Abstract
The human poliovirus receptor (PVR) is a cell surface protein with a multitude of functions in human biology. PVR was initially identified as the receptor for the human poliovirus and recent discoveries have given a greater insight into both its morphology and its function. Alternative splicing of the PVR gene results in a total of 4 alternatively spliced isoforms. Two of these isoforms lack a complete transmembrane domain and are considered soluble and block viral infection; the remaining two transmembrane isoforms differ only at their extreme C-terminal domains resulting in differential localization in epithelia and polarity of viral infection. In addition to its role as a receptor for the human poliovirus, several native biological functions have also been uncovered. PVR is an important cell adhesion protein and is involved in the transendothelial migration of leukocytes. Through its interactions with CD226 and TIGIT, transmembrane proteins found on leukocytes, PVR is a key regulator of the cell-mediated immune response. As PVR is differentially regulated in a broad spectrum of cancers, it has a strong potential for clinical use as a biomarker. PVR is also a possible target for novel cancer therapies. Utilizing its natural tropism for PVR, a genetically modified form of the live attenuated poliovirus vaccine is currently being tested for its ability to locate and destroy certain tumors. These recent studies emphasize the importance of PVR in human biology and demonstrate its utility beyond being a viral receptor protein.
Keywords: Human poliovirus receptor (PVR), CD155, poliovirus, immunomodulation
1. Introduction
The objective of this review is to summarize both historical and contemporary research on poliovirus and its receptor, PVR. The human poliovirus receptor (PVR) has a plethora of names due to disagreements in naming conventions between different fields of research and multiple independent discoveries of both the protein and its corresponding gene. Names include Cluster of Differentiation 155 (CD155), Poliovirus Sensitivity gene (PVS), Herpesvirus entry mediator D (HVED), Nectin-like molecule 5 (NECL5 or Necl-5), and Tumor-Associated Glycoprotein E4 (TAGE4), however, the human poliovirus receptor (PVR) remains dominant in the literature and will be used throughout the review (Baury et al., 2001; Masson et al., 2001; Nixdorf et al., 1999; Siddique et al., 1988; Takai et al., 2008). PVR was found to be an integral membrane protein at a period when the polio vaccine was still in its infancy and initial research focused on its role in poliovirus infection. (Holland and Mc, 1961). However, as poliomyelitis gradually transitioned from global pandemic to the verge of eradication, interest in human poliovirus and its eponymous receptor protein waned. The actual identity of the PVR protein was elucidated in 1989 by Mendelsohn et al and enormous strides have since been made concerning its many native functions (Mendelsohn et al., 1989). Recent research has demonstrated PVR to be broadly relevant to cell adhesion and migration, adaptive immunity, and cancer.
1.1 Poliomyelitis
Poliomyelitis is a devastating neurologic disease that has greatly impacted humanity for millennia (Falconer and Bollenbach, 2000; Paul, 1971). It is propagated by the human poliovirus, a positive sense single stranded RNA virus (Ryan and Ray, 2014). Because this virus is transmitted primarily by the fecal-oral route, it has historically been widespread in regions with high population densities and subpar sanitation systems (Kew et al., 2005). Poliovirus infection progresses to poliomyelitis when the virus invades the central nervous system. By attacking the motor functions of the spinal cord, the disease begins weakening muscle function, which can lead to widespread muscle paralysis and even death, usually due to loss of respiratory muscle function. Often this paralysis is irreversible, permanently disabling survivors of poliomyelitis (Mueller et al., 2005). Since major vaccination campaigns began in the 1950’s, the incidence rate of poliomyelitis has dramatically decreased. In 2015, there were fewer than 100 reported cases of poliomyelitis with only two countries, Afghanistan and Pakistan, reporting endemic transmission (Cochi et al., 2016). Although political instability has limited full vaccination efforts, it is a strong possibility that wild-type poliovirus transmission could be eradicated within the next decade (Cochi et al., 2016; Kennedy et al., 2015).
1.2. PVR: an overview
While poliomyelitis is no longer a highly prevalent medical condition, there has been a substantial volume of research published on the poliovirus in the past 20 years. Particular interest has been given to the cell surface protein that functions as the primary receptor for human poliovirus. PVR is a cell adhesion protein that facilitates the binding and entry of poliovirus into susceptible cells (Koike et al., 1991; Oda et al., 2004; Strauss et al., 2015). Like many cell surface proteins, and viral receptors such as the Coxsackievirus and Adenovirus Receptor (CAR) and CD46, PVR undergoes alternative splicing, generating 4 unique splice forms (Fig. 1, Table 1) (Excoffon et al., 2014; Koike et al., 1990). Two of these splice forms lack a complete transmembrane domain, rendering them as secreted or soluble isoforms (Baury et al., 2003). The other two splice forms have a complete transmembrane domain and are often referenced as the transmembrane isoforms (Koike et al., 1990). Although PVR was originally described as a viral receptor, recent literature has focused primarily on its endogenous functions in host biology. PVR has been shown to function as a cell adhesion protein as well as a facilitator of transendothelial migration (TEM) (Oda et al., 2004; Sullivan et al., 2013). PVR also has a vital role in regulating the cell mediated response of the immune system (Stanietsky et al., 2009; Tahara-Hanaoka et al., 2005). Finally, PVR is often differentially regulated in neoplastic and cancerous cells (Brown et al., 2014; Gong et al., 2014). Studies have demonstrated the efficacy of using a genetically modified strain of the human poliovirus to target PVR positive cancerous cells (Brown et al., 2014; Brown and Gromeier, 2015; Dobrikova et al., 2008; Gromeier et al., 2000). As many of PVR’s newly discovered functions have direct clinical applications, it is critical that we have a deeper understanding of PVR and its unique role in human biology.
