Abstract
Background:
Stem cell-based therapy is a novel strategy for the treatment of neurodegenerative diseases. The transplantation of fully differentiated cells instead of stem cells in order to decrease serious adverse complications of stem cell therapy is a new idea. In this study, the effect of lithium chloride on dopaminergic differentiation of human immortalized RenVm cells was investigated in order to access a population of fully differentiated cells for transplantation in Parkinson disease.
Methods:
The immortalized RenVm cells were induced to dopaminergic differentiation using a neurobasal medium supplemented with N2 and different concentrations (1, 3, 6 mM) of Lithium Chloride (LiCl) for 4, 8 and 12 days. The efficiency of dopaminergic differentiation was evaluated using immunocytochemistry and western blot techniques for tyrosine hydroxylase and β-catenin marker expression.
Results:
Our results indicated that LiCl can promote dopaminergic differentiation of RenVm cells in a dose-dependent manner.
Conclusion:
It can be concluded that LiCl is able to facilitate dopaminergic differentiation of cultured cells by affecting Wnt-frizzled signaling pathway.
Keywords: Beta catenin, Cell differentiation, Lithium, Wnt proteins
Introduction
The conventional therapies for neurodegenerative diseases are based on either immunomodulation/anti inflammation or neurotransmitter replacement 1–4. Although these pharmacological therapies can partially reverse neuronal disturbances, these methods result in some side effects. Recently, cell-based therapy is proposed as a novel paradigm for treatment of neurodegenerative diseases such as multiple sclerosis 5, Parkinson 6, Huntington’s 7 and Alzheimer’s disease 8. Although the principal mechanism responsible for these therapeutic effects of stem cell transplantation is not clear, but it seems to be due to trophic effects and differentiation potential of stem cells into functional neurons 5, 9.
Unlike the studies which offered the beneficial potential of stem cell therapy, the serious adverse events of this method such as tumorigenic potential cannot be denied. The differentiation degree of the transplanted cells is one of the most important factors which is involved in tumorigenesis. Therefore, molecular pathways involved in signaling cell differentiation have been studied by many researchers in order to access a population of fully differentiated cells.
Wnt proteins are a group of cysteine-rich glycosylated proteins that are able to stimulate the growth of specific tissues and play an important role in the regulation of various cellular processes such as cell proliferation and differentiation 10–12.
Wnt1 is a class of these proteins which consists of several members including Wnt1, Wnt2, Wnt3, Wnt8 proteins 13. The biological activity of these proteins is mediated via specific receptors, including the frizzled transmembrane receptor and lipoprotein related protein 5 and 6 (LRP-5/6) 14. According to current published data, Wnt1 proteins when binding to their receptors are able to activate another intracellular pathway termed canonical Wnt/ß-catenin pathway which leads to inhibition of the downstream glycogen synthase kinase-3ß (GSK-3ß). Thus, ß-catenin phosphorylation, ubiquitination and subsequent degradation thorough proteasomes will be suppressed. As a result, ß-catenin can translocate into the cell nucleus and bind to DNA which can trigger gene transcription. Overall, several particular biological functions such as specific precursor cells regulation, cellular differentiation and nervous tissue development may occur through canonical Wnt-frizzled signaling pathway 15–18. Therefore, the induction of cell differentiation is possible using specific factors with the potential to trigger Wnt-Frizzled signaling pathway.
Lithium Chloride (LiCl) is an agent which is widely used to treat manic depressive illness 19,20. This chemical compound can affect the central nervous system in a variety of ways including inhibition of glycogen synthase kinase 3 β (GSK3β) and mimicking the effects of Wnt signaling on gene expression, cell proliferation and differentiation 21–23. In addition, lithium possesses additional beneficial effects including neuroprotective effects through increasing the brain-derived neurotrophic factor 24,25 and vascular endothelial growth factor expression 26,27, anti-apoptotic effects by inducing up-regulation of B-cell lymphoma protein-2 (bcl-2) as well as suppressing the calcium-dependent activation of pro-apoptotic signaling pathways 28,29.
