Abstract
Contrast-induced acute kidney injury (CI-AKI) is a common complication of many diagnostic and therapeutic cardiovascular procedures. It is associated with longer in-hospital stay, more complicated hospitalization course, and higher in-hospital morbidity and mortality. With increasing use of contrast media in various diagnostic and interventional procedures, the prevalence of CI-AKI is expected to rise. Although pre-hydration with intravenous normal saline is recommended in patients with elevated risk of CI-AKI, this approach is often not feasible in many clinical settings. Remote ischemic conditioning (RIC), elicited by application of one or more, brief, non-injurious episodes of ischemia and reperfusion of a limb, is a promising therapy for preventing or attenuating the deleterious effects of contrast media on the kidney. Although the mechanisms of protection by RIC have not been completely defined, complex humoral, neural, and inflammatory pathways have been hypothesized to be in play. Given that RIC is non-invasive and cheap, it is attractive from clinical and economic perspective as a therapy to protect the kidney from CI-AKI. In this succinct review, we highlight the unifying mechanisms of CI-AKI and provide an overview of proposed biological mechanisms of renal protection by RIC. Emerging pre-clinical and clinical evidence in interventional cardiology are also discussed.
Keywords: remote ischemic conditioning, acute kidney injury, contrast-induced nephropathy, cardiac catheterization
Introduction
Contrast-induced acute kidney injury (CI-AKI) defined as relative increase in serum creatinine within 48–72 hours is a common complication of intravenous, iodinated contrast medium that is widely used for diagnostic and therapeutic cardiovascular interventions [1–10]. CI-AKI predicts elevated risk of heart attack, longer in-hospital stay, more complicated hospitalization course, and higher in-hospital morbidity and mortality [2,6–8]. It is one of the leading causes of hospital-acquired AKI, and the third most common cause of AKI after hypovolemia, and nephrotoxic medications [11]. The incidence of CI-AKI varies substantially (14.8 to 55%) among studies depending on how CI-AKI is defined, and the presence of risk factors such as chronic renal insufficiency, diabetes mellitus and heart failure [1–10, 12]. The risk of CI-AKI is especially high in the setting of emergent cardiac procedures such as primary percutaneous coronary intervention (PCI) for acute myocardial infarction [2,12]. In the US, approximately 1.4 million cardiac catheterization procedures are performed each year, and other contrast-enhanced diagnostic imaging studies are performed for various purposes [13,14]. With increasing use of contrast medium in diagnostic and interventional procedures, the incidence of CI-AKI may rise in the next few decades. Although pre-hydration with intravenous normal saline is recommended in patients with elevated risk of CI-AKI, this approach is often not feasible in many clinical settings including patients with heart failure and acute ST elevation myocardial infarction (STEMI) requiring emergent primary PCI.
Emerging evidence suggests that remote ischemic conditioning (RIC), elicited by brief episodes of ischemia and reperfusion at a distant vascular bed, may protect vital organs including the kidney from subsequent injury [15–19]. This strategy appears to be protective when applied prior to ischemic injury (pre-conditioning), during ischemic injury (peri-conditioning) or at the onset of reperfusion (post-conditioning) [17]. Classically, RIC is performed in humans by inflating a blood pressure (BP) cuff placed around one limb to a pressure above systolic BP for 5 minutes followed by deflation for another 5 minutes to allow reperfusion; this cycle is usually repeated 3–4 times [16,17]. Given that RIC is non-invasive and cheap, it is attractive from clinical and economic perspective as a therapy to protect the kidney from CI-AKI. In this succinct review, we highlight the unifying mechanisms of CI-AKI and provide an overview of proposed biological mechanisms of renal protection by RIC. Emerging pre-clinical and clinical evidence are also discussed.
Contrast media and contrast-induce acute kidney injury risk
Iodinated contrast medium is a substance used to enhance the visibility of vascular structures and organs during radiographic procedures. As detailed in Table 1, contrast media may be either ionic or non-ionic; or be of high or low osmolarity. Evidence indicates that type of contrast agents plays a role in the development CI-AKI [20–22]. In one study, for example, administration of a nonionic, low-osmolality contrast agent led to an 18% reduction in creatinine clearance [20]. In comparison, an ionic high-osmolality contrast medium produced a greater reduction in creatinine clearance (42%) [20]. Among patients with normal or mildly depressed renal function, use of a non-ionic, low-osmolality contrast medium minimized nephrotoxicity as measured by reductions in creatinine clearance after coronary angiography [21]. In the meta-analysis of 45 trials, greater increase in serum creatinine after administration of high- compared with low- osmolality contrast medium was seen in patients with pre-existing renal failure [22]. Thus, nonionic and low osmolality contrast media are preferred in patients at high risk for developing CI-AKI. The volume of contrast medium is another major modifiable risk factor for CI-AKI [12,23,24]. Although minimizing contrast volume is the preferred strategy for the prevention of CI-AKI, some studies have shown that relatively low volume of contrast (less than 100 ml) can still induce permanent renal injury and the need for dialysis in patients with diabetes and pre-existing chronic kidney disease [25,26].
