Abstract
Interleukin-33 (IL-33) is a member of the IL-1 cytokine family that has been widely studied since its discovery in 2005 for its dichotomous functions in homeostasis and inflammation. IL-33, along with its receptor suppression of tumorigenicity 2 (ST2), has been shown to modulate both the innate and adaptive immune system. Originally, the IL-33/ST2 signaling axis was studied in the context of inducing type 2 immune responses with the expression of ST2 by T helper 2 (TH2) cells. However, the role of IL-33 is not limited to TH2 responses. Rather, IL-33 is a potent activator of TH1 cells, group 2 innate lymphoid cells (ILC2s), regulatory T (Treg) cells, and CD8+ T cells. The intestine is uniquely important in this discussion, as the intestinal epithelium is distinctively positioned to interact with both pathogens and the immune cells housed in the mucosa. In the intestine, IL-33 is expressed by the pericryptal fibroblasts and its expression is increased particularly in disease states. Moreover, IL-33/ST2 signaling aberrancy is implicated in the pathogenesis of inflammatory bowel disease (IBD). Accordingly, for this review, we will focus on the role of IL-33 in the regulation of intestinal immunity, involvement in intestinal disease, and implication in potential therapeutics.
Keywords: Interleukin-33, intestinal immunity, T cells, inflammatory bowel disease, microbiome
1. Introduction
Interleukin-33 (IL-33) is a member of the IL-1 family, a group of cytokines that includes IL-1α, IL-1β, and IL-18, which act to initiate inflammatory responses secondary to local stress [1]. IL-33 notably behaves as both an extracellular, traditional cytokine and nuclear transcription factor [2]. When it was first discovered just over 10 years ago, it was found secondary to its unique receptor, suppression of tumorigenicity 2 (ST2). At the time, IL-33 was established to be a potent activator of type 2 immune responses integral to adaptive immunity [3]. However, it is now understood IL-33 behaves as a key player in both innate and adaptive immunity. ST2 is expressed by several important immune cells, including but not limited to T helper 1 (TH1) cells, TH2 cells, group 2 innate lymphoid cells (ILC2s), regulatory T (Treg) cells, and CD8+ T cells. Moreover, IL-33 is released by epithelial and endothelial cells in response to cell injury and necrosis, thereby acting as an alarmin to initiate the innate immune response. The inherent properties of both IL-33 and ST2 demarcate their unique position in mucosal immunity, both during homeostasis and inflammation. With the expression of IL-33 by the epithelium of mucosal barriers and the expression of ST2 by several immune cells, IL-33/ST2 signaling is critical in understanding intestinal immunity. Accordingly, the focus of the review will be the role of IL-33 signaling in this setting.
In this review, we will first describe the molecular and cellular characteristics of IL-33 and its receptor ST2. We will then discuss the IL-33/ST2 signaling axis as it pertains to intestinal immunity. It is in this context, we will define the role of IL-33 in the maintenance of the intestinal epithelium, the activation of the immune system secondary to local inflammation, and the communication of the intestine with the microbiome. We will then describe the relationship between IL-33 and several cell types that comprise adaptive immunity, including TH2 cells, Tregs, and Th17 cells. We will conclude our discussion by describing the implications of IL-33/ST2 signaling aberrancy in inflammatory bowel disease.
2. IL-33/ST2 Expression and Signaling Overview
IL-33/ST2 signaling has been recently detailed in broader contexts in several excellent reviews [4–6]. We will briefly describe the molecular and cellular fundamentals of IL-33 and its receptor ST2. In this respect, we will also highlight IL-33 and ST2 expression and signaling in the intestine.
2.1. IL-33 expression and post-translational modification
The cytokine IL-33 was first described as a novel member of the IL-1 cytokine family in 2005 [1]. IL-33 is encoded by a gene located on chromosome 9 in humans and chromosome 19 in mice [4, 7]. The gene product is a 30 kD protein that has the characteristic 12 β sheet and tetrahedron structure shared by all members of the IL-1 cytokine family [5]. Unlike traditional inducible cytokines, IL-33 is constitutively expressed by several cells including human endothelial cells and epithelial cells and stored in the nucleus of these cells [8, 9].
While the gene product is biologically active (referred to as full length IL-33), the potency of IL-33 increases significantly (up to 30x) after post-translational cleavage at the N-terminus by inflammatory proteases such as neutrophil serine proteases, cathepsin G, and elastase, referred to as mature IL-33 [10, 11]. Accordingly, this mechanism of IL-33 activation is particularly advantageous when IL-33 is released as the full gene product in the extracellular space in the setting of cell injury or death by endothelial and epithelial cells. Appropriately, in this setting of barrier integrity compromise, immune cells can then secrete proteases to cleave IL-33 to its more active form [5, 11]. In its activated form, IL-33 can more efficiently promote the local immune processes in mucosal disruption via epithelial cell injury and death. Furthermore, extracellular proteolytic processing of IL-33 by inflammatory proteases potentiates IL-33 activity.
2.2. Intracellular IL-33
IL-33 has been demonstrated to act as both an extracellular ligand and an intracellular signaling molecule [5, 7]. The role of intracellular IL-33 independent of the presence of the IL-33 receptor ST2 has been studied most extensively in endothelial cells [9, 12–16]. Upon synthesis, full-length IL-33 that has not undergone activation by proteolytic processing is able to translocate to the nucleus via the presence of a nuclear localization sequence and bind to heterochromatin [9, 12–16]. Notably, mature IL-33 that has undergone proteolytic processing is unable to translocate to the nucleus in direct contrast to full-length IL-33 [7, 17].
In endothelial cells, IL-33 is constitutively expressed [12, 14, 16] and is believed to behave as a transcriptional repressor [12]. Moreover, nuclear IL-33 is an indicator of endothelial cell quiescence [14, 15]. Intracellular IL-33 expression was noted when endothelial cells entered the G1/G0 state with a concomitant loss of Ki-67 expression, a marker of cellular proliferation, suggesting that IL-33 was behaving as a transcriptional repressor [14] via the Notch signaling pathway [15].
However, the role of nuclear IL-33 is not limited to endothelial cells and may influence transcription in a multitude of cell types. Recent studies have demonstrated nuclear IL-33 is important in synovial fibroblasts [18], skin keratinocytes [19], and bone-marrow-derived mast cells [20]. For example, synovial fibroblasts of rheumatoid arthritis patients stimulated by TNF-α resulted in increased expression of IL-33 localized to the nucleus with minimal detection in the supernatant of the cells, suggesting IL-33 is working as a transcriptional regulator in this cell population and not as a traditional secreted cytokine [18]. More studies evaluating the role of nuclear IL-33 in regulating transcription other cell types are necessary.
An important study by Bessa et al highlighted the importance of the subcellular compartmentalization of IL-33 [21]. In this study, mice were generated with the N-terminus of IL-33 mutated such that there was a loss of nuclear localization and consequent chromatin association, referred to as IL33tm1/+ mice [21]. Importantly, these mice had increased circulating IL-33 as a consequence of basal gene transcription, not secondary to exogenous recombinant protein administration or protein overexpression [21]. These IL33tm1/+ mice also developed a prominent pro-inflammatory response with multi-organ involvement, including but not limited to splenomegaly, lymphadenopathy, and enterocolitis [21]. Cellularly, these mice had infiltrates of neutrophils, dendritic cells, macrophages, and eosinophils [21]. Yet, ST2−/−IL33tm1/+ mice, in which IL-33 receptor ST2 was absent, did not have this inflammatory phenotype, suggesting that the interaction of IL-33 and ST2 was integral for these downstream effects [21]. This study not only highlights the existence of IL-33 nuclear localization, but demonstrates that without an appropriate stimulating event in acute cell injury and subsequent resolution, IL-33 can be implicated in chronic, non-resolving inflammation if not properly localized to the nucleus [21]. Despite its clear interaction with genetic material, it is uncertain how IL-33 behaves as a direct regulator of gene expression, including its own expression. Further investigations are necessary to determine the mechanisms by which IL-33 regulates its own expression and secretion.