An immortalized cell line refers to a population of undifferentiated cells that are characterized by high self-renewal ability and multi-potency. Thus, these cells can be grown in vitro for long periods and can create more cells. In addition, these cells are capable to differentiate into other cells lineage such as neurons, astrocytes and oligodendrocytes. Therefore, these cells are very important tools for research in cell biology and cell based therapy. RenVM is one of the immortalized human neural stem cell lines which is isolated from 10 week fetal neural ventral mesencephalon and was established with the V-myc oncogene by retroviral transduction.
With respect to the broad beneficial effects of lithium in signaling cell pathways, in the current study, the effects of several doses of lithium chloride were evaluated on dopaminergic differentiation of human immortalized RenVm cells.
Materials and Methods
RenVm cells culture
All chemicals, unless specified otherwise, were purchased from Sigma-Aldrich, St. Louis, MO, USA. In addition, the human immortalized RenVm cell lines were purchased from the Regeneron company. The immortalized RenVm cells were seeded at the concentration of 25,000 cell/CM2 in 25 cm2 flasks and propagated in the pre-differentiation medium consisting of Dulbecco’s Modified Eagles Medium (DMEM) supplemented with B27 (gibco), gentamicin 50 ng/ml, bFGF 10 ng/ml, EGF 20 ng/ml, and heparin 10 U/ml in a 37°C humidified incubator with a 5% CO2 environment. At approximately 80% confluency, these cells were passaged and then seeded at approximately 10000 cells /CM2 in laminin coated 96 wells.
Dopaminergic differentiation of RenVm cells
Differentiation stage was initiated by discharge of growth factors once the cells reached a confluency of 90%. Immortalized RenVm cells were dissociated using trypsin (500 μg/ml) solution and seeded at a density of 10000 cells/cm2 on laminin coated 96 wells. Cells were maintained in differentiation medium comprised of neurobasal medium (gibco) supplemented with N2 (gibco), gentamicin, and different concentrations (1, 3, 6 mM) of LiCl for 4, 8 and 12 days. For control group, cells were maintained in differentiation medium without lithium. In addition, for Control-DMSO (C-DMSO) cells were maintained in differentiation medium supplemented with 0.1% DMSO. The media changed every other day with the ratio of 80%. The cells were fixed on days 4, 8 and 12 for further experiments.
Immunocytochemistry
Four, eight and twelve days after neural induction, the cells were fixed with 4% paraformaldehyde for half an hour. The cells were permeabilized with 10% v/v normal donkey serum in PBS-Triton 0.3% v/v for 1 hr at room temperature. Fixed cells were washed 3–4 times in PBS and were incubated with primary antibodies (mouse anti TH, 1:500 dilution; anti TuJ1, 1:1000 dilution) overnight at 37°C. After washing with PBS, the cells were exposed to 1 hr with a 1:100 dilution of Alexa 488-conjugated goat anti mouse IgG secondary antibody. Finally, cells were observed using fluorescence microscope (Nikon Inc., Melville, NY).
For quantitative analysis, total number of positive cells was counted on each acquired image by ImageJ, and the ratio to the number of nuclei was analyzed for each antigen and the number of immunopositive cells was counted in a minimum total of 200 cells per slide. In addition, all immunocytochemistry studies were repeated at least twice.
Western blot
Differentiated cells were subjected to Western blot analysis. Briefly, the samples were lysed using lysis buffer, centrifuged at 14,000 g for 30 min at 4°C and the supernatant was used for immunodetection with anti TH (1:500 dilution), anti TuJ1(1 μg/ml) and anti β-catenin (0.25 μg/ml). Finally appropriate secondary antibodies conjugated with alkaline phosphatase were used and the relative expression levels of TH, TuJ1and β-catenin proteins were assessed. In addition, all western blot analysis was repeated at least twice.