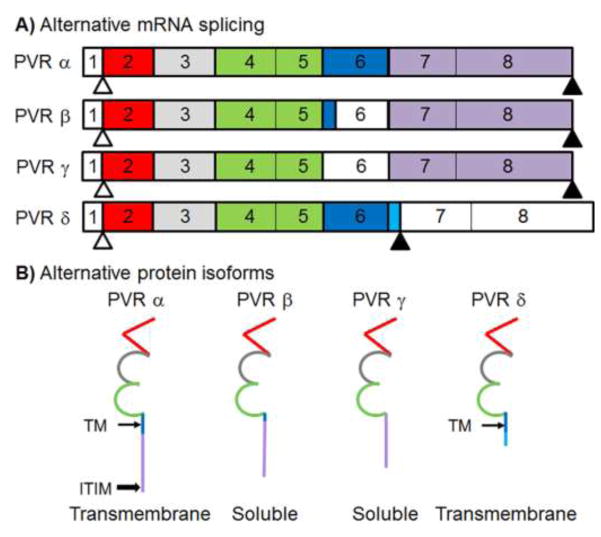
Fig 1. Schematic of exon map for PVR A) RNA spliceforms and B) protein isoforms.
Unshaded exons are not expressed in the protein. Exons are color coded to match the portions of the protein that they encode: red = Ig-like domain 1, grey = Ig-like domain 2, green = Ig-like domain 3, dark blue = transmembrane domain (thin arrow/TM), light blue = unique sequence in δ isoform, and gold = C-terminal domain. Start codons appear as white triangles and stop codons appear as shaded triangles. Compared to the canonical isoform PVRα, soluble isoforms PVRβ and γ contain splicing events in exon 6 which result in partial (β) or complete (γ) loss of exon 6. There is an alternative splicing event between transmembrane isoforms PVRα and PVRδ in which an additional eight residues and a stop codon are incorporated at the end of exon 6, resulting in exons 7 and 8 not being translated in PVRδ. The immunoreceptor tyrosine-based inhibitory motif (ITIM) of PVRα is indicated (block arrow).
Table 1.
Protein isoforms of PVR
| Isoform | Exons encoding protein sequence (Baury et al., 2003) | Soluble or Transmembrane (Baury et al., 2003) | Location in polarized cells (Ohka et al., 2001) | Interaction with Poliovirus (Baury et al., 2003; Koike et al., 1991) |
|---|---|---|---|---|
| PVRα | 1–8 | Transmembrane | Normally basolateral, tissue dependent | Binds to poliovirus, promotes viral entry |
| PVRβ | 1–5, partial 6, 7–8 | Soluble | Secreted | Binds to poliovirus, decreases viral entry |
| PVRγ | 1–5, 7–8 | Soluble | secreted | Binds to poliovirus, decreases viral entry |
| PVRδ | 1–6, alternative C-terminus | Transmembrane | Non-specific localization, found at both apical and basolateral surface | Binds to poliovirus, promotes viral entry |
2. Morphology and Splice form Diversity of PVR
PVR is a transmembrane glycoprotein in the immunoglobulin superfamily. Located on human chromosome 19 (NC_000019.10), the PVR gene is transcribed into a 20kb mRNA sequence composed of 8 different exons (Koike et al., 1990; Mendelsohn et al., 1989; Siddique et al., 1986; Speir et al., 2016) (Fig. 1). Exon 1 codes for the 5′ UTR and a signal peptide domain that functions as a leader sequence. Exon 2 is translated into the first of three immunoglobulin-like domains. The first immunoglobulin-like domain is a V domain while the second and third immunoglobulin-like domains are C2 domains, encoded by exon 3 or exons 4 and exon 5, respectively (Baury et al., 2003; Koike et al., 1990; Koike et al., 1991). Exon 6 and exon 7 become the transmembrane domain and the cytoplasmic domain, respectively. Finally exon 8 is translated into the C-terminus region and the 3′UTR. In total, PVR is 417 amino acids when all 8 exons are translated in full.
2.1 Soluble PVR
PVR expresses as two soluble isoforms, PVRβ and PVRγ, both of which lack all or part of the transmembrane region encoded by exon 6, causing them to be secreted from cells. Exons 1–5 as well as 7–8 on both soluble isoforms are identical to the canonical PVRα. PVRβ, the longer and more common of the two soluble splice forms, contains a small, truncated, fragment of the first part of exon 6; PVRγ lacks exon 6 in its entirety (Baury et al., 2003). Soluble and transmembrane isoforms of PVR can be found in tissues that are susceptible to poliovirus infection, such as the organs of the gastrointestinal tract and nervous tissue, as well as in tissues that are not, including the kidney, lung, liver, and testes. Soluble PVR (sPVR) isoforms can be found in a variety of bodily fluids, including: blood serum, cerebrospinal fluid, and urine (Baury et al., 2003; Iguchi-Manaka et al., 2016). As the extracellular domains of the sPVR isoforms are identical to the extracellular domain of transmembrane PVR, they can compete with transmembrane PVR for the canyon-like receptor binding site of poliovirus (Baury et al., 2003). Therefore when sPVR is overexpressed in poliovirus susceptible HeLa cells, it significantly reduces viral entry and viral infectivity (Baury et al., 2003).
