Summary
Objective
To examine the effects of dietary weight loss, with and without exercise, on selected soluble biomarkers in overweight and obese older adults with symptomatic knee OA.
Design
Blood samples were analyzed from 429 participants in the IDEA trial randomized to either an 18 month exericse control group (E), weight loss diet (D), or D+E. C1M, C2M, C3M and CRPM biomarkers and IL-6 were quantitated using ELISAs. Radiographic progression was defined as a decrease in joint space width of ≥ 0.7mm. Statistical modeling of group means and associations used mixed models adjusted for visit, baseline BMI, gender, and baseline values of the outcome.
Results
Compared to the E control group, C1M was significantly lower in the D and D+E groups at both 6 and 18 months while C3M was significantly lower in D and D+E at 6 months and in D+E at 18 months. C2M did not change in any group. Using data from all groups, change in C1M (p<0.0001), C3M (p<0.0001), as well as CRPM (p=0.0004) from baseline to 18 months was positively associated with change in weight. No marker was associated with change in WOMAC pain or radiographic progression. C3M (p=0.008) and CRPM (p=0.028) were positively associated with change in WOMAC function. Change in IL-6 was positively associated with change in C1M, C3M, and CRPM.
Conclusion
Overweight and obese adults with knee OA who lost weight from diet and diet plus exercise reduced serum markers of interstitial matrix turnover and inflammation but not type II collagen degradation.
Keywords: Osteoarthritis, biomarkers, collagen, weight loss, exercise
Introduction
Exercise and weight loss are effective in reducing pain and improving function in overweight and obese adults with osteoarthritis (OA) of the knee1, 2. Based on evidence from a number of successful randomized clinical trials, guidelines for the management of knee OA, including those from the Osteoarthritis Research Society International (OARSI)3, the American College of Rheumatology4, as well as several other groups (reviewed in5), include exercise and weight loss as key non-pharmacologic recommendations. Although there is substantial evidence in support of exercise and weight loss for symptomatic improvement, it is not clear if these interventions affect joint tissue metabolism or alter structural progression..
A seminal trial in support of the combined approach of exercise and weight loss was the Intensive Diet and Exercise for Arthritis (IDEA) trial2. The IDEA trial demonstrated that, compared to an exercise control group, an 18 month intervention that combined dietary-induced weight loss with exercise resulted in significant improvements in pain and function, measured using the WOMAC instrument, as well as a significant reduction in plasma IL-6 as a marker of inflammation. The diet only group also exhibited a significant decrease in knee compressive forces. Despite these improvements, there were no significant differences in changes in knee joint structural outcomes, including radiographic joint space narrowing and, in a subset of participants, MRI measures6.
In addition to imaging, there is significant interest in developing biochemical markers that could be used to monitor the effects of an intervention on the joint tissues affected by OA, including the articular cartilage, bone, synovium, and other soft-tissue structures7. These include markers of type II collagen and aggrecan degradation and synthesis, as well as degradation of other extracellular matrix (ECM) proteins such as cartilage oligomeric protein (COMP)8 and type III collagen9. The degradation of ECM proteins in OA joints is primarily mediated by enzymes of the matrix metalloproteinase (MMP) family. MMP-mediated degradation occurs at specific sites within ECM proteins resulting in protein fragments that can be recognized by site-specific “neoepitope” antibodies10. To date, two of the most commonly studied ECM degradation markers have been type II collagen, which is predominately found in the articular cartilage, and type I collagen, found in bone and fibrous soft-tissues7, 8.
Although a number of biochemical markers of joint tissue turnover have been developed and tested, most studies have been limited to cross-sectional studies of people with and without OA or observational studies of OA progression11. The objectives of our study were to examine the change in levels of biochemical markers of collagen degradation and tissue inflammation in blood samples that had been obtained from participants during the IDEA trial and to determine the association of selected clinical outcomes, including radiographic progression, with the change in biomarker levels over the course of the interventions. For this study, which represents a secondary analysis of the IDEA trial, markers of MMP-mediated degradation of type I (C1M), type II (C2M), and type III (C3M) collagen were measured, as well as a marker of C-reactive protein (CRP) degradation (CRPM). These markers have been suggested to measure inflammation-mediated tissue destruction including that caused by synovitis12, 13.
