Abstract
Candida albicans is a human fungal pathogen capable of causing lethal systemic infections. The plasma membrane plays key roles in virulence because it not only functions as a protective barrier, it also mediates dynamic functions including secretion of virulence factors, cell wall synthesis, invasive hyphal morphogenesis, endocytosis, and nutrient uptake. Consistent with this functional complexity, the plasma membrane is composed of a wide array of lipids and proteins. These components are organized into distinct domains that will be the topic of this review. Some of the plasma membrane domains that will be described are known to act as scaffolds or barriers to diffusion, such as MCC/eisosomes, septins, and sites of contact with the endoplasmic reticulum. Other zones mediate dynamic processes, including secretion, endocytosis, and a special region at hyphal tips that facilitates rapid growth. The highly organized architecture of the plasma membrane facilitates the coordination of diverse functions and promotes the pathogenesis of C. albicans.
Introduction
The human fungal pathogen Candida albicans is found in a wide range of niches. In healthy individuals it is commonly found as a commensal organism on the skin and mucosa. However, under certain conditions it is capable of causing severe infections of the oral mucosa or life-threatening systemic infections (Kullberg and Arendrup, 2015). Changes in medical care are increasing the pool of susceptible patients (Brown, et al., 2012, Kullberg and Arendrup, 2015, Pfaller and Diekema, 2010). For example, prophylactic use of anti-bacterial antibiotics allows the endogenous commensal forms of C. albicans to overgrow and disseminate. Similarly, increased use of indwelling medical devices, such as catheters, provides sites for biofilm formation that can release a large inoculum of C. albicans into the host. Surgical procedures and catheterization facilitate infection by enabling C. albicans to cross the skin or mucosal barriers. Patients with a compromised immune system are at much greater risk of developing a severe infection. Current therapeutic strategies have limited effectiveness once a severe infection has developed and drug resistant strains are emerging (Cowen, et al., 2015). Thus, more research is needed to define the mechanisms of C. albicans pathogenesis.
The pathogenic effects of C. albicans are caused by its ability to grow in the host and disseminate to internal organs. The plasma membrane plays critical roles in promoting virulence because this essential barrier mediates secretion of virulence factors, endocytosis, cell wall synthesis and invasive hyphal morphogenesis. Proteins in the plasma membrane also mediate nutrient transport and sense pH, osmolarity, nutrients, and other factors in the extracellular environment. The critical role of the plasma membrane in virulence is highlighted by the fact that it is the target of the most widely used antifungal drugs. Amphotericin binds ergosterol and forms pores in the plasma membrane, azoles inhibit ergosterol production, and echinocandins block the β-glucan synthase that forms the major component of the cell wall (Kullberg and Arendrup, 2015, Odds, et al., 2003). The plasma membrane also contains proteins that can confer resistance to fluconazole and other drugs by pumping them out of the cell (Cowen, et al., 2015). Complement and some antimicrobial peptides are also likely to act on the plasma membrane.
Consistent with its complex function, the plasma membrane is composed of a diverse array of proteins and lipids (Martin and Arkowitz, 2014, Schuberth and Wedlich-Soldner, 2015). These constituents are organized into specialized domains that are thought to coordinate different plasma membrane functions (Malinsky, et al., 2010, van Meer, et al., 2008). The definition of what defines a plasma membrane domain is not that restrictive, as it could be anything from a small transient cluster of lipids or proteins, to a structure as large as a yeast bud (Ziolkowska, et al., 2012). Some domains, such as lipid rafts, are controversial because they are too small to be readily observed, may exist only transiently, and are difficult to purify (Munro, 2003). Therefore, this review will primarily focus on six different domains that were selected because they carry out functions that are critical for virulence and because they are protein-organized domains that are stable enough and large enough to be detected by electron or fluorescence microscopy. The first section will describe a set of three domains that are thought to act as barriers to diffusion or scaffolds that organize the plasma membrane (MCC/eisosomes, septins, and sites of contact between the plasma membrane and the endoplasmic reticulum). The next section will describe three examples of dynamic domains, including zones of secretion, endocytosis, and the fast-growing zone at hyphal tips. Another section will describe emerging data on other types of domains and clusters of proteins that indicate further levels of organization in the plasma membrane. A longer description of newer domains, such as MCC/eisosomes, will be presented, whereas shorter summaries will be presented for other zones in the plasma membrane that are better known.
Barriers and scaffolds in the plasma membrane: MCC/eisosomes, septins, and sites of ER contact
Membrane domains that act as barriers to diffusion or scaffolds to recruit specific proteins play key roles in defining the architecture of the plasma membrane. Three domains that have these properties will be described in this section: eisosomes, septins, and sites of ER contact. In addition to these domains, it is likely that the cell wall also helps to form barriers in the fungal plasma membrane. Many proteins that are crosslinked into the cell wall have a transmembrane domain or GPI anchor that can create a barrier to diffusion (Klis, et al., 2010).
MCC/Eisosome domains – stable islands in the plasma membrane
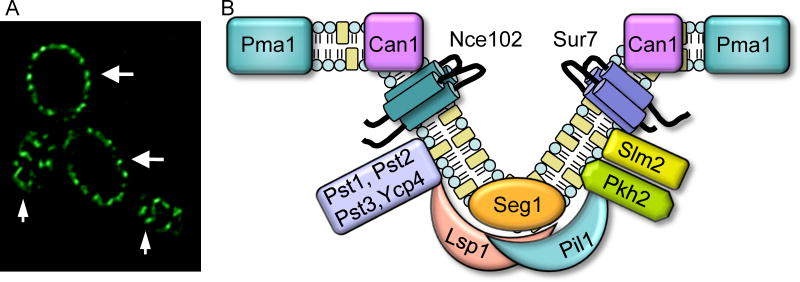
Recent studies with S. cerevisiae have indicated that fungal plasma membranes are composed of two types of domains. One major domain has been termed the Membrane Compartment of Pma1 (MCP), the plasma membrane ATPase. This domain includes proteins that diffuse in the plasma membrane and dynamic regions that mediate secretion and endocytosis. Another domain has been called the Membrane Compartment of Can1 (MCC) because it contains the arginine transporter Can1 (Douglas and Konopka, 2014, Grossmann, et al., 2007, Malinsky, et al., 2010, Murphy and Kim, 2012, Olivera-Couto and Aguilar, 2012). The MCC domains are distinctive because they are comprised of about 50 punctate patches that are very stable in the plasma membrane (Fig. 1A). Initial studies showed that the resident proteins, such as Sur7 and Can 1, did not colocalize with actin patches or sites of endocytosis (Malinska, et al., 2004, Malinska, et al., 2003, Malinsky, et al., 2010, Young, et al., 2002). Other proteins discovered in the MCC include the uracil and tryptophan nutrient symporters (Brach, et al., 2011, Frohlich, et al., 2009, Grossmann, et al., 2007), and two different families of tetraspan proteins that are predicted to have four transmembrane domains (Alvarez, et al., 2008, Frohlich, et al., 2009, Grossmann, et al., 2008). One family of tetraspanners includes Sur7 and its paralogs Fmp45, Pun1, and Ynl194c. The other family includes Nce102 and Fhn1.
Figure 1. MCC/eisosome domains in the plasma membrane of C. albicans.
(A) MCC/eisosome domains visualized by fluorescence microscopy of C. albicans cells producing an Lsp1-GFP fusion protein. Small arrows point to regions at the top of cells where MCC/eisosome domains can be visualized as punctate patches in the plasma membrane. Larger arrows point to regions where the mid-section of the cell is in focus, in which MCC/eisosomes appear as a series of patches around the perimeter of the cell.
(B) Model for MCC/eisosome structure. Note that only a representative set of the >30 proteins that localize to MCC/eisosomes is shown.
Subsequent studies revealed that the peripheral membrane proteins Pil1 and Lsp1 form a complex on the cytoplasmic side of the MCC (Walther, et al., 2006). This cluster of peripheral membrane proteins was termed the eisosome, a fusion of the Greek “eis”, meaning into or portal, and “soma”, meaning body, as they were initially thought to correspond to sites of endocytosis (Walther, et al., 2006). The MCC and eisosome are connected parts of the same overall structure, as is diagrammed in Figure 1B. At least 17 proteins appear to localize to eisosomes (Aguilar, et al., 2010, Deng, et al., 2009, Frohlich, et al., 2009, Grossmann, et al., 2008, Ho, et al., 2002, Yu, et al., 2008), a subset of which is shown in Figure 1B. Pil1 and its paralog Lsp1 are the most abundant proteins in eisosomes, and will be described in more detail below, as they promote MCC/eisosome formation by forming filaments and binding membranes through their BAR (Bin/Amphiphysin/Rvs) domains (Karotki, et al., 2011).
MCC/eisosomes correspond to furrows in the plasma membrane
High-resolution electron microscopy of freeze-etched S. cerevisiae cells demonstrated that MCC/eisosomes correspond to invaginations in the plasma membrane (Stradalova, et al., 2009). The invaginations form furrows that are about 50 nm deep and 200 to 300 nm long. This was an important advance in the study of plasma membrane domains, as it linked the MCC/eisosomes to furrows in the yeast plasma membrane that were discovered in the early 1960s (Moor and Muhlethaler, 1963). Although the existence of furrows had been known for a long time, their significance was unclear other than that they were distinct from the finger-like projections that form during endocytosis (Barug and de Groot, 1985, Buser and Drubin, 2013, Gross, et al., 1978, Moor and Muhlethaler, 1963, Mulholland, et al., 1994, Takeo, 1984). A recent study showed that membrane furrows can be found in a wide range of organisms with cell walls, including fungi, algae, and lichens, although the size and shape can be quite variable in these different organisms (Lee, et al., 2015).
Formation of MCC/eisosomes and the associated furrows in S. cerevisiae requires the Pil1 protein (Aguilar, et al., 2010, Grossmann, et al., 2008, Loibl, et al., 2010, Walther, et al., 2006). Lsp1 is 70% identical and also contributes, but cannot function on its own, most likely because it binds less efficiently to the plasma membrane (Olivera-Couto, et al., 2011, Walther, et al., 2006). Determination of the high-resolution structure of Lsp1 provided another major breakthrough by showing that Pil1 and Lsp1 are related to BAR domain proteins that bind membranes and promote curvature (Olivera-Couto, et al., 2011, Zimmerberg and McLaughlin, 2004, Ziolkowska, et al., 2011). The ability of Pil1 and Lsp1 to assemble into long filaments, led to a landmark study that proposed a “half-pipe” model for eisosomes formation in which filaments of Pil1 and Lsp1 align their BAR domains to curve the plasma membrane and form furrows (Karotki, et al., 2011).