Recent studies have established a relationship between sPVR expression and cancer progression. In animals, fibrosarcoma cells transduced with the ECD of mouse PVR were implanted into Balb/c mice, and the amount of sPVR produced by resulting tumors had a strong positive correlation with tumor size (Iguchi-Manaka et al., 2016). In humans, sPVR is found in higher serum concentrations compared to healthy donors across a broad spectrum of cancer patients, including lung, gastrointestinal, breast, ovarian, and colorectal cancers (Iguchi-Manaka et al., 2016; Masson et al., 2001). Interestingly, in later stage cancers (stage 3 and 4), the expression of sPVR is demonstrably higher than in early stage cancers (stage 1 and stage 2) (Iguchi-Manaka et al., 2016). Thus, sPVR is a potential biomarker for monitoring the progression of colorectal and gastric cancers, two forms of cancer that are currently difficult to diagnose and treat. As the transmembrane isoforms of PVR are known targets for the activation of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs), it is possible that overproduction of sPVR isoforms could be a cancer-specific defense mechanism against the cell mediated immune response (Lozano et al., 2012; Paschen et al., 2009). By masking the signaling effect of transmembrane isoforms of PVR, sPVR overexpression could facilitate the evasion of a cell mediated immune response, allowing the cancer to progress to later stages.
2.2. PVRα and PVRδ: transmembrane isoforms of PVR
In addition to two soluble isoforms, PVR contains two transmembrane isoforms: PVRα and PVRδ (Table 1). Both contain complete and identical copies of the first 6 exons. PVRδ utilizes a cryptic splice site within exon 6, which incorporates additional nucleotides at the end of exon 6 and introduces a stop codon (Mendelsohn et al., 1989). This alternative splicing leads to two separate transmembrane isoforms in which the last 32 amino acids encoded by exons 7–8 in PVRα are replaced by 8 unique amino acids in PVRδ that are encoded by additional nucleotides at the end of exon 6 (Oda et al., 2004; Speir et al., 2016). These last few amino acids interact differentially with key regulatory proteins, facilitating distinct localizations in polarized cells. In PVRα, these last few amino acids encode an immunoreceptor tyrosine-based inhibitory motif (ITIM). When this ITIM motif interacts with μ1B, a clathrin adaptor complex subunit protein, PVRα localizes to the basolateral surface (Ohka et al., 2001). In cell lines where μ1B is expressed, PVRα localizes to the basolateral surface; in cells that lack μ1B expression, i.e. LLCPK1 cells, PVRα localizes at both the basolateral and apical surfaces (Ohka et al., 2001). As PVRδ lacks the critical ITIM motif on its C-terminal domain, it does not have a regulatory interaction with μ1B and localizes to both the basolateral and apical surface regardless of the presence or absence of μ1B (Ohka et al., 2001).
The presence of different C-terminal sequences promoting localization of different PVR transmembrane isoforms is analogous to the differential localization observed between Coxsackie and adenovirus receptor (CAR) isoforms. The 8 exon isoform of CAR (CAREx8) localizes to the apical surface, whereas the 7 exon isoform, (CAREx7) localizes to the basolateral surface in polarized epithelial cells. This is due to the presence of different PDZ binding motif sequences in the C-termini of each isoform that facilitate differential interactions with PDZ-domain containing scaffolding proteins (Excoffon et al., 2010; Excoffon et al., 2004; Kolawole et al., 2012; Yan et al., 2015).
3. PVR as a Viral Receptor
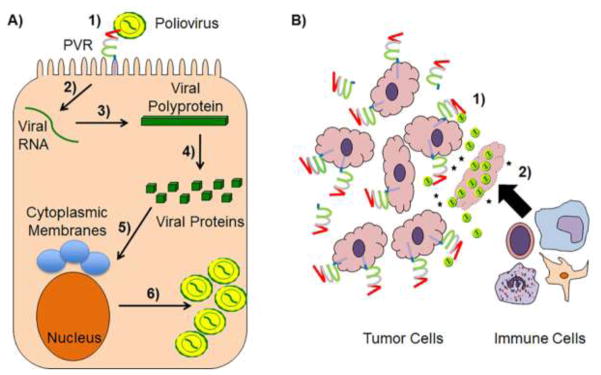
The extracellular domain of PVR binds to the poliovirus leading to viral entry and infection. As poliovirus depends on the fecal-oral route of transmission, the virus must interact with the complex microbiome of the gastrointestinal tract (Kuss et al., 2011). Poliovirus binds to bacterial lipopolysaccharide (LPS) in the gut. The interaction between poliovirus and LPS promotes virion stability and cell attachment by preventing premature RNA release and enhancing the binding affinity between poliovirus and PVR (Robinson et al., 2014). Thus, the more LPS producing bacteria present in the GI tract, the more susceptible a host is to poliovirus infection (Kuss et al., 2011). Poliovirus initially exists in a collapsed form (160S) in the extracellular fluid (De Sena and Mandel, 1977; Tsang et al., 2001). This collapsed form of the virus depends on the presence of a hydrophobic protein core in the VP1 subunit called the pocket factor (Filman et al., 1989). Polioviruses have an attachment site at a depression on their surface, known as a canyon-like receptor (Tuthill et al., 2010). While previous studies have shown PVR binding near the five-fold axis of the canyon-like receptor, more recent studies have suggested that the initial binding of the poliovirus with PVR occurs at the quasi threefold axis close to the location of the pocket factor (Koike et al., 1991; Strauss et al., 2015). Of the 3 extracellular immunoglobulin like domains of PVR, the first V domain is required for the initial binding interaction and is essential for poliovirus entry (Koike et al., 1991). Upon binding to PVR, the hydrophobic pocket factor is lost and poliovirus takes on an extended 135S conformation (Butan et al., 2014). It is likely at this stage that the PVR binding site shifts to the five-fold axis of the poliovirus. Once the poliovirus has converted to the expanded 135S form, pores open in the 2 fold and 3 fold axes of the virus, which form openings in the host cell (Strauss et al., 2015). The RNA genome of the virus passes through these pores into endocytic vesicles. Once the RNA genome is inside the host cell, it can begin utilizing the host cell machinery for viral replication (Fig 2A) (Leveque and Semler, 2015).
