Abstract
Pain and depression often co-occur, but the underlying mechanisms have not been elucidated. Here, we used the spared nerve injury (SNI) model in mice to induce both neuropathic pain and depression-like behavior. We investigated whether brain IL-1 signaling and activity of kynurenine 3-monoxygenase (KMO), a key enzyme for metabolism of kynurenine into the neurotoxic NMDA receptor agonist quinolinic acid, are necessary for comorbid neuropathic pain and depression-like behavior.
SNI mice showed increased expression levels of Il1b and Kmo mRNA in the contralateral side of the brain. The SNI-induced increase of Kmo mRNA was associated with increased KMO protein and elevated quinolinic acid and reduced kynurenic acid in the contralateral hippocampus. The increase in KMO-protein in response to SNI mostly took place in hippocampal NeuN-positive neurons rather than microglia.
Inhibition of brain IL-1 signaling by intracerebroventricular administration of IL-1RA after SNI prevented the increase in Kmo mRNA and depression-like behavior measured by forced swim test. However, inhibition of brain IL-1 signaling has no effect on mechanical allodynia. In addition, intracerebroventricular administration of the KMO inhibitor Ro 61-8048 abrogated depression-like behavior without affecting mechanical allodynia after SNI.
We show for the first time that the development of depression-like behavior in the SNI model requires brain IL-1 signaling and activation of neuronal KMO, while pain is independent of this pathway. Inhibition of KMO may represent a promising target for treating depression.
Keywords: comorbidity, pain, depression, interleukin-1, kynurenine 3-monooxygenase, hippocampus, kynurenine pathway, quinolinic acid, psychoneuroimmunology
1. Introduction
The lifetime prevalence of major depressive disorder in the general population is in the range of 10–20% (Demyttenaere, et al. 2004; Kessler, et al. 2012). However, the prevalence of depression is significantly higher in individuals presenting with chronic pain (20–80%), indicating that chronic pain is an important risk factor for developing depression (Bair, et al. 2003; Gustorff, et al. 2008; Leo 2005; Poole, et al. 2009). The treatment of major depression remains a major challenge. Only a small portion of patients with major depression responds to treatments targeting monoamine reuptake (Souery, et al. 2011; Thase, et al. 2001). Moreover, patients with chronic pain and depression are poorly responsive to current antidepressant treatments (Karp, et al. 2005).
Depression is associated with activation of the innate immune system (Capuron, et al. 2002; Dowlati, et al. 2010; Miller and Raison 2016). Preclinical studies have shown that peripheral inflammation induces depression-like behavior in addition to sickness in rodents (Dantzer, et al. 2008). The development of inflammation-induced depression-like behavior is mediated by activation of the tryptophan degrading enzyme indoleamine 2,3-dioxygenase IDO1 (O’Connor, et al. 2009). IDO1 initiates the metabolism of tryptophan into kynurenine (KYN). Kynurenine can be further converted to 3-hydroxy-kynurenine (3-HK) by kynurenine 3-monooxygenase (KMO), and ultimately transformed into quinolinic acid (QA) by kynureninase (KYNU) and 3-hydroxyanthralinic acid dioxygenase (HAAO) (Schwarcz and Stone 2017). Quinolinic acid is a neurotoxic N-methyl-D-aspartate receptor (NMDAR) agonist (Guillemin 2012; Santamaria and Rios 1993). Elevations of QA concentrations have been reported in serum and CSF of patients with symptoms of depression (Baranyi, et al. 2015; Bay-Richter, et al. 2015; Raison, et al. 2010; Savitz, et al. 2015b; Vogelgesang, et al. 1996). Interestingly, elevated QA is associated with smaller hippocampal volume in unmedicated individuals with major depression (Savitz, et al. 2015a), which is important because reduced hippocampal volume is a hallmark of major depressive disorder (Arnone, et al. 2016). Modulating the kynurenine pathways has been proposed to represent a potential novel strategy to treat depression (Dantzer, et al. 2011; Parrott and O’Connor 2015).
The role of inflammation in the pathophysiology of depression is usually studied in rodents injected with the potent pro-inflammatory cytokine inducer lipopolysaccharide (LPS). LPS induces a transient episode of depression-like behavior followed by a return to baseline within 48 hours (Dantzer, et al. 2008). In this model, the development of depression-like behavior is dependent on the activation of NMDA receptors by elevated QA (Walker, et al. 2013). Kynurenine metabolism is strongly skewed toward production of neurotoxic KMO-dependent metabolites in the hippocampus following LPS challenge (Parrott, et al. 2016a). Genetic deletion of Kmo or Haao, the downstream enzyme of KMO responsible for QA production, prevents depression-like behavior in response to LPS (Parrott, et al. 2016b). Depression-like behavior is also observed in animal models of neuropathic pain induced by peripheral nerve injury (Kontinen, et al. 1999; Norman, et al. 2010; Zhou, et al. 2015). In contrast to LPS, nerve injury induces a long-lasting (if not permanent) depression-like behavior together with a low grade peripheral inflammation, which closely reflects what is observed clinically.
In a previous study, we demonstrated that depression-like behavior in mice submitted to spared nerve injury (SNI) is mediated by activation of peripheral but not brain IDO1 in association with increased circulating KYN (Zhou, et al. 2015). Circulating KYN can enter the brain (Kita, et al. 2002) where it can be metabolized further into neuroprotective kynurenic acid (KYNA) or neurotoxic 3-HK and QA metabolites. This last metabolic pathway is downstream of activation of KMO. We hypothesized that the development of depression-like behavior in SNI mice is dependent on brain KMO activity which is the rate limiting step for the metabolism of brain KYN into neurotoxic QA. Very little is known about the cellular basis of this metabolic step. It is commonly accepted that inflammation activates KMO in microglia (Corona, et al. 2010; Gonzalez-Pena, et al. 2016; Guillemin, et al. 2003). However, IL-1β has been shown in vitro to increase the expression of Kmo mRNA in primary cultures of hippocampal neurons (Zunszain, et al. 2012). As IL-1β is up-regulated in the brain of rodents subjected to SNI (del Rey, et al. 2011; Norman, et al. 2010) it could also up-regulate the expression of Kmo in neurons.