Methods
Study Participants
Blood samples and radiographs used for our study were collected from participants in the IDEA trial (NCT00381290) which was an 18-month prospective, single-blind, randomized controlled trial that enrolled 454 overweight and obese (27.0 ≤ BMI ≤ 40.5 kg/m2), older (age ≥ 55 yrs) adults with knee pain and radiographic evidence of tibiofemoral osteoarthritis (Kellgren Lawrence grade = 2 or 3) with or without mild or moderate patellofemoral OA. Details of the inclusion and exclusion criteria and study design, as well as the main outcomes, have been previously published2, 14. Participants were allowed to use non-steroidal anti-inflammatory drugs (NSAIDs) or discontinue use during the course of the study in consultation with the physician managing their OA. Data on NSAID use was only available at baseline.
Participants were randomized to one of three 18-month interventions: intensive dietary weight loss-only; intensive dietary weight loss-plus-exercise; or exercise-only control. Blood samples were collected at baseline, 6 and 18 months and knee radiographs at baseline and 18 months. Blood was collected in the early morning after a 10-hour fast and processed to produce serum (used for the C1M, C2M, C3M, and CRPM assays) and plasma (used for the IL-6 assays), which were stored as aliquots at −80°C until used for analysis. The 6- and 18-month samples were collected at least 24 hours after the last acute bout of exercise training and sampling was postponed (1–2 weeks after recovery from symptoms) in the event of an acute respiratory, urinary tract, or other infection. Bilateral, posteroanterior, weight-bearing, knee x-rays were obtained with the participants’ knees flexed at 15 degrees using a positioning device (Synaflexer) as described6.
Analysis of biochemical markers
The four biochemical markers (CRPM, C1M, C2M and C3M) were assessed using solid phase competitive ELISAs as previously described15–18. In brief, a 96-well streptavidin plate was coated with the appropriate biotinylated synthetic peptide dissolved in assay buffer and incubated for 30 min at 20°C. Peptide calibrator or sample was added to appropriate wells, and then 100 uL of a conjugated monoclonal antibody against the target sequence was added to wells and incubated. The mix of primary antibody and samples was incubated for 2 hours at 20°C or 20 hours at 4°C. After thorough washing of the microtiter plate, 100μl tetramethylbenzinidine (TMB) (KemEn-Tec cat.438OH) was added and the plate was incubated for 15 minutes at 20°C in the dark. All the above incubation steps included shaking at 300 rpm. After each incubation step, the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). The TMB reaction was stopped by adding 100ul of stopping solution (1% HCl) and measured at 450 nm with 650 nm as the reference. The samples were assessed in duplicates including five quality control samples on each microtiter plate. The measurement range of the assays were in the nanomole range for C1M, C3M and CRPM and in the picomole range for C2M, and the intra- and inter-assay CVs were below 15%.
Details of the analysis of plasma IL-6 and the results from the IDEA trial have been previously reported2.
Radiographic measure of OA progression
The change in joint space width from baseline to 18 months was used as a radiographic measure of disease progression. The methods used to measure joint space width in the IDEA study have been published6 as well as the criteria used to define progressors and non-progressors19. In brief, the minimum joint space width (mJSW) was measured in digitized radiographic images using an automated software application. A radiographic “progressor” was defined as exhibiting a decrease in JSW of ≥ 0.7mm in the medial tibiofemoral compartment from baseline to 18 months, which was based on the OARSI-OMERACT definition of relevant radiological progression20. A “non-progressor” was defined as a decrease in JSW of ≤0.35mm. From IDEA participants who had radiographs available at both baseline and 18 months, 76 progressors and 180 non-progressors were available for analysis.
Statistical Analyses
The present biomarker study represents a secondary analysis of data from the IDEA trial. Descriptive statistics were used for baseline characteristics of participants with biomarker data. Biomarker values were log-transformed for all analyses. Treatment group means and pairwise differences were assessed using mixed models adjusted for visit, treatment, the visit by treatment interaction, baseline BMI, gender, and baseline values of the outcome at 6 and 18 months, with visit-specific comparisons estimated using contrast statements. Cohen’s d effect sizes were estimated for diet and diet plus exercise groups compared to exercise alone. Associations between changes in biomarker values and clinical values including weight, pain, and function at 18 months were assessed using multivariable linear regression models adjusted for baseline BMI, gender, and baseline values of the biomarker. Finally, we compared biomarker changes across OA progression status using ANCOVA at 18 months adjusted for baseline BMI, gender, treatment arm, and baseline value of the outcome. Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC), and all statistical comparisons were deemed significant when p<0.05 without adjustment for multiple comparisons.