Fig 2. Schematic of the poliovirus life cycle and oncolytic viral therapy.
A) 1) Poliovirus binds to PVR at the cell surface, which may be facilitated by LPS, to enter the target cell. 2) The poliovirus positive single-stranded RNA genome is translated by host cellular machinery to produce a large polyprotein. 3) The polyprotein is then autocleaved into distinct viral proteins that 4) induce cytoplasmic relocalization of nuclear proteins and the formation of membranous structures with distinct lipid and protein compositions. 6) These events then allow the viral RNA-dependent RNA polymerase to replicate its genome and progeny virions to form. B) Several cancerous cell types upregulate transmembrane and soluble PVR isoforms making cells more susceptible to oncolytic poliovirus. This method combats tumors by 1) direct lysis of neoplastic cells and 2) the release immunostimulatory molecules, such as cytokines and cancer antigens, (black stars) that recruit immune effector cells into the tumor microenvironment, effectively training the immune system to destroy the tumor.
4. PVR as a cell adhesion protein and regulator of transendothelial migration (TEM)
Endogenous functions of PVR include the regulation of cell adhesion and cell motility (Sloan et al., 2004). PVR contains an ITIM domain that is regulated by Src kinase phosphorylation. Upon phosphorylation of this ITIM domain, PVR can freely interact with Src homology region 2 domain-containing phosphatase (SHP-2), a cell signaling protein involved in regulating cell migration (Billadeau and Leibson, 2002; Freeman et al., 1992). The interaction with SHP-2 has a plethora of physiological effects on cell adhesion and cell motility. SHP-2 binding to phosphorylated PVR inhibits cell matrix adhesion, suppressing PVR+ cells from binding to fibronectin coated plates. PVR, however, has a high natural affinity for vitronectin. This interaction is powerful enough to allow PVR+ cells to bind to vitronectin containing plates even with cell matrix adhesion inhibited (Lange et al., 2001; Oda et al., 2004). In the absence of vitronectin, the interaction between PVR and SHP-2 reduces cell-cell adhesion and increases the mobility of activated cells (Oda et al., 2004).
In addition to its involvement in cell matrix adhesion, PVR has a key role in facilitating transendothelial migration (TEM). TEM is the process by which leukocytes migrate from the blood stream through cell-cell junctions towards danger signals at the epithelial surface (Muller, 2009). This process depends on both homophilic and heterophilic protein interactions between proteins found on either leukocytes, endothelial cells, or both cell types. For example, platelet endothelial cell adhesion molecule (PECAM-1 or CD31) on endothelial cells interacts with PECAM-1 on leukocytes in a homophilic interaction during TEM (Muller et al., 1993). Likewise, CD99 is involved in a similar homophilic binding process to promote successful TEM (Schenkel et al., 2002). By contrast, PVR is involved in a heterophilic interaction with DNAX Accessory Molecule-1 (DNAM-1 or CD226) in which PVR on endothelial cells binds with CD226 on leukocytes (Reymond et al., 2004). Interestingly, PECAM-1, CD99, and PVR all localize to the lateral border recycling compartment during TEM (Mamdouh et al., 2008; Sullivan et al., 2013). This lateral border recycling compartment regulates the expression of these three cell adhesion proteins on endothelial cells during TEM. PECAM-1 is expressed first for homophilic adhesion, followed by the heterophilic interaction between PVR and CD226, and finally CD99 is expressed for a homophilic adhesion interaction before TEM is completed (Sullivan et al., 2013). While both PECAM-1 and CD99 expression are important for TEM, the knockout of the interaction between PVR and CD226 completely abrogates TEM (Sullivan et al., 2013).
5. PVR as a regulator of immune function
PVR exerts a major role in regulating the cell-mediated immune response by interacting with CD226 and T cell immunoreceptor with IG and ITIM domains (TIGIT). CD226 is a cytotoxicity receptor found on a variety of cytotoxic T-lymphocytes (CTLs), such as natural killer (NK) cells, CD8+ T cells, and cytokine induced killer (CIK) cells (Shibuya et al., 1996). CD226 induces the release of cytokines that ultimately lead to the apoptosis of target cells (Shibuya et al., 1996). PVR expression is enhanced by the DNA damage response, a process that is upregulated during viral infection and tumorigenesis (Ardolino et al., 2011). Because it is associated with damaged cells, PVR is often used as a natural marker for inducing apoptosis (Cerbonil et al., 2014). The cell-mediated immune response is activated when CD226 binds with PVR expressed in these defective cells, and ultimately results in CTL mediated destruction of the infected cell (de Andrade et al., 2014; Tahara-Hanaoka et al., 2005; Tahara-Hanaoka et al., 2004). PVR also interacts with TIGIT, a receptor protein found on CTLs that inhibits the cell mediated response (Joller et al., 2011; Li et al., 2014). TIGIT activation reduces the proliferation of CTLs and inhibits the production and release of cytokines, thereby diminishing the effectiveness of the cell mediated immune response (Zhang et al., 2016). By interacting with both CD226 and TIGIT, PVR can either activate or inhibit the cell mediated response. Thus, PVR expression impacts the immune response in a complex manner dependent on tissue tropism and disease pathology.
In HIV-1 infected cells, two viral specific proteins, Vpu and Nef, reduce the expression of PVR (Bolduan et al., 2014; Matusali et al., 2012). The reduction of PVR expression facilitates HIV-1 evasion of the immune response by lessening the probability that CD226+ CTLs will interact with PVR on infected cells. In graft versus host disease (GVHD), reducing the expression of CD226 on CTLs inhibits the host immune response, leading to better outcomes in murine models (Nabekura et al., 2010). However, the converse is not true. Upon inhibition of PVR, a natural ligand for CD226, GVHD is aggravated, hastening host mortality (Seth et al., 2011). Because PVR expression is associated with a reduced immune response in GVHD, it is likely that PVR preferentially interacts with TIGIT in GVHD and lessens the immune response. As nectin-2 (CD112) is also a ligand for CD226, it is possible that in some tissues PVR enhances the cell-mediated immune response, while in other tissues CD112 upregulates cytokine release (Tahara-Hanaoka et al., 2004).