Results
RenVm cells characterization before and after differentiation
The morphology of the RenVm cells depended on mitogenic factors (EGF and FGF-2) used for their expansion. The cells expanded as islands (or clusters) of cells, and appeared to have bipolar cell morphology (Figure 1A). Under growth conditions, they displayed an undifferentiated neural morphology. In addition, they showed rosette-like formations when reaching confluency. After differentiation, ReNcell VM cells formed networks in which initial extensions elongated and became more complex leading to a dense cellular network. Over time, ReNcell VM cells started to form more complex cell connections (Figures 1B and 1C).
Figure 1.
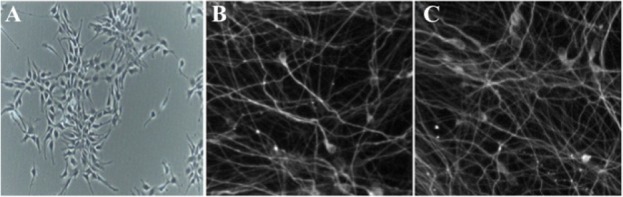
Phase contrast and fluorescence images of cultured and differentiated RenVm cells. RenVm cells at the beginning of the neural differentiation (A). The differentiated RenVm cells four (B) and eight (C) days after the beginning of differentiation. Scale bars represent 200 μm in A and 100 μm in B and C.
Immunocytochemistry study of dopaminergic differentiation
Immunocytochemistry staining with cell type-specific markers was used to recognize the phenotype of differentiated cells. Fluorescence microscopic analysis four, eight and twelve days post neural induction showed that the mean percentage of cells which expressed TH and Tuj1 markers was different in all of the studied groups (Figures 2 and 3). In addition, in one of the lithium concentrations (3 ng/ml) on the fourth day, a high percentage of cells expressed TH marker which was significant when compared with other groups (Figure 4).
Figure 2.
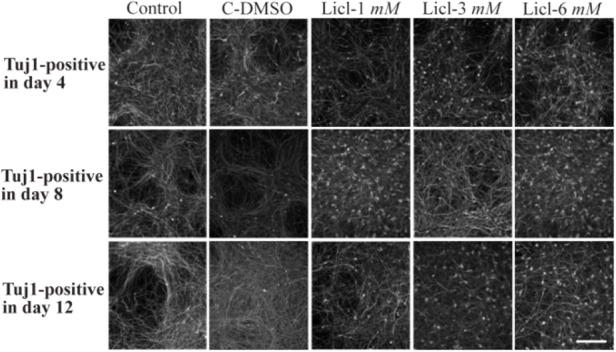
Immunocytochemistry images of differentiated cells which expressed Tuj1 marker in different concentrations of lithium (1 mM, 3 mM, 6 mM), control and in DMSO control (C-DMSO) groups. Scale bar represents 100 μm.
Figure 3.
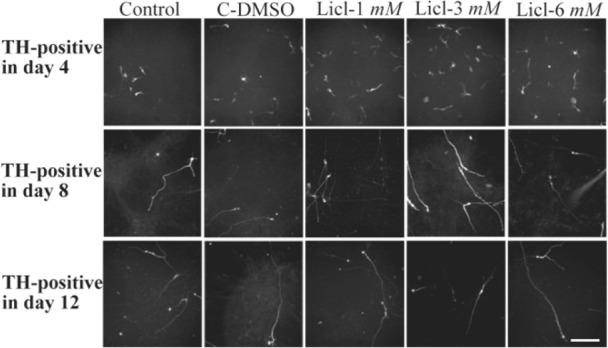
Immunocytochemistry images of differentiated cells which expressed tyrosine hydroxylase marker in different lithium concentrations (1, 3, 6 mM), control and in DMSO control (C-DMSO) groups. Scale bar represents 100 μm.
Figure 4.