The goal of this study was to determine the contribution of brain IL-1β signaling and KMO to SNI-induced mechanical allodynia and depression-like behavior.
2. Materials and Methods
2.1. Animals
Male C57BL/6J mice (10–16 weeks old, The Jackson Laboratory, Bar Harbor, ME) were individually housed on a reversed light/dark cycle (lights on at 10:00 pm and off at 10:00 am). Water and food were available ad libitum. The study was conducted in accordance with NIH guidelines for the care and use of animals and under protocols approved by the Institutional Animal Care and Use Committee.
2.2. Surgery
SNI surgery was performed as described (Bourquin, et al. 2006; Laumet, et al. 2015). Briefly, the sural common peroneal and tibial branches of the left sciatic nerve were exposed under isoflurane anesthesia. The tibial and common peroneal nerves were transected. The sural nerve was kept intact. For sham surgery, nerves were exposed but not transected. I.c.v. cannula implantation was performed 7–9 days prior to SNI surgery under ketamine (100 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (10 mg/kg; LLOYD Laboratories, Shenandoah, IA) anesthesia. For intracerebroventricular cannulation, mice were secured in a stereotaxic frame, (Stoelting, Wood Dale, IL), a burr hole was drilled 0.6 mm posterior to and 1.5 mm lateral to Bregma. A guide cannula (PlasticsOne, Roanoke, VA) with projection length of 1.3 mm was inserted into the ventricle. Animals were terminated on the 8th day after SNI for tissue collection after ice-cold PBS perfusion.
2.3. Behavior
Mechanical allodynia was monitored using von Frey hairs. The 50% paw withdrawal threshold was calculated using the up-and-down method as previously described (Chaplan, et al. 1994; Laumet, et al. 2015).
Depression-like behavior was measured by increased immobility in the forced swim test (FST) (Norman, et al. 2010; Walker, et al. 2013). FST was performed on day 7 after SNI surgery. Behavior was recorded for 5 min after mice were placed for 1 min in a bucket of water at 25 ± 0.2°C.
Spontaneous locomotor activity was measured on day 7 after SNI surgery. The mice were individually placed into a new empty cage devoid of litter. The cage was divided into four virtual parts, and locomotor activity was measured by counting the number of line crossings over a 5-min period.
2.4. Drug Administration
The type I IL-1 receptor antagonist IL-1RA (R&D Systems, Minneapolis, MN) was given i.c.v. (40 ng/4 μL/day in saline) on days 6 and 7 after SNI surgery (Norman, et al. 2010). The KMO inhibitor Ro 61-8048 (Sigma-Aldrich, St. Louis, MO) was given i.c.v on days 6 and 7 after SNI surgery (0.4 μg/2 μl/day in 20 % DMSO in saline)(Hamann, et al. 2008; Rover, et al. 1997). Mice were given a 4-hour interval between i.c.v. injection of drugs and behavioral tests. The analgesic retigabine (#R-100, Alomone laboratory, Jerusalem, Israel) was administered intraperitoneally at a dose of 10 mg/kg in PBS 20 min before behavioral testing (Krukowski, et al. 2017).
2.5. Real-Time PCR
Total RNA was extracted in TRIzol reagent (Life Tech., Carlsbad, CA) from ipsilateral and contralateral prefrontal cortex (defined as the cortex part of the frontal lobe), hippocampi and lumbar spinal cord. One μg was reverse transcribed to cDNA using a high capacity cDNA reverse transcription kit (Life Tech., Foster City, CA)2. Real-time qPCR was performed on an Applied Biosystems ViiA using PrimeTime qPCR assays for IL-1β (exon3-4, NM_008361 3–4), Kmo (exon10-11, NM_133809 10–11), Kynu (exon9-11, NM_001032998), Haao (exon4-5, NM_012205) and Gapdh (exon2-3, NM_008084 2–3); all from Integrated DNA Technologies, Coraville, IA. Amplifications without template were included as negative controls. Relative quantitative measurement of target gene levels corrected for GAPDH was performed using the ΔΔCt method.
2.6. Western-blot
Ipsilateral and contralateral hippocampi were homogenized in 250 μl RIPA buffer (Pierce) in the presence of proteinase inhibitor cocktail P8340 (Sigma-Aldrich, St. Louis, MO). Lysates were centrifuged at 16,000 g for 10 min at 4°C. The supernatant was collected, and the protein concentration was measured using Bradford assay kit (Bio-Rad, Hercules, CA). Fifty μg of total proteins of each sample was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were transferred to nitrocellulose membranes. The membranes were incubated overnight at 4°C with anti-KMO (#10698, Proteintech, Rosemont, IL) diluted 1/1,000 in a 5 % of blocking buffer solution, followed by horseradish peroxidase–conjugated goat anti-rabbit secondary antibody (#111-035-144, Jackson ImmunoResearch, West Grove, PA) diluted 1/4,000 for 1 h at room temperature. The protein band was revealed with an ECL (GE Healthcare), and the protein band intensity was quantified by using the ImageQuant LAS4000 (GE Healthcare). The results were normalized for β-actin (#A3854, Sigma Aldrich, St Louis, MO diluted 1/5,000 in blocking buffer) used as a protein loading control.
2.7. High-Performance Liquid chromatography and mass-spectrometry (HPLC-MS)
We used HPLC-MS to determine brain levels of tryptophan and kynurenine metabolites as previously described (Walker, et al. 2013; Zhou, et al. 2015). Hippocampus samples were homogenized. Resultant samples were then filtered using a 3 kDa 0.5 mL Millipore Amicon Ultra filter which was spun down at 13,500 g for 60 min at 4°C. Injection of the resulting solution was performed in triplicate for analysis of each sample.
Standard curves were prepared using pure compounds (KYN, KYNA, and quinolinic acid (QA) purchased from Sigma dissolved in 0.2% acetic acid. Internal standards (13C6-KYN,) were added to each standard and sample to examine and correct for sample matrix and instrument variation.