Regulation of MCC/eisosome formation
The spatial location of MCC/eisosomes is regulated because their formation is restricted to growing buds and does not occur in mother cells under normal conditions (Moreira, et al., 2009, Young, et al., 2002). Eisosomes are not present at the tips of emerging buds, in part because the expression of PIL1 is cell cycle regulated and because it takes time for eisosomes to assemble (Moreira, et al., 2009). The binding of Pil1 to the plasma membrane appears to be the limiting step in eisosome formation. Seg1 promotes eisosome formation by recruiting Pil1 to the plasma membrane (Moreira, et al., 2012). Additional proteins needed for MCC/eisosome formation or stability include the MCC protein Nce102 and the eisosome proteins Slm1/2 (Frohlich, et al., 2009). These and other proteins that influence eisosome formation are thought to mediate their effect by regulating the synthesis of lipids, especially sphingolipids and ergosterol (Aguilar, et al., 2010, Frohlich, et al., 2009, Grossmann, et al., 2008, Stradalova, et al., 2009). Although the MCC/eisosome patches appear to be distributed in a random pattern in the plasma membrane, they do not overlap, suggesting there is a mechanism that allows eisosomes to sense the presence of a neighbor (Moreira, et al., 2009).
Interestingly, MCC/eisosomes do not diffuse in the plasma membrane (Malinska, et al., 2003, Walther, et al., 2006, Young, et al., 2002). This stability does not appear to be due to a direct connection to the cell wall, actin filaments, or microtubules (Malinska, et al., 2004). Perhaps the immobility of MCC/eisosomes is due to the formation of furrows at these sites combined with the attachment to the plasma membrane of stable filaments comprised of thousands of copies of Pil1 and Lsp1. BAR domain proteins have been shown to promote formation of stable lipid domains by "freezing" phosphoinositides, suggesting Pil1 and Lsp1 could do the same (Zhao, et al., 2013). Other MCC/eisosome proteins do not show such high stability, as Nce102 and Slm1/2 move in and out of these domains in response to sphingolipid levels (Frohlich, et al., 2009). Can1 and the other nutrient transporters can also move in and out of these domains (Brach, et al., 2011, Frohlich, et al., 2009, Grossmann, et al., 2007).
Pil1 and Lsp1 are phosphorylated in vivo by a pair of redundant protein kinases Pkh1 and Pkh2, that localize to eisosomes (Walther, et al., 2007, Zhang, et al., 2004), but the role of this modification on eisosome assembly/disassembly is not yet clear (Deng, et al., 2009, Luo, et al., 2008, Mascaraque, et al., 2013, Walther, et al., 2007). The conclusion that Pil1 phosphorylation promotes eisosome disassembly was based on mutating sites that reside on the predicted membrane-binding surface of Pil1 (Ser45 Ser59 Ser230 Thr233), where the negatively charged phosphate groups would be expected to interfere with membrane association and prevent eisosome formation (Frohlich, et al., 2009, Walther, et al., 2007, Ziolkowska, et al., 2011). The opposite conclusion that phosphorylation promotes eisosome assembly was based on mutating residues that face away from the plasma membrane (Ser6 Thr27 Ser59 Thr233 Ser273 Ser299), and could therefore be involved in promoting or stabilizing filament formation (Luo, et al., 2008, Ziolkowska, et al., 2011). However, different interpretations have also been made concerning the effects of mutating just residues Ser230 Thr233 (Deng, et al., 2009, Mascaraque, et al., 2013), suggesting that the mutant strains may also be sensitive to growth conditions or other factors. The Slt2 MAP kinase has also been implicated in phosphorylating Pil1 on Ser230 and Thr233, indicating that other kinases contribute to MCC/eisosome regulation (Mascaraque, et al., 2013).
MCC/eisosome functions in S. cerevisiae
Mutations affecting S. cerevisiae MCC/eisosome proteins can cause broad defects in plasma membrane organization, endocytosis, and the cell wall (Douglas and Konopka, 2014). Eisosomes were initially proposed to act as stable sites of endocytosis (Walther, et al., 2006), but other studies failed to detect endocytosis at these sites (Kabeche, et al., 2011, Reijnst, et al., 2011, Seger, et al., 2011, Vangelatos, et al., 2010, Walther, et al., 2006, Young, et al., 2002). However, eisosomes may play an indirect role in endocytosis because they influence lipid homeostasis as described further below (Murphy, et al., 2011). On the other hand, because of their stability in the plasma membrane, MCC/eisosomes have been proposed to act as protected islands that prevent plasma membrane proteins from endocytosis (Grossmann, et al., 2008). Although it is controversial whether Can1-GFP is stabilized by association with eisosomes (Brach, et al., 2011, Grossmann, et al., 2008), it is likely that proteins stably associated with MCC/eisosomes are protected from endocytosis. For example, Sur7 is one of the most stable proteins in the cell (Thayer, et al., 2014).
MCC/eisosomes have been implicated in regulating the homeostasis of different kinds of lipids. In one proposed mechanism, the MCC protein Nce102 and the eisosome proteins Slm1/2 regulate sphingolipid homeostasis (Frohlich, et al., 2009). Membrane stress, including decreased sphingolipids or membrane stretching, causes Slm1/2 to move out of the eisosomes and associate with TORC2 (Berchtold, et al., 2012, Kamble, et al., 2011). The Slm1/2 proteins then recruit Ypk1 to the plasma membrane, it is phosphorylated by TORC2, and then Ypk1 phosphorylates the Orm1/2 proteins to prevent them from negatively regulating the Lcb1/2 enzymes that initiate sphingolipid synthesis (Breslow, et al., 2010, Han, et al., 2010, Niles, et al., 2012, Roelants, et al., 2011, Sun, et al., 2012). A fuller description of sphingolipid homeostasis is beyond the scope of this review, but can be found in other recent publications (Epstein and Riezman, 2013, Niles and Powers, 2012). Eisosomes have also been shown to be important for regulation of phosphatidylinositol 4,5-bisphosphate (PI4,5P2) (Frohlich, et al., 2014, Kabeche, et al., 2014). S. cerevisiae eisosomes recruit the phosphatases Inp51 and Inp52 to the plasma membrane, thereby promoting conversion of PI4,5P2 to PI4P (Frohlich, et al., 2014, Murphy, et al., 2011).
Roles of MCC/eisosomes in C. albicans virulence
MCC/eisosomes in C. albicans are similar to those in S. cerevisiae, as GFP-tagged versions of Sur7, Fmp45, Lsp1, Pil1, Slm2, and Seg1 all localize to stable punctate patches in the plasma membrane ((Alvarez, et al., 2008, Bernardo and Lee, 2010, Reijnst, et al., 2011, Wang, et al., 2011), and unpublished data). However, there are interesting differences, such as the fact that the MCC protein Sur7 plays a more important role in C. albicans than in S. cerevisiae (Alvarez, et al., 2008). Another MCC protein, Nce102, also has distinctive properties in C. albicans (Douglas, et al., 2013). In addition, studies in C. albicans have shown that members of the flavodoxin-like protein (FLP) family of quinone reductases localize to eisosomes and are important for resistance to oxidative stress (Li, et al., 2015). The function of Sur7, Nce102, and FLP quinone reductases in C. albicans will be described below, with an emphasis on their roles in virulence.
C. albicans sur7Δ mutants have strong morphogenesis defects, including abnormal hyphae, wider buds, and they frequently fail to complete cytokinesis (Alvarez, et al., 2008). One distinctive defect is that sur7Δ mutants contain deep invaginations of cell wall growth (Alvarez, et al., 2008) that are more extreme than the cell wall invaginations that are observed in an S. cerevisiae pil1Δ mutant (Walther, et al., 2006). Electron microscopy of sur7Δ mutant cells showed that these invaginations are typically in the form of long tubes (Alvarez, et al., 2008). This is interesting as it suggests that the cell wall invaginations are linked to abnormal regulation of PI4,5P2. Similar types of tubular cell wall invaginations were observed in a C. albicans inp51Δ mutant, which lacks a PI5-specific PI4,5P2 phosphatase (Badrane, et al., 2012). Consistent with the morphogenesis defects, actin and septin proteins were mislocalized in the sur7Δ cells (Alvarez, et al., 2008). Actin patches were readily detected in the mother cell and were not restricted to the growing buds as expected. In addition, septins were not restricted to the bud neck and were instead found to be present at other sites, often forming small ectopic rings (Douglas, et al., 2005). Abnormal septin localization could contribute to the altered cell wall synthesis, as the septins recruit cell wall synthesis machinery to the bud neck during septation (Bridges and Gladfelter, 2015). This possibility is supported by the fact that some S. cerevisiae septin mutants form abnormal cell wall invaginations (Roh, et al., 2002, Schmidt, et al., 2003).
Although the sur7Δ mutant appeared to form thicker cell walls than the wild type control cells, the cell walls were apparently weaker as indicated by increased sensitivity to Calcofluor White and other factors that exacerbate cell wall defects (Alvarez, et al., 2008, Bernardo and Lee, 2010, Wang, et al., 2011). Analysis of the composition of the cell wall in the sur7Δ mutant suggested that this defect was due in part to decreased levels of β-glucan, a major component of the cell wall that is important for cell wall rigidity (Wang, et al., 2011). Sur7 presumably influences β-glucan synthesis indirectly, since the β-1,3-glucan synthase enzyme is mobile and often associated with cortical actin patches rather than eisosomes (Drgonova, et al., 1996, Qadota, et al., 1996, Utsugi, et al., 2002).
The sur7Δ mutant showed a strong virulence defect in a mouse model of systemic C. albicans infection (Douglas, et al., 2012). One underlying reason is that sur7Δ cells are defective in forming hyphal filaments that promote invasive growth into tissues in vivo and biofilm formation (Alvarez, et al., 2008, Bernardo and Lee, 2010, Douglas, et al., 2012, Wang, et al., 2011). The sur7Δ mutant cells are also more sensitive to a variety of stresses, including growth at high temperature and exposure to copper. Remarkably, the sur7Δ cells are ~2,000-fold more sensitive to copper, which appears to contribute to the poor growth of the mutant cells in macrophages (Douglas, et al., 2012). These virulence defects highlight the potential significance of MCC/eisosomes as novel drug targets.
Nce102 localization and function is somewhat different in C. albicans than in S. cerevisiae, as C. albicans Nce102 was only partially enriched in MCC/eisosomes during log phase growth and became tightly localized to MCC/eisosomes only in older stationary phase cultures (Douglas, et al., 2013). A unique phenotype of the nce102Δ mutant is that it failed to undergo invasive hyphal growth into low concentrations of agar, but invaded well into higher concentrations of agar. This was surprising, since mutants studied previously typically showed greater difficulty invading into a denser agar matrix (Warenda and Konopka, 2002). This suggests that the nce102Δ cells received a second signal from the denser agar matrix that enabled them to undergo invasive hyphal growth. This unique invasive growth defect of nce102Δ cells appears to be due to a partial defect in actin organization (Douglas, et al., 2013). Consistent with these defects, the nce102Δ mutant was less virulent in mice (Douglas, et al., 2013).