2.3. Extracellular IL-33
The role of IL-33 as a traditional cytokine is better understood; however, the mechanisms by which IL-33 is released into the extracellular space are under active investigation [22]. As a traditional cytokine, binding of extracellular IL-33 to its receptor ST2 initiates several cellular signaling pathways. However, like other members of the IL-1 family, IL-33 release is not regulated by a traditional export pathway through the endoplasmic reticulum and Golgi apparatus [23]. Multiple studies have proposed that cell injury or death are the dominant mechanisms by which IL-33 reaches the extracellular environment, such that in a steady state, IL-33 is not actively secreted by cells [5, 24, 25]. However, because IL-33 is expressed constitutively by endothelial and epithelial cells and exists in the nuclei of these cell types fully translated, IL-33 is immediately available to the extracellular microenvironment secondary to cell injury and necrosis [22]. As such, IL-33 is an important and early herald for the immune system when there is a breach in mucosal integrity secondary to epithelial cell damage [25].
IL-33 release may also be influenced by extracellular ATP concentration [26–28]. One such study found ATP was released by intestinal epithelial cells following parasitic infection [26]. This increase in extracellular ATP resulted in the activation of mast cells and subsequent IL-33 secretion by mast cells [26]. This in turn induced IL-13-producing ILC2, culminating in goblet cell hyperplasia and mucin production necessary for helminth eradication [26].
Like mast cells, corneal epithelial cells [27] and astrocytes [28] release IL-33 in response to the presence of pathogen-associated molecular patterns (PAMPs), which was potentiated by the presence of increased extracellular ATP [28]. This is advantageous as these cell types can appropriately respond to microbial infection to induce an inflammatory response via IL-33.
Lastly, IL-33 secretion can be increased under mechanical stress. Cardiomyocytes, in response to mechanical deformation secondary to cardiac muscle contraction, secrete physiologic IL-33 into the extracellular space [29–32]. In the gut, it is plausible that IL-33 release from intestinal epithelial cells is a consequence of deformation by muscle contraction and relaxation during peristalsis. Further studies are required to definitively identify the circumstances under which IL-33 is released independent of cell injury and death.
2.4. IL-33/ST2 signaling
2.4.1. ST2 receptor expression and isoforms
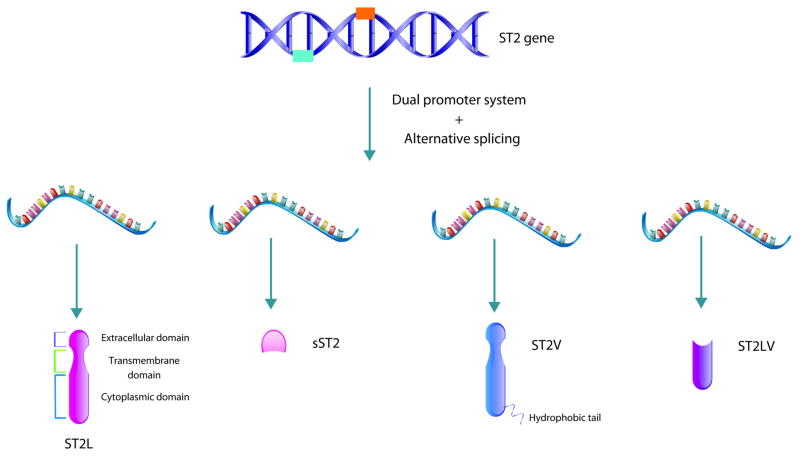
The IL-1 family orphan receptor, suppression of tumorigenicity 2 (ST2) was classified as the receptor for IL-33 in 2005, but was originally identified as an orphan cytokine receptor in 1993 [1, 33] (Figure 2). ST2 has two promoter region sites that result in the coding of two distinct proteins, sST2 and ST2L [34]. sST2 is the soluble form of ST2 that shares the extracellular components of ST2L, including the ligand binding domain, but lacks the transmembrane and intracellular components of ST2L [6, 34]. ST2L is a transmembrane receptor that shares structural similarity to other interleukin receptors [34]. Furthermore, ST2L has two additional isoforms created by alternative splicing, ST2V and ST2LV [35, 36]. One isoform, termed ST2V, shares the extracellular and transmembrane of ST2L, but contains a hydrophobic C-terminus which is in contrast to the hydrophilic C-terminus of ST2L [36]. This ST2V isoform is notably enriched in the GI tract, including the stomach, small intestine, and colon, detected by Southern blotting evaluating human tissue [6, 37]; however, the signaling implications of these C-terminus differences between ST2V and ST2L are presently unknown. However, it is interesting to consider how this structural difference of the ST2 isoform enriched in the intestine could impact the function and role of the IL-33/ST2 signaling axis in homeostasis, immune function, and disease. The final isoform of ST2, termed ST2LV, lacks the transmembrane domain of ST2L; however, maintains the intracellular domain, suggesting it exists as a soluble protein [35]. The function and expression of this isoform is still a matter of active investigation and it will be important to identify these ST2 receptor isoforms as they may have implications in intestinal homeostasis and disease.
Figure 2. Isoforms of IL-33 receptor ST2.
There are four isoforms of ST2 encoded by the gene ST2 located on human chromosome 2q12. The two most prominent isoforms include transmembrane ST (ST2L also known as IL1RL1-b) and soluble ST (sST2, IL1RL1-a). They are the consequence of a dual promotor system that results in differential mRNA expression. ST2L, like other type 1 IL-1 receptors, is comprised of an extracellular domain, transmembrane domain, and cytoplasmic domain, whereas sST2 does not possess the transmembrane and cytoplasmic domains and thus exists as a soluble protein. Moreover, alternative splicing results in the formation of ST2V and ST2LV. ST2V shares the same extracellular and transmembrane domains as ST2L, but is notable for its unique hydrophobic tail and it is particularly enriched in the gastrointestinal tract. Lastly, ST2LV remarkably lacks the transmembrane domain of ST2L but maintains the intracellular domain, suggesting it exists as a soluble protein.
2.4.2. IL-33/ST2 signaling axis
IL-33 activates the intracellular signaling cascade in target cells by first binding to the transmembrane isoforms of ST2 (ST2L and ST2V, heretofore referred to as ST2 in this review) [4, 5]. The complex of IL-33 and ST2 leads to the recruitment of a co-receptor, most commonly IL-1 receptor accessory protein (IL-1RAcP) [1, 38–40]. The heterodimer of the two transmembrane receptors (ST2 and IL-1RAcP) further recruits intracellular scaffold proteins and kinases, including myeloid differentiation response protein (MyD88), IL-1R associated kinase, TNF receptor-associated factor 6, mitogen-activated protein kinases (including JNK, p38, and ERK), and nuclear factor-kappa B (NF-κB) [7, 41, 42]. The activation of these signaling programs can then influence various cellular processes, including the secretion of other cytokines, as well as cell replication and survival [4, 7].
The interaction of NF-κB and IL-33 was explored in murine bone marrow-derived mast cells [43]. In this study, they found exposure to IL-33 stimulated ICAM-1 expression in mast cells and this effect was lost with NF-κB inhibition [43]. Thus, they concluded that in response to IL-33, in the setting of early skin inflammation, mast cells increase ICAM-1 expression to activate LFA-1-expressing cells in an NF-κB dependent manner [43]. This study demonstrates the ability of IL-33 to influence complex signaling pathways, particularly NF-κB, to produce an array of cellular responses.
However, full-length, intracellular IL-33 is also able to interact with transcription factor NF-κB via the N-terminus of IL-33 and the p65 subunit of NF-κB [2]. As a consequence of this complex, NF-κB had a decreased ability to binding its associated DNA, thereby impairing p-65-activated transactivation [2]. Notably, IL-33 overexpression resulted in reduced and delayed expression of NF-κB target genes, including IκBα, TNF-α, and C-REL upon stimulation by rhIL-1β, suggesting nuclear IL-33 is able to sequester NF-κB, thereby dampening NF- κB-induced pro-inflammatory signaling [2]. In this context, intracellular IL-33 behaves as a transcriptional repressor to decrease inflammation. Moreover, in keratinocytes, nuclear IL-33 via its interaction with NF-κB is able to promote wound healing [19]. In this study, IL-33 knockout mice had delayed wound healing, whereas intraperitoneal injection of NF-κB inhibitor improved this delayed wound healing [19]. Importantly, these effects were believed to be mediated by nuclear IL-33 and not IL-33 behaving as a traditional cytokine [19]. Thus, IL-33 is able to behave as a traditional cytokine and influence NF-κB signaling upon binding to its receptor ST2 as well as intracellularly.