Samples were analyzed with a Waters Acquity HPLC system equipped with an YMC ODS AQ 2×100 mm, 3 μm particle column which provided separation of the kynurenine metabolites prior to detection by a Waters Quattro Premier XE triple quadrupole mass spectrometer operating in the MS/MS configuration. Full loop injections with a 3 time overfill were performed with a 5 μL loop requiring a total sample volume of 15 μL. Column and pre-column tubing were maintained at 40°C while eluting kynurenine metabolites
This resulted in the following conditions: capillary voltage 1.5 V, cone voltage 15 V, source temperature 150°C and desolvation temperature 500°C.
2.8. Immunofluorescence
Mice were perfused with ice-cold 4% para-formaldehyde/0.01M PBS (pH 7.4). Frozen perfusion-fixed brains were sliced on Leica CM3050 S Cryostat. Coronal sections (10 μm) were labeled with goat anti-mouse Iba1 (1:500) (Abcam #ab5076, Cambridge, UK) and/or mouse anti-mouse NeuN (1:50) (Millipore, Temecula, CA) and rabbit anti-mouse KMO (1:50) (Proteintech, Rosemoont, IL) antibodies followed by secondary goat anti-mouse and goat anti-rabbit fluorescent antibodies (1:1000) (Alexa Fluor, Eugene, OR). Brain slices from KMO knock-out mice (Heisler and O’Connor 2015) were used as negative control. Omission of the primary antibody served as negative control. Images were captured with a Leica confocal microscope (Leica, CTR4000).
2.9. Statistical Analysis
The data are expressed as means ± SEM. Testing of statistical significance was performed using two-way ANOVA or two-way repeated-measures ANOVA for mechanical allodynia experiments followed by Bonferroni’s multiple test correction.
3. Results
3.1. Depression-like behavior in SNI mice is not dependent on mechanical allodynia
SNI mice displayed mechanical allodynia and increased duration of immobility in FST (Norman, et al. 2010; Zhou, et al. 2015). To ensure that increased duration of immobility as a measure of depression-like behavior is not biased by increased mechanical allodynia, we examined the effect of the analgesic retigabine (10 mg/kg) on FST. First, we confirmed that 10 mg/kg retigabine reversed mechanical allodynia after SNI (Figure S1A). Sham- and SNI-treated mice were injected with PBS or retigabine and submitted 20 min later to the FST. Retigabine did not affect the enhanced immobility time of SNI-treated mice in the FST, indicating that retigabine-induced acute pain relief has no antidepressant effect (Figure S1B). Retigabine also had no effect on general activity of mice (Figure S1C). In the SNI model of depression-like behavior, pain was not driving the increased immobility in the FST nor affected spontaneous locomotor activity.
3.2. Peripheral nerve injury upregulates brain KMO expression and activity
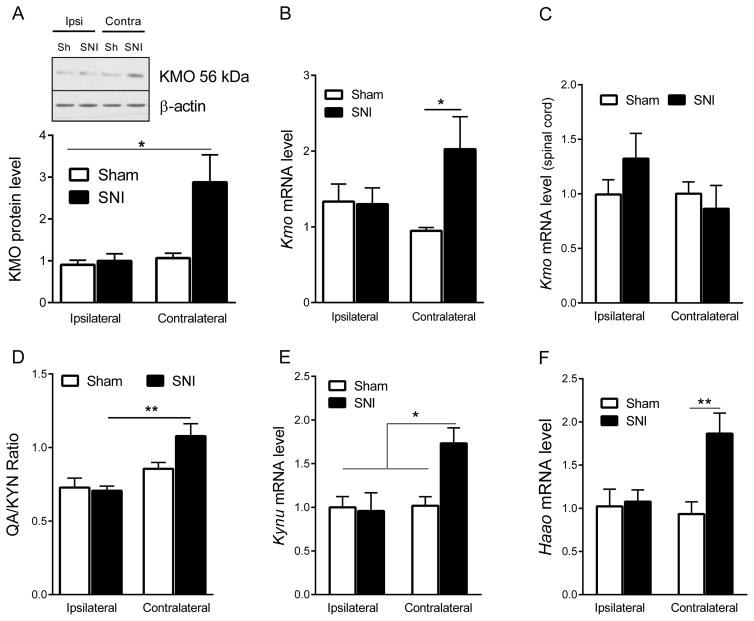
SNI upregulated KMO protein level in the contralateral but not the ipsilateral hippocampus (two-way ANOVA side x surgery, F=4.29, p=0.04) (Fig. 1A). Quantitative RT-PCR analysis confirmed that Kmo mRNA expression was increased in the contralateral as compared to the ipsilateral hippocampus of SNI mice (two-way ANOVA side × surgery, F=4.37, p=0.044) (Fig. 1B). SNI did not affect Kmo mRNA expression in the spinal cord (Fig. 1C). To determine whether the increase in hippocampal KMO mRNA and protein level was associated with an increase in KMO enzymatic activity, we assessed the ratio of QA to KYN as a proxy of KMO activity. SNI increased the ratio of QA to KYN in the contralateral but not the ipsilateral hippocampus (two-way ANOVA side x surgery, F=7.33, p=0.011), indicating an increase in KMO activity in the contralateral hippocampus (Fig. 1D). There were no statistically significant group differences for Kyn, QA, and KynA individually (Table 1). As KYN can also be metabolized to the neuroprotective metabolite kynurenic acid (KYNA), we also measured the levels of KYNA in the hippocampus. The level of KYNA was not significantly changed in response to SNI (Table 1). Downstream of KMO, KYNU and HAAO are necessary to produce QA. The expression of Kynu and Haao mRNA levels increased in contralateral hippocampus in response to SNI (Kynu two-way ANOVA side x surgery, F=5.66, p=0.02; Haao two-way ANOVA side x surgery, F=5.75, p=0.02) (Fig. 1E, F).
Figure 1. Effect of SNI on brain kynurenine monooxygenase (KMO) expression.