A family of quinone reductases that localize to eisosomes in C. albicans was also discovered to be important for virulence (Li, et al., 2015). Pst1, Pst2, Pst3, and Ycp4 belong to the Flavodoxin-Like Protein (FLP) family of quinone reductases that have been shown in bacteria and plants to act as NAD(P)H quinone oxidoreductases (Carey, et al., 2007). Consistent with this, a C. albicans quadruple mutant lacking all four genes (pst1Δ pst2Δ pst3Δ ycp4Δ) was more sensitive to benzoquinone. Surprisingly, this quadruple mutant was also more sensitive to H2O2 and a variety of other oxidants including linolenic acid, a polyunsaturated fatty acid that can auto-oxidize and promote lipid peroxidation (Li, et al., 2015). These results suggested that FLPs reduce ubiquinone (coenzyme Q), enabling it to serve as an antioxidant in the plasma membrane. In agreement with this, a C. albicans coq3Δ mutant that fails to synthesize ubiquinone was also highly sensitive to oxidative stress. It is not clear why the FLPs localize to eisosomes, but their presence suggests that these domains may be preferentially exposed to oxidative stress. FLPs are critical for survival in the host, as the quadruple mutant was avirulent in a mouse model of systemic candidiasis under conditions where infection with wild type C. albicans was lethal (Li, et al., 2015). These studies demonstrate that FLPs and ubiquinone represent important new antioxidant mechanisms that are critical for virulence.
Septins act as barriers and scaffolds at sites of cytokinesis
Septin proteins were first discovered in S. cerevisiae for their role in cell septation (Gladfelter, et al., 2001). These GTP-binding proteins assemble into filaments that attach to the inner surface of the plasma membrane and are now known to play key roles in cytokinesis, cell polarization, and membrane remodeling (Douglas, et al., 2005). The septins are highly conserved in many other eukaryotes, such as fungi and animals, but are not found in plants and certain protozoans.
Septin localization in buds and hyphae
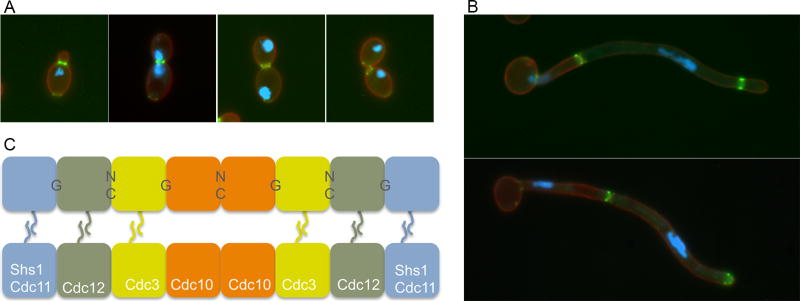
Septins are important for morphogenesis, and they have emerged as very useful landmarks for studying the progression of the cell cycle in S. cerevisiae, C. albicans and other fungi (Gladfelter, 2010, McMurray and Thorner, 2009). Some of the major stages of septin localization during the C. albicans cell cycle are shown in Fig. 2A. Similar to the pioneering studies of septins carried out in S. cerevisiae, one of the earliest events in the C. albicans cell cycle is that a patch of septins in the plasma membrane develop into a ring about 15 min prior to bud emergence. The growing bud then forms through the septin ring. As the bud develops the septin ring undergoes a transition into a more extended hourglass configuration, and the nuclear DNA divides across the septin ring when cells undergo mitosis. After completion of mitosis, the septin architecture undergoes a dramatic change into a double ring structure, cytokinesis occurs between the rings, and then the septins disperse as the bud separates from the mother cell.
Figure 2. Septin localization in C. albicans.
(A) and (B) show septin structures in green that were visualized by fluorescence microscopy of cells that produce a fusion between the Cdc10 septin and GFP. The blue color indicates the position of the nucleus due to staining with Hoechst dye. The red color corresponds to the plasma membrane that was detected by a Pleckstrin Homology (PH) domain fused to RFP.
(A) Septin ring forms at the bud neck, converts to an hourglass, and then splits into a double ring prior to cytokinesis, and then disassembles after cell septation.
(B) Septin localization in C. albicans hyphal cells. Note that the older septin rings remain stable for a longer period of time in hyphae following cell division in the growing hyphae.
(C) Octomer model for septin filament organization in S. cerevisiae. Note that either Cdc11 or Shs1 (Sep7) can occupy the terminal positions
Septin rings also form at sites of cytokinesis during C. albicans hyphal growth (Fig. 2B) (Martin and Konopka, 2004, Sudbery, 2001, Warenda and Konopka, 2002). Septins initially associate with the growing hyphal tip, and then form a ring that remains at a fixed position and does not move with the elongating hyphal tip. Mitosis then takes place across the septin ring. Subsequently, the septins split into a double ring formation and cytokinesis occurring in between the rings. Interestingly, the septin rings do not quickly disassemble on the mother cell side of the hyphae as they do in budding cells. This likely occurs because the mother cell is delayed in reentering the cell cycle (Veses, et al., 2009). The mother cell vacuole swells up to deliver cellular constituents to mediate rapid tip growth, so there is a delay before the mother cell regenerates and delivers a signal to disassemble septins and start a new cell cycle.
Septin filament formation
The ability of septins to form 10 nm filaments has been well studied in S. cerevisiae, which encodes five mitotic septins, Cdc3, Cdc10, Cdc11, Cdc12, and Shs1/Sep7. An octomer model has been proposed in which eight septin subunits are organized in the order shown in Fig. 2C (McMurray and Thorner, 2009). Two octomers associate in parallel via their coiled-coil domains in the septin C-terminal tails, except for Cdc10 which lacks this domain. The upper octomer shown in Fig. 2C is labeled to indicate whether the septin monomers associate via their GTP-binding domains (G interface) or if the interface involves the N- and C-termini of the septin protein (NC interface). These structural predictions for septin interaction were greatly aided by the crystal structure of a human SEPT2-SPET6-SEPT7 complex (Sirajuddin, et al., 2007).
Septins function as scaffolds and barriers
One key function of the septins is to act as a scaffold or framework for recruiting proteins to the bud neck. About 60 different proteins have been identified to localize to the bud neck in a septin-dependent manner in S. cerevisiae, although only a few have been shown to bind directly to septin proteins (Bridges and Gladfelter, 2015, Douglas, et al., 2005). In addition to septum formation, the proteins recruited to the bud neck are involved in a variety of processes. This includes the Bud proteins that act to select the future site of bud emergence, Bni4 that recruits chitin synthase to form a chitin ring at the future bud site, Swe1 that acts in a cell cycle checkpoint, and proteins involved in sensing spindle orientation (Cid, et al., 2002, Merlini and Piatti, 2011, Oh and Bi, 2011).
Septins also act as barriers to diffusion in the plasma membrane. One way they do this is to prevent proteins in the bud from diffusing back into the mother cell (Barral, et al., 2000, Takizawa, et al., 2000). This is important for maintaining polarized growth in the bud. Septins also act as a barrier at the mother bud neck to prevent the cortical ER from leaving the mother cell and entering the bud. As described further below, the Scs2 protein present in the ER binds to the Shs1 septin and prevents the ER from crossing the septin ring to enter the bud (Chao, et al., 2014). A second way the septins act as a barrier is to restrict cell wall deposition during cytokinesis (Dobbelaere and Barral, 2004). The septins form a double ring at the bud neck late in the cell cycle that restricts diffusion of the components that mediate synthesis of the septum. For example, the polarisome, the exocyst subunit Sec3, chitin synthase II, and the actomyosin ring become trapped between the septin rings to help restrict formation of the septum to this region (Bridges and Gladfelter, 2015, Oh and Bi, 2011). With further progression of cytokinesis, the actomyosin ring contracts, and secretory vesicles fuse to create the septum.
Septins play key roles in C. albicans hyphal morphogenesis
C. albicans septins play special roles in forming elongated hyphal cells that are capable of invasive growth into tissues in vivo (Sudbery, 2011). Septins have also been implicated in formation of chlamydospores, which are large thick-walled cells that form at the ends of filamentous hyphal cells (Martin, et al., 2005). These results indicate that septins are important for more than just cytokinesis. For example, the cdc10Δ and cdc11Δ mutants developed abnormally curved hyphal filaments, indicating a role guiding polarized tip growth that is perhaps mediated by a patch of septins at the leading edge of hyphal growth (Blankenship, et al., 2014, Warenda and Konopka, 2002). The cdc10Δ, cdc11Δ, and a cdc12-6ts mutant were also found to have defects in selecting a site for the emergence of germ tubes that are the initial outgrowths that turn into hyphae. Usually mother cells form a second germ tube at a site distal to the first. However, septin mutants often form a second germ tube adjacent to the first, which would limit the ability to disseminate the infection by sending out a hypha in a new direction (Li, et al., 2012, Warenda and Konopka, 2002). Consistent with this, there is a patch of septins at the neck of the mother cell and germ tube that does not correspond to a site of cytokinesis, suggesting it acts as a barrier to prevent formation of a second germ tube nearby (Sudbery, 2001, Warenda and Konopka, 2002). Septin mutants are also defective in invasive growth in a mouse model of candidiasis (Warenda, et al., 2003).
The special roles of septins during hyphal morphogenesis have also led to the discovery of new types of septin regulation. For example, the ability of cytoplasmic septin subunits to exchange with those in septin filaments appears to be distinct in hyphal cells (Gonzalez-Novo, et al., 2008). Also, the Sep7 septin appears to play a special role in preventing cytokinesis from occurring in the elongating hyphae so that elongated multicellular chains of filamentous cells can form (Gonzalez-Novo, et al., 2008). There are also different mechanisms used by protein kinases to regulate septins during hyphal growth, including a role for cyclin-dependent kinases in regulating Cdc11 (Sinha, et al., 2007, Wang, 2009).
ER-Plasma membrane contact sites
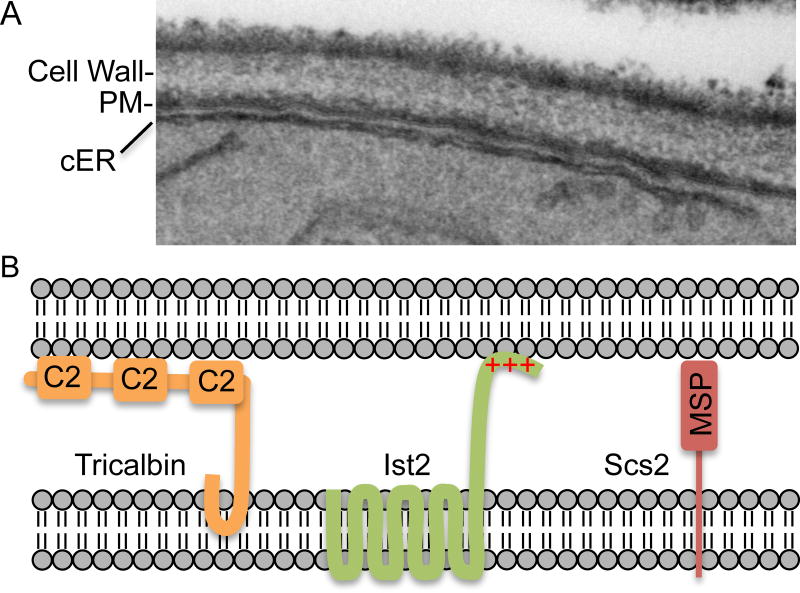
Sites of direct contact between the ER and plasma membrane create another type of specialized membrane domain that greatly influences the architecture and function of the plasma membrane (Fig. 3A). Although the ER is best known for its role in mediating the early stages of the secretory pathway, the ER also forms sites of direct contact with the plasma membrane, mitochondria, and other membrane bound organelles (Henne, et al., 2015, Wong, et al., 2014). This is an understudied area in C. albicans, so most of what is known comes from studies on S. cerevisiae and mammalian cells. The plasma membrane associated ER is often called the cortical ER to distinguish it from the ER extending through the cytoplasm or the perinuclear ER. These cortical ER contact sites with the plasma membrane have significant influence, as EM studies with S. cerevisiae have indicated the ER covers 20–40% of the plasma membrane (Schuck, et al., 2009, West, et al., 2011), and live cell studies with fluorescently tagged ER proteins suggest that about 65% of the plasma membrane is covered by ER (Stradalova, et al., 2012).