Results
The demographic characteristics of the 429 IDEA participants from whom blood samples were available at baseline and 18 months (Table 1) were similar to the 454 individuals reported in the main outcomes paper2. On average, 58% of the participants were using NSAIDs at the baseline visit. Patellofemoral OA was present in at least one knee in 71% of the participants and 86% had bilateral tibiofemoral knee OA. There were no differences in these characteristics among the three intervention groups.
Table 1.
Baseline characteristics of participants
| Exercise Only | Diet Only | Diet+Exercise | All | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) | N | Mean ± SD or n (%) |
|
| ||||||||
| Age (yrs) | 145 | 65.62 ± 6.42 | 144 | 65.94 ± 6.24 | 140 | 65.72 ± 6.04 | 429 | 65.76 ± 6.22 |
|
| ||||||||
| Female | 145 | 104 (71.7) | 144 | 101 (70.1) | 140 | 101 (72.1) | 429 | 306 (71.3) |
|
| ||||||||
| White Race | 145 | 118 (81.4) | 144 | 124 (86.1) | 140 | 113 (80.7) | 429 | 355 (82.8) |
|
| ||||||||
| BMI (kg/m2) | 145 | 33.49 ± 3.73 | 144 | 33.63 ± 3.79 | 140 | 33.49 ± 3.70 | 429 | 33.54 ± 3.73 |
|
| ||||||||
| Weight (kg) | 145 | 92.30 ± 14.52 | 144 | 93.39 ± 15.48 | 140 | 92.68 ± 14.38 | 429 | 92.79 ± 14.77 |
|
| ||||||||
| Height (cm) | 145 | 165.78 ± 8.96 | 144 | 166.31 ± 9.38 | 140 | 166.11 ± 9.02 | 429 | 166.07 ± 9.10 |
|
| ||||||||
| Gait Speed (m/s) | 145 | 1.22 ± 0.18 | 144 | 1.18 ± 0.18 | 140 | 1.19 ± 0.19 | 429 | 1.20 ± 0.18 |
|
| ||||||||
| KL Grade 2 | 145 | 72 (49.7) | 144 | 70 (48.6) | 140 | 67 (47.9) | 429 | 209 (48.7) |
|
| ||||||||
| Bilateral OA | 145 | 128 (88.3) | 144 | 118 (81.9) | 140 | 121 (86.4) | 429 | 367 (85.5) |
|
| ||||||||
| PF OA | 145 | 104 (71.7) | 144 | 104 (72.2) | 140 | 96 (68.6) | 429 | 304 (70.9) |
|
| ||||||||
| NSAID use | 145 | 86 (59.3) | 144 | 83 (57.6) | 140 | 82 (58.6) | 429 | 251 (58.5) |
|
| ||||||||
| SF-36 Physical | 144 | 36.68 ± 9.07 | 141 | 35.81 ± 8.78 | 139 | 36.45 ± 9.48 | 424 | 36.31 ± 9.10 |
|
| ||||||||