Table 1.
Commonly used iodinated contrast media
| Name | Type | Iodine content | Osmolality | |
|---|---|---|---|---|
| Nonionic | ||||
| iohexol (Omnipaque 350) | Monomer | 350 mgI/ml | 884 | Low |
| iopamidol (Isovue 370) | Monomer | 370 mgI/ml | 796 | Low |
| iopromide (Ultravist 370) | Monomer | 370 mgI/ml | 774 | Low |
| ioxilan (Oxilan 350) | Monomer | 350 mgI/ml | 695 | Low |
| iodixanol (Visipaque 320) | Dimer | 320 mgI/ml | 290 | Low |
| Ionic | ||||
| metrizoate (Isopaque 370) | Monomer | 370 mgI/ml | 2100 | High |
| diatrizoate (Hypaque 50) | Monomer | 300 mgI/ml | 1550 | High |
| ioxaglate (Hexabrix) | Dimer | 320 mgI/ml | 580 | Low |
Many risk factors predict increased risk for developing CI-AKI including: pre-existing kidney disease, older age, female gender, diabetes, heart failure, use of nephrotoxic drugs, renal artery stenosis, nephrotic syndrome, multiple myeloma, and renal transplant [4–6,12]. Diabetes mellitus increases the risk of CI-AKI in patients undergoing cardiac catheterization partly because of impaired nitric oxide generation [25,27]. Also, older age is an independent predictor of CI-AKI for multiple reasons including age-related changes in renal function such as diminished glomerular filtration rate, tubular secretion, and concentrating ability of the kidnes [5,12]. Periprocedural hypotension is also a major risk factor for the development of CI-AKI, and even a relatively short period of hypotension is hazardous [12,28]. Detailed analyses of these risk factors have been previously published [12]. Mehran et al. combined these risk factors to develop a risk classification system for prediction of CI-AKI in patients undergoing coronary angiography [12].
Mechanism of contrast-induced acute kidney injury
The pathogenesis of CI-AKI has been proposed to include vascular and tubular pathways. A detailed review of pathophysiological mechanisms of CI-AKI is outside the scope of this review, and has been extensively reviewed [29–33]. Of the various proposed pathways, the major pathophysiological concepts of CI-AKI are: 1) contrast medium–induced vasoconstriction and reduction in renal blood flow resulting in renal ischemic injury; 2) iodinated contrast agent and oxygen free radical–mediated direct renal tubular toxicity [29–33].
Vasoconstriction and renal ischemic injury
It well established in the literature that contrast agents induce natriuresis and diuresis, which activate the tubuloglomerular feedback response, resulting in vasoconstriction of the glomerular afferent arterioles causing a decrease in glomerular filtration rate [33–36]. The best data related to the pathogenesis of contrast-induced vasoconstriction come from animal models. Studies have shown evidence of acute tubular necrosis (ATN) following contrast agent infusion in animal models, though the mechanisms are still not completely understood [34–36]. The most favored theory is that ATN is caused by renal vasoconstriction resulting in medullary hypoxia, possibly mediated by alterations in nitric oxide, endothelin, and/or adenosine. Given that the renal medullary vascular bed is composed of long vessels of small diameter, reduction in renal medullary blood flow may also be due to increased viscosity of contrast agent and enhanced tubular interstitial pressure. The outer medulla, in particular appears to be susceptible to injury due to reductions in renal blood flow [35]. This increased susceptibility results from baseline borderline hypoxic conditions in the outer medulla, which are due in part to the high oxygen requirements for active sodium transport and countercurrent flow [36].