2.4.3. Physiologic inhibition of IL-33/ST2 signaling
Inhibition of IL-33 signaling is important to moderate IL-33 activity and maintain homeostasis [4]. Before nuclear IL-33 can be released into the extracellular space, it is subjected to processing by caspases [4, 17, 44, 45]. This occurs during apoptosis, in which active proteases, most importantly caspases 3 and 7, act to cleave IL-33 into inactive protein fragments, to potentially suppress its pro-inflammatory profile during apoptosis where inflammation is counterproductive [4, 17, 44, 45]. This is in direct contrast to cell necrosis, in which IL-33 is released into the extracellular space, which can then initiate inflammatory responses [22]. Accordingly, intracellular processing of IL-33 via caspases is one method of inhibiting IL-33 secretion and subsequent activity (Figure 1).
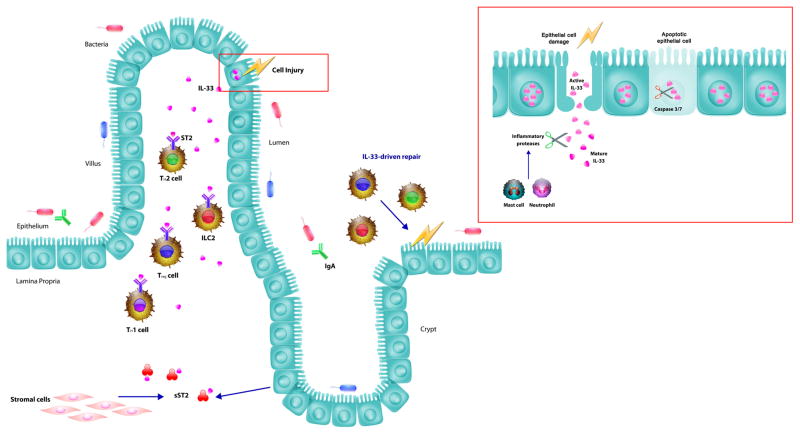
Figure 1. The role of IL-33 in intestinal immunity.
IL-33 is expressed by several epithelial cell types including the epithelial cells of the intestinal mucosa. In response to cell injury, IL-33 is released from the injured cell to recruit the immune system, thereby behaving as an alarmin. The IL-33 receptor, ST2, is expressed on several immune cells of the intestine, including TH2 cells, ILC2s, Tregs, and TH1 cells. Upon activation of these cells, there are increased efforts to repair the epithelium. However, the stromal cells and epithelial cells are capable of releasing sST2, which acts as a receptor decoy, thereby limiting IL-33 induced inflammation. The intestine also readily cooperates with commensal bacteria and pathogenic bacteria. IL-33 has been shown to interact with the gut microbiome and IgA to promote intestinal immunity.
On a cellular level, IL-33 is released from the epithelial cell as active IL-33, which can then be acted upon by inflammatory proteases from neutrophils and mast cells to produce mature IL-33. Mature IL-33 is significantly more potent in this state and can accordingly activate the immune system more efficiently. However, there are several mechanisms in place to limit its response to cell injury and death. One such example is cleavage by caspases 3 and 7 by apoptotic cells to deactivate IL-33 before its release. This inhibits IL-33 activity and the subsequent inflammatory response.
Another such method of restricting IL-33/ST2 signaling is the metabolism of the ligand, IL-33 in the extracellular space. IL-33 can exist for a few hours in the extracellular environment as a competent signaling molecule, but will eventually become oxidized [46]. This oxidation results in conformational changes that prevent IL-33 from binding to the ST2 receptor. Additionally, some of the same proteases that can cleave IL-33 into its most active form during tissue injury can also process it into inactive fragments, thereby limiting the amount of time potent IL-33 will be available to augment local inflammation [47].
The ST2 receptor itself is also subject to regulation. Like many transmembrane receptors, ST2 can be ubiquitinated at the membrane and targeted for destruction in a phosphorylation-dependent mechanism [48]. Moreover, IL-33/ST2 signaling can also be limited after the ligand has bound the receptor. Single immunoglobulin domain IL-Ir related molecule (SIGIRR) inhibits IL-33 signaling by binding the IL-33/ST2 complex to prevent the active co-receptor IL-1RAcP from binding and recruiting the intracellular molecules after IL-33 binds to ST2 [49].
Lastly, one of the more unique mechanisms of IL-33/ST2 signaling regulation is via the extracellular sST2 “decoy” receptor [4, 6]. sST2 is an excreted form of the extracellular domain of the ST2L receptor that can bind IL-33 in the extracellular environment. sST2 functions as a competitive inhibitor of the ST2 transmembrane receptor as its binds and prevents IL-33 from binding ST2 and initiating the aforementioned intracellular signaling programs [4, 6, 30, 50]. Elevated sST2 levels have been reported in the settings of acute HIV infection, graft versus host disease, and inflammatory bowel disease [51–53]. It is currently unknown if elevated sST2 is a cause of unregulated inflammation in the intestine or secondary to an ongoing systemic inflammatory process.
3. IL-33: an alarmin in the intestine
As an alarmin, or an endogenous molecule released as consequence of tissue injury to promote innate immunity activation, IL-33 is one of the first molecules that “sounds the alarm” to indicate there has been a breach in the primary defenses of the intestinal epithelium against pathogens and other threats [5]. In this section, we will detail how IL-33 functions in the homeostasis of intestinal epithelial barrier function. We will also describe how IL-33 recruits cells of the innate immune system when barrier integrity has been compromised.
3.1. Maintenance of the intestinal epithelium
The gut epithelium serves as one of the first lines of defense of the intestine against ingested pathogens, antigens, and toxins [54]. In order to maintain the integrity of this mucosal barrier, the intestinal epithelium undergoes swift and continual self-renewal to replace damaged and dying cells. This process is mediated by a well-established pathway in which intestinal stem cells from the crypts mature and differentiate into one of many cell types that make up the mucosal barrier [25]. A recent study by Mahapatro et al. demonstrated that intestinal IL-33 expression is localized to the pericryptal fibroblasts during homeostasis and is increased during infection [55]. These authors have also elucidated a role for IL-33/ST2 signaling in the differentiation of stem cells in organoid culture. Activation of the IL-33/ST2 pathway in epithelial progenitor cells leads to inhibition of Notch signaling and results in the differentiation of stem cells towards a secretory intestinal cell lineage [55]. These secretory cells include Paneth cells, goblet cells, and enteroendocrine cells. Specifically, goblet cells are known to play an important role in intestinal immunity by producing mucin, the main constituent of mucus, an important barrier mechanism to decrease the interaction between the epithelium and pathogenic bacteria [4, 54]. Notably, mice injected with IL-33 in vivo produce more colonic mucin [56]. Taken together, these observations demonstrate an important role of IL33/ST2 signaling in maintaining the physical barrier of the intestinal mucosal epithelium as the first line of defense against pathogens.
3.2. IL-33 and activation of local inflammation
While IL-33 is necessary for maintaining the intestinal barrier, once the barrier is breached, IL-33 continues to fight against pathogens by recruiting and activating innate immune cells to promote a type 2 inflammatory response [5]. A type 2 inflammatory response is characterized by the involvement of TH2 cells and the presence of specific cytokines, namely IL-4, -5, -9, and 13, produced by both innate and adaptive immune cells with the ultimate goal of tissue repair and barrier function maintenance following tissue destruction. [46]. This is in opposition to the type 1 immune response via TH1 cells with the molecular signature involving cytotoxic T cells and IFN-γ to eliminate intracellular pathogens [57]. The type 2 inflammatory response has been characterized as an important defense mechanism against parasitic infection and regulator of type 1 immune responses to prevent autoimmunity [46–48].