(A) Effect of SNI on KMO (56 kD) protein level in the ipsilateral and contralateral hippocampus is shown by Western blot representative image and quantification (sham n=5, SNI n=8). Data were analyzed by two-way ANOVA (side x surgery, F=4.29, p=0.04) followed by Bonferroni’s multiple comparison test. **= p<0.01 indicates significant difference between groups. (B) Effect of SNI on mRNA expression of Kmo in the ipsilateral and contralateral hippocampus (Sham n=9, SNI n=9). *= p<0.05 using two-way ANOVA (Side × surgery, F=4.37, p=0.044) followed by Bonferroni’s multiple comparison test. (C) (Effect of SNI on mRNA expression of Kmo in the ipsilateral and contralateral spinal cord (Sham n=5, SNI n=5). Two-way ANOVA (Side × surgery, F=1.69, p=0.21). (D) Effect of SNI on the hippocampal level of quinolinic acid-to-kynurenine ratio (QA/KYN) in the contralateral and ipsilateral hippocampus measured by HPLC-MS (Sham n=7, SNI n=10). **= p<0.01 data were analyzed by two-way ANOVA followed by Bonferroni’s multiple test correction (Side x surgery, F=7.33, p=0.011). (E) Effect of SNI on mRNA expression of Kynu in the ipsilateral and contralateral hippocampus (Sham n=8, SNI n=8). *= p<0.05 using two-way ANOVA (Side × surgery, F=5.66, p=0.02) followed by Bonferroni’s multiple comparison test. (F) Effect of SNI on mRNA expression of Haao in the ipsilateral and contralateral hippocampus (Sham n=8, SNI n=8). **= p<0.01 using two-way ANOVA (Side × surgery, F=5.75, p=0.02) followed by Bonferroni’s multiple comparison test. Data are normalized to sham contralateral =1 and presented as mean ± SEM.
Table 1. Effect of SNI surgery on kynurenine, quinolinic acid and kynurenic acid concentration in the hippocampus.
Kynurenine (KYN), quinolinic acid (QA), and kynurenic acid (KYNA) concentrations were determined by high-performance liquid chromatography mass spectrometry (HPLC-MS) in contralateral and ipsilateral hippocampus measured by HPLC-MS (Sham n=7, SNI n=10). The kynurenine metabolite concentration were calculated per mg of wet weight. Data are presented as mean ± SEM.
| Sham | SNI | |||
|---|---|---|---|---|
|
| ||||
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
|
| ||||
| KYN (pg/mg) | 20.29 ± 0.77 | 17.32 ± 0.82 | 18.49 ± 0.51 | 16.14 ± 0.73 |
| QA (ng/mg) | 14.59 ± 1.06 | 14.87 ± 1.20 | 12.98± 0.50 | 17.67 ± 1.23 |
| KYNA (pg/mg) | 0.75 ± 0.13 | 0.70 ± 0.13 | 0.85 ± 0.12 | 0.60 ± 0.06 |
3.3. Nerve injury induces KMO in hippocampal neurons
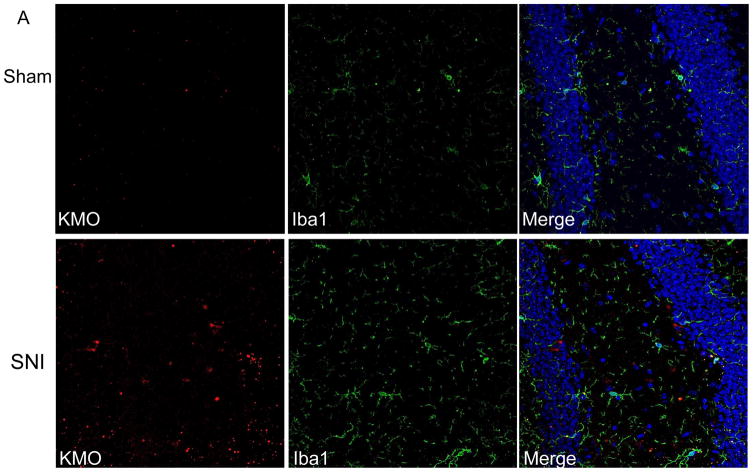
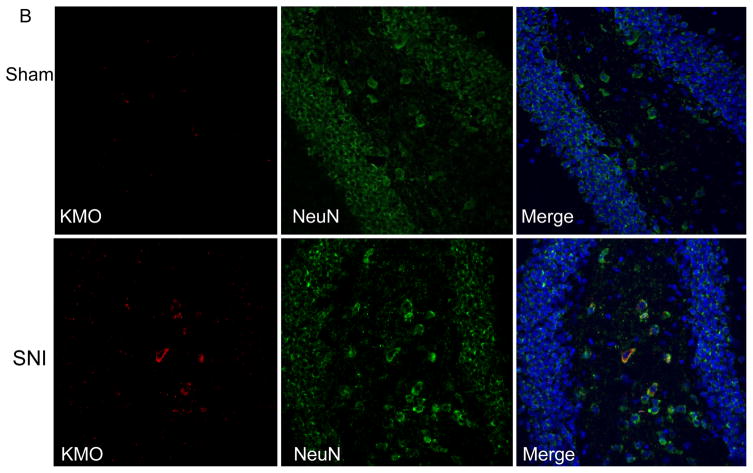
In the context of systemic inflammation, the increase in brain KMO protein mainly occurs in microglia (Corona, et al. 2010; Gonzalez-Pena, et al. 2016; Guillemin, et al. 2003). We determined whether SNI also increases KMO protein in hippocampal microglia. Double immunofluorescence analysis for KMO and the microglial marker Iba1 on brain sections showed that SNI increased Iba1 levels in the contralateral hippocampus, indicative of microglial activation in response to SNI (Figure 2B). However, the majority of the SNI-induced increase in KMO protein did not take place in microglia. (Fig. 2A). Additional analysis using the neuronal nucleus marker NeuN revealed that KMO-positive cells co-labeled for NeuN in the contralateral hippocampus of SNI mice (Fig. 2B). Thus, the SNI-induced increase in KMO protein occurs mainly in NeuN-positive neurons in the dentate gyrus of the contralateral hippocampus. The specificity of the KMO antibody was validated using brain sections from KMO knock-out mice (Fig. S2).
Figure 2. Effect of SNI on cellular distribution of KMO expression in the hippocampus.
Immunofluorescence analysis of KMO protein in the dentate gyrus of the hippocampus. (A) Double immunostaining of KMO and Iba1 in the hippocampus of Sham- or SNI-treated mice. (B) Double immunostaining of KMO and NeuN in the hippocampus of Sham- or SNI-treated mice. Images presented are representatives of pictures taken from 3 mice per group.