| SF-36 Mental | 144 | 56.40 ± 8.44 | 141 | 55.75 ± 8.44 | 139 | 57.57 ± 6.41 | 424 | 56.57 ± 7.85 |
|
| ||||||||
| WOMAC Pain (0–20) | 144 | 6.15 ± 2.94 | 144 | 6.63 ± 2.99 | 140 | 6.65 ± 3.41 | 428 | 6.48 ± 3.12 |
|
| ||||||||
| WOMAC Function (0–68) | 145 | 23.34 ± 10.21 | 144 | 25.03 ± 10.47 | 140 | 24.27 ± 11.71 | 429 | 24.21 ± 10.80 |
| IL-6 (pg/ml) | 145 | 2.96 ± 2.05 | 143 | 3.19 ± 2.35 | 140 | 3.29 ± 2.23 | 428 | 3.15 ± 2.21 |
BMI, body mass index; KL, Kellgren Lawrence; PF, patellofemoral; NSAID, non-steroidal anti-inflammatory drug; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; IL-6, interleukin-6
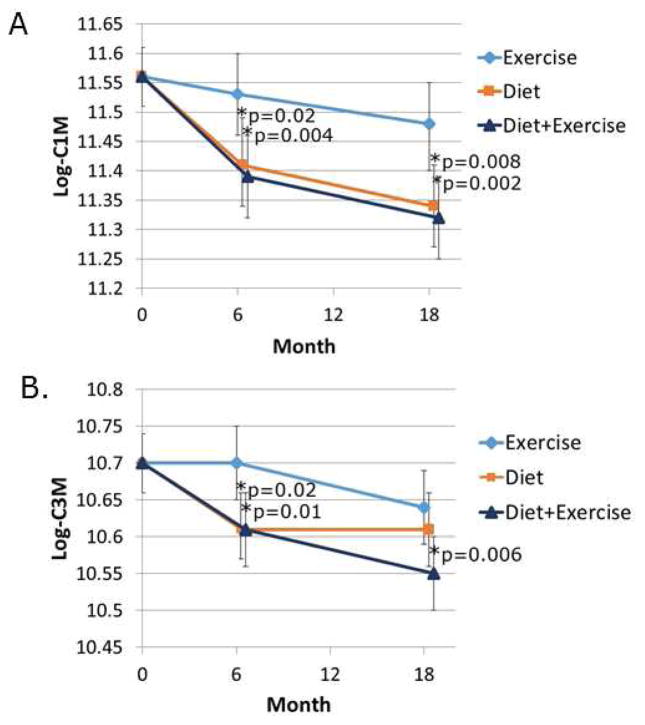
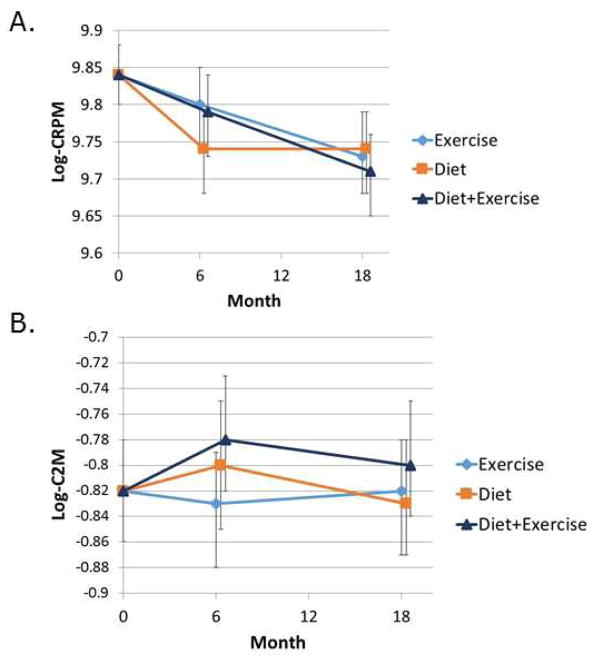
The C1M, C2M and C3M biomarkers recognize neoepitopes in types I, II, and III collagen, respectively, that are generated by MMP cleavage of collagen in the soft connective tissues (C1M and C3M) including synovium, tendons and ligaments but not in bone and the articular cartilage (C2M)12, 13. The CRPM biomarker recognizes a fragment of CRP and is thought to be a marker of chronic inflammation in OA12. The level of C1M declined in all three intervention groups over the course of the study with significantly lower levels at 6 and 18 months when the diet and the diet plus exercise groups were compared to the exercise only control group (Fig. 1A). Compared to exercise only, the effect sizes were −0.26 for diet only and −0.29 for diet plus exercise (Table 2). Likewise, C3M levels declined more in the diet and diet plus exercise groups compared to the exercise control group with significant differences noted at 6 months while at 18 months only diet plus exercise was significantly lower than the exercise controls with an effect size of −0.24 (Fig. 1B and Table 2). CRPM declined in all three groups over 18 months but there was no significant difference among the interventions (Fig. 2A) while C2M did not change in any of the three groups over the course of the study (Fig. 2B).
Fig. 1.