Figure 3. Sites of membrane contact between the ER and the plasma membrane.
(A) Photo of a section of a cell analyzed by transmission electron microscopy. Note the close association between a segment of cortical ER and the plasma membrane.
(B) Model for three proteins that play key roles in forming sites of contact between the ER and the plasma membrane (Henne, et al., 2015, Manford, et al., 2012).
Three types of proteins participate in forming ER-plasma membrane contact sites in S. cerevisiae (Fig. 3B). One class of proteins includes the tricalbans (Tcb1, Tcb2, Tcb3) that contain an N-terminal domain that inserts into the outer leaflet of the ER and a C-terminal domain that binds to lipids in the plasma membrane via multiple C2 domains. A second type of protein, Ist2, has multiple transmembrane domains that anchor it in the ER, and a C-terminal region rich in basic amino acids that bind to acidic lipids in the plasma membrane. The third type of protein is represented by Scs2, which has a transmembrane domain that anchors it in the ER and an N-terminal MSP domain that binds to the plasma membrane. Scs2 has also been shown to play a key role in restricting ER to the mother cell during bud morphogenesis by interacting with the septin ring at the bud neck (Chao, et al., 2014, Wong, et al., 2014).
The ER is in very close apposition to the plasma membrane at these sites, as they are about 33 nM apart (Pichler, et al., 2001, Schuck, et al., 2009). This suggested that the cortical ER acts as a barrier that blocks access of cytoplasmic components to the plasma membrane. Consistent with this the ER-plasma membrane contact sites lack ribosomes, and secretion and endocytosis do not occur at these sites (Stradalova, et al., 2012). Although the cortical ER covers a significant portion of the plasma membrane, live cell imaging revealed significant movement over a 20 s time course, indicating that most areas of the plasma membrane eventually get exposed to the cytoplasm (Stradalova, et al., 2012). One exception was that eisosomes were not observed to be covered by ER (Stradalova, et al., 2012). It was suggested that the membrane furrows projecting inward may disrupt the formation of the ER-plasma membrane contact sites.
The sites of ER-plasma membrane contact are thought to play important roles in lipid synthesis and the homeostasis of ions and lipids (Henne, et al., 2015). ER-plasma membrane contact sites represent a way to transfer lipids, proteins, and signals directly from the ER to the plasma membrane, thereby bypassing the need to flow through the Golgi and secretory vesicles. For example, synthesis of sphingolipids and phospholipids is thought to occur at these sites (Henne, et al., 2015, Pichler, et al., 2001, Tavassoli, et al., 2013). Another example is that the Sac1 lipid phosphatase is anchored in the ER but acts on the plasma membrane. Sac1 dephosphorylates phosphatidyl inositol phosphate (PI4P), thereby depleting this lipid and preventing it from being converted into the important regulatory lipid PI4,5P2. As a result, mutants defective in forming ER-plasma membrane contact sites have higher levels of PI4P, similar to mutants lacking Sac1 (Manford, et al., 2012). Sac1 also acts in concert with Osh6 to promote non-vesicular trafficking of phosphatidylserine from its site of synthesis in the ER to the plasma membrane. Osh6 binds phosphatidylserine in the ER and then transports it to the plasma membrane. At the plasma membrane, Osh6 then exchanges phosphatidylserine for PI4P, which it then transports to the ER. In this case, Sac1 is thought to primarily dephosphorylate PI4P in the ER to create a gradient of PI4P at the ER-plasma membrane interface that drives the transport of phosphatidylserine to the plasma membrane (Maeda, et al., 2013, Moser von Filseck, et al., 2015). A sac1Δ mutant in C. albicans is very defective in cell wall integrity and virulence (Zhang, et al., 2015).
Dynamic zones in the plasma membrane
Sites of secretion – the exocyst complex
The transport of secretory vesicles to specific sites in the plasma membrane is essential for lipid homeostasis, polarized growth, and secretion of proteins, including many that are involved in virulence. The stages of the secretory pathway have been mapped out in S. cerevisiae, including a series of landmark studies by Randy Scheckman who was awarded a Nobel Prize in 2013 for his contributions to defining the secretory pathway (Bonifacino, 2014). Only a brief overview of this complex process will be presented here, as there have been several recent reviews on the different stages of secretion that involve insertion of material into the ER, vesicle trafficking from the ER to the Golgi complex, and finally the delivery to the plasma membrane (Heider and Munson, 2012, Munson and Novick, 2006).
After mature secretory vesicles are released from the Golgi network, a complex of proteins known as the exocyst mediates the delivery of the vesicles to appropriate target sites in the plasma membrane. The vesicles are efficiently directed along actin cables to the plasma membrane by the myosin-V motor protein, Myo2p. Upon delivery to the target site, the exocyst tethers the vesicle to the membrane, as SNARE proteins mediate fusion (Donovan and Bretscher, 2015, Heider and Munson, 2012, Luo, et al., 2014, Munson and Novick, 2006).
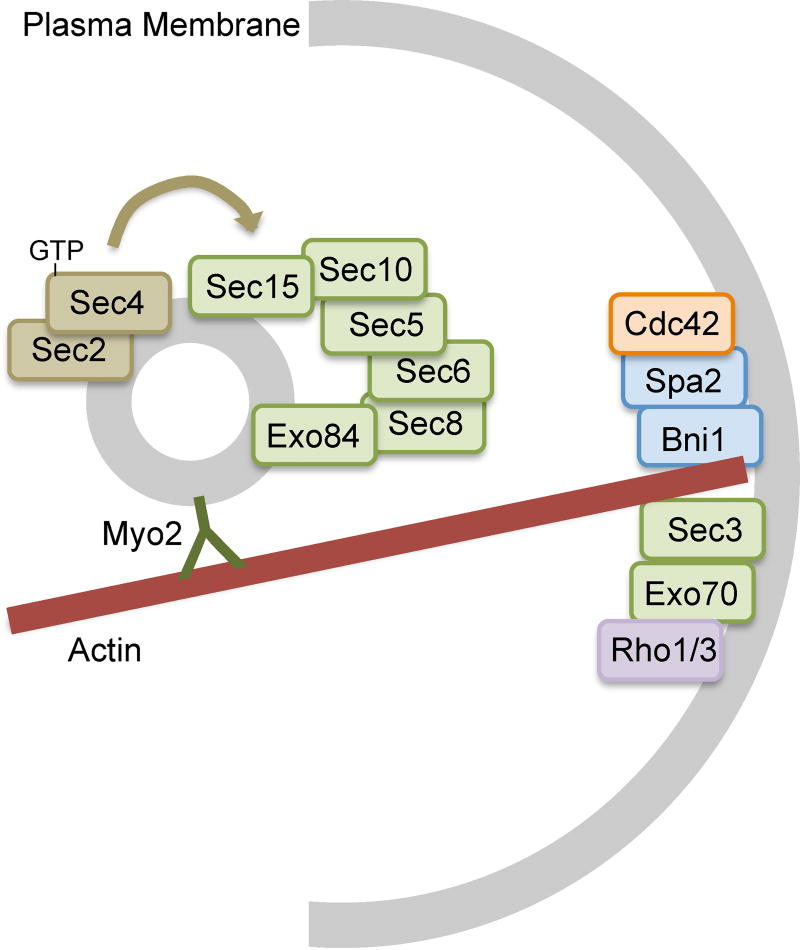
The exocyst is a conserved complex comprised of eight subunits that was first identified in S. cerevisiae (Fig. 4). The subunits include Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 (Heider and Munson, 2012). Sec3 and a portion of Exo70 are recruited to the plasma membrane independently, whereas the other subunits traffic together with secretory vesicles to the plasma membrane.
Figure 4. Model for exocyst complex that mediates secretion.
The exocyst is defined as a complex of 8 proteins (shaded green). Exo70 and Sec3 localize to the plasma membrane to define the future site of vesicle fusion. The other six exocyst proteins traffic along with the secretory vesicles. The eight members of the exocyst form a complex at the plasma membrane that activates the SNARE complex to promote vesicle fusion.
Sec3 and Exo70 serve as key landmarks that create a special domain, since they arrive in the plasma membrane first. Both of these proteins associate with the plasma membrane in part by interacting with PI4,5P2; Sec3 through a Pleckstrin Homology domain, and Exo70 through several binding sites in its C-terminal region (Pleskot, et al., 2015). Small GTPases are also needed to recruit Sec3 and Exo70 to the target site, including Rho1, Rho3 and Cdc42 (Guo, et al., 2001, Munson and Novick, 2006, Zhang, et al., 2001).
The other exocyst proteins are loaded onto secretory vesicles in a manner that is initiated by the Sec4 GTPase, which is regulated by its guanine nucleotide exchange factor Sec2. Sec4-GTP promotes association with Sec15, and thereby subsequent recruitment of the other exocyst components. Sec4 GTPase activity is further thought to mediate assembly of the exocyst complex, whose architecture creates a “Y-shape” that facilitates its tethering function (Heider and Munson, 2012, Luo, et al., 2014).
Studies of the exocyst in C. albicans have confirmed its key role in secretion, and also revealed some specialized functions during hyphal growth. Interestingly, Sec3 was not needed for the initial polarized growth to start a hyphae (germ tube formation), but was needed to maintain polarized hyphal growth (Li, et al., 2007). The Sec3-dependent phase started after the septin ring formed, indicating an interaction between exocyst domains and septins. Another interesting aspect is that efficient hyphal extension requires constitutive phosphorylation of Exo84 throughout mitosis by Cdk1 in complex with the hyphal-specific cyclin Hgc1 (Caballero-Lima and Sudbery, 2014, Zheng and Wang, 2004). This contrasts with regulation of Exo84 in S. cerevisiae, in which phosphorylation of the exocyst component Exo84 by Cdk1-Clb2 during mitosis causes the exocyst to disassemble. Other studies have shown that Sec6 and Sec15 have distinct roles in mediating polarized secretion and filamentation in C. albicans (Chavez-Dozal, et al., 2015, Chavez-Dozal, et al., 2015).