3.4. Brain IL-1 signaling mediates increased expression of KMO after nerve injury
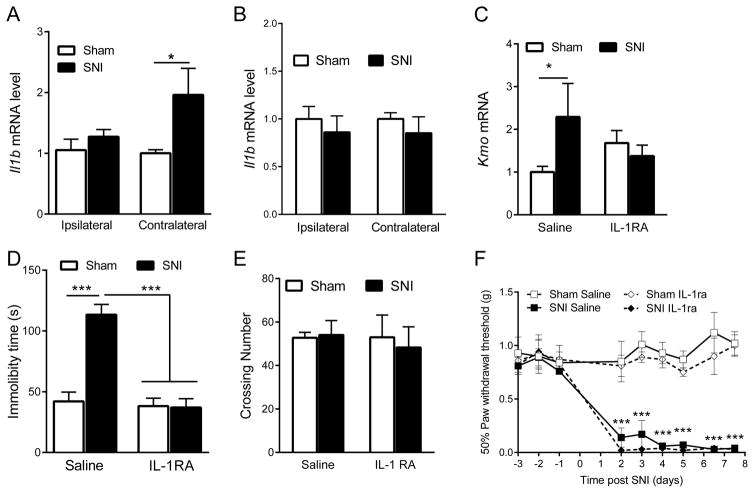
IL-1β has been reported to up-regulate Kmo mRNA expression in neuronal cells in vitro (Zunszain, et al. 2012). Quantitative-RT-PCR analysis showed that Il1b mRNA expression is up-regulated in the contralateral prefrontal cortex after SNI (two-way ANOVA side x surgery, F=3.69, p=0.04) (Fig. 3A) but not in the hippocampus (Fig. 3B). Next, we determined the contribution of IL-1 signaling to the SNI-induced increase in brain KMO. I.c.v. administration of IL-1RA inhibited the SNI-induced increase in Kmo mRNA in the contralateral hippocampus (two-way ANOVA surgery x drug, F=4.26, p=0.047) (Fig. 3C). At the behavioral level, i.c.v. administration of IL-1RA prevented the increased immobility in the forced swim test that occurred in SNI mice, indicating that brain IL-1 signaling is required for SNI-induced depression-like behavior, possibly via a KMO-mediated pathway (two-way ANOVA surgery x drug, F=23.45, p=0.0002) (Fig. 3D). Neither IL-1 RA nor SNI affected locomotor activity indicating that the observed effects on immobility in the forced swim test cannot be attributed to overall alterations in activity (Fig. 3E). Mechanical allodynia in response to SNI was not affected by i.c.v. IL-1RA injection (two-way ANOVA surgery/drug x time, F=8.4, p<0.0001) (Fig. 3F).
Figure 3. Effect of i.c.v. IL-1RA administration on Kmo expression and behavior.
(A) Effect of SNI on Il1b mRNA expression in the ipsilateral and contralateral prefrontal cortex (Sham n=7, SNI n=11). *= p<0.05 indicates significant difference between groups using two-way ANOVA followed by Bonferroni’s multiple comparison test (side x surgery, F=3.69, p=0.04). (B) Effect of SNI on Il1b mRNA expression in the ipsilateral and contralateral hippocampus (Sham n=8, SNI n=8). (C) Effect of i.c.v. IL-1RA injection (40 ng/4μl/days on day 6 and 7 after surgery) on Kmo mRNA expression in the contralateral hippocampus of Sham and SNI-treated mice (Sham n=6, SNI n=11). *= p<0.05 data were analyzed by two-way ANOVA followed by Bonferroni’s multiple test correction (Surgery x drug, F=4.26, p=0.047). (D) Effect of i.c.v. IL-1RA injection on immobility time (n=5/group). ***= p<0.001 indicate significant difference between groups using two-way ANOVA repeated measures followed by Bonferroni’s multiple test (Surgery x drug, F=23.45, p=0.0002). (E) Effect of SNI on locomotor activity (n=4/group). (F) Effect of i.c.v. IL-1RA injection on mechanical allodynia (n=4/group). ***= p<0.001 indicates difference between Sham + IL-1RA and SNI + IL-1RA groups using two-way ANOVA followed by Bonferroni’s multiple comparison test (Surgery/drug x time, F=8.4, p<0.0001). All data are presented as mean ± SEM.
3.5. KMO inhibition reverses nerve injury-induced depression-like behavior
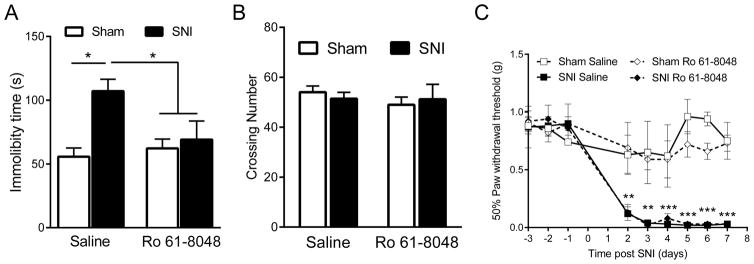
To further establish the contribution of brain KMO activity to depression-like behavior in response to SNI, we administered i.c.v. the inhibitor of KMO, Ro 61-8048 (Hamann, et al. 2008; Rover, et al. 1997), on days 6 and 7 after SNI. Ro 61-8048 significantly blocked the increased immobility time in the forced swim test in SNI mice (two-way ANOVA surgery x drug, F=4.99, p=0.04) (Fig. 4A). Neither SNI nor Ro 61-8048 administration affected spontaneous locomotor activity which means that these findings were not biased by alterations in motor activity (Fig. 4B). Consistent with the lack of effect of i.c.v. IL-1RA on mechanical allodynia, i.c.v. Ro 61-8048 did not affect mechanical allodynia in SNI mice (Fig. 4C). In sham-operated control mice immobility time in forced swim test and mechanical pain thresholds were not affected by i.c.v. administration of the KMO inhibitor.
Figure 4. Effect of i.c.v. injection of KMO inhibitor Ro 61-8048 on depression-like behavior and mechanical allodynia.