Effects of dietary weight loss and exercise interventions on biochemical markers of type I (C1M) and type III (C3M) collagen degradation. Serum samples from baseline, 6 month and 12 month blood collections were assayed for the indicated biochemical markers. The data (expressed as ng/ml) was log transformed and adjusted for baseline values and baseline BMI and gender. Mixed models were used to assess treatment group means and pairwise differences.
Table 2.
Effects of exercise and diet interventions on biomarker levels.
| Exercise Only | Diet Only | Diet & Exercise | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 18 months | Change | 18 months | Change | 18 months | Change | ||||
| Description | Mean, SE (95% CI) | Mean, SE (95% CI) | Mean, SE (%) | Mean, SE (95% CI) | Mean, SE (%) | Effect Size | Mean, SE (95% CI) | Mean, SE (%) | Effect Size | P-value (18 months) |
| Log C1M Mean Result | 11.56, 0.03 (11.51, 11.61) | 11.48, 0.04 (11.40, 11.55) | −0.08, 0.04 (−0.66) | 11.34, 0.04 (11.27, 11.41) | −0.21, 0.04 (−1.82) | −0.26 | 11.32, 0.04 (11.25, 11.39) | −0.23, 0.04 (−2.00) | −0.29 | 0.0035 |
| Log C2M Mean Result | −0.82, 0.02 (−0.86, −0.78) | −0.82, 0.02 (−0.87, −0.78) | 0.01, 0.02 (−0.84) | −0.83, 0.02 (−0.87, −0.78) | 0.00, 0.02 (−0.35) | −0.01 | −0.80, 0.02 (−0.84, −0.75) | 0.03, 0.02 (−4.20) | 0.07 | 0.5565 |
| Log C3M Mean Result | 10.70, 0.02 (10.66, 10.74) | 10.64, 0.02 (10.59, 10.69) | −0.05, 0.02 (−0.49) | 10.61, 0.03 (10.56, 10.66) | −0.09, 0.03 (−0.82) | −0.09 | 10.55, 0.02 (10.50, 10.60) | −0.15, 0.02 (−1.37) | −0.24 | 0.0208 |
| Log CRPM Mean Result | 9.84, 0.02 (9.80, 9.88) | 9.73, 0.03 (9.68, 9.79) | −0.08, 0.03 (−0.84) | 9.74, 0.03 (9.68, 9.79) | −0.08, 0.03 (−0.83) | 0.00 | 9.71, 0.03 (9.65, 9.76) | −0.11, 0.03 (−1.12) | −0.06 | 0.6922 |
All 18 month means adjusted for baseline values, baseline BMI and gender. Effect size is based on comparison to the exercise only group.
Fig. 2.
Effects of dietary weight loss and exercise interventions on biochemical markers of CRP (CRPM) and type II collagen (C2M) degradation. Serum samples from baseline, 6 month and 12 month blood collections were assayed for the indicated biochemical markers. The data (expressed as ng/ml) was log transformed and adjusted for baseline values and baseline BMI and gender. Mixed models were used to assess treatment group means and pairwise differences.
The decline in C1M and C3M in the diet and diet plus exercise groups compared to exercise alone suggested an association with weight loss since we had found that participants in the diet group lost on average 9.5% of their baseline body weight and the diet plus exercise group lost 11.4% while the exercise only group lost just 2.2% of their body weight2. Indeed, using the combined data from all three intervention groups, there was a highly significant positive association between change in weight from baseline to 18 months and change in C1M and C3M (p<0.0001) and CRPM (p=0.0004), however, change in weight was negatively associated with change in C2M (p=0.0065).(Table 3). We also explored the associations between change in the four biomarkers with change in WOMAC pain and function and found positive associations for change in C3M and CRPM with WOMAC function (Table 2).
Table 3.