Sites of endocytosis in the plasma membrane
Endocytosis is critical for the turnover and homeostasis of lipids and proteins in the plasma membrane, and also for bringing substances into the cell (Goode, et al., 2015, Kaksonen, et al., 2005, Weinberg and Drubin, 2012). This complex process occurs when the plasma membrane forms an invagination that pinches off to form a vesicle. The most well understood endocytic pathway is known as clathrin-mediated endocytosis. In S. cerevisiae, this pathway involves the recruitment of >50 proteins in a temporally ordered and hierarchial manner. The choreographed steps that mediate endocytosis include target protein recognition and clustering, membrane remodeling, and force-generating actin-filament assembly and turnover to drive membrane invagination and vesicle scission.
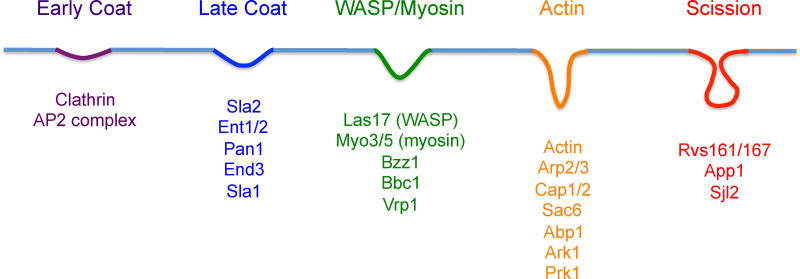
Current models divide the endocytic process into several modules; the Early, the Coat, which includes early, intermediate, and late stages, the WASP/Myo, the Actin, and finally the Scission module (Fig. 5). The Early module begins as a patch in the plasma membrane at a selected site that is away from the MCC/eisosomes, exocyst, and sites of ER contact. This site develops into the Early Coat module when a key component, clathrin, forms a lattice on the inner surface of the plasma membrane that begins the process of invaginating the membrane to develop a vesicle (Avinoam, et al., 2015). Another important Early Coat factor is the AP2 complex, which acts as an adaptor to recruit specific cargo proteins (Weinberg and Drubin, 2012).
Figure 5. Overview of events that occur in the plasma membrane during endocytosis.
Proteins that mediate endocytosis are recruited to plasma membrane in a defined order. An early coat complex forms with cargo proteins clathrin and the AP2 complex to initiate the process. The subsequent intermediates form with the recruitment of the indicated proteins. The process culminates with the scission phase that releases an endocytic vesicle.
Additional proteins are recruited as the nascent endocytic domain transitions through an Intermediate Coat stage to the Late Coat stage (Fig 5). These proteins have diverse roles in stabilizing the scaffold of proteins, binding the plasma membrane, and contacting the actin cytoskeleton (Skruzny, et al., 2012). Transition to the WASP/MYO module takes place with the recruitment of Las17, the WASP protein in yeast, which serves as the chief inducer of the Arp2/3 complex nucleation of actin filament polymerization. The type 1 myosin, Myo3p/Myo5p and its regulators also join the endocytic patch at this stage. The next major event is the arrival of actin and its accompanying factors to form the Actin module. Induction of the Arp2/3 complex by Las17p begins actin nucleation and polymerization, providing the driving force for membrane invagination (Goode, et al., 2015, Weinberg and Drubin, 2012). Actin patches in the cortical region of the plasma membrane are one of the hallmarks of sites of endocytosis in cell biology studies. The Scission phase then occurs to release the invagination as an endocytic vesicle. The BAR-domain proteins Rvs1p and Rvs167p are key players in this process as they promote membrane curvature at the neck that forms the scission site.
Studies in C. albicans have confirmed that many of the orthologous proteins play important roles in endocytosis (Borth, et al., 2010, Douglas, et al., 2009, Epp, et al., 2013, Oberholzer, et al., 2006, Walther and Wendland, 2004). Interestingly, a clathrin and Arp2,3-independent pathway for endocytosis was discovered in C. albicans, highlighting the complexity of this process (Epp, et al., 2013). Analysis of endocytosis mutants has also shown that they are defective in hyphal morphogenesis (Borth, et al., 2010, Douglas, et al., 2009, Epp, et al., 2013, Oberholzer, et al., 2006, Walther and Wendland, 2004). This is likely due in part to a role for endocytosis in shaping hyphal tip growth that will be described further below (Jones and Sudbery, 2010). In addition, it is also likely due to the interrelationship between actin function in both endocytosis and morphogenesis. Consistent with a key role for endocytosis in hyphal growth, the late coat complex protein Sla1 is differentially regulated by phosphorylation during budding and hyphal growth (Zeng, et al., 2012). The hyphal defect of C. albicans mutants lacking RVS161 and RVS167, which are defective in scission phase of endocytosis, correlated with reduced virulence in a mouse model of candidiasis and a defect in invasive growth in vivo (Douglas, et al., 2009).
Hyphal Tip – a region of rapid highly polarized growth
The rapidly growing tip of hyphal cells represents a special plasma membrane zone because it requires a high degree of coordination between expansion of the cell wall, organization of the cytoskeleton to guide targeted deposition of secretory vesicles, and endocytosis (Sudbery, 2011). These processes must be tightly regulated to promote highly polarized hyphal growth. In addition, novel control mechanisms are needed, as hyphal cells can relocate the site of polarized growth in response to encountering physical barriers (thigmotropism), which is thought to be important for invasive growth (Brand, et al., 2008). That a higher degree of regulation is required to maintain filamentous hyphal growth is supported by analysis of a number of different mutants that can form buds efficiently, but are not capable of forming true hyphae. Some examples of the special forms of regulation that contribute to hyphal growth will be described below.
Hyphal tip growth is mediated by many of the same pathways that regulate bud growth. For example, the small GTPase Cdc42 promotes polarized recruitment to the hyphal tip of polarisome components, including Spa2 and Bni1, which induce formation of actin filaments to guide the secretory vesicles to appropriate exosome sites in the plasma membrane (Sudbery, 2011). However, one difference is that a special hyphal-specific cyclin (Hgc1) is needed to direct cyclin dependent kinase activity toward substrate proteins at the hyphal tip that regulate polarized growth (Bishop, et al., 2010, Caballero-Lima and Sudbery, 2014, Wang, 2009, Zheng and Wang, 2004, Zheng, et al., 2007). Hgc1-CDK kinase phosphorylates the Rga2 GTPase activating protein that regulates Cdc42, thereby preventing Rga2 from localizing at hyphal tips and resulting in maintenance of strong Cdc42 activity at the tip (Zheng, et al., 2007). Other roles for the Hgc1-CDK include phosphorylation of Sec2, a guanyl-nucleotide exchange factor (GEF) for the small G-protein Sec4 that promotes polarized delivery of secretory vesicles (Bishop, et al., 2010), phosphorylation of the Exo84 subunit of the exocyst complex (Caballero-Lima and Sudbery, 2014), and phosphorylation of septin proteins (Sinha, et al., 2007).
A major difference between bud and hyphal growth is that a zone enriched in membrane vesicles termed the Spitzenkörper lies underneath the hyphal tip (Fig. 6). The Spitzenkörper is thought to provide a steady supply of new material to maintain rapid growth at hyphal tips (Crampin, et al., 2005). The Spitzenkörper was first discovered in filamentous fungi, which have a similar challenge of needing a special mechanism to deliver a constant supply of new vesicles along the length of the cell to promote rapid tip growth. In contrast, the Spitzenkörper has not been observed in budding cells that switch to isotropic growth and do not require such an extended form of vesicle trafficking along the length of filamentous growth.
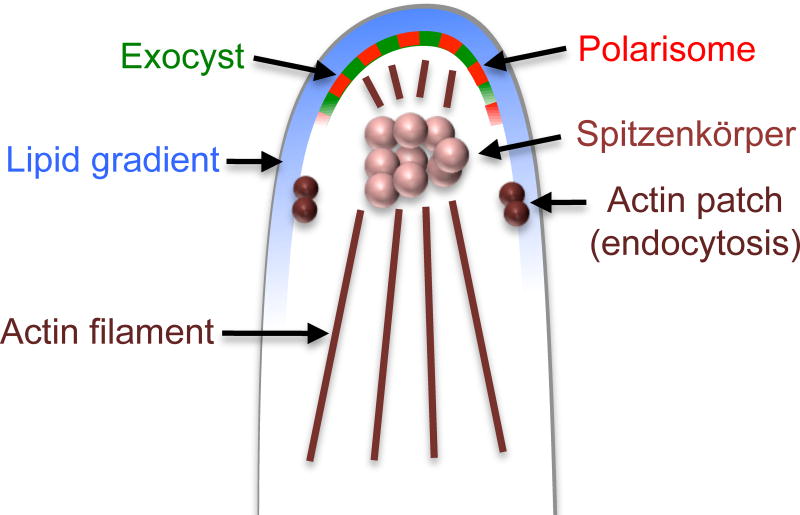
Figure 6. Model for region of highly polarized growth at hyphal tips.
The hyphal tips have a polarized distribution of lipids in the plasma membrane, including enrichment of PI4,5P2 (Vernay, et al., 2012). This region also stains more strongly with the ergosterol binding agent filipin, indicating a change in ergosterol levels or increased accessibility of filipin (Martin and Konopka, 2004). The hyphal tip region is also enriched in polarisome and exocyst domains that mediate highly polarized growth. Note the presence of actin patches at a subapical region where they promote endocytosis to help maintain tip growth (Caballero-Lima, et al., 2013). Underlying the plasma membrane is a complex of vesicles termed the Spitzenkörper, which is thought to promote the rapid growth at the tip (Jones and Sudbery, 2010). Actin filaments guide secretory vesicles to the hyphal tip.
Polarization of lipids also occurs at hyphal tips and is thought to help reinforce the recruitment of morphogenesis proteins to this region. Hyphal tips were found to stain with filipin, a fluorescent ergosterol-binding compound, suggesting that this zone is enriched in ergosterol (Alvarez, et al., 2007, Martin and Konopka, 2004). However, it has also been suggested that increased filipin staining could indicate that the lipid environment is changed in other ways that promote better access of filipin (Jin, et al., 2008). Either way, these results indicate that there is a special lipid zone at hyphal tips. More recent studies have shown that phosphatidylinositol lipids are enriched at hyphal tips. A Pleckstrin Homology (PH) domain fused to GFP that binds to the phosphatidyl bisphosphate PI4,5P2 was found to be highly enriched at hyphal tips (Vernay, et al., 2012). PI4,5P2 is known to promote polarized cell growth by recruiting morphogenesis proteins to the plasma membrane. PI4P, which is involved in vesicle trafficking and membrane dynamics, is also enriched at hyphal tips (Ghugtyal, et al., 2015). In addition, regulation of sphingolipid synthesis plays an important role as cells lacking the Lag1 ceramide synthase, which are defective in forming inositol-containing sphingolipids, fail to form hyphae (Cheon, et al., 2012).
Coordination between zones of exocytosis and endocytosis at hyphal tips is also important for polarized growth. While the polarisome and exocyst components are located in a crescent at the apex of the hyphal tip, the sites of endocytosis occur at a slightly subapical zone (Jones and Sudbery, 2010). This was detected because actin patches, which mark sites of endocytosis, are clustered in the subapical zone. Molecular modeling indicates that this orientation of sites of exocytosis and endocytosis helps to promote polarized hyphal tip growth (Caballero-Lima, et al., 2013).