(A) Immobility time in FST (n=5/group). *= p<0.05 using two-way ANOVA followed by Bonferroni’s test for multiple comparison (Surgery x drug, F=4.99, p=0.04). (B) Locomotor activity (n=5/group). (C) Mechanical allodynia (n=5/group), **= P<0.01 and ***= p<0.001 indicate significant difference between Sham + Ro 61-8048 and SNI + Ro 61-8048 groups analyzed by two-way ANOVA repeated measures followed by Bonferroni’s multiple comparison test (Surgery/drug x time, F=5.09, p<0.0001). All data are presented as mean ± SEM.
4. Discussion
Using SNI as a model of chronic pain and comorbid depression, we show that nerve injury upregulated neuronal KMO mRNA and protein levels in the contralateral hippocampus. SNI also increased expression of Kynu and Haao, and the ratio of QA-to-KYN in the contralateral hippocampus, indicating activation of the entire KMO branch of the kynurenine pathway. The SNI-induced upregulation of neuronal KMO was dependent of brain IL-1 signaling as it was abrogated by i.c.v administration of IL-1RA. Inhibition of KMO enzymatic activity by i.c.v. administration of Ro 61-8048 blocked depression-like behavior but not mechanical allodynia in response to SNI. We previously showed that nerve injury-induced depression, but not mechanical allodynia is dependent on peripheral IDO1 activation (Zhou, et al. 2015). We now extend these findings by showing that SNI-induced depression-like behavior also requires IL-1β-mediated activation of the KMO branch of the kynurenine pathway.
We used several approaches (qRT-PCR, western blotting and immunofluorescence analysis) to demonstrate that SNI upregulates KMO in the brain. This SNI-induced increase in brain KMO was limited to the hemisphere contralateral to the injured nerve indicating a role of an ascending, potentially nociceptive, pathways from the site of injury to the brain. Consistent with our present data, a previous study reported that brain IL-1β is increased in the contralateral hemisphere in response to chronic constriction injury (Alberati-Giani, et al. 1996) as well as to SNI in rat models (del Rey, et al. 2011). Our results indicate that SNI induced an increase in IL-1β in the contralateral prefrontal cortex, but not in the hippocampus. Conversely, KMO was increased in the contralateral hippocampus, but not in the prefrontal cortex. Nevertheless, i.c.v. administration of IL-1RA inhibited the increase in hippocampal KMO mRNA, indicating that IL-1β signaling is responsible for the increase in KMO. The finding that Il1b mRNA was increased in the prefrontal cortex does not necessarily mean that IL-1β action is restricted to this part of the brain; it may well have effects at distance, for example by activating neurons projecting to other parts of the brain including the hippocampus. Such action at a distance has already been demonstrated for activation of hypothalamic paraventricular CRH-containing neurons in response to IL-1β expressed in the lateral medulla (Ericsson, et al. 1994). We focused our efforts in this study on the hippocampus because this is a critical hub for depression and enhanced level of QA has been observed in the hippocampus of depressed patients (Savitz, et al. 2015a). However, KMO expression can be dysregulated in other brain regions as well as changes in Kmo mRNA level have been reported in the prefrontal cortex of patients with bipolar disorders (Lavebratt, et al. 2014).
It is commonly accepted that the increase in brain KMO in response to peripheral inflammation takes place in microglia. However, this notion is based on in vitro analysis of cellular responses to LPS or cytokine administration (Corona, et al. 2010; Guillemin, et al. 2003) (Chiarugi, et al. 2001; Schwarcz and Stone 2017). Consistent with a recent report (Liu, et al. 2017), we also observed activation of microglia in the hippocampus in response to SNI. However, we observed that in response to SNI, KMO protein was mainly increased in NeuN-positive neuronal cells in the hippocampal dentate gyrus and not in the microglia. There is evidence for expression of Kmo mRNA in microglia (Corona, et al. 2010; Guillemin, et al. 2003), but according to RNA sequencing data the level is very low in microglia from untreated mice (Gonzalez-Pena, et al. 2016). This low expression of microglial KMO under baseline conditions likely explains why we did not detect KMO protein in microglia. In line with our findings, the Human Protein Atlas shows that immunolabeling for KMO is more intense in neuronal cells than in glial cells in the hippocampus (http://www.proteinatlas.org/ENSG00000117009-KMO/tissue/primary+data). The SNI-induced increase in KMO protein was mainly detected in the polymorphic layer of the dentate gyrus of the hippocampus, suggesting that KMO-positive cells are late stage progenitors. Interestingly, it has been shown that IL-1β induces KMO expression in primary cultures of hippocampal neuronal cells (Zunszain, et al. 2012). Zunszain et al., also reported that IL-1β impaired hippocampal neurogenesis via KMO. IL-1β has been implicated in inhibition of neurogenesis and reduction in sucrose preference in chronic stress model of depression (Koo and Duman 2008)., but a possible role of KMO has not been investigated in this condition. As reduction of hippocampal neurogenesis has been observed in a mouse model of neuropathic pain (Dimitrov, et al. 2014; Mutso, et al. 2012), it would be interesting to determine whether it is also KMO-dependent. In order to understand better the relationship between IL-1β and KMO, future work should aim at identifying the cellular source of IL-1β and QA in the SNI model of depression.
We have previously reported that SNI induces the expression of Ido1 in the liver but not in the brain (Zhou, et al. 2015). The kynurenine that is generated at the periphery in response to SNI-induced inflammation can then enter the brain (Kita, et al. 2002) where it is metabolized into neurotoxic kynurenine metabolites including QA by the KMO branch of the kynurenine pathways. (Alberati-Giani, et al. 1996; Guillemin, et al. 2003; Walker, et al. 2013). The role of QA in inflammation-induced depression has already been proposed at the clinical level and demonstrated at the preclinical level (Bay-Richter, et al. 2015; Savitz, et al. 2015b; Walker, et al. 2013) (Parrott, et al. 2016b). In the present study we reported an increase of the QA-to-KYN ratio only in the contralateral hippocampus which is consistent with the increased expression of KMO and the two downstream enzymes (Kynu and Haao) of the KMO branch in the contralateral hippocampus. Identification of the cellular origin of these two enzymes can shed light on the cellular source of the QA that is formed in the brain of SNI mice. The abrogation of SNI-induced depression-like behavior by i.c.v. injection of the KMO-inhibitor Ro 61-8048 indicates that KMO-activity is necessary for the development of depressive symptoms in response to SNI. Although inhibition of KMO should reduce QA level it can also increase KYNA (Clark, et al. 2005; Giorgini, et al. 2013; Rover, et al. 1997) that acts as NMDAR antagonist (Stone and Darlington 2002). This means that the effects of Ro 61-8048 administration must be interpreted with caution in the absence of measurement of its effect on brain kynurenine metabolites.