Change in biomarkers over 18 months and associations with change in weight, WOMAC scores and IL-6*
| Predictor (change per SD) | Outcome change | Slope (95% CI) | Partial correlation | P-value |
|---|---|---|---|---|
| Weight | Log-C1M | 0.12 (0.08, 0.16) | 0.30 | <0.0001 |
| Weight | Log-C2M | −0.04 (−0.07, −0.01) | −0.15 | 0.0065 |
| Weight | Log-C3M | 0.08 (0.05, 0.11) | 0.28 | <0.0001 |
| Weight | Log-CRPM | 0.06 (0.03, 0.09) | 0.20 | 0.0004 |
| Log-C1M | WOMAC Pain | 0.13 (−0.20, 0.46) | 0.04 | 0.43 |
| Log-C2M | WOMAC Pain | 0.09 (−0.24, 0.42) | 0.03 | 0.59 |
| Log-C3M | WOMAC Pain | 0.20 (−0.13, 0.53) | 0.07 | 0.24 |
| Log-CRPM | WOMAC Pain | 0.26 (−0.07, 0.58) | 0.09 | 0.12 |
| Log-C1M | WOMAC Function | 0.65 (−0.38, 1.67) | 0.07 | 0.22 |
| Log-C2M | WOMAC Function | −0.36 (−1.39, 0.67) | −0.04 | 0.49 |
| Log-C3M | WOMAC Function | 1.16 (0.13, 2.19) | 0.13 | 0.027 |
| Log-CRPM | WOMAC Function | 1.15 (0.14, 2.16) | 0.13 | 0.026 |
| Log-IL-6 | Log-C1M | 0.15 (0.11, 0.19) | 0.37 | <0.0001 |
| Log-IL-6 | Log-C2M | −0.03 (−0.06, −0.00) | −0.11 | 0.045 |
| Log-IL-6 | Log-C3M | 0.08 (0.05, 0.11) | 0.28 | <0.0001 |
| Log-IL-6 | Log-CRPM | 0.07 (0.04, 0.10) | 0.23 | <0.0001 |
Model-adjusted estimates of associations between 18-month change in weight (1SD = 8.05 kg) vs. 18-month change in biomarkers or between change in biomarkers and WOMAC pain and function subscales. Associations adjusted for baseline BMI, gender, and baseline value of biomarker.
In a previous study using data from the IDEA trial, we reported that changes in body weight and regional fat mass were positively associated with the change in IL-621 and so we determined whether the change in IL-6 was associated with change in the biomarkers. There were significant positive associations between change in IL-6 and change in C1M, C3M, and CRPM but not C2M (Table 3) which trended toward a negative, rather than a positive association.
Finally, we looked for differences in the change in the biomarkers between radiographic progressors and non-progressors but did not find any significant association with the biomarkers or IL-6 (Table 4).
Table 4.
Change in biomarker levels at 18 months in radiographic progressors compared to non-progressors.*
| Variable Mean change | Non-progressors N=180 | Progressors N=76 | P-value |
|---|---|---|---|
|
| |||
| Log C1M | −0.21 (−0.28, −0.15) | −0.21 (−0.30, −0.11) | 0.91 |
|
| |||
| Log C2M | 0.03 (−0.01, 0.07) | 0.03 (−0.02, 0.09) | 0.82 |
|
| |||
| Log C3M | −0.13 (−0.17, −0.09) | −0.09 (−0.15, −0.03) | 0.29 |
|
| |||
| Log CRPM | −0.12 (−0.17, −0.07) | −0.09 (−0.16, −0.02) | 0.53 |
| Log IL-6 | −0.20 (−0.29, −0.12) | −0.15 (−0.28, −0.02) | 0.44 |
Tested using ANCOVA at 18 months, adjusted for baseline BMI, gender, randomization group, and baseline value of the outcome. Progressors defined as change in JSW < −0.7mm, while non-progressors had change in JSW > −0.35.
Discussion
There is significant interest in developing biochemical markers that can be used to measure the effects of interventions for knee OA on joint tissues22. In the present study, two potential markers of joint tissue inflammation (C1M and C3M) decreased over the course of an 18-month clinical trial in overweight and obese adults with knee OA assigned to dietary-induced weight loss or exercise plus weight loss interventions compared to an exercise without diet intervention that served as a control group. The decline in C1M and C3M appeared to be driven by the decrease in weight which, as previously reported2 was significantly greater in the two groups that received the dietary intervention.