Group 2 innate lymphoid cells (ILC2s) are a class of innate immune cells that are involved in IL-33 signaling, specifically in the promotion of type 2 immune responses [4, 58–62]. ILCs are enhanced at barrier surfaces and have been demonstrated to be integral to mucosal repair in the setting of infection [63–66]. Recent studies have shown that IL-33 activates ILC2s in the gut to produce the growth factor amphiregulin (AREG) more dramatically than ILC2s found at other mucosal sites [56]. AREG binds to the epidermal growth factor receptor (EGFR) to promote tissue repair during inflammation to restore mucosal integrity [56, 65]. Disruption of the AREG/EGFR signaling axis has been implicated in human patients and murine models of inflammatory bowel disease [67–69]. In animal models of superficial epithelial injury in the gut, such as dextran sulfate sodium (DSS)-induced intestinal injury, there is a significant increase in the number of AREG-expressing ILC2s [56]. Furthermore, administration of exogenous IL-33 to mice with DSS-mediated intestinal epithelial injury resulted in the activation of ILC2 cells and amelioration of the clinical manifestations of intestinal inflammation, such as weight loss and rectal bleeding [56]. Additionally, these IL-33-treated mice subjected to DSS had improved restoration of normal colonic crypt architecture and increased expression of genes involved in tight junction formation as compared to PBS-treated mice subjected to DSS colitis [56], further supporting the involvement of IL-33 in tissue repair. These studies suggest that IL-33 treatment can be an important mediator of tissue repair in the setting of intestinal inflammation in an ILC2/AREG-dependent manner. However, this is strictly in the setting of exogenous IL-33 administration in mouse models of intestinal inflammation. It is has been shown that in the setting of helminth intestinal infection, ATP released by parasite-infected cells stimulates local mast cells to produce IL-33, which then activates IL-13-producing ILC2s necessary for helminth expulsion [26]. This is a physiologic response to intestinal infection by helminths, raising the speculation that IL-33 stimulation of ILC2s in intestinal inflammation outside the setting of infection could be beneficial. Accordingly, the physiologic and potential advantageous role of IL-33 with respect to ILC2s stimulation during intestinal inflammation requires further investigation.
3.3. IL-33 and neuroimmune interactions
Emerging literature has implicated the enteric nervous system (ENS) in playing an important role in regulating inflammatory responses in the bowel [70–73]. Specifically, enteric glial cells express Toll-like receptors (TLRs) and have been demonstrated to be important in barrier immune regulation in the setting of intestinal inflammation [74, 75]. Neurosphere-derived glial cells respond to alarmins IL-33 and IL-1β in addition to TLR2 and TLR4, inducing production of innate IL-22 and controlling neurotrophic factors in a MYD88-dependent manner [73, 76]. This particular finding is one aspect of the novel glial cell-ILC3-intestinal epithelial cell relationship highlighted in this study, in which neurotrophic factors are defined to be the molecular link between glial cells and intestinal epithelial defense via production of IL-22 [76]. Further studies are necessary to understand the role of IL-33 in the context of glial cells, ILC3s, and intestinal epithelial cells.
Additionally, IL-33 induces enteric glia to secrete neurotrophic factors termed GFLs that have also been demonstrated to play an important role in gut epithelial barrier homeostasis by maintaining tight junctions and negatively regulating local inflammatory response [73, 76–78]. Moreover, IL-33 influences the ENS to induce hypermotility of the gut as an attempt to expel invading parasites from the intestine [4]. Thus, IL-33 induces smooth muscle contractions in an ENS-dependent mechanism, further demonstrating the important crosstalk between the ENS and inflammatory reactions in the bowel [4, 79]. The neuroimmune interactions in the bowel remain an active area of research and further investigation will continue to advance our understanding of the impacts of this important collaboration in gut inflammation.
3.4. IL-33 and gut microbiota
Immunity at mucosal surfaces is notable for the presence of commensal bacteria [80]. In the gut, the microbiome has been established to be a significant mediator of metabolism and local inflammation [81–85]. A recent study demonstrated that IL-33 is an important regulator of the components of the microbiome [86]. Mice deficient in IL-33 are dysbiotic, or have a higher concentration of pro-inflammatory bacteria that comprise their microbiome compared to their wild-type (WT) controls [86]. Specifically, IL-33-deficient mice have more segmented filamentous bacteria that have also been found in higher concentrations in mouse models of inflammatory bowel disease as well as increased Akkermansia muciniphila, which can degrade mucus [86–88]. Consequently, this dysbiosis promoted release of IL-1α, which drives colitis and even tumor formation [86].
Previous studies have demonstrated that immunoglobulin A (IgA), the immunoglobulin primarily involved in mucosal humoral immunity, preferentially binds to colitogenic bacteria, bacteria involved in the development of colitis [87]. More recent work has discovered a role for IL-33 in regulating this IgA-dependent immune reaction [86]. Compared to WT controls, mice lacking IL-33 were specifically deficient in IgA, but had equivalent levels of other immunoglobulins [86]. The lack of IgA in IL-33-deficient mice resulted in the continued presence of colitogenic bacteria that would normally be identified for removal by the binding of IgA [86–88]. These findings demonstrate the integral role IL-33 plays in modulating intestinal inflammation through interactions with the gut microbiome.
4. IL-33 and Adaptive Immunity
4.1. IL-33, Th2 cells, and type 2 immune responses
In addition to the role of IL-33 in regulating innate or non-specific immune responses, IL-33 also interacts with the cells of the adaptive immune system. In the gut, this has been best observed in the activation of TH2 cells in the presence of helminth or other parasitic infections [4, 6, 59, 89]. TH2 cells were discovered to express ST2 almost two decades ago [3, 4, 90], which they express at baseline as compared to non-differentiated T cells or TH1 cells [90, 91]. Furthermore, ST2 receptor expression is upregulated secondary to IL-33 signaling and requires the transcription factor GATA3 [91], the master transcriptional regulator of TH2 cell differentiation. Importantly, IL-33 also indirectly activates TH2 cells by binding to ST2 expressing-antigen presenting cells (APCs), which can then induce TH2 polarization [4, 92, 93]. The net result of these combined efforts of the IL-33/ST2 axis on TH2 cells is the secretion of type 2 immune response cytokines (IL-4, IL-5, and IL-13) to further enhance the reaction initiated by IL-33-induced ILC2 cells to fight helminth infection in the gut and other organs [4, 93–95].
In this way, IL-33 is important mediator in the induction of type 2 immune responses such as TH2 secretion of IL-4, IL-5 and IL-14, in addition to activation of ILCs, basophils, eosinophils, and mast cells of the innate immune system [4, 96]. However, IL-33 is not the only cytokine to induce type 2 responses. Rather, IL-33 acts in synergy with IL-25 and thymic stromal lymphopoietin (TSLP) derived from epithelial cells to induce a type 2 immune response [4, 61, 96–98]. The overlapping roles of IL-25 and IL-33 in the induction of type 2 responses have been studied in various tissues [97]. In the lung, mouse models of asthma exhibited a more prominent role for IL-33 in airway hyperreactivity and induction of IL-13-producing ILC2s. IL-25 mediated responses were more gradual and weaker in this setting [97]. Similarly, IL-25 and IL-33 had partially redundant roles in helminth intestinal infection [61]. In this study, nuocytes were identified to be innate immune cells involved in type 2 immune responses during helminth infection via secretion of IL-13 and IL-15 [61]. This cell population was able to expand in the presence of IL-25 and IL-33; however, IL-25 was the predominant cytokine to expand the nuocyte cell population [61]. These studies demonstrate the collective role of IL-33 and IL-25 as stimulators of type 2 immune responses, which are tissue and disease specific.
4.2. IL-33 signaling in TH17 cells and Tregs
The balance between the canonically pro-inflammatory TH17 cells and the anti-inflammatory T regulatory cells (Tregs) has been theorized to be an important regulator of inflammation in the gut during homeostasis and disease [99, 100]. This is particularly intriguing as both cell types are known to be enriched in the mucosal surface of the gut. TH17 cells promote inflammation to help combat invading pathogens, but are also implicated in colitis and autoimmunity [101–105]. In contrast, Tregs are integral to the anti-inflammatory response of the immune system, particularly in the prevention of autoimmunity. [103]. Interestingly, the composition of commensal bacterial that comprise the gut microbiome are postulated to play an important role in the balance of TH17 and Tregs [99, 100]. The density of TH17 cells in the gut is dependent on exposure to bacteria, as there is a relative paucity of TH17 cells in mice raised in germ free conditions [106–109]. Remarkably, IL-33 appears to influence both Tregs and TH17 cells to reduce inflammation and protect the gut mucosa from self-inflicted damage.
A recent study has now elucidated a role for IL-33 in regulating TH17 cells in the gut by downregulating pro-inflammatory cytokine expression and limiting expansion of this cell population [110]. These authors demonstrated in their model of intestinal inflammation, via injection of anti-CD3 antibody into mice, IL-33 is released by epithelial cells of the small intestine [110]. Importantly, they found intestinal epithelial cells were the predominant source of IL-33 in the small intestine during inflammation [110]. This finding is important because it highlights the context-dependent expression of IL-33. In the steady state, the intestinal epithelium is not a principle source of IL-33, but in the setting of inflammation, intestinal epithelial cells are indeed significant sources of IL-33. Moreover, while TH17 cells do not express the ST2 receptor at baseline, TH17 cells from mice injected with anti-CD3 express ST2 [110]. Once TH17 cells become competent for IL-33 signaling, the IL-33/ST2 axis induces a downregulation of pro-inflammatory markers and an upregulation of the anti-inflammatory cytokine IL-10 [110]. Furthermore, mice deficient in the ST2 receptor have a more robust expansion of the TH17 cell population in the setting of inflammation compared to WT controls [110]. These data indicate that via the suppression of TH17 cells, IL-33 is an important modulator of inflammation in the small intestine and can help attenuate inflammation in the setting of TH17-mediated cell injury.