6. PVR and Cancer
Recent studies have shown a strong correlation between increased PVR expression and cancer, suggesting that PVR may be involved in cancer pathology. In lung, colorectal, gastric, breast, ovarian, melanoma, and glioblastoma cancers, PVR expression is upregulated (Casado et al., 2009; Chan et al., 2012; Gromeier et al., 2000; Iguchi-Manaka et al., 2016; Masson et al., 2001; Nakai et al., 2010; Ochiai et al., 2004). In colorectal cancer, sPVR expression is directly correlated to tumor size and cancer progression (Iguchi-Manaka et al., 2016). The increased expression of PVR across a broad spectrum of cancers suggests that it plays a role in the pathology of cancer. It is possible that cancerous cells increase PVR expression to manipulate its role in CTL activation or inhibition, cell adhesion, or in other immune functions. Since PVR is upregulated in a plethora of cancers, it is likely that it could have clinical significance as a biomarker for detecting and monitoring cancer progression (Iguchi-Manaka et al., 2016).
Due to its interaction with two key regulators of the cell-mediated immune response, CD226 and TIGIT, PVR is intricately involved in the response of the immune system to cancer. In many cancer cells where PVR is overexpressed, it functions as a ligand for CD226, activating the immune response against cancer (Altomonte et al., 2009; Iguchi-Manaka et al., 2008; Tahara-Hanaoka et al., 2005). By recruiting and activating CD226+ NK cells and CD8+ cells, PVR can be used to train the immune system to recognize neoplastic cells and begin systematically destroying the tumor (Ishiyama et al., 2006). Hepatocellular carcinoma (HCC) cells manage to avoid this immune response by downregulating PVR expression (Erickson et al., 2006; Qu et al., 2015). Utilizing the unfolded protein response (UPR), HCC reduces PVR expression, lessening the probability that NK cells will detect and destroy the cancerous cells (Gong et al., 2014). Alternatively, melanoma cells manipulate the expression of TIGIT on CTLs to convert PVR into an immunosuppressive agent (Inozume et al., 2016; Pauken and Wherry, 2014; Stanietsky et al., 2009). In the presence of IFNγ+ melanoma tumors, CTL cells experience CD226 suppression and TIGIT activation (Lozano et al., 2012). Increased TIGIT expression on CTLs combined with enhanced PVR expression on melanoma cells leads to the suppression of the cell mediated immune response, thus facilitating the progression of the melanoma tumor (Chauvin et al., 2015; Inozume et al., 2016).
Understanding the relationship between PVR expression and cancer has led to a novel method for targeting cancer cells using oncolytic polioviruses. As many cancerous cells overexpress the poliovirus receptor (PVR), it is possible to use the preexisting tropism of an attenuated poliovirus to target cancer cells for destruction (Brown et al., 2014). Oncolytic viruses cannot infect all of the cells in a tumor. However, by initiating a local infection, oncolytic viruses can recruit leukocytes to a tumor site and thereby train the immune system to attack and destroy cancerous cells (Prestwich et al., 2009) (Fig. 2B). Preclinical studies have been conducted using a replication deficient form of the poliovirus modeled after the live attenuated form of the poliovirus vaccine (Campbell et al., 2005; Lashkevich, 2013). This strain, known as the attenuated poliovirus (Sabin)-rhinovirus IRES PV open reading frame (PVSRIPO) strain is a genetically engineered viral vector created by replacing the internal ribosomal entry site (IRES) of the Sabin strain of the poliovirus with the IRES from the human rhinovirus type 2 (HRV2) (Brown and Gromeier, 2015; Dobrikova et al., 2008). By replacing the IRES site, PVSRIPO is replication deficient in non-cancerous neuronal cells. While the original objective in developing an attenuated poliovirus may have been to reduce viral neuropathogenicity in order to make a safer vaccine strain, PVSRIPO demonstrated early promise in targeting glioblastoma cells in an in vitro setting (Brown et al., 2014; Gromeier et al., 1996; Gromeier et al., 2000). Phase I clinical trials (NCT01491893) using PVSRIPO against recurrent glioblastoma have thus far shown promising results. As of May 2016, PVSRIPO has been elevated to the Breakthrough Therapy designation at the FDA and researchers are hopeful that efficacy may be found against a broad range of cancers. Interestingly, as wild-type poliovirus is on the verge of being eradicated, a genetically modified form of the poliovirus vaccine has the potential to become a next generation cancer treatment.
7. Conclusion
PVR is an important cell surface protein, involved in a multitude of biological processes. This diversity of physiological functions is partially due to its 4 unique isoforms. Its soluble isoforms can be secreted from cells and can travel through interstitial fluid; its transmembrane forms are critical for viral attachment and cell signaling. Additionally, its interactions with CD226 and TIGIT have widespread implications. From transendothelial migration to the cell mediated immune response, PVR’s complex interaction with these two proteins is critical to elucidating PVR’s role in a broad spectrum of cellular mechanisms. Special interest has been given to PVR in order to unravel its relationship with cancer. As PVR is often upregulated in cancerous cells, it has strong potential for use as a biomarker for detecting and monitoring the progression of cancer. Contemporary research has even demonstrated that manipulating the tropism of the live attenuated poliovirus vaccine to target and destroy cancer cells may be an effective next generation treatment against many forms of cancer. Over the past several decades, research on PVR has produced important findings in many diverse fields. As it is likely that PVR is involved in processes outside of the scope of the current literature, time will continue to reveal the functions of PVR beyond its role as a viral receptor.