Direct renal tubular cytotoxicity
Direct tubular injury, due to direct cytotoxic effects of contrast medium or in association with the generation of oxygen free radicals, contributes to CI-AKI [37,38]. Importantly, direct cytotoxicity may also be exacerbated by and act in concert with renal artery vasoconstriction. Sustained reduction in renal blood flow, because of vasoconstriction, may last for several hours. These results in concentration of iodinated contrast medium in the renal tubules and collecting ducts. This stasis of contrast in the kidney allows for direct cellular injury and death to renal tubular cells [37,38]. The degree of cytotoxicity to renal tubular cells is directly related to the length of exposure those cells [29–33,37,38]. Similarly, the sustained reduction in renal blood flow to the outer medulla, as previously discussed, may lead to medullary hypoxia, ischemia, and tubular cell death.
In summary, the contrast media used for diagnostic and therapeutic interventions can induce renal ischemic injury, possibly caused by contrast medium–mediated vasoconstriction and free radical–mediated direct renal tubular toxicity [29–38]. The combination of these sets of processes result in CI-AKI, although other processes including inflammation and oxidative stress may play further role.
The concept of remote ischemic conditioning
The endogenous protective phenomenon of “ischemic conditioning,” in which an organ is put into a protected state by subjecting it to one or more brief non-injurious episodes of ischemia and reperfusion, was initially shown to have the potential of attenuating myocardial ischemia and injury [15]. This strategy was first described in 1986 as the protection conferred to ischemic myocardium by preceding brief periods of non-injurious ischemia separated by periods of reperfusion [15]. In this report, four five-minute episodes of regional ischemia in canine myocardium induced by reversible coronary artery ligation, each followed by a five-minutes of reperfusion, protected against myocardial infarction during a subsequent prolonged period of ischemia, resulting in a 75 percent reduction in infarct size compared to a control group [15]. This early observation initiated research interest in ischemic conditioning, however, the invasive nature of this approach hampered further research in humans and translation into clinical practice.
Intriguingly, shortly after this initial report on ischemic conditioning, it was discovered that ischemic conditioning stimulus applied to one vascular bed, or organ or tissue, conferred global protection and rendered a remote tissue and organ resistant to ischemia and ischemia-reperfusion injury [16–19]. This strategy, termed “remote ischemic conditioning (RIC),” rejuvenated research interest in this field [16]. In 1993, it was demonstrated in anesthetized dogs that 4 episodes of 5-min ischemia and reperfusion in the left circumflex coronary territory, followed by a 1-hour occlusion of the left anterior descending coronary artery, significantly reduced myocardial infarct size [16]. RIC was proven to be cardio-protective in this preclinical study, and subsequent reports have confirmed that other organs such as brain, liver, kidney can be protected in a similar manner [39–43].
Mechanism of renal protection by remote ischemic conditioning
The mechanisms of protection by RIC are not completely understood but several studies have implicated complex humoral, anti-inflammatory and neuronal signaling pathways. The details of these pathways, particularly in relation to cardio-protection, have been previously reviewed [17,44,45].
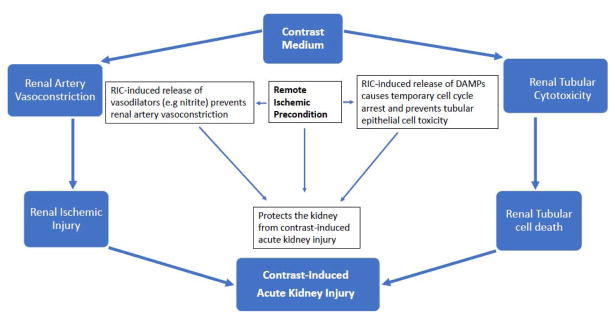
With regards to the kidney, we propose that RIC prevents CI-AKI via two major pathways: 1) generation of NO/nitrite, and 2) release of damage associated proteins (DAMPS) and activation temporary cell cycle arrest in renal tubular epithelial cells [45]. As shown in Figure 1, these processes ultimately enhance the ability of the kidney to withstand contrast-induced vasoconstriction and tubular toxicity, the two major mechanisms of CI-AKI.
Figure 1.
Proposed Mechanism of Protection Against Contrast-induced Acute Kidney Injury by Remote Ischemic Conditioning.
Generation of nitric oxide/nitrite
Studies suggest that several signal transduction pathways are activated by RIC, including generation of endogenous protective biomarkers in many organs, such as nitrite [46,47]. Recent translational studies in rats and humans found nitrite, the single electron oxidation product of NO, to be increased by about 50% with a single cycle of RIC when normal reperfusion accompanied by reactive hyperemia was performed [46]. Further experiments suggested that nitrite was both necessary and sufficient to confer cardio-protection when human plasma after RIC was given to rat hearts in an ex vivo cardiac ischemia reperfusion model [46]. Thus plasma nitrite, which correlates strongly to endothelial nitric oxide synthase (eNOS) activity, NO generation and vasodilatation, may represent a means by which RIC prevents renal vasoconstriction.