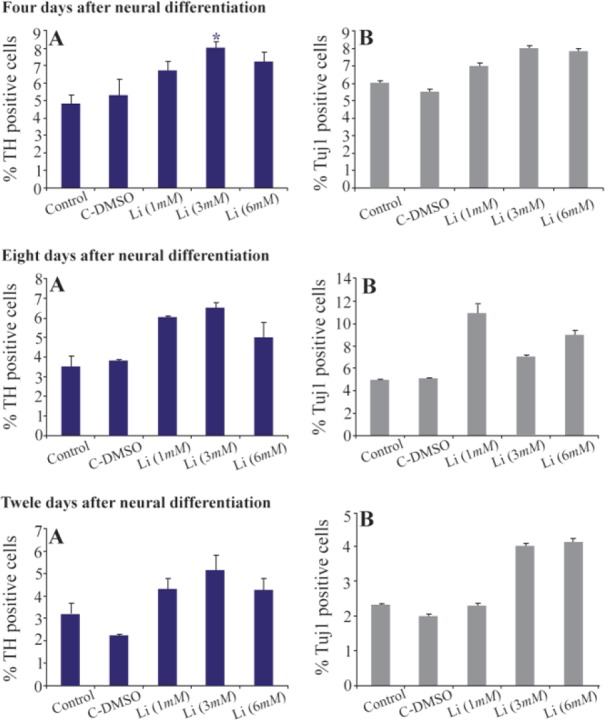
The mean percentage of differentiated cells which expressed TH and Tuj1 markers. In the 3 ng/ml concentration of lithium on the fourth day, the mean percentage of TH positive cells significantly increased compared to other groups (p≤0.05)
Western blot analysis
Four, eight and twelve days post neural induction, protein was extracted from differentiated cells for western blot assays. To this end, β-actin was used as a control marker. Our finding showed that the expression of TuJ1 marker was almost identical in all groups, but in line with immunocytochemistry results, the expression of TH in 3 ng/ml of lithium on the fourth day was also higher when compared to other groups (Figure 5).
Figure 5.
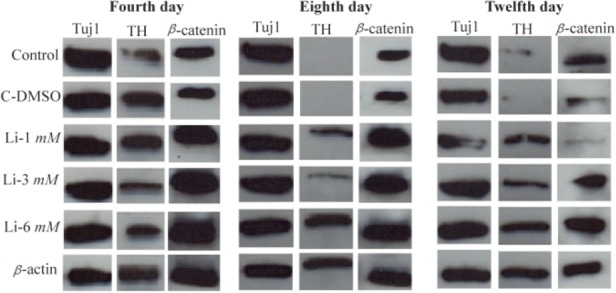
Western analysis of differentiated cells in different concentrations of LiCL for TH, Tuj1 and β-catenin markers in 4, 8 and 12 days. β-actin was used as a control marker.
Discussion
There is a lot of concern about the safety of cell-based therapy in treatment of neurodegenerative diseases 30. In order to reduce the side effects of this procedure, the transplantation of fully differentiated cells instead of stem cells has been suggested. To this end, the present study was done in order to promote human immortalized RenVm cells differentiation into dopaminergic neuron using LiCl. LiCl is one of the important factors which could alter gene expression through affecting cellular signaling and thus determine cell fate 21–23. The results of present study revealed that LiCl can promote differentiation of RenVm cells and induce the expression of TH in cultured cells. As shown in figure 4, the induction of the TH expression in RenVm cells is dose-dependent and time-dependent. In particular, 3.0 mM LiCl administration after four days showed the highest effect on TH marker expression than other LiCl concentrations. Additionally, western blot results demonstrated that TH expression level increased in all groups treated with LiCl especially in four days. β-catenin expression level also increased in the groups treated with LiCl compared to the control and DMSO groups. Therefore, a hypothesis can be considered in which LiCl may participate in promotion of dopaminergic differentiation by inhibition of the GSK-3ß activity. As a result, the inhibition of β-catenin phosphorylation occurs and the cytoplasmic level of this factor increases. In conclusion, β-catenin can enter the nucleus and by binding to DNA, is able to increase the transcription of specific genes involved in dopaminergic differentiation.
Conclusion
Overall, it can be concluded that LiCl is able to affect a variety of cell signaling pathways such as Wnt-Frizzled signaling pathway. This agent within the therapeutic range is able to inhibit both GSK-3ß activity and β-catenin phosphorylation and facilitates differentiation of RenVm cells to dopaminergic neuronal cells.
Acknowledgments
The authors would like to thank the Isfahan University of Medical Sciences, Iran.