Highlights.
Human PVR (CD155) is both a viral receptor and immunomodulatory protein
PVR function depends on expression of alternative protein isoforms
Poliovirus is a candidate oncolytic virus in clinical trials
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the NIH Award 2 R15 AI090625-02A1, a Wright State University Research Initiation Award, and a Biology Award for Research Excellence (JRB). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Wright State University.
Footnotes
Ethical approval:
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest:
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altomonte J, Wu L, Meseck M, Chen L, Ebert O, Garcia-Sastre A, Fallon J, Mandeli J, Woo SLC. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer gene therapy. 2009;16(3):266–278. doi: 10.1038/cgt.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, Santoni A. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood. 2011;117(18):4778–4786. doi: 10.1182/blood-2010-08-300954. [DOI] [PubMed] [Google Scholar]
- Baury B, Geraghty RJ, Masson D, Lustenberger P, Spear PG, Denis MG. Organization of the rat Tage4 gene and herpesvirus entry activity of the encoded protein. Gene. 2001;265(1–2):185–194. doi: 10.1016/s0378-1119(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Baury B, Masson D, McDermott BM, Jr, Jarry A, Blottiere HM, Blanchardie P, Laboisse CL, Lustenberger P, Racaniello VR, Denis MG. Identification of secreted CD155 isoforms. Biochem Biophys Res Commun. 2003;309(1):175–182. doi: 10.1016/s0006-291x(03)01560-2. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Leibson PJ. ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Invest. 2002;109(2):161–168. doi: 10.1172/JCI14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduan S, Reif T, Schindler M, Schubert U. HIV-1 Vpu mediated downregulation of CD155 requires alanine residues 10, 14 and 18 of the transmembrane domain. Virology. 2014;464:375–384. doi: 10.1016/j.virol.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Dobrikova EY, Dobrikov MI, Walton RW, Gemberling SL, Nair SK, Desjardins A, Sampson JH, Friedman HS, Friedman AH, Tyler DS, Bigner DD, Gromeier M. Oncolytic polio virotherapy of cancer. Cancer. 2014;120(21):3277–3286. doi: 10.1002/cncr.28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Gromeier M. Cytotoxic and immunogenic mechanisms of recombinant oncolytic poliovirus. Curr Opin Virol. 2015;13:81–85. doi: 10.1016/j.coviro.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butan C, Filman DJ, Hogle JM. Cryo-Electron Microscopy Reconstruction Shows Poliovirus 135S Particles Poised for Membrane Interaction and RNA Release. Journal of virology. 2014;88(3):1758–1770. doi: 10.1128/JVI.01949-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SA, Lin J, Dobrikova EY, Gromeier M. Genetic determinants of cell type-specific poliovirus propagation in HEK 293 cells. Journal of virology. 2005;79(10):6281–6290. doi: 10.1128/JVI.79.10.6281-6290.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado JG, Pawelec G, Morgado S, Sanchez-Correa B, Delgado E, Gayoso I, Duran E, Solana R, Tarazona R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immun. 2009;58(9):1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbonil C, Fionda C, Soriani A, Zingoni A, Doria M, Cippitelli M, Santoni A. The DNA damage response: a common pathway in the regulation of NKG2D and DNAM-1 ligand expression in normal infected, and cancer cells. Front Immunol. 2014:4. doi: 10.3389/fimmu.2013.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CJ, Andrews DM, Smyth MJ. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol. 2012;24(2):246–251. doi: 10.1016/j.coi.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Chauvin JM, Pagliano O, Fourcade J, Sun ZJ, Wang H, Sander C, Kirkwood JM, Chen THT, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125(5):2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochi SL, Hegg L, Kaur A, Pandak C, Jafari H. The Global Polio Eradication Initiative: Progress, Lessons Learned, And Polio Legacy Transition Planning. Health Aff (Millwood) 2016;35(2):277–283. doi: 10.1377/hlthaff.2015.1104. [DOI] [PubMed] [Google Scholar]
- de Andrade LF, Smyth MJ, Martinet L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol Cell Biol. 2014;92(3):237–244. doi: 10.1038/icb.2013.95. [DOI] [PubMed] [Google Scholar]
- De Sena J, Mandel B. Studies on the in vitro uncoating of poliovirus. II. Characteristics of the membrane-modified particle. Virology. 1977;78(2):554–566. doi: 10.1016/0042-6822(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Dobrikova EY, Broadt T, Poiley-Nelson J, Yang XY, Soman G, Giardina S, Harris R, Gromeier M. Recombinant Oncolytic Poliovirus Eliminates Glioma In Vivo Without Genetic Adaptation to a Pathogenic Phenotype. Molecular Therapy. 2008;16(11):1865–1872. doi: 10.1038/mt.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson BM, Thompson NL, Hixson DC. Tightly regulated induction of the adhesion molecule necl-5/CD155 during rat liver regeneration and acute liver injury. Hepatology. 2006;43(2):325–334. doi: 10.1002/hep.21021. [DOI] [PubMed] [Google Scholar]
- Excoffon KJ, Bowers JR, Sharma P. Alternative splicing of viral receptors: A review of the diverse morphologies and physiologies of adenoviral receptors. Recent research developments in virology. 2014;9:1–24. [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Gansemer ND, Mobily ME, Karp PH, Parekh KR, Zabner J. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PloS one. 2010;5(3):e9909. doi: 10.1371/journal.pone.0009909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J Cell Sci. 2004;117(Pt 19):4401–4409. doi: 10.1242/jcs.01300. [DOI] [PubMed] [Google Scholar]