Recent investigations demonstrate that the IL-33 signaling axis promotes an anti-inflammatory Treg phenotype in the setting of colonic inflammation in mice as a means of protecting the large intestinal epithelium from ongoing damage [111]. ST2 is highly expressed on Tregs of the colon and IL-33 promotes expansion of this Treg population and also acts as a “homing beacon” for ST2-expressing Tregs in the setting of inflammation [111]. Intriguingly, IL-23, a pro-inflammatory cytokine with a clearly defined pathogenic role in IBD, inhibits the IL-33/ST2 signaling axis, thereby interrupting the body’s ability to discriminate self from non-self. These data suggest that IL-33 plays an important role in preventing secondary tissue damage in inflammatory reactions, particularly in the large intestine. These combined studies also highlight IL-33 tissue specificity, in which it is important to discriminate between the small and large intestine with respect to inflammation and immune responses.
Moreover, a recent study demonstrated that IL-33 activity can also lead to long term immunosuppression following a systemic inflammatory response via expansion of the Treg population [112]. In an experimental mouse model of septic peritonitis, IL-33 stimulated ILC2-mediated M2 macrophage polarization via type 2 cytokine production, which lead to IL-10 release, activation of Tregs, and ultimately immunosuppression in sepsis survivors [112]. ST2-deficient mice that survived the experimental sepsis model had decreased type 2 cytokine (IL-4, IL-13) release and Treg expansion, with no subsequent immunosuppression and improved survival following secondary infection by L. pneumophila [112]. Furthermore, WT mice following sepsis treated with exogenous sST2 had improved survival with this secondary infection compared to WT mice without sST2, suggesting that depleting IL-33 prevents long-term immune dysfunction [112]. These data correlate with human data that shows survivors of severe sepsis and septic shock had elevated serum levels of IL-33, IL-10, and Tregs [112]. The integration of these mouse and human studies suggest that in the setting of systemic inflammation, IL-33 acts as an alarmin to provide acute initial protection, but also long-term immunosuppression and immune dysfunction via stimulation of Tregs. These studies demonstrate the importance of understanding the interaction between IL-33 signaling and Tregs in the regulation of immune responses in different settings of inflammation, which have therapeutic implications in various tissues.
5. IL-33/ST2 Signaling Axis in Inflammatory Bowel Disease
5.1. Inflammatory bowel disease: Crohn’s and UC
Inflammatory bowel disease (IBD) is an autoimmune disease characterized by chronic inflammation in the bowel [113]. There are two types of IBD, ulcerative colitis (UC), which is characterized by superficial ulcers and epithelial inflammation in continuous stretches of the large intestine and rectum, and Crohn’s disease (CD), which is identified by full thickness ulceration that can be present anywhere along the gastrointestinal tract, including the small and large intestines. Despite being grouped as part of the same disease entity, it has been hypothesized that UC and CD are distinguished on the molecular and immunologic level. The mechanisms of disease pathogenesis in both UC and CD have yet to be fully elucidated. However, both diseases are secondary to autoimmune tissue destruction and are consequently treated with immunomodulators and immunosuppressants. Significant research is underway to investigate the mechanisms of these chronic, debilitating diseases in order to identify new potential targets for treatment.
5.2. Immunological differences between UC and CD
The differences between UC and CD are evident when evaluating the immunologic profiles of these two distinct entities [114]. Although there is overlap in immune cells and cytokines involved in these diseases, there are some trends worth noting. CD is noted for having cytokines indicative of TH1 polarization, whereas UC has a cytokine profile more characteristic for TH2 polarization [114]. A recent clinical study evaluating mRNA expression from diseased intestinal tissue of patients with IBD found increased IL-17, IL-23, and IL-32 in patients with CD compared to control and UC patients, potentially indicative of a more prominent role of TH17 cells in CD in addition to TH1 cells [115]. They also found increased mRNA expression of IL-5, IL-13, IL-15, and IL-33 in patients with UC compared to control and CD, suggestive of a prominent TH2 immune response in UC [115]. Given the increase in mRNA expression of IL-33 in UC, it is suggestive that IL-33 may play a more significant role in UC; however, this requires further investigating [115, 116]. The similarities and differences between these immunologic profiles yield valuable clues on the pathogenesis of IBD. Moreover, they need to be accounted for in the study of experimental models of IBD. Moving forward, we will discuss IL-33 in the context of IBD with specific attention to UC.
5.3. IL-33 and UC
The role of IL-33 as either a pro-inflammatory cytokine behaving as an endogenous alarmin or a nuclear transcriptional repressor of inflammation in the setting of IBD is presently under investigation; nonetheless, studies suggest IL-33 plays in a role in UC. In a study evaluating patients with active UC and patients in remission, they found a significant increase in mucosal IL-33 mRNA expression in patients with acute UC compared to healthy patients [116], corroborating the findings of prior studies that found increased IL-33 expression in the mucosa of the large intestine in active UC [52, 117–121]. In this study, biopsies of active UC lesions were noteworthy for the selective expression of nuclear IL-33 in enterocytes of the intestinal epithelium, specifically in the crypts [116]. This was lost with normalization of mucosal tumor necrosis factor (TNF) expression, which is indicative of disease remission following treatment with infliximab, an anti-TNF immunomodulator, in addition to a significant decrease in mucosal IL-33 expression [116]. These findings suggest IL-33 expression is increased in active UC, specifically by intestinal epithelial cells, that decreases upon disease remission. While this does not define the role IL-33 plays in UC pathogenesis, it warrants further studies in larger patient cohorts. Additionally, the cellular sources of increased mucosal IL-33 expression vary among these studies. Accordingly, future studies necessitate specific attention to cellular and subcellular localization of IL-33.
Moreover, IL-33 receptor ST2 is increased in the mucosa and serum in patients with active UC [52, 120, 122, 123]. It is presently unclear if this is the consequence of increased IL-33 expression during active disease or if this is an inherent defect in IL-33/ST2 signaling unique to IBD. However, its role as a potential serum marker in disease evolution, treatment response, and disease remission is currently being studied [122, 123]. Broadly speaking, these studies provide correlations between dysregulated IL-33 signaling and UC without clear causation. Importantly, however, human studies provide valuable insights in the possible role of IL-33/ST2 signaling in UC pathogenesis and provide context in the study of IL-33 in experimental models of colitis.
5.4. IL-33 Signaling Aberrancy in Experimental Colitis
Despite evidence that IL-33 expression is elevated during active UC, it is unclear if IL-33 signaling has a causative role in pathogenesis or is a consequence of chronic intestinal inflammation during UC. Accordingly, many experimental animal models of colitis exist to study UC, including but not limited to DSS- and 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis.
Given that intestinal IL-33 expression is elevated in UC, it is possible IL-33 is directly involved in the development of intestinal inflammation, behaving as an alarmin and instigating a pro-inflammatory response. Thus, inhibiting IL-33 signaling would be protective in colitis, whereas administration of IL-33 would exacerbate disease. One study utilizing both DSS- and TNBS-induced colitis found genetic ablation of ST2 was protective [124]. Moreover, inhibition of IL-33 signaling using an ST2 neutralizing antibody ameriolated DSS-induced colitis, whereas IL-33 treatment resulted in increased epithelial barrier permeability [124]. Additionally, treatment with recombinant IL-33 (rIL-33) exacerbated DSS-induced colitis via TH2 polarization and inhibition of TH1 immune responses [125–128]. This IL-33-mediated TH2 immune response was corroborated in two models of experimental colitis with upregulation GATA-3, which is necessary for TH2 differentiation [126]. In this respect, the exacerbation of DSS-induced colitis by rIL-33 was absent in IL-4−/− BALB/c mice, reaffirming the role of IL-33 in type 2 immune responses and that inhibition of these immune responses is protective in colitis [125]. These studies suggest that IL-33 signaling and the subsequent type 2 responses exacerbate colitis such that rIL-33 is deleterious in this setting.