The C1M and C3M markers, which are elevated in the serum of patients with rheumatoid arthritis, were reported to decline in response to treatment with the anti-IL-6 antibody tocilizumab in combination with methotrexate15 suggesting they could be markers of synovial inflammation. In support of this, both markers were increased in the conditioned media of OA synovial explants after treatment with the cytokines TNFα or IL-1β13. The finding that the change in IL-6 was associated with the change in both markers in the present study also suggests that these markers are responsive to a change in an inflammatory process. However, we were unable to determine if the change in C1M or C3M was due to a change in synovitis since MRI measures of synovitis were obtained from only 105 of the IDEA participants and synovitis was not found to differ among the three intervention groups at 18 months6.
Adipose tissue in obese individuals is more inflamed than in non-obese individuals and is an important contributor to systemic inflammation, referred to as “metainflammation”23. We had previously shown a significant correlation between reduction in fat mass and decline in plasma IL-6 in the IDEA participants21. The present finding that declines in C1M, C3M, as well as CRPM were strongly associated with weight loss and with the decline in IL-6 suggests that metainflammation was reduced in the IDEA participants who lost weight over the 18 month intervention. The negative association between change in weight and change in C2M, albeit weak, is more difficult to explain and may represent a spurious result given that C2M levels did not change significantly in any of the intervention groups over the course of the study.
Finding biochemical markers that correlate with symptoms and disease progression in individuals with OA has been a challenge. However, recent work measuring 18 biomarkers in longitudinal samples from the observational Osteoarthritis Initiative (OAI) study revealed eight biomarkers that predicted OA cases defined as those with knee OA who exhibited worsening of radiographic changes and pain between the 24 month and 48 month study visits11. In that study, the two strongest predictors of case status were urinary markers for type II collagen degradation, uC2C-HUSA and uCTX-II, while serum C2C to measure type II collagen degradation and serum C1,2C for types I and II collagen degradation were not predictive. In addition, serum CTX-I for type I collagen degradation, which reflects bone resorption, was modestly predictive (odds ratio 1.28). This shows that different neoepitopes of the same protein may provide independent information. However, no markers reflecting synovial or connective tissue turnover, other than bone and cartilage, were measured in the OAI cohort, thus it had not been shown whether markers of tissue inflammation would be associated with OA progression.
In the present study, we did not find any relationship between change in the serum markers and radiographic progression, measured as change in joint space width. Also, none of the markers measured were associated with the change in WOMAC pain. However, C3M and CRPM were significantly (albeit weakly) associated with change in WOMAC function. The lack of an association with radiographic progression could be due to the inclusion of only individuals with KL 2–3 OA and small numbers of progressors (n=76) who could be compared to the 180 non-progressors. In addition, the choice of a decrease in JSW of ≤0.35mm as the cut off for a non-progressor is less than the smallest detectable difference which could lead to misclassification and bias towards the null. However, the findings are consistent with our previous report that the interventions did not alter structural progression assessed by JSW on radiographs or cartilage loss by MRI in the subset of 105 participants6.
There are limitations to this study. This was a secondary analysis using blood samples and data collected from a prior randomized controlled trial that was not designed or powered specifically for biomarker studies. Also the study could not fully evaluate the effects of exercise alone on the biomarkers because the control group in the IDEA trial received an exercise intervention. This was due to prior studies demonstrating the benefits of exercise and the thought that exercise should be part of the standard of care for knee OA. Although the IDEA trial showed marked improvement in pain and function in the diet and diet plus exercise groups, the change in biomarkers did not show strong correlations with the improvement in symptoms other than associations with change in C3M and CRPM with WOMAC function. One potential reason is that the biomarkers are measured in serum samples and so reflect systemic activity while the symptoms reported by the participants using the WOMAC instruments reflect local symptoms. Another is that many factors beyond what is happening in the joint influence how people perceive and report symptoms. This may be why symptoms and radiographic severity of OA do not show strong correlations in most studies.
In summary, using longitudinal data from an 18-month clinical trial of exercise and weight loss for knee OA, serum biomarkers of type I and type III collagen degradation were found to decrease in response to weight loss or exercise plus weight loss interventions when compared to an exercise only group. The decrease in these markers over the course of the study and two systemic markers of inflammation, CRPM and IL-6, were strongly associated with weight loss indicating that overweight and obese adults with knee OA who lose weight experience reduced inflammation at a systemic as well as tissue level.