The spared nerve injury model of neuropathic pain induces both chronic pain and depression-like behavior. However, little is known about the common mechanisms underlying the comorbidity of pain and depression. Despite the limitations mentioned before, activation of NMDA receptors by QA could be one of these mechanisms. Indeed, there is evidence that NMDA receptor antagonists such as ketamine have antidepressant effects (Berman, et al. 2000; Sofia and Harakal 1975), and analgesic effects (Conseiller, et al. 1970; Sigtermans, et al. 2009). However, it is important to note that these effects do not occur in the same anatomical location. Inhibition of NMDA receptor activation in the brain reduces depression while NMDA receptors in the spinal cord mediate allodynia (Berman, et al. 2000; Collins, et al. 2010). The fact that i.c.v. injection of Ro 61-8048 blocked the depression-like behavior but did not alleviate mechanical allodynia is in accordance with this difference in anatomical location. Intrathecal injection of Ro 61-8048 can reduce mechanical allodynia in a nerve injury model of neuropathic pain (Rojewska, et al. 2016). However, when administered i.c.v., Ro 61-8084 is unlikely to reach the spinal cord in sufficient quantities to be active there. The same dichotomy, i.e. no effect on mechanical allodynia but inhibition of depression-like behavior was observed in response to i.c.v IL-1RA. Further dissociation between pain and depression was identified by the lack of effect of the analgesic retigabine on depression-like behavior as measured by increased immobility in the FST. Such a dissociation had already been reported. For example, the reduced motivation to obtain a food reward observed in SNI mice was not reversed by analgesic treatment (Schwartz, et al. 2014). In the same manner, depression-like behavior persisted after resolution of evoked-pain hypersensitivity in a transient model of nerve injury based on sciatic nerve cuffing (Dimitrov, et al. 2014).
In conclusion, the results of the present study provide clear evidence that spared nerve injury is associated with the brain expression of KMO in neuronal cells and the activation of the KMO branch of the kynurenine pathway in the contralateral hippocampus in an IL-1b-dependent manner. The enhanced KMO activity is responsible for the development of depression-like behavior. These findings indicate that brain KMO represents a promising new target for the development of new molecules aiming to treat depression comorbid with chronic pain.
Supplementary Material
Supplementary Figure 1. Effect of pain relieved induced by retigabine 10 mg/kg injection on depression-like behavior and mechanical allodynia. (A) Mechanical allodynia (n=5/group), *= P<0.05 and **= p<0.01 indicate significant effect of SNI surgery and retigabine treatment analyzed by two-way ANOVA repeated measures followed by Bonferroni’s multiple comparison test (Surgery x drug, F=5.12, p<0.038). (B) Immobility time in FST after SNI or sham surgery in WT mice measured 20 min after PBS or retigabine injection (i.p. 10 mg/kg) (n= 6/group). * = p<0.05 using two-way ANOVA followed by Bonferonni’s’s correction (surgery x drug interaction, F=0.19, p=0.66; main factor surgery F=16.9, p=0.0005). (C) Effect of SNI and retigabine on spontaneous locomotor activity (n=5/group). All data are presented as mean ± SEM.
Supplementary Figure 2. Immunostaining of KMO and the nuclear marker Hoescht 33342 (blue) in the hippocampus of SNI-treated KMO knock-out mice.
Highlights.
Nerve injury induces depression and upregulates KMO expression and activity
KMO is upregulated in neurons in the contralateral hippocampus and not in microglia
Upregulation of KMO is downstream of cerebral IL-1 signaling
Inhibition of brain KMO reverses depression but not allodynia after nerve injury
Acknowledgments
This work was supported by the National Institutes of Health R01 NS073939, R01 NS074999 and R01 MH090127, a STAR grant from The University of Texas Systems (A.K.), and a Cyrus Scholar Award (G.L.).
