestingly, nonmitochondrial respiration also increased following the intervention (3.15-fold increase, p = .043) (Fig. 1). Individual variations were observed in all subjects in nonmitochondrial respiration, basal respiration, and spare respiratory capacity. No significant correlations were observed between measures of VO2MAX and mitochondrial function (nonmitochondrial respiration, basal respiration, proton leak, respiratory capacity, or spare respiratory capacity) either pre- or postintervention (rho −0.571 to 0.536, p ≥ .18 for all).
FIG. 1.
Comparison of mitochondrial respiration before and after aerobic exercise intervention.
Aerobic exercise in HIV-infected individuals on stable ART increased VO2MAX and systemic mitochondrial respiration. Aerobic exercise demonstrated a significant median VO2MAX increase of 3.4 ml kg−1 min−1 (∼14%) after a 12-week intervention, which is ∼1 metabolic equivalent. O'Brien et al. found an improvement of 2.63 ml kg−1 min−1 following aerobic training estimated from 14 studies on HIV-infected patients included in their meta-analysis and concluded that an increase of 2.0 ml kg−1 min−1 was clinically significant.6
This study shows a significant increase in respiratory capacity of mitochondria with no change in basal respiration, resulting in an increase in calculated spare respiratory capacity (the ability of substrate supply and electron transport to respond to an increase in energy demand).7,8 A threefold increase in nonmitochondrial respiration and a nonsignificant increase in proton leak were found. The observed increase in nonmitochondrial oxygen consumption could be explained by an increase in overall cellular oxygen consumption (mitochondrial and nonmitochondrial) due to an increased overall demand for oxygen within the cell. In leukocytes, nonmitochondrial respiration is attributed to enzymes associated with inflammation, including cyclo-oxygenases, lipoxygenases, and NADPH oxidases.
Limitations to our study include the high dropout rate. However, similar participation rates ranging from 45% to 87% of participants have been reported in the literature.2,9 We nominally reimbursed participants and this was a successful incentive. Both VO2MAX and mitochondrial respiratory parameters improved with training, and the lack of correlations between the two may simply be secondary to the small sample size. Additionally, the small sample could not allow for adjustments for prior ZDV or d4T use. There was no matching HIV seronegative arm for comparison and only respiration within PBMCs was assessed.
Our pilot study provides the first direct evidence that aerobic exercise significantly increases not only VO2MAX but also PBMC mitochondrial respiration in HIV-infected individuals on suppressive ART. Our study is consistent with the U.S. Department of Health and Human Services' Physical Activity Guidelines that exercise is effective in managing HIV-related complications and improving quality of life.10 Further research is warranted to examine the impact of respiration within different types of immune cells and to assess whether exercise-induced improvement in cellular respiration has functional consequences on immune cells.
Acknowledgments
This work was supported by the Department of Health and Human Services National Institutes of Health grants U54RR026136, U54MD007584, R01HL095135 (M.G. and C.M.S.), and MD000173 (M.G. and C.M.S.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gomes-Neto M, Conceicao CS, Oliveira Carvalho V, Brites C: A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo) 2013;68:1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shephard RJ: Physical impairment in HIV infections and AIDS: Responses to resistance and aerobic training. J Sports Med Phys Fitness 2015;55:1013–1028 [PubMed] [Google Scholar]
- 3.Ehrman J: ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription. 6th ed. Wolters Kluwer/Lippincott Williams & Williams, Philadelphia, PA, 2010 [Google Scholar]
- 4.International Material Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) Section 2 1007HS Laboratory Processing. In: International Material Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT), 2013
- 5.Takemoto JK, Miller TL, Wang J, Jacobson DL, Geffner ME, Van Dyke RB, et al. : Insulin resistance in HIV-infected youth is associated with decreased mitochondrial respiration. AIDS 2017;31:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien K, Nixon S, Tynan AM, Glazier R: Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev 2010:CD001796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, Juel Rasmussen L: Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res 2012;2012:192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand MD, Nicholls DG: Assessing mitochondrial dysfunction in cells. Biochem J 2011;435:297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL: A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immunodeficiency virus: A randomised controlled trial. Aust J Physiother 2006;52:185–190 [DOI] [PubMed] [Google Scholar]
- 10.Yarasheski KE, Roubenoff R: Exercise treatment for HIV-associated metabolic and anthropomorphic complications. Exerc Sport Sci Rev 2001;29:170–174 [DOI] [PubMed] [Google Scholar]