However, it is important to distinguish between acute and chronic intestinal inflammation in the evaluation of IL-33 signaling. In one study evaluating DSS-induced colitis, IL-33−/− mice had improved viability in the acute setting, but at days 14 and 15, the viability of IL-33−/− and IL-33+/+ mice were comparable, suggesting IL-33 is an important pro-inflammatory cytokine in the acute setting of DSS-induced inflammation, which is not present with chronic inflammation [62]. Moreover, while IL-33 exacerbated acute experimental colitis, in chronic DSS-induced colitis, there was improved intestinal regeneration with decreased bacterial translocation and the induction of type 2 immune responses with IL-33 administration [129].
Therefore, IL-33 signaling has the potential to be protective in colitis and several studies suggest that this may be the case. Despite the increase in IL-33 expression in experimental colitis, one study found decreased intestinal tissue injury and clinical symptoms with administration of rIL-33 as well as an increase in TH2 cytokines and Tregs [130]. Notably, ST2 is expressed by colonic Tregs [111]. In this study, they found that Tregs promoted by IL-33 signaling can ameliorate inflammation, which is inhibited by IL-23 [111]. However, the protective effects of IL-33 signaling in experimental colitis is not limited to Tregs. Gut-associated ILC2s respond to IL-33 with the expression of growth factor amphiregulin (AREG) such that in DSS-induced colitis, exogenous IL-33 or ILC2 transfer attenuated intestinal inflammation in an AREG-dependent manner [56].
In other studies, IL-33 was protective in colitis by inducing polarization of macrophages to M2 macrophages, which are typically associated with TH2 cytokines and promote resolution of inflammation [131, 132]. In one study, these M2 macrophages, which they termed alternatively activated macrophages, were isolated from IL-33-treated mice and transferred to mice with TNBS-induced colitis [131]. This conferred protection to recipient mice, in which there was a significant decrease in intestinal disease and inflammatory markers [131]. A similar finding was found in which peritoneal injection of IL-33 in experimental colitis induced M2 macrophage polarization, which subsequently resulted in goblet cell differentiation and attenuated inflammation [132]. Taken together, these studies suggest IL-33 signaling may be protective in experimental colitis via induction of type 2 immune responses and induction of immune cells involved in mitigating intestinal inflammation.
Presently, it is unclear if IL-33/ST2 signaling in protective in experimental colitis and UC, however there are some trends evident in these studies. IL-33 expression is increased in inflamed mucosa of UC and experimental colitis. Exogenous IL-33 may attenuate or provoke intestinal inflammation; however, its effects are predominantly secondary to the ability of IL-33 to induce type 2 immune responses via activation of TH2 cells. Timing of inflammation is also important in understanding the effects of IL-33, such that distinguishing acute and chronic inflammation is necessary, and several questions remain and require further investigating. Specifically, it is ambiguous if IL-33 is a consequence of intestinal inflammation or if IL-33 is a key instigator in promoting an inflammatory response. It remains unclear how IL-33 signaling is dysregulated in UC and experimental colitis. Lastly, the precise mechanism of protection by exogenous IL-33 remains unknown. Moreover, it is important to discern supplementation with exogenous IL-33 in treatment of colitis from the role of endogenous IL-33 in disease patheogenesis and treatment. However, our understanding of IL-33 in experimental colitis and UC has expanded greatly over the past few years and warrants further studies to answer these questions.
6. Conclusions
IL-33 is a unique cytokine defined by its duality, acting as both a traditional extracellular cytokine as well as a nuclear transcription factor. IL-33 readily interacts with both the innate immune system and adaptive immune system. It is both admired for its maintenance of tissue homeostasis and provides a promising target for disease therapeutics. In this review, we hope to have highlighted its remarkable importance in intestinal immunity. IL-33 with its receptor ST2 are positioned to interact with major components of the intestine, which include the epithelial cells in response to cell injury, the microbiome composed of commensal bacteria, ingested pathogenic bacteria, and immune cells of the mucosa, notably TH2 cells, Tregs, and TH17 cells. In this respect, IL-33 behaves as an alarmin in response to epithelial cell injury to initiate the innate immune response. It is important to highlight the role of IL-33 is context dependent, and thus in a steady state, does not behave in the same manner as its more defined role in epithelial cell injury. It also behaves as an important inducer of TH2 and thus type 2 immune responses that aim to combat helminth infections and reduce type 1 immune responses to maintain tissue integrity. Given its implication in intestinal immunity, the role of IL-33/ST2 signaling in IBD is an area of active investigation. Presently, it is unclear whether aberrancy of IL-33 signaling drives IBD pathogenesis or is secondary to the inflammatory state of the disease. However, there is a clear need to further study IL-33 in this context for the potential development of immunomodulatory therapeutics in IBD. The goal of this review was to highlight the role of IL-33 in intestinal immunity with the hopes of identifying important studies to drive further investigations into this distinct cytokine.
Highlights.
IL-33 plays an important role in intestinal barrier immunity via activation of the innate immune system in the setting of cell injury
IL-33 interacts with the microbiota of the intestine to facilitate homeostasis
IL-33 is a critical regulator of adaptive immunity and Th17/Treg balance
The IL-33/ST2 signaling axis has been implicated in the pathophysiology of inflammatory bowel disease
Acknowledgments
Funding Sources
MG is supported by grants K08DK101608 and R03DK111473 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17-79, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, and the Department of Pediatrics at Washington University School of Medicine, St. Louis. EMS received support from the NIH Medical Scientist Training Program Training Grant T32 GM07200.
Abbreviations
- IL
Interleukin
- IL-1RAcP
IL-1 receptor accessory protein
- DAMP
Damage associated molecular patterns
- TLRs
Toll-like receptors
- ILC
Innate lymphoid cells
- AREG
Amphiregulin
- EGFR
Epidermal growth factor receptor
- IBD
Inflammatory bowel disease
- UC
Ulcerative colitis
- CD
Crohn’s Disease
- IEC
Intestinal epithelial cells
- Treg
Regulatory T cells
- DSS
Dextran sodium sulfate
- MyD88
Myeloid differentiation primary response protein 88
- Tc
Cytotoxic T cells
Footnotes
Disclosures: The authors have nothing to disclose and no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Ali S. The dual function cytokine IL-33 interacts with the transcription factor NF-kB to dampen NF-kB-stimulated gene transcription. J Immunol. 2011;187:1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 3.Lohning M. T1/ST2 is preferentially expressed on murine TH2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for TH2 effector function. Proc Natl Acad Sci USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 5.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nature immunology. 2016;17(2):122–31. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 6.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7(10):827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin MU. Special aspects of interleukin-33 and the IL-33 receptor complex. Semin Immunol. 2013;25(6):449–57. doi: 10.1016/j.smim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Pichery M. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 9.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3(10):e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefrancais E. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(43):15502–7. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104(1):282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9(10):1006–12. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173(4):1229–42. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundlisaeter E, Edelmann RJ, Hol J, Sponheim J, Kuchler AM, Weiss M, Udalova IA, Midwood KS, Kasprzycka M, Haraldsen G. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am J Pathol. 2012;181(3):1099–111. doi: 10.1016/j.ajpath.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Baekkevold ES, Roussigné M, Yamanaka T, Johansen F-E, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard J-P. Molecular Characterization of NF-HEV, a Nuclear Factor Preferentially Expressed in Human High Endothelial Venules. The American Journal of Pathology. 2003;163(1):69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali S, Nguyen DQ, Falk W, Martin MU. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem Biophys Res Commun. 2010;391(3):1512–6. doi: 10.1016/j.bbrc.2009.12.107. [DOI] [PubMed] [Google Scholar]
- 18.Kunisch E, Chakilam S, Gandesiri M, Kinne RW. IL-33 regulates TNF-alpha dependent effects in synovial fibroblasts. Int J Mol Med. 2012;29(4):530–40. doi: 10.3892/ijmm.2012.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshio T, Komine M, Tsuda H, Tominaga SI, Saito H, Nakae S, Ohtsuki M. Nuclear expression of IL-33 in epidermal keratinocytes promotes wound healing in mice. J Dermatol Sci. 2017;85(2):106–114. doi: 10.1016/j.jdermsci.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol. 2009;183(12):7890–7. doi: 10.4049/jimmunol.0802449. [DOI] [PubMed] [Google Scholar]