Release of DAMPS and activation of temporary cell cycle arrest
Studies have shown that RIC may attenuate AKI by causing release of (DAMPS) during brief ischemia, that are filtered by the kidney and signal through toll-like receptors in the proximal tubules epithelia, which may then induce natural defenses such as bio-energetic down-regulation and temporary cell cycle arrest [41,45]. These defenses, once engaged, can then protect the kidney during subsequent ischemic stress and exposure to toxins. Specifically, in the kidney, RIC-induced generation of tissue inhibitor of metalloproteinases 2 (TIMP-2) and insulin-like growth factor–binding protein 7 (IGFBP7), both markers of cell cycle arrest, have been previously reported [41,45]. Recent research indicates that the product of urinary TIMP-2 and IGFBP7 (TIMP2*IGFBP7) is a predictor of AKI when they increase after exposures such as cardiac surgery [41,48,49], whereas increases in TIMP2*IGFBP7 in response to RIC protect the kidney from AKI due to cardiac surgery [41,45].
Given that renal ischemic injury and tubular toxicity are the most common pathophysiological concepts of CI-AKI, it stands to reason that RIPC may prevent CI-AKI via nitrite-induced vasodilation and TIMP2*IGFBP7-mediated temporary cell cycle arrest of the renal tubular epithelial cells at the time of exposure to contrast medium. Although the process of identifying the precise molecular mechanisms of RIC has been slow, it is important that these efforts continue- it may be possible to target identified molecular pathways pharmacologically. Thus, RIC-mediated protective pathways may become target for therapeutic interventions in the nearest future.
Overview of clinical evidence of renal protection by remote ischemic conditioning
Preliminary clinical studies in cardiac surgery patients on RIC showed promise for renal protection [41–43], however recent large randomized clinical trials have yielded neutral results in patients undergoing cardiac surgery [50,51]. With regards to CI-AKI in the context of coronary angiography, few clinical trials and observational studies have shown promising results in various clinical settings [52–54].
In the landmark, randomized Pilot Renal Protection Trial (RenPro) involving 100 patients with impaired renal function undergoing elective coronary angiography, Er et al. showed reduced rate of CI-AKI (12% versus 40%) in patients that received RIC compared to control [52]. Multivariable analysis adjusting for contrast media volume and diabetes mellitus status revealed that RIC was a strong independent correlate for prevention of CI-AKI [52]. Following this report in high risk patients, Igarashi et al. assessed the incidence of CI-AKI based on urinary liver-type fatty acid-binding protein (L-FABP) level (defined as L-FABP >17.4μg/g) and >25% increase in serum creatinine level in a small sample of patients (n=60) with moderate renal disease (eGFR 30–60Ml/min per 1.73m2) undergoing elective coronary angiography [53]. They observed that RIC was associated with reduced incidence of CI-AKI determined by L-FABP (7.7% vs 26.9%; P = 0.038) but not serum creatinine. Subsequent meta-analysis by Pei et al. explored the effect of RIC on CI-AKI in five clinical trials (n=1153 subjects) in patients undergoing elective PCI [54]. They observed a reduced risk of CI-AKI in RIC group (OR 0.61; 95% CI 0.38 to 0.98; P = 0.04) with no significant heterogeneity (I2 = 39.0%) [54].
In the context of STEMI, two studies assessing the effect of RIC of the limb on CI-AKI have been performed. In an elegant randomized controlled trial involving STEMI patients (n=94) in Japan, Yamanaka et al. showed that RIC applied before PCI significantly reduced CI-AKI (10% versus 36%) compared with PCI alone [55]. Recent analysis of data from National Cardiovascular Data Registry (NCDR) Acute Coronary Treatment and Intervention Outcomes Network Registry®-Get With the Guidelines™ (ACTION Registry–GWTG) from two PCI-hospitals in the United States utilizing RIC during helicopter transport showed that STEMI patients who received RIC during inter-facility helicopter transport were less likely to have CI-AKI compared to patients who did not receive RIC (8.7% versus 18.5%; unadjusted OR=0.42, 95% CI 0.18– 0.94, p=0.036) [56]. Further subgroup analyses revealed that the magnitude and direction of the RIC effect on incidence of CI-AKI were generally consistent among different subgroups, except for those with morbid obesity (BMI>35) [56].