Other domains in the plasma membrane
The special zones and domains described above represent only a subset of the organized architecture of the plasma membrane. Studies with S. cerevisiae have identified other membrane microdomains within the MCP that are likely to exist in C. albicans and other fungi. One example of this is the Target of Rapamycin Complex 2 (TORC2), which forms patches within the plasma membrane. This complex contains the conserved Target of Rapamycin (TOR) kinase, which regulates actin polymerization, cell polarity, and ceramide synthesis (Berchtold and Walther, 2009). TORC2 forms patches in the plasma membrane that are mobile and spatially distinct from the MCC/eisosomes. Patchy localization in the plasma membrane that is independent of actin and eisosomes has also been detected for the Stt4 lipid kinase that generates PI4P and the Mss4 kinase that generates PI4,5P2, and the two kinase complexes show very little colocalization (Audhya and Emr, 2002, Baird, et al., 2008). Proteins involved in sensing extracellular pH (Rim9, Dfg16, and Rim21) were similarly found in patches that were independent of eisosomes (Obara, et al., 2012). Although the role of clustering these proteins into patches is not clear, it is significant that the proteins in these complexes are important for viability or virulence of C. albicans (Davis, 2009, Ghugtyal, et al., 2015).
To examine the complexity of plasma membrane protein organization in S. cerevisiae, a study was carried out in which 46 plasma membrane proteins were tagged with fluorescent proteins and analyzed by TIRF microscopy (Schuberth and Wedlich-Soldner, 2015, Spira, et al., 2012). Interestingly, the proteins appeared in a range of patterns from punctate patches to large networks. Most of these proteins did not overlap, even if they showed a similar degree of patchiness in the plasma membrane. For integral membrane proteins, analysis of chimeric proteins indicated that the transmembrane segments were confer specificity for how a specific protein is targeted to a specific subdomain. Further studies will be needed to determine the role protein segregation in plasma membrane function.
Summary
Organization of the plasma membrane into different domains helps to coordinate the diverse functions carried out by this organelle that are needed for C. albicans virulence. Recent findings, such as discovery of MCC/ eisosomes and new roles for septins, indicate that there is still much more to be learned about plasma membrane architecture and its role in fungal pathogenesis. Further studies will not only be necessary for providing a better understanding of the mechanisms of plasma membrane function, they will also be key for developing new therapeutic approaches for treating C. albicans. The importance of the plasma membrane makes it a promising target for the development of novel therapeutic drugs. Furthermore, new insights into plasma membrane function will help to better understand the mechanism of antifungal drugs, since the most commonly used drugs target the plasma membrane (e.g. Amphotericin, Fluconazole, Caspofungin) (Odds, et al., 2003). Research on the plasma membrane is also expected to reveal new insights into the mechanisms used by the immune system to attack C. albicans with complement and antimicrobial peptides. Future studies on C. albicans will therefore also have significance by providing an important model for defining the role of plasma membrane function in other fungal pathogens.
Acknowledgments
We thank the members of our lab for their help and advice. The research in our lab on plasma membrane organization in C. albicans was supported by grants from the National Institutes of Health awarded to J.B.K. (RO1AI047837 and R21DE025200).
References
- Aguilar PS, Frohlich F, Rehman M, Shales M, Ulitsky I, Olivera-Couto A, Braberg H, Shamir R, Walter P, Mann M, Ejsing CS, Krogan NJ, Walther TC. A plasma-membrane E-MAP reveals links of the eisosome with sphingolipid metabolism and endosomal trafficking. Nat. Struct. Mol. Biol. 2010;17:901–908. doi: 10.1038/nsmb.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Douglas LM, Konopka JB. Sterol-rich plasma membrane domains in fungi. Eukaryot. Cell. 2007;6:755–763. doi: 10.1128/EC.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Douglas LM, Rosebrock A, Konopka JB. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol. Biol. Cell. 2008;19:5214–5225. doi: 10.1091/mbc.E08-05-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Avinoam O, Schorb M, Beese CJ, Briggs JA, Kaksonen M. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science. 2015;348:1369–1372. doi: 10.1126/science.aaa9555. [DOI] [PubMed] [Google Scholar]
- Badrane H, Nguyen MH, Blankenship JR, Cheng S, Hao B, Mitchell AP, Clancy CJ. Rapid redistribution of phosphatidylinositol-(4,5)-bisphosphate and septins during the Candida albicans response to caspofungin. Antimicrob. Agents Chemother. 2012;56:4614–4624. doi: 10.1128/AAC.00112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird D, Stefan C, Audhya A, Weys S, Emr SD. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J. Cell Biol. 2008;183:1061–1074. doi: 10.1083/jcb.200804003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintainence of cell polarity in yeast. Molec. Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Barug D, de Groot K. Effect of the imidazole derivative lombazole on the ultrastructure of Staphylococcus epidermidis and Candida albicans. Antimicrob. Agents Chemother. 1985;28:643–647. doi: 10.1128/aac.28.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat. Cell Biol. 2012;14:542–547. doi: 10.1038/ncb2480. [DOI] [PubMed] [Google Scholar]
- Berchtold D, Walther TC. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol. Biol. Cell. 2009;20:1565–1575. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo SM, Lee SA. Candida albicans SUR7 contributes to secretion, biofilm formation, and macrophage killing. BMC Microbiol. 2010;10 doi: 10.1186/1471-2180-1110-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A, Lane R, Beniston R, Chapa-y-Lazo B, Smythe C, Sudbery P. Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase. Embo J. 2010;29:2930–2942. doi: 10.1038/emboj.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Cheng S, Woolford CA, Xu W, Johnson TM, Rogers PD, Fanning S, Nguyen MH, Clancy CJ, Mitchell AP. Mutational analysis of essential septins reveals a role for septin-mediated signaling in filamentation. Eukaryot. Cell. 2014;13:1403–1410. doi: 10.1128/EC.00127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS. Vesicular transport earns a Nobel. Trends Cell Biol. 2014;24:3–5. doi: 10.1016/j.tcb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borth N, Walther A, Reijnst P, Jorde S, Schaub Y, Wendland J. Candida albicans Vrp1 is required for polarized morphogenesis and interacts with Wal1 and Myo5. Microbiology. 2010;156:2962–2969. doi: 10.1099/mic.0.041707-0. [DOI] [PubMed] [Google Scholar]
- Brach T, Specht T, Kaksonen M. Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J. Cell Sci. 2011;124:328–337. doi: 10.1242/jcs.078519. [DOI] [PubMed] [Google Scholar]
- Brand A, Vacharaksa A, Bendel C, Norton J, Haynes P, Henry-Stanley M, Wells C, Ross K, Gow NA, Gale CA. An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans. Eukaryot Cell. 2008;7:712–720. doi: 10.1128/EC.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges AA, Gladfelter AS. Septin Form and Function at the Cell Cortex. J. Biol. Chem. 2015;290:17173–17180. doi: 10.1074/jbc.R114.634444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Buser C, Drubin DG. Ultrastructural imaging of endocytic sites in Saccharomyces cerevisiae by transmission electron microscopy and immunolabeling. Microsc. Microanal. 2013;19:381–392. doi: 10.1017/S1431927612014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Lima D, Kaneva IN, Watton SP, Sudbery PE, Craven CJ. The spatial distribution of the exocyst and actin cortical patches is sufficient to organize hyphal tip growth. Eukaryot. Cell. 2013;12:998–1008. doi: 10.1128/EC.00085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Lima D, Sudbery PE. In Candida albicans, phosphorylation of Exo84 by Cdk1-Hgc1 is necessary for efficient hyphal extension. Mol. Biol. Cell. 2014;25:1097–1110. doi: 10.1091/mbc.E13-11-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J, Brynda J, Wolfova J, Grandori R, Gustavsson T, Ettrich R, Smatanova IK. WrbA bridges bacterial flavodoxins and eukaryotic NAD(P)H:quinone oxidoreductases. Protein Sci. 2007;16:2301–2305. doi: 10.1110/ps.073018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JT, Wong AK, Tavassoli S, Young BP, Chruscicki A, Fang NN, Howe LJ, Mayor T, Foster LJ, Loewen CJ. Polarization of the endoplasmic reticulum by ER-septin tethering. Cell. 2014;158:620–632. doi: 10.1016/j.cell.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Chavez-Dozal AA, Bernardo SM, Rane HS, Herrera G, Kulkarny V, Wagener J, Cunningham I, Brand AC, Gow NA, Lee SA. The Candida albicans Exocyst Subunit Sec6 Contributes to Cell Wall Integrity and Is a Determinant of Hyphal Branching. Eukaryot. Cell. 2015;14:684–697. doi: 10.1128/EC.00028-15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chavez-Dozal AA, Bernardo SM, Rane HS, Lee SA. Functional Analysis of the Exocyst Subunit Sec15 in Candida albicans. Eukaryot. Cell. 2015;14:1228–1239. doi: 10.1128/EC.00147-15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cheon SA, Bal J, Song Y, Hwang HM, Kim AR, Kang WK, Kang HA, Hannibal-Bach HK, Knudsen J, Ejsing CS, Kim JY. Distinct roles of two ceramide synthases, CaLag1p and CaLac1p, in the morphogenesis of Candida albicans. Mol. Microbiol. 2012;83:728–745. doi: 10.1111/j.1365-2958.2011.07961.x. [DOI] [PubMed] [Google Scholar]
- Cid VJ, Jimenez J, Molina M, Sanchez M, Nombela C, Thorner JW. Orchestrating the cell cycle in yeast: sequential localization of key mitotic regulators at the spindle pole and the bud neck. Microbiology. 2002;148:2647–2659. doi: 10.1099/00221287-148-9-2647. [DOI] [PubMed] [Google Scholar]
- Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb Perspect Med. 2015;5:a019752. doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampin H, Finley K, Gerami-Nejad M, Court H, Gale C, Berman J, Sudbery P. Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 2005;118:2935–2947. doi: 10.1242/jcs.02414. [DOI] [PubMed] [Google Scholar]
- Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr. Opin. Microbiol. 2009;12:365–370. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Deng C, Xiong X, Krutchinsky AN. Unifying fluorescence microscopy and mass spectrometry for studying protein complexes in cells. Mol. Cell. Proteomics. 2009;8:1413–1423. doi: 10.1074/mcp.M800397-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- Donovan KW, Bretscher A. Tracking individual secretory vesicles during exocytosis reveals an ordered and regulated process. J. Cell Biol. 2015;210:181–189. doi: 10.1083/jcb.201501118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LM, Alvarez FJ, McCreary C, Konopka JB. Septin function in yeast model systems and pathogenic fungi. Eukaryot. Cell. 2005;4:1503–1512. doi: 10.1128/EC.4.9.1503-1512.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LM, Konopka JB. Fungal membrane organization: the eisosome concept. Annu. Rev. Microbiol. 2014;68:377–393. doi: 10.1146/annurev-micro-091313-103507. [DOI] [PubMed] [Google Scholar]