- 21.Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, Cote-Sierra J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun. 2014;55:33–41. doi: 10.1016/j.jaut.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Lefrancais E, Cayrol C. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. Eur Cytokine Netw. 2012;23(4):120–7. doi: 10.1684/ecn.2012.0320. [DOI] [PubMed] [Google Scholar]
- 23.Carta S, Lavieri R, Rubartelli A. Different Members of the IL-1 Family Come Out in Different Ways: DAMPs vs. Cytokines? Frontiers in Immunology. 2013;4:123. doi: 10.3389/fimmu.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaczmarek A, Vandenabeele P, Krysko Dmitri V. Necroptosis: The Release of Damage-Associated Molecular Patterns and Its Physiological Relevance. Immunity. 2013;38(2):209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2012;24 doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimokawa C, Kanaya T, Hachisuka M, Ishiwata K, Hisaeda H, Kurashima Y, Kiyono H, Yoshimoto T, Kaisho T, Ohno H. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity. 2017;46(5):863–874 e4. doi: 10.1016/j.immuni.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol. 2011;43(9):1383–91. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84(3):631–43. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a Mechanically Responsive Cytokine Secreted by Living Cells. The Journal of biological chemistry. 2012;287(9):6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Chen Q, Li H, Myerburg M, Spannhake EW, Natarajan V, Zhao Y. Lysophosphatidic acid increases soluble ST2 expression in mouse lung and human bronchial epithelial cells. Cell Signal. 2012;24(1):77–85. doi: 10.1016/j.cellsig.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. The Journal of clinical investigation. 2007;117(6):1538–49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen WY, Hong J, Gannon J, Kakkar R, Lee RT. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(23):7249–54. doi: 10.1073/pnas.1424236112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS letters. 1993;318(1):83–7. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 34.Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, Katashima R, Itakura M, Tominaga S. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264(2):397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 35.Iwahana H, Hayakawa M, Kuroiwa K, Tago K, Yanagisawa K, Noji S, Tominaga S. Molecular cloning of the chicken ST2 gene, a novel variant form of the ST2 gene product, ST2LV. Biochim Biophys Acta. 2004;1681(1):1–14. doi: 10.1016/j.bbaexp.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Tominaga S, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Komatsu N. Presence and expression of a novel variant form of ST2 gene product in human leukemic cell line UT-7/GM. Biochemical and biophysical research communications. 1999;264(1):14–8. doi: 10.1006/bbrc.1999.1469. [DOI] [PubMed] [Google Scholar]
- 37.Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, Tominaga S. Tissue distribution, subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochemical and biophysical research communications. 2001;285(5):1377–83. doi: 10.1006/bbrc.2001.5306. [DOI] [PubMed] [Google Scholar]
- 38.Lingel A, Weiss TM, Niebuhr M, Pan B, Appleton BA, Wiesmann C, Bazan JF, Fairbrother WJ. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors--insight into heterotrimeric IL-1 signaling complexes. Structure. 2009;17(10):1398–410. doi: 10.1016/j.str.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer G, Lipsky BP, Smithgall MD, Meininger D, Siu S, Talabot-Ayer D, Gabay C, Smith DE. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine. 2008;42(3):358–64. doi: 10.1016/j.cyto.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Hammel M, He Y, Tainer JA, Jeng US, Zhang L, Wang S, Wang X. Structural insights into the interaction of IL-33 with its receptors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(37):14918–23. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. Journal of immunology. 2007;179(4):2551–5. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 42.Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18660–5. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Numata T, Ito T, Maeda T, Egusa C, Tsuboi R. IL-33 promotes ICAM-1 expression via NF-kB in murine mast cells. Allergol Int. 2016;65(2):158–65. doi: 10.1016/j.alit.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):9021–6. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31(1):84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Cohen ES, Scott IC, Majithiya JB, Rapley L, Kemp BP, England E, Rees DG, Overed-Sayer CL, Woods J, Bond NJ, Veyssier CS, Embrey KJ, Sims DA, Snaith MR, Vousden KA, Strain MD, Chan DT, Carmen S, Huntington CE, Flavell L, Xu J, Popovic B, Brightling CE, Vaughan TJ, Butler R, Lowe DC, Higazi DR, Corkill DJ, May RD, Sleeman MA, Mustelin T. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Commun. 2015;6:8327. doi: 10.1038/ncomms9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. The Journal of biological chemistry. 2012;287(11):8205–13. doi: 10.1074/jbc.M111.295055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J, Wei J, Mialki RK, Mallampalli DF, Chen BB, Coon T, Zou C, Mallampalli RK, Zhao Y. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nature immunology. 2012;13(7):651–8. doi: 10.1038/ni.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bulek K, Swaidani S, Qin J, Lu Y, Gulen MF, Herjan T, Min B, Kastelein RA, Aronica M, Kosz-Vnenchak M, Li X. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. Journal of immunology. 2009;182(5):2601–9. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochemical and biophysical research communications. 2002;299(1):18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- 51.Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, Koehn BH, Pfeifer D, Taylor PA, Prinz G, Dierbach H, Stickel N, Beck Y, Warncke M, Junt T, Schmitt-Graeff A, Nakae S, Follo M, Wertheimer T, Schwab L, Devlin J, Watkins SC, Duyster J, Ferrara JL, Turnquist HR, Zeiser R, Blazar BR. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125(20):3183–92. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107(17):8017–22. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehraj V, Jenabian MA, Ponte R, Lebouche B, Costiniuk C, Thomas R, Baril JG, LeBlanc R, Cox J, Tremblay C, Routy JP. t.C.L.-T.N.-P.S.G. Montreal Primary Hiv Infection, The plasma levels of soluble ST2 as a marker of gut mucosal damage in early HIV infection. Aids. 2016;30(10):1617–27. doi: 10.1097/QAD.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 55.Mahapatro M, Foersch S, Hefele M, He G-W, Giner-Ventura E, McHedlidze T, Kindermann M, Vetrano S, Danese S, Günther C, Neurath Markus F, Wirtz S, Becker C. Programming of Intestinal Epithelial Differentiation by IL-33 Derived from Pericryptal Fibroblasts in Response to Systemic Infection. Cell Reports. 2016;15(8):1743–1756. doi: 10.1016/j.celrep.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 56.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin–EGFR interactions. Proceedings of the National Academy of Sciences. 2015;112(34):10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. The Journal of allergy and clinical immunology. 2015;135(3):626–35. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–82. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 59.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J-i, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 60.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, Sudo K, Okumura K, Saito H, Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107(43):18581–6. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nature immunology. 2016;17(7):765–74. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 64.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology. 2011;12(11):1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314(5806):1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- 66.Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM, Jr, Wilson KT, Polk DB. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. The Journal of clinical investigation. 2011;121(6):2242–53. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sipos F, Molnar B, Zagoni T, Berczi L, Tulassay Z. Growth in epithelial cell proliferation and apoptosis correlates specifically to the inflammation activity of inflammatory bowel diseases: ulcerative colitis shows specific p53- and EGFR expression alterations. Diseases of the colon and rectum. 2005;48(4):775–86. doi: 10.1007/s10350-004-0831-5. [DOI] [PubMed] [Google Scholar]
- 69.Sipos F, Muzes G, Valcz G, Galamb O, Toth K, Leiszter K, Krenacs T, Tulassay Z, Molnar B. Regeneration associated growth factor receptor and epithelial marker expression in lymphoid aggregates of ulcerative colitis. Scandinavian journal of gastroenterology. 2010;45(4):440–8. doi: 10.3109/00365521003624144. [DOI] [PubMed] [Google Scholar]
- 70.Veiga-Fernandes H, Pachnis V. Neuroimmune regulation during intestinal development and homeostasis. Nature immunology. 2017;18(2):116–122. doi: 10.1038/ni.3634. [DOI] [PubMed] [Google Scholar]
- 71.Chow AK, Gulbransen BD. Potential roles of enteric glia in bridging neuroimmune communication in the gut. American journal of physiology. Gastrointestinal and liver physiology. 2017;312(2):G145–G152. doi: 10.1152/ajpgi.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Margolis KG, Gershon MD. Enteric Neuronal Regulation of Intestinal Inflammation. Trends Neurosci. 2016;39(9):614–24. doi: 10.1016/j.tins.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nature reviews. Gastroenterology & hepatology. 2017;14(3):143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- 74.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. The Journal of clinical investigation. 2015;125(3):918–25. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D’Alessandro A, Cammarota M, Giuliano M, Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut. 2014;63(1):105–15. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- 76.Ibiza S, Garcia-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, Eberl G, Grice EA, Veiga-Fernandes H. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535(7612):440–3. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang DK, He FQ, Li TK, Pang XH, Cui DJ, Xie Q, Huang XL, Gan HT. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol. 2010;222(2):213–22. doi: 10.1002/path.2749. [DOI] [PubMed] [Google Scholar]