Abbreviations
- 3-HK
3-hydroxy-kynurenine
- FST
forced swim test
- HAAO
3-hydroxyanthralinic acid dioxygenase
- i.c.v
intracerebroventricular
- IDO
indoleamine 2,3-dioxygenase
- IL
interleukin
- KMO
kynurenine 3-monooxygenase
- KYN
kynurenine
- KYNA
Kynurenic acid
- KYNU
kynureninase (KYNU)
- NMDA
N-methyl-D-aspartate receptor
- QA
quinolinic acid
- SNI
spared nerve injury
- RA
receptor antagonist
Footnotes
Declaration of interest
The work of Drs. Kavelaars, Heijnen, Dantzer, and O’Connor is supported by the NIH. Drs. Heijnen and Kavelaars received a grant from Acetylon Inc. for work not related to the present study. Dr. Dantzer has received an honorarium from Danone Nutricia Research, France. Drs Lee and Budac were employed by Lundbeck Research USA at the time the analysis of kynurenine metabolites was carried out. The authors have no other potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberati-Giani D, et al. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J Neurochem. 1996;66(3):996–1004. doi: 10.1046/j.1471-4159.1996.66030996.x. [DOI] [PubMed] [Google Scholar]
- Arnone D, et al. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp. 2016;37(4):1393–404. doi: 10.1002/hbm.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Baranyi A, et al. Quinolinic Acid Responses during Interferon-alpha-Induced Depressive Symptomatology in Patients with Chronic Hepatitis C Infection - A Novel Aspect for Depression and Inflammatory Hypothesis. PLoS One. 2015;10(9):e0137022. doi: 10.1371/journal.pone.0137022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay-Richter C, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–7. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bourquin AF, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122(1–2):14e1–14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Capuron L, et al. Treatment of cytokine-induced depression. Brain Behav Immun. 2002;16(5):575–80. doi: 10.1016/s0889-1591(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, et al. Synthesis and release of neurotoxic kynurenine metabolites by human monocyte-derived macrophages. J Neuroimmunol. 2001;120(1–2):190–8. doi: 10.1016/s0165-5728(01)00418-0. [DOI] [PubMed] [Google Scholar]
- Clark CJ, et al. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect Immun. 2005;73(8):5249–51. doi: 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, et al. NMDA receptor antagonists for the treatment of neuropathic pain. Pain Med. 2010;11(11):1726–42. doi: 10.1111/j.1526-4637.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- Conseiller C, Levante A, Vourc’h G. Ketamine, a new anesthetic agent. Anesth Analg (Paris) 1970;27(1):1–28. [PubMed] [Google Scholar]
- Corona AW, et al. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, et al. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36(3):426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, et al. Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152(12):2827–35. doi: 10.1016/j.pain.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291(21):2581–90. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, et al. Anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity. J Neurosci. 2014;34(37):12304–12. doi: 10.1523/JNEUROSCI.0312-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14(2):897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini F, et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288(51):36554–66. doi: 10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pena D, et al. Microglia Transcriptome Changes in a Model of Depressive Behavior after Immune Challenge. PLoS One. 2016;11(3):e0150858. doi: 10.1371/journal.pone.0150858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279(8):1356–65. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, et al. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–12. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- Gustorff B, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008;52(1):132–6. doi: 10.1111/j.1399-6576.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- Hamann M, Sander SE, Richter A. Effects of the kynurenine 3-hydroxylase inhibitor Ro 61-8048 after intrastriatal injections on the severity of dystonia in the dt sz mutant. Eur J Pharmacol. 2008;586(1–3):156–9. doi: 10.1016/j.ejphar.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Heisler JM, O’Connor JC. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun. 2015;50:115–24. doi: 10.1016/j.bbi.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591–7. doi: 10.4088/jcp.v66n0508. [DOI] [PubMed] [Google Scholar]
- Kessler RC, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–84. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, et al. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem. 2002;82(2):258–68. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, et al. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80(1–2):341–6. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158(6):1126–1137. doi: 10.1097/j.pain.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumet G, et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci. 2015;18(12):1746–55. doi: 10.1038/nn.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C, et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. 2014;19(3):334–41. doi: 10.1038/mp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403–12. doi: 10.1007/s11940-005-0032-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. TNF-alpha Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury. J Neurosci. 2017;37(4):871–881. doi: 10.1523/JNEUROSCI.2235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32(17):5747–56. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, et al. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15(4):404–14. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, O’Connor JC. Kynurenine 3-Monooxygenase: An Influential Mediator of Neuropathology. Front Psychiatry. 2015;6:116. doi: 10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, O’Connor JC. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation. 2016a;13(1):124. doi: 10.1186/s12974-016-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, et al. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016b;6(10):e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole H, et al. Depression in chronic pain patients: prevalence and measurement. Pain Pract. 2009;9(3):173–80. doi: 10.1111/j.1533-2500.2009.00274.x. [DOI] [PubMed] [Google Scholar]
- Raison CL, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojewska E, et al. Pharmacological kynurenine 3-monooxygenase enzyme inhibition significantly reduces neuropathic pain in a rat model. Neuropharmacology. 2016;102:80–91. doi: 10.1016/j.neuropharm.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Rover S, et al. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl) benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem. 1997;40(26):4378–85. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- Santamaria A, Rios C. MK-801, an N-methyl-D-aspartate receptor antagonist, blocks quinolinic acid-induced lipid peroxidation in rat corpus striatum. Neurosci Lett. 1993;159(1–2):51–4. doi: 10.1016/0304-3940(93)90796-n. [DOI] [PubMed] [Google Scholar]
- Savitz J, et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015a;40(2):463–71. doi: 10.1038/npp.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, et al. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun. 2015b;46:55–9. doi: 10.1016/j.bbi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology. 2017;112(Pt B):237–247. doi: 10.1016/j.neuropharm.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, et al. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345(6196):535–42. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigtermans MJ, et al. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145(3):304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Sofia RD, Harakal JJ. Evaluation of ketamine HCl for anti-depressant activity. Arch Int Pharmacodyn Ther. 1975;214(1):68–74. [PubMed] [Google Scholar]
- Souery D, et al. Switching antidepressant class does not improve response or remission in treatment-resistant depression. J Clin Psychopharmacol. 2011;31(4):512–6. doi: 10.1097/JCP.0b013e3182228619. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1(8):609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178:234–41. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- Vogelgesang SA, et al. Quinolinic acid in patients with systemic lupus erythematosus and neuropsychiatric manifestations. J Rheumatol. 1996;23(5):850–5. [PubMed] [Google Scholar]
- Walker AK, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38(9):1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, et al. Peripheral indoleamine 2,3-dioxygenase 1 is required for comorbid depression-like behavior but does not contribute to neuropathic pain in mice. Brain Behav Immun. 2015;46:147–53. doi: 10.1016/j.bbi.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–49. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Effect of pain relieved induced by retigabine 10 mg/kg injection on depression-like behavior and mechanical allodynia. (A) Mechanical allodynia (n=5/group), *= P<0.05 and **= p<0.01 indicate significant effect of SNI surgery and retigabine treatment analyzed by two-way ANOVA repeated measures followed by Bonferroni’s multiple comparison test (Surgery x drug, F=5.12, p<0.038). (B) Immobility time in FST after SNI or sham surgery in WT mice measured 20 min after PBS or retigabine injection (i.p. 10 mg/kg) (n= 6/group). * = p<0.05 using two-way ANOVA followed by Bonferonni’s’s correction (surgery x drug interaction, F=0.19, p=0.66; main factor surgery F=16.9, p=0.0005). (C) Effect of SNI and retigabine on spontaneous locomotor activity (n=5/group). All data are presented as mean ± SEM.
Supplementary Figure 2. Immunostaining of KMO and the nuclear marker Hoescht 33342 (blue) in the hippocampus of SNI-treated KMO knock-out mice.