- Douglas LM, Martin SW, Konopka JB. BAR domain proteins Rvs161 and Rvs167 contribute to Candida albicans endocytosis, morphogenesis, and virulence. Infect. Immun. 2009;77:4150–4160. doi: 10.1128/IAI.00683-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LM, Wang HX, Keppler-Ross S, Dean N, Konopka JB. Sur7 Promotes Plasma Membrane Organization and Is Needed for Resistance to Stressful Conditions and to the Invasive Growth and Virulence of Candida albicans. MBio. 2012;3:e00254–00211. doi: 10.1128/mBio.00254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LM, Wang HX, Konopka JB. The MARVEL Domain Protein Nce102 Regulates Actin Organization and Invasive Growth of Candida albicans. MBio. 2013:4. doi: 10.1128/mBio.00723-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova J, Drgon T, Tanaka K, Kollar R, Chen GC, Ford RA, Chan CS, Takai Y, Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Epp E, Nazarova E, Regan H, Douglas LM, Konopka JB, Vogel J, Whiteway M. Clathrin- and Arp2/3-Independent Endocytosis in the Fungal Pathogen Candida albicans. MBio. 2013;4:e00476–00413. doi: 10.1128/mBio.00476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S, Riezman H. Sphingolipid signaling in yeast: potential implications for understanding disease. Front. Biosci. 2013;5:97–108. doi: 10.2741/e599. [DOI] [PubMed] [Google Scholar]
- Frohlich F, Christiano R, Olson DK, Alcazar-Roman A, DeCamilli P, Walther TC. A role for eisosomes in maintenance of plasma membrane phosphoinositide levels. Mol. Biol. Cell. 2014;25:2797–2806. doi: 10.1091/mbc.E13-11-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, Moreira K, Aguilar PS, Hubner NC, Mann M, Walter P, Walther TC. A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J. Cell Biol. 2009;185:1227–1242. doi: 10.1083/jcb.200811081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghugtyal V, Garcia-Rodas R, Seminara A, Schaub S, Bassilana M, Arkowitz RA. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. Proc. Natl. Acad. Sci. U S A. 2015;112:8644–8649. doi: 10.1073/pnas.1504259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS. Guides to the final frontier of the cytoskeleton: septins in filamentous fungi. Curr. Opin. Microbiol. 2010;13:720–726. doi: 10.1016/j.mib.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Novo A, Correa-Bordes J, Labrador L, Sanchez M, Vazquez de Aldana CR, Jimenez J. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol. Biol. Cell. 2008;19:1509–1518. doi: 10.1091/mbc.E07-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eskin JA, Wendland B. Actin and endocytosis in budding yeast. Genetics. 2015;199:315–358. doi: 10.1534/genetics.112.145540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H, Kuebler O, Bas E, Moor H. Decoration of specific sites on freeze-fractured membranes. J. Cell Biol. 1978;79:646–656. doi: 10.1083/jcb.79.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann G, Malinsky J, Stahlschmidt W, Loibl M, Weig-Meckl I, Frommer WB, Opekarova M, Tanner W. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J. Cell Biol. 2008;183:1075–1088. doi: 10.1083/jcb.200806035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 2007;26:1–8. doi: 10.1038/sj.emboj.7601466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- Han S, Lone MA, Schneiter R, Chang A. Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. U S A. 2010;107:5851–5856. doi: 10.1073/pnas.0911617107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Liou J, Emr SD. Molecular mechanisms of inter-organelle ER-PM contact sites. Curr. Opin. Cell Biol. 2015;35:123–130. doi: 10.1016/j.ceb.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams SL, Millar A, Taylor P, Bennett K, Boutilier K, Yang L, Wolting C, Donaldson I, Schandorff S, Shewnarane J, Vo M, Taggart J, Goudreault M, Muskat B, Alfarano C, Dewar D, Lin Z, Michalickova K, Willems AR, Sassi H, Nielsen PA, Rasmussen KJ, Andersen JR, Johansen LE, Hansen LH, Jespersen H, Podtelejnikov A, Nielsen E, Crawford J, Poulsen V, Sorensen BD, Matthiesen J, Hendrickson RC, Gleeson F, Pawson T, Moran MF, Durocher D, Mann M, Hogue CW, Figeys D, Tyers M. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 2002;415:180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
- Jin H, McCaffery JM, Grote E. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J. Cell. Biol. 2008;180:813–826. doi: 10.1083/jcb.200705076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Sudbery PE. Spitzenkorper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties. Eukaryot Cell. 2010;9:1455–1465. doi: 10.1128/EC.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeche R, Baldissard S, Hammond J, Howard L, Moseley JB. The filament-forming protein Pil1 assembles linear eisosomes in fission yeast. Mol. Biol. Cell. 2011;22:4059–4067. doi: 10.1091/mbc.E11-07-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeche R, Roguev A, Krogan NJ, Moseley JB. A Pil1-Sle1-Syj1-Tax4 functional pathway links eisosomes with PI(4,5)P2 regulation. J. Cell Sci. 2014;127:1318–1326. doi: 10.1242/jcs.143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kamble C, Jain S, Murphy E, Kim K. Requirements of Slm proteins for proper eisosome organization, endocytic trafficking and recycling in the yeast Saccharomyces cerevisiae. J. Biosci. 2011;36:79–96. doi: 10.1007/s12038-011-9018-0. [DOI] [PubMed] [Google Scholar]
- Karotki L, Huiskonen JT, Stefan CJ, Ziolkowska NE, Roth R, Surma MA, Krogan NJ, Emr SD, Heuser J, Grunewald K, Walther TC. Eisosome proteins assemble into a membrane scaffold. J. Cell Biol. 2011;195:889–902. doi: 10.1083/jcb.201104040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis FM, Brul S, De Groot PW. Covalently linked wall proteins in ascomycetous fungi. Yeast. 2010;27:489–493. doi: 10.1002/yea.1747. [DOI] [PubMed] [Google Scholar]
- Kullberg BJ, Arendrup MC. Invasive Candidiasis. N. Engl. J. Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- Lee JH, Heuser JE, Roth R, Goodenough U. Eisosome Ultrastructure and Evolution in Fungi, Microalgae and Lichens. Eukaryot. Cell. 2015 doi: 10.1128/EC.00106-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Lee RT, Wang YM, Zheng XD, Wang Y. Candida albicans hyphal morphogenesis occurs in Sec3p-independent and Sec3p-dependent phases separated by septin ring formation. J. Cell Sci. 2007;120:1898–1907. doi: 10.1242/jcs.002931. [DOI] [PubMed] [Google Scholar]
- Li L, Naseem S, Sharma S, Konopka JB. Flavodoxin-Like Proteins Protect Candida albicans from Oxidative Stress and Promote Virulence. PLoS Pathog. 2015;11:e1005147. doi: 10.1371/journal.ppat.1005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang C, Konopka JB. A Candida albicans Temperature-Sensitive cdc12-6 Mutant Identifies Roles for Septins in Selection of Sites of Germ Tube Formation and Hyphal Morphogenesis. Eukaryot Cell. 2012;11:1210–1218. doi: 10.1128/EC.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loibl M, Grossmann G, Stradalova V, Klingl A, Rachel R, Tanner W, Malinsky J, Opekarova M. C terminus of Nce102 determines the structure and function of microdomains in the Saccharomyces cerevisiae plasma membrane. Eukaryot. Cell. 2010;9:1184–1192. doi: 10.1128/EC.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC. The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. J. Biol. Chem. 2008;283:10433–10444. doi: 10.1074/jbc.M709972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zhang J, Guo W. The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol. Biol. Cell. 2014;25:3813–3822. doi: 10.1091/mbc.E14-04-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, Gavin AC. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- Malinska K, Malinsky J, Opekarova M, Tanner W. Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J. Cell Sci. 2004;117:6031–6041. doi: 10.1242/jcs.01493. [DOI] [PubMed] [Google Scholar]
- Malinska K, Malinsky J, Opekarova M, Tanner W. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol. Biol. Cell. 2003;14:4427–4436. doi: 10.1091/mbc.E03-04-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky J, Opekarova M, Tanner W. The lateral compartmentation of the yeast plasma membrane. Yeast. 2010;27:473–478. doi: 10.1002/yea.1772. [DOI] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev. Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Martin SG, Arkowitz RA. Cell polarization in budding and fission yeasts. FEMS Microbiol Rev. 2014;38:228–253. doi: 10.1111/1574-6976.12055. [DOI] [PubMed] [Google Scholar]
- Martin SW, Douglas LM, Konopka JB. Cell cycle dynamics and quorum sensing in Candida albicans chlamydospores are distinct from budding and hyphal cells. Eukaryot Cell. 2005;4:1191–1202. doi: 10.1128/EC.4.7.1191-1202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SW, Konopka JB. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell. 2004;3:675–684. doi: 10.1128/EC.3.3.675-684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SW, Konopka JB. SUMO modification of septin-interacting proteins in Candida albicans. J. Biol. Chem. 2004;279:40861–40867. doi: 10.1074/jbc.M406422200. [DOI] [PubMed] [Google Scholar]
- Mascaraque V, Hernaez ML, Jimenez-Sanchez M, Hansen R, Gil C, Martin H, Cid VJ, Molina M. Phosphoproteomic analysis of protein kinase C signaling in Saccharomyces cerevisiae reveals Slt2 mitogen-activated protein kinase (MAPK)-dependent phosphorylation of eisosome core components. Mol. Cell. Proteomics. 2013;12:557–574. doi: 10.1074/mcp.M112.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MA, Thorner J. Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast. Cell cycle. 2009;8:195–203. doi: 10.4161/cc.8.2.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L, Piatti S. The mother-bud neck as a signaling platform for the coordination between spindle position and cytokinesis in budding yeast. Biol. Chem. 2011;392:805–812. doi: 10.1515/BC.2011.090. [DOI] [PubMed] [Google Scholar]
- Moor H, Muhlethaler K. Fine structure in frozen-etched yeast cells. J. Cell Biol. 1963;17:609–628. doi: 10.1083/jcb.17.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira KE, Schuck S, Schrul B, Frohlich F, Moseley JB, Walther TC, Walter P. Seg1 controls eisosome assembly and shape. J. Cell Biol. 2012;198:405–420. doi: 10.1083/jcb.201202097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira KE, Walther TC, Aguilar PS, Walter P. Pil1 controls eisosome biogenesis. Mol. Biol. Cell. 2009;20:809–818. doi: 10.1091/mbc.E08-03-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser von Filseck J, Copic A, Delfosse V, Vanni S, Jackson CL, Bourguet W, Drin G. INTRACELLULAR TRANSPORT. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–436. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J. Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: Elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Boxberger J, Colvin R, Lee SJ, Zahn G, Loor F, Kim K. Pil1, an eisosome organizer, plays an important role in the recruitment of synaptojanins and amphiphysins to facilitate receptor-mediated endocytosis in yeast. Eur. J. Cell Biol. 2011;90:825–833. doi: 10.1016/j.ejcb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Kim KT. Insights into eisosome assembly and organization. J. Biosci. 2012;37:295–500. doi: 10.1007/s12038-012-9206-6. [DOI] [PubMed] [Google Scholar]
- Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T. Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc. Natl. Acad. Sci. U S A. 2012;109:1536–1541. doi: 10.1073/pnas.1117563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles BJ, Powers T. Plasma membrane proteins Slm1 and Slm2 mediate activation of the AGC kinase Ypk1 by TORC2 and sphingolipids in S. cerevisiae. Cell cycle. 2012;11:3745–3749. doi: 10.4161/cc.21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Yamamoto H, Kihara A. Membrane protein Rim21 plays a central role in sensing ambient pH in Saccharomyces cerevisiae. J. Biol. Chem. 2012;287:38473–38481. doi: 10.1074/jbc.M112.394205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer U, Nantel A, Berman J, Whiteway M. Transcript profiles of Candida albicans cortical actin patch mutants reflect their cellular defects: contribution of the Hog1p and Mkc1p signaling pathways. Eukaryot. Cell. 2006;5:1252–1265. doi: 10.1128/EC.00385-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC, Brown AJ, Gow NA. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003;11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Oh Y, Bi E. Septin structure and function in yeast and beyond. Trends Cell. Biol. 2011;21:141–148. doi: 10.1016/j.tcb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera-Couto A, Aguilar PS. Eisosomes and plasma membrane organization. Mol. Genet. Genomics. 2012;287:607–620. doi: 10.1007/s00438-012-0706-8. [DOI] [PubMed] [Google Scholar]
- Olivera-Couto A, Grana M, Harispe L, Aguilar PS. The eisosome core is composed of BAR domain proteins. Mol. Biol. Cell. 2011;22:2360–2372. doi: 10.1091/mbc.E10-12-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur. J. Biochem. 2001;268:2351–2361. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- Pleskot R, Cwiklik L, Jungwirth P, Zarsky V, Potocky M. Membrane targeting of the yeast exocyst complex. Biochim. Biophys. Acta. 2015;1848:1481–1489. doi: 10.1016/j.bbamem.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Qadota H, Python CP, Inoue SB, Arisawa M, Anraku Y, Zheng Y, Watanabe T, Levin DE, Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Reijnst P, Walther A, Wendland J. Dual-colour fluorescence microscopy using yEmCherry-/GFP-tagging of eisosome components Pil1 and Lsp1 in Candida albicans. Yeast. 2011;28:331–338. doi: 10.1002/yea.1841. [DOI] [PubMed] [Google Scholar]
- Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U S A. 2011;108:19222–19227. doi: 10.1073/pnas.1116948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh DH, Bowers B, Schmidt M, Cabib E. The septation apparatus, an autonomous system in budding yeast. Mol. Biol. Cell. 2002;13:2747–2759. doi: 10.1091/mbc.E02-03-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Varma A, Drgon T, Bowers B, Cabib E. Septins, under Cla4p regulation, and the chitin ring are required for neck integrity in budding yeast. Mol. Biol. Cell. 2003;14:2128–2141. doi: 10.1091/mbc.E02-08-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuberth C, Wedlich-Soldner R. Building a patchwork - The yeast plasma membrane as model to study lateral domain formation. Biochim Biophys Acta. 2015;1853:767–774. doi: 10.1016/j.bbamcr.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 2009;187:525–536. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger S, Rischatsch R, Philippsen P. Formation and stability of eisosomes in the filamentous fungus Ashbya gossypii. J. Cell Sci. 2011;124:1629–1634. doi: 10.1242/jcs.082487. [DOI] [PubMed] [Google Scholar]
- Sinha I, Wang YM, Philp R, Li CR, Yap WH, Wang Y. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev. Cell. 2007;13:421–432. doi: 10.1016/j.devcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Skruzny M, Brach T, Ciuffa R, Rybina S, Wachsmuth M, Kaksonen M. Molecular basis for coupling the plasma membrane to the actin cytoskeleton during clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. U S A. 2012;109:E2533–2542. doi: 10.1073/pnas.1207011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira F, Mueller NS, Beck G, von Olshausen P, Beig J, Wedlich-Soldner R. Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat. Cell Biol. 2012;14:640–648. doi: 10.1038/ncb2487. [DOI] [PubMed] [Google Scholar]
- Stradalova V, Blazikova M, Grossmann G, Opekarova M, Tanner W, Malinsky J. Distribution of cortical endoplasmic reticulum determines positioning of endocytic events in yeast plasma membrane. PloS one. 2012;7:e35132. doi: 10.1371/journal.pone.0035132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradalova V, Stahlschmidt W, Grossmann G, Blazikova M, Rachel R, Tanner W, Malinsky J. Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J. Cell Sci. 2009;122:2887–2894. doi: 10.1242/jcs.051227. [DOI] [PubMed] [Google Scholar]
- Sudbery PE. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol. Microbiol. 2001;41:19–31. doi: 10.1046/j.1365-2958.2001.02459.x. [DOI] [PubMed] [Google Scholar]
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- Sun Y, Miao Y, Yamane Y, Zhang C, Shokat KM, Takematsu H, Kozutsumi Y, Drubin DG. Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Mol. Biol. Cell. 2012;23:2388–2398. doi: 10.1091/mbc.E12-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo K. Lack of invaginations of the plasma membrane during budding and cell division of Saccharomyces cerevisiae and Schizosaccharomyces pombe. FEMS Microbiol. Lett. 1984;22:97–100. [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Tavassoli S, Chao JT, Young BP, Cox RC, Prinz WA, de Kroon AI, Loewen CJ. Plasma membrane--endoplasmic reticulum contact sites regulate phosphatidylcholine synthesis. EMBO Rep. 2013;14:434–440. doi: 10.1038/embor.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer NH, Leverich CK, Fitzgibbon MP, Nelson ZW, Henderson KA, Gafken PR, Hsu JJ, Gottschling DE. Identification of long-lived proteins retained in cells undergoing repeated asymmetric divisions. Proc Natl Acad Sci U S A. 2014;111:14019–14026. doi: 10.1073/pnas.1416079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T, Minemura M, Hirata A, Abe M, Watanabe D, Ohya Y. Movement of yeast 1,3-beta-glucan synthase is essential for uniform cell wall synthesis. Genes Cells. 2002;7:1–9. doi: 10.1046/j.1356-9597.2001.00495.x. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangelatos I, Roumelioti K, Gournas C, Suarez T, Scazzocchio C, Sophianopoulou V. Eisosome organization in the filamentous ascomycete Aspergillus nidulans. Eukaryot. Cell. 2010;9:1441–1454. doi: 10.1128/EC.00087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernay A, Schaub S, Guillas I, Bassilana M, Arkowitz RA. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J. Cell Biol. 2012;198:711–730. doi: 10.1083/jcb.201203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veses V, Richards A, Gow NA. Vacuole inheritance regulates cell size and branching frequency of Candida albicans hyphae. Mol Microbiol. 2009;71:505–519. doi: 10.1111/j.1365-2958.2008.06545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Wendland J. Polarized hyphal growth in Candida albicans requires the Wiskott-Aldrich Syndrome protein homolog Wal1p. Eukaryot Cell. 2004;3:471–482. doi: 10.1128/EC.3.2.471-482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Aguilar PS, Frohlich F, Chu F, Moreira K, Burlingame AL, Walter P. Pkh-kinases control eisosome assembly and organization. EMBO J. 2007;26:4946–4955. doi: 10.1038/sj.emboj.7601933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- Wang HX, Douglas LM, Aimanianda V, Latge JP, Konopka JB. The Candida albicans Sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strength. Eukaryot. Cell. 2011;10:72–80. doi: 10.1128/EC.00167-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. CDKs and the yeast-hyphal decision. Curr. Opin. Microbiol. 2009;12:644–649. doi: 10.1016/j.mib.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Warenda AJ, Kauffman S, Sherrill TP, Becker JM, Konopka JB. Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 2003;71:4045–4051. doi: 10.1128/IAI.71.7.4045-4051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenda AJ, Konopka JB. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell. 2002;13:2732–2746. doi: 10.1091/mbc.E02-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Drubin DG. Clathrin-mediated endocytosis in budding yeast. Trends Cell Biol. 2012;22:1–13. doi: 10.1016/j.tcb.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M, Zurek N, Hoenger A, Voeltz GK. A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. J. Cell Biol. 2011;193:333–346. doi: 10.1083/jcb.201011039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, Chao JT, Loewen CJ. Barriers to uniformity within the endoplasmic reticulum. Curr. Opin. Cell Biol. 2014;29:31–38. doi: 10.1016/j.ceb.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Young ME, Karpova TS, Brugger B, Moschenross DM, Wang GK, Schneiter R, Wieland FT, Cooper JA. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol. Cell. Biol. 2002;22:927–934. doi: 10.1128/MCB.22.3.927-934.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, Hirozane-Kishikawa T, Gebreab F, Li N, Simonis N, Hao T, Rual JF, Dricot A, Vazquez A, Murray RR, Simon C, Tardivo L, Tam S, Svrzikapa N, Fan C, de Smet AS, Motyl A, Hudson ME, Park J, Xin X, Cusick ME, Moore T, Boone C, Snyder M, Roth FP, Barabasi AL, Tavernier J, Hill DE, Vidal M. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Wang YM, Wang Y. Cdc28-Cln3 phosphorylation of Sla1 regulates actin patch dynamics in different modes of fungal growth. Mol. Biol. Cell. 2012;23:3485–3497. doi: 10.1091/mbc.E12-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yu Q, Jia C, Wang Y, Xiao C, Dong Y, Xu N, Wang L, Li M. The actin-related protein Sac1 is required for morphogenesis and cell wall integrity in Candida albicans. Fungal Genet. Biol. 2015;81:261–270. doi: 10.1016/j.fgb.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschutz JH, Guo W. Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lester RL, Dickson RC. Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1p and downstream signaling pathways Pkc1p and Ypk1p. J. Biol. Chem. 2004;279:22030–22038. doi: 10.1074/jbc.M400299200. [DOI] [PubMed] [Google Scholar]
- Zhao H, Michelot A, Koskela EV, Tkach V, Stamou D, Drubin DG, Lappalainen P. Membrane-sculpting BAR domains generate stable lipid microdomains. Cell Rep. 2013;4:1213–1223. doi: 10.1016/j.celrep.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XD, Lee RT, Wang YM, Lin QS, Wang Y. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 2007;26:3760–3769. doi: 10.1038/sj.emboj.7601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J, McLaughlin S. Membrane curvature: How BAR domains bend bilayers. Curr. Biol. 2004;14:R250–R252. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- Ziolkowska NE, Christiano R, Walther TC. Organized living: formation mechanisms and functions of plasma membrane domains in yeast. Trends in cell biology. 2012;22:151–158. doi: 10.1016/j.tcb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Ziolkowska NE, Karotki L, Rehman M, Huiskonen JT, Walther TC. Eisosome-driven plasma membrane organization is mediated by BAR domains. Nat. Struct. Mol. Biol. 2011;18:854–856. doi: 10.1038/nsmb.2080. [DOI] [PubMed] [Google Scholar]