Acknowledgments
Funding
Supported by grants from National Institute of Arthritis, Musculoskeletal and Skin Disease (R01 AR052528 and P60 AR064166), and the National Institute on Aging (P30 AG21332).
We thank the IDEA research staff for data and sample collection and Karin Murphy for assistance with sample processing.
Footnotes
Author contributions
Study conception and design: all authors
Analysis and interpretation of the data: all authors
Drafting of the article: RL, DB, and AB-J who also take responsibility for the integrity of the work as a whole
Critical revision of the article for important intellectual content: all authors
Final approval of the article: all authors
Conflict of interests
Drs. Anne Bay-Jensen and Morton Karsdal are employees of and hold stock/stock options in Nordic Biosciences. Dr. Ali Guermazi holds stock/stock options in Boston Imaging Core Lab, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 2.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:455–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 5.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative Semin. Arthritis Rheum. 2014;43:701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Hunter DJ, Beavers DP, Eckstein F, Guermazi A, Loeser RF, Nicklas BJ, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage. 2015;23:1090–1098. doi: 10.1016/j.joca.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28:61–71. doi: 10.1016/j.berh.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–1763. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bay-Jensen AC, Reker D, Kjelgaard-Petersen CF, Mobasheri A, Karsdal MA, Ladel C, et al. Osteoarthritis year in review 2015: soluble biomarkers and the BIPED criteria. Osteoarthritis Cartilage. 2016;24:9–20. doi: 10.1016/j.joca.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76:186–195. doi: 10.1136/annrheumdis-2016-209252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siebuhr AS, Petersen KK, Arendt-Nielsen L, Egsgaard LL, Eskehave T, Christiansen C, et al. Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthritis Cartilage. 2014;22:44–50. doi: 10.1016/j.joca.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Kjelgaard-Petersen C, Siebuhr AS, Christiansen T, Ladel C, Karsdal M, Bay-Jensen AC. Synovitis biomarkers: ex vivo characterization of three biomarkers for identification of inflammatory osteoarthritis. Biomarkers. 2015;20:547–556. doi: 10.3109/1354750X.2015.1105497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messier SP, Legault C, Mihalko S, Miller GD, Loeser RF, DeVita P, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet Disord. 2009;10:93. doi: 10.1186/1471-2474-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bay-Jensen AC, Platt A, Byrjalsen I, Vergnoud P, Christiansen C, Karsdal MA. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin Arthritis Rheum. 2014;43:470–478. doi: 10.1016/j.semarthrit.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Bay-Jensen AC, Byrjalsen I, Siebuhr AS, Christiansen C, Platt A, Karsdal MA. Serological biomarkers of joint tissue turnover predict tocilizumab response at baseline. J Clin Rheumatol. 2014;20:332–335. doi: 10.1097/RHU.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siebuhr AS, Bay-Jensen AC, Leeming DJ, Plat A, Byrjalsen I, Christiansen C, et al. Serological identification of fast progressors of structural damage with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R86. doi: 10.1186/ar4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bay-Jensen AC, Platt A, Siebuhr AS, Christiansen C, Byrjalsen I, Karsdal MA. Early changes in blood-based joint tissue destruction biomarkers are predictive of response to tocilizumab in the LITHE study. Arthritis Res Ther. 2016;18:13. doi: 10.1186/s13075-015-0913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeser RF, Pathmasiri W, Sumner SJ, McRitchie S, Beavers D, Saxena P, et al. Association of urinary metabolites with radiographic progression of knee osteoarthritis in overweight and obese adults: an exploratory study. Osteoarthritis Cartilage. 2016;24:1479–1486. doi: 10.1016/j.joca.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ornetti P, Brandt K, Hellio-Le Graverand MP, Hochberg M, Hunter DJ, Kloppenburg M, et al. OARSI-OMERACT definition of relevant radiological progression in hip/knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:856–863. doi: 10.1016/j.joca.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beavers KM, Beavers DP, Newman JJ, Anderson AM, Loeser RF, Jr, Nicklas BJ, et al. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis Cartilage. 2015;23:249–256. doi: 10.1016/j.joca.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraus VB, Blanco FJ, Englund M, Henrotin Y, Lohmander LS, Losina E, et al. OARSI Clinical Trials Recommendations: Soluble biomarker assessments in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23:686–697. doi: 10.1016/j.joca.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]