- 78.Meir M, Flemming S, Burkard N, Bergauer L, Metzger M, Germer CT, Schlegel N. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. American journal of physiology. Gastrointestinal and liver physiology. 2015;309(8):G613–24. doi: 10.1152/ajpgi.00357.2014. [DOI] [PubMed] [Google Scholar]
- 79.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. Journal of immunology. 2003;171(2):948–54. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 80.Oh JE, Kim BC, Chang DH, Kwon M, Lee SY, Kang D, Kim JY, Hwang I, Yu JW, Nakae S, Lee HK. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(6):E762–71. doi: 10.1073/pnas.1518589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belkaid Y, Harrison OJ. Homeostatic Immunity and the Microbiota. Immunity. 2017;46(4):562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 84.van den Elsen LW, Poyntz HC, Weyrich LS, Young W, Forbes-Blom EE. Embracing the gut microbiota: the new frontier for inflammatory and infectious diseases. Clin Transl Immunology. 2017;6(1):e125. doi: 10.1038/cti.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 86.Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti T-D. IL-33 regulates the IgA-microbiota axis to restrain IL-1α–dependent colitis and tumorigenesis. The Journal of clinical investigation. 2016;126(12):4469–4481. doi: 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–10. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stephens WZ, Round JL. IgA targets the troublemakers. Cell Host Microbe. 2014;16(3):265–7. doi: 10.1016/j.chom.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, Harris NL. IL-1β Suppresses Innate IL-25 and IL-33 Production and Maintains Helminth Chronicity. PLOS Pathogens. 2013;9(8):e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187(5):787–94. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13463–8. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. The Journal of allergy and clinical immunology. 2009;123(5):1047–54. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hussaarts L, Yazdanbakhsh M, Guigas B. Priming dendritic cells for th2 polarization: lessons learned from helminths and implications for metabolic disorders. Front Immunol. 2014;5:499. doi: 10.3389/fimmu.2014.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, Herbert DR. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):282–7. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson S, Jones FM, Fofana HK, Landoure A, Kimani G, Mwatha JK, Sacko M, Vennervald BJ, Dunne DW. A late IL-33 response after exposure to Schistosoma haematobium antigen is associated with an up-regulation of IL-13 in human eosinophils. Parasite Immunol. 2013;35(7–8):224–8. doi: 10.1111/pim.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 97.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, McKenzie AN. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132(4):933–41. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 99.Omenetti S, Pizarro TT. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luo A, Leach ST, Barres R, Hesson LB, Grimm MC, Simar D. The Microbiota and Epigenetic Regulation of T Helper 17/Regulatory T Cells: In Search of a Balanced Immune System. Front Immunol. 2017;8:417. doi: 10.3389/fimmu.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 103.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 104.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–59. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 105.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 106.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 107.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 109.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pascual-Reguant A, Bayat Sarmadi J, Baumann C, Noster R, Cirera-Salinas D, Curato C, Pelczar P, Huber S, Zielinski CE, Lohning M, Hauser AE, Esplugues E. TH17 cells express ST2 and are controlled by the alarmin IL-33 in the small intestine. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.5. [DOI] [PubMed] [Google Scholar]
- 111.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Lohning M, Belkaid Y, Fallon PG, Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513(7519):564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nascimento DC, Melo PH, Pineros AR, Ferreira RG, Colon DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, Borges MC, Zamboni DS, Liew FY, Cunha FQ, Alves-Filho JC. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–42. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 114.Muzes G, Molnar B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18(41):5848–61. doi: 10.3748/wjg.v18.i41.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nemeth ZH, Bogdanovski DA, Barratt-Stopper P, Paglinco SR, Antonioli L, Rolandelli RH. Crohn’s Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus. 2017;9(4):e1177. doi: 10.7759/cureus.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gundersen MD, Goll R, Hol J, Olsen T, Rismo R, Sorbye SW, Sundnes O, Haraldsen G, Florholmen J. Loss of interleukin 33 expression in colonic crypts - a potential marker for disease remission in ulcerative colitis. Sci Rep. 2016;6:35403. doi: 10.1038/srep35403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45(10):999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 118.Seidelin JB. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80–85. doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 119.Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarstrom C, Loos T, Kasprzycka M, Sorensen DR, Nilsen HR, Kuchler AM, Vatn MH, Haraldsen G. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177(6):2804–15. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beltran CJ, Nunez LE, Diaz-Jimenez D, Farfan N, Candia E, Heine C, Lopez F, Gonzalez MJ, Quera R, Hermoso MA. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(7):1097–107. doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- 121.Latiano A, Palmieri O, Pastorelli L, Vecchi M, Pizarro TT, Bossa F, Merla G, Augello B, Latiano T, Corritore G, Settesoldi A, Valvano MR, D’Inca R, Stronati L, Annese V, Andriulli A. Associations between genetic polymorphisms in IL-33, IL1R1 and risk for inflammatory bowel disease. PLoS One. 2013;8(4):e62144. doi: 10.1371/journal.pone.0062144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Diaz-Jimenez D, De la Fuente M, Dubois-Camacho K, Landskron G, Fuentes J, Perez T, Gonzalez MJ, Simian D, Hermoso MA, Quera R. Soluble ST2 is a sensitive clinical marker of ulcerative colitis evolution. BMC Gastroenterol. 2016;16:103. doi: 10.1186/s12876-016-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boga S, Alkim H, Koksal AR, Ozagari AA, Bayram M, Tekin Neijmann S, Sen I, Alkim C. Serum ST2 in inflammatory bowel disease: a potential biomarker for disease activity. J Investig Med. 2016;64(5):1016–24. doi: 10.1136/jim-2016-000062. [DOI] [PubMed] [Google Scholar]
- 124.Sedhom MA, Pichery M, Murdoch JR, Foligne B, Ortega N, Normand S, Mertz K, Sanmugalingam D, Brault L, Grandjean T, Lefrancais E, Fallon PG, Quesniaux V, Peyrin-Biroulet L, Cathomas G, Junt T, Chamaillard M, Girard JP, Ryffel B. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62(12):1714–23. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pushparaj PN, Li D, Komai-Koma M, Guabiraba R, Alexander J, McSharry C, Xu D. Interleukin-33 exacerbates acute colitis via interleukin-4 in mice. Immunology. 2013;140(1):70–7. doi: 10.1111/imm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seidelin JB, Coskun M, Kvist PH, Holm TL, Holgersen K, Nielsen OH. IL-33 promotes GATA-3 polarization of gut-derived T cells in experimental and ulcerative colitis. J Gastroenterol. 2015;50(2):180–90. doi: 10.1007/s00535-014-0982-7. [DOI] [PubMed] [Google Scholar]
- 127.Zhu J, Xu Y, Zhu C, Zhao J, Meng X, Chen S, Wang T, Li X, Zhang L, Lu C, Liu H, Sun X. IL-33 induces both regulatory B cells and regulatory T cells in dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2017;46:38–47. doi: 10.1016/j.intimp.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 128.Zhu J, Yang F, Sang L, Zhai J, Zhang X, Yue D, Li S, Li Y, Lu C, Sun X. IL-33 Aggravates DSS-Induced Acute Colitis in Mouse Colon Lamina Propria by Enhancing Th2 Cell Responses. Mediators Inflamm. 2015;2015:913041. doi: 10.1155/2015/913041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grobeta P, Doser K, Falk W, Obermeier F, Hofmann C. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis. 2012;18(10):1900–9. doi: 10.1002/ibd.22900. [DOI] [PubMed] [Google Scholar]
- 130.Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F, Fang M. IL-33 ameliorates experimental colitis through promoting Th2/Foxp3(+) Treg responses in mice. Mol Med. 2012;18 doi: 10.2119/molmed.2011.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tu L, Chen J, Xu D, Xie Z, Yu B, Tao Y, Shi G, Duan L. IL-33-induced alternatively activated macrophage attenuates the development of TNBS-induced colitis. Oncotarget. 2017 doi: 10.18632/oncotarget.15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seo DH, Che X, Kwak MS, Kim S, Kim JH, Ma HW, Kim DH, Kim TI, Kim WH, Kim SW, Cheon JH. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep. 2017;7(1):851. doi: 10.1038/s41598-017-00840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]