References
- 1. Aisen PS. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol 2002; 1 (5): 279– 284. [DOI] [PubMed] [Google Scholar]
- 2. Palmer AM. New and emerging immune-targeted drugs for the treatment of multiple sclerosis. Br J Clin Pharmacol 2014; 78 (1): 33– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Non-steroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 2007; 69 (19): 1836– 1842. [DOI] [PubMed] [Google Scholar]
- 4. Seedat S, Kesler S, Niehaus DJ, Stein DJ. Pathological gambling behaviour: emergence secondary to treatment of Parkinson’s disease with dopaminergic agents. Depress Anxiety 2000; 11 (4): 185– 186. [DOI] [PubMed] [Google Scholar]
- 5. Ghasemi N, Razavi S, Mardani M, Esfandiari E, Salehi H, Zarkesh Esfahani SH. Transplantation of human adipose-derived stem cells enhances remyelination in lysolecithin-induced focal demyelination of rat spinal cord. Mol Biotechnol 2014; 56 (5): 470– 478. 24570177 [Google Scholar]
- 6. Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006; 24 (3): 781– 792. [DOI] [PubMed] [Google Scholar]
- 7. Bantubungi K, Blum D, Cuvelier L, Wislet-Gendebien S, Rogister B, Brouillet E, et al. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington’s disease. Mol Cell Neurosci 2008; 37 (3): 454– 470. [DOI] [PubMed] [Google Scholar]
- 8. Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci 2009; 106 (32): 13594– 13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Neurochem 2008; 107 (1): 141– 151. [DOI] [PubMed] [Google Scholar]
- 10. Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004; 303 (5663): 1483– 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol 2000; 10 (3): 392– 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol 2004; 19 (2): 495– 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol 1995; 15 (5): 2625– 2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsieh JC. Specificity of WNT-receptor interactions. Front Biosci 2004; 9: 1333– 1338. [DOI] [PubMed] [Google Scholar]
- 15. Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 2001; 128 (21): 4189– 4201. [DOI] [PubMed] [Google Scholar]
- 16. Michiue T, Fukui A, Yukita A, Sakurai K, Danno H, Kikuchi A, et al. XIdax, an inhibitor of the canonical Wnt pathway, is required for anterior neural structure formation in Xenopus. Dev Dyn 2004; 230 (1): 79– 90. [DOI] [PubMed] [Google Scholar]
- 17. Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol 2005; 277 (2): 287– 295. [DOI] [PubMed] [Google Scholar]
- 18. Panhuysen M, Vogt Weisenhorn DM, Blanquet V, Brodski C, Heinzmann U, Beisker W, et al. Effects of Wnt1 signaling on proliferation in the developing mid/hind-brain region. Mol Cell Neurosci 2004; 26 (1): 101– 111. [DOI] [PubMed] [Google Scholar]
- 19. Li X, Ketter TA, Frye MA. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J Affect Disord 2002; 69 (1–3): 1– 14. [DOI] [PubMed] [Google Scholar]
- 20. Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull 2000; 35 (2): 5– 49. [PubMed] [Google Scholar]
- 21. Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 2010; 31 (1): 24– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med 2004; 6 (21): 1– 18. [DOI] [PubMed] [Google Scholar]
- 23. Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev 2007; 31 (6): 920– 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 2001; 158 (1): 100– 106. [DOI] [PubMed] [Google Scholar]
- 25. Jacobsen JP, Mørk A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res 2004; 1024 (1): 183– 192. [DOI] [PubMed] [Google Scholar]
- 26. Guo S, Arai K, Stins MF, Chuang DM, Lo EH. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke 2009; 40 (2): 652– 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol 2006; 40 (1): 138– 147. [DOI] [PubMed] [Google Scholar]
- 28. Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem 1999; 72 (2): 879– 882. [DOI] [PubMed] [Google Scholar]
- 29. Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression A prominent role in neuroprotection against excitotoxicity. J Biol Chem 1999; 274 (10): 6039– 6042. [DOI] [PubMed] [Google Scholar]
- 30. Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 2009; 6 (2): e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]