- Falconer M, Bollenbach E. Late functional loss in nonparalytic polio. Am J Phys Med Rehab. 2000;79(1):19–23. doi: 10.1097/00002060-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Filman DJ, Syed R, Chow M, Macadam AJ, Minor PD, Hogle JM. Structural Factors That Control Conformational Transitions and Serotype Specificity in Type-3 Poliovirus. Embo Journal. 1989;8(5):1567–1579. doi: 10.1002/j.1460-2075.1989.tb03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RM, Jr, Plutzky J, Neel BG. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci U S A. 1992;89(23):11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Fang L, Liu R, Wang Y, Xing J, Chen Y, Zhuang R, Zhang Y, Zhang C, Yang A, Zhang X, Jin B, Chen L. UPR decreases CD226 ligand CD155 expression and sensitivity to NK cell-mediated cytotoxicity in hepatoma cells. Eur J Immunol. 2014;44(12):3758–3767. doi: 10.1002/eji.201444574. [DOI] [PubMed] [Google Scholar]
- Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A. 1996;93(6):2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97(12):6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JJ, Mc LL. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, Yasui T, Kikutani H, Shibuya K, Shibuya A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205(13):2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi-Manaka A, Okumura G, Kojima H, Cho Y, Hirochika R, Bando H, Sato T, Yoshikawa H, Hara H, Shibuya A, Shibuya K. Increased Soluble CD155 in the Serum of Cancer Patients. PloS one. 2016;11(4):e0152982. doi: 10.1371/journal.pone.0152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma Cells Control Antimelanoma CTL Responses via Interaction between TIGIT and CD155 in the Effector Phase. J Invest Dermatol. 2016;136(1):255–263. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43(2):362–372. doi: 10.1002/hep.21035. [DOI] [PubMed] [Google Scholar]
- Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting Edge: TIGIT Has T Cell-Intrinsic Inhibitory Functions. J Immunol. 2011;186(3):1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J, Mckee M, King L. Islamist insurgency and the war against polio: a cross-national analysis of the political determinants of polio. Globalization Health. 2015:11. doi: 10.1186/s12992-015-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. The Poliovirus Receptor Protein Is Produced Both as Membrane-Bound and Secreted Forms. Embo Journal. 1990;9(10):3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Ise I, Nomoto A. Functional domains of the poliovirus receptor. Proc Natl Acad Sci U S A. 1991;88(10):4104–4108. doi: 10.1073/pnas.88.10.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolawole AO, Sharma P, Yan R, Lewis KJ, Xu Z, Hostetler HA, Ashbourne Excoffon KJ. The PDZ1 and PDZ3 domains of MAGI-1 regulate the eight-exon isoform of the coxsackievirus and adenovirus receptor. Journal of virology. 2012;86(17):9244–9254. doi: 10.1128/JVI.01138-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334(6053):249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R, Peng X, Wimmer E, Lipp M, Bernhardt G. The poliovirus receptor CD155 mediates cell-to-matrix contacts by specifically binding to vitronectin. Virology. 2001;285(2):218–227. doi: 10.1006/viro.2001.0943. [DOI] [PubMed] [Google Scholar]
- Lashkevich VA. History of development of the live poliomyelitis vaccine from Sabin attenuated strains in 1959 and idea of poliomyelitis eradication. Vopr Virusol. 2013;58(1):4–10. [PubMed] [Google Scholar]
- Leveque N, Semler BL. A 21st century perspective of poliovirus replication. PLoS pathogens. 2015;11(6):e1004825. doi: 10.1371/journal.ppat.1004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xia PY, Du Y, Liu SW, Huang GL, Chen J, Zhang HL, Hou N, Cheng X, Zhou LY, Li PF, Yang X, Fan ZS. T-cell Immunoglobulin and ITIM Domain (TIGIT) Receptor/Poliovirus Receptor (PVR) Ligand Engagement Suppresses Interferon-gamma Production of Natural Killer Cells via beta-Arrestin 2-mediated Negative Signaling. Journal of Biological Chemistry. 2014;289(25):17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 Axis Regulates Human T Cell Function. J Immunol. 2012;188(8):3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. Journal of Experimental Medicine. 2008;205(4):951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49(2):236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusali G, Potesta M, Santoni A, Cerboni C, Doria M. The Human Immunodeficiency Virus Type 1 Nef and Vpu Proteins Downregulate the Natural Killer Cell-Activating Ligand PVR. Journal of virology. 2012;86(8):4496–4504. doi: 10.1128/JVI.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Mueller S, Wimmer E, Cello J. Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus Res. 2005;111(2):175–193. doi: 10.1016/j.virusres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of Transendothelial Migration of Leukocytes. Circulation research. 2009;105(3):223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng XH, Phillips DM. Pecam-1 Is Required for Transendothelial Migration of Leukocytes. Journal of Experimental Medicine. 1993;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Shibuya K, Takenaka E, Kai H, Shibata K, Yamashita Y, Harada K, Tahara-Hanaoka S, Honda S, Shibuya A. Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. P Natl Acad Sci USA. 2010;107(43):18593–18598. doi: 10.1073/pnas.1005582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai R, Maniwa Y, Tanaka Y, Nishio W, Yoshimura M, Okita Y, Ohbayashi C, Satoh N, Ogita H, Takai Y, Hayashi Y. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010;101(5):1326–1330. doi: 10.1111/j.1349-7006.2010.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf R, Schmidt J, Karger A, Mettenleiter TC. Infection of Chinese hamster ovary cells by pseudorabies virus. Journal of virology. 1999;73(10):8019–8026. doi: 10.1128/jvi.73.10.8019-8026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H, Moore SA, Archer GE, Okamura T, Chewning TA, Marks JR, Sampson JH, Gromeier M. Treatment of intracerebral neoplasia and neoplastic meningitis with regional delivery of oncolytic recombinant poliovirus. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(14):4831–4838. doi: 10.1158/1078-0432.CCR-03-0694. [DOI] [PubMed] [Google Scholar]
- Oda T, Ohka S, Nomoto A. Ligand stimulation of CD155alpha inhibits cell adhesion and enhances cell migration in fibroblasts. Biochem Biophys Res Commun. 2004;319(4):1253–1264. doi: 10.1016/j.bbrc.2004.05.111. [DOI] [PubMed] [Google Scholar]
- Ohka S, Ohno H, Tohyama K, Nomoto A. Basolateral sorting of human poliovirus receptor alpha involves an interaction with the mu1B subunit of the clathrin adaptor complex in polarized epithelial cells. Biochem Biophys Res Commun. 2001;287(4):941–948. doi: 10.1006/bbrc.2001.5660. [DOI] [PubMed] [Google Scholar]
- Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, Steinle A, Schadendorf D, Ugurel S. Differential Clinical Significance of Individual NKG2D Ligands in Melanoma: Soluble ULBP2 as an Indicator of Poor Prognosis Superior to S100B. Clinical Cancer Research. 2009;15(16):5208–5215. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. TIGIT and CD226: Tipping the Balance between Costimulatory and Coinhibitory Molecules to Augment the Cancer Immunotherapy Toolkit. Cancer Cell. 2014;26(6):785–787. doi: 10.1016/j.ccell.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Paul JR. A history of poliomyelitis. Yale University Press; New Haven: 1971. p. xv.p. 486. Yale studies in the history of science and medicine. [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, Vile RG. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20(10):1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Huang X, Zhou X, Lu Z, Liu F, Shi Z, Lu L, Wu Y, Chen Y. Loss of CD155 expression predicts poor prognosis in hepatocellular carcinoma. Histopathology. 2015;66(5):706–714. doi: 10.1111/his.12584. [DOI] [PubMed] [Google Scholar]
- Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. Journal of Experimental Medicine. 2004;199(10):1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe. 2014;15(1):36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KJ, Ray CG. Sherris medical microbiology. 6. McGraw-Hill Education Medical; New York: 2014. [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3(2):143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- Seth S, Ravens I, Lee CW, Glage S, Bleich A, Forster R, Bernhardt G, Koenecke C. Absence of CD155 aggravates acute graft-versus-host disease. P Natl Acad Sci USA. 2011;108(10):E32–E33. doi: 10.1073/pnas.1017969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- Siddique T, Bartlett R, Mckinney R, Hung WY, Bruns G, Mohandas TK, Wilfert C, Roses A. Poliovirus Sensitivity (Pvs) Gene Is on the Short Arm of Chromosome-19. Neurology. 1986;36(4):137–137. doi: 10.1016/0888-7543(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Siddique T, McKinney R, Hung WY, Bartlett RJ, Bruns G, Mohandas TK, Ropers HH, Wilfert C, Roses AD. The poliovirus sensitivity (PVS) gene is on chromosome 19q12----q13.2. Genomics. 1988;3(2):156–160. doi: 10.1016/0888-7543(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag LL, Jay DG. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir ML, Zweig AS, Rosenbloom KR, Raney BJ, Paten B, Nejad P, Lee BT, Learned K, Karolchik D, Hinrichs AS, Heitner S, Harte RA, Haeussler M, Guruvadoo L, Fujita PA, Eisenhart C, Diekhans M, Clawson H, Casper J, Barber GP, Haussler D, Kuhn RM, Kent WJ. The UCSC Genome Browser database: 2016 update. Nucleic Acids Res. 2016;44(D1):D717–D725. doi: 10.1093/nar/gkv1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. P Natl Acad Sci USA. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M, Filman DJ, Belnap DM, Cheng NQ, Noel RT, Hogle JM. Nectin-Like Interactions between Poliovirus and Its Receptor Trigger Conformational Changes Associated with Cell Entry. Journal of virology. 2015;89(8):4143–4157. doi: 10.1128/JVI.03101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DP, Seidman MA, Muller WA. Poliovirus Receptor (CD155) Regulates a Step in Transendothelial Migration between PECAM and CD99. Am J Pathol. 2013;182(3):1031–1042. doi: 10.1016/j.ajpath.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Miyamoto A, Hara A, Honda S, Shibuya K, Shibuya A. Identification and characterization of murine DNAM-1 (CD226) and its poliovirus receptor family ligands. Biochem Biophys Res Commun. 2005;329(3):996–1000. doi: 10.1016/j.bbrc.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16(4):533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nature reviews Molecular cell biology. 2008;9(8):603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Tsang SK, McDermott BM, Racaniello VR, Hogle JM. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. Journal of virology. 2001;75(11):4984–4989. doi: 10.1128/JVI.75.11.4984-4989.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill TJ, Groppelli E, Hogle JM, Rowlands DJ. Picornaviruses. Curr Top Microbiol Immunol. 2010;343:43–89. doi: 10.1007/82_2010_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Sharma P, Kolawole AO, Martin SC, Readler JM, Kotha PL, Hostetler HA, Excoffon KJ. The PDZ3 domain of the cellular scaffolding protein MAGI-1 interacts with the Coxsackievirus and adenovirus receptor (CAR) The international journal of biochemistry & cell biology. 2015;61:29–34. doi: 10.1016/j.biocel.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BF, Zhao WN, Li HZ, Chen YY, Tian H, Li LT, Zhang LZ, Gao C, Zheng JN. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol Immun. 2016;65(3):305–314. doi: 10.1007/s00262-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]