In summary, while most studies assessing the effect of RIC on CI-AKI have shown positive results, studies of the effect of RIC on AKI in surgical and medical patients without the use of contrast media have yielded mixed results. Importantly, the location and nature of RIC protocol as well as the definition of AKI varied among these studies making comparison of study findings difficult. Although RIC is safe, cheap and readily available, widespread adoption into clinical practice has been hindered by mixed results of clinical data and heterogeneity among existing studies including- different disease processes, patient populations, clinical settings and method of delivering RIC. Therefore, for RIC to become standard of care in clinical practice, more well-designed randomized controlled trials using standardized RIC protocol are needed.
Application and timing of remote ischemic conditioning
RIC is commonly performed in humans by inflating a BP cuff placed around upper or lower limb to a pressure above systolic BP (usually 200 mmHg) for 5 minutes followed by deflation for another 5 minutes to allow reperfusion; this cycle is usually repeated 3–4 times [16,17]. RIC can be applied prior to ischemic injury (pre-conditioning), during ischemic injury (peri-conditioning) or after ischemic injury and at the onset of reperfusion (post-conditioning) [17]. For patients undergoing elective cardiac catheterization, remote ischemic pre-conditioning is the most commonly employed strategy. In relation to acute myocardial ischemia, pre-conditioning is almost impossible, unless performed on daily basis, because the timing of coronary occlusion and acute cardiac ischemic events cannot be predicted. Post-conditioning, on the hand, may be too late to prevent ischemic injury and the early part of reperfusion injury. As such, remote ischemic peri-conditioning appears to be the most feasible RIC method of cardio-protection in the setting of STEMI and NSTEMI. In relation to CI-AKI, both pre-conditioning and peri-conditioning are feasible when performed before cardiac catheterization and contrast load. Given the complexity of STEMI patients and the need for emergent revascularization, RIC is best performed during inter-facility transport of STEMI patients to prevent interference with primary PCI during cardiac catheterization. Our group has previously shown the feasibility of remote ischemic peri-conditioning application during inter-facility air transport of STEMI patients in United States [56–58].
Conclusion
Over the last three decades, several preclinical and small-size clinical studies have shown that RIC may protect the kidney from CI-AKI. Although, the underlying mechanisms of this reno-protective effect have not been fully elucidated, it appears to be partially related to RIC-induced renal vasodilatation and temporary cell cycle arrest of renal tubular epithelial cells. Further studies are needed to establish the reno-protective effects of RIC, determine the optimal dosing, and examine novel molecular mechanisms that may serve as target for therapeutic interventions.
Emerging evidence suggests prevention of contrast-induced acute kidney injury by remote ischemic conditioning.
Conditioning-induced renal vasodilatation and temporary cell cycle arrest of renal tubular epithelial cells appear to play a role.
Identifying novel molecular mechanisms of ischemic conditioning may help develop therapeutic interventions.
Further studies are needed to establish the reno-protective effects of remote ischemic conditioning.
Acknowledgments
Funding: This study was funded in part by National Institutes of Health (5K12HL109068-04).
Footnotes
Disclosures: All authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Rumsfeld JS, Spertus JA, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–5. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 3.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 4.Lautin EM, Freeman NJ, Schoenfeld AH, Bakal CW, Haramati N, Friedman AC, et al. Radiocontrast-associated renal dysfunction: incidence and risk factors. AJR Am J Roentgenol. 1991;157:49–58. doi: 10.2214/ajr.157.1.2048539. [DOI] [PubMed] [Google Scholar]
- 5.Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older: a prospective study. Arch Intern Med. 1990;150:1237–42. [PubMed] [Google Scholar]
- 6.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006;17:2871–7. doi: 10.1681/ASN.2006030301. [DOI] [PubMed] [Google Scholar]
- 8.Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113–9. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 9.Sudarsky D, Nikolsky E. Contrast-induced nephropathy in interventional cardiology. Int J Nephrol Renovasc Dis. 2011;4:85–99. doi: 10.2147/IJNRD.S21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–16. doi: 10.1161/CIRCULATIONAHA.110.970160. [DOI] [PubMed] [Google Scholar]
- 11.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 13.Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes. 2011;4:193–7. doi: 10.1161/CIRCOUTCOMES.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Palevsky PM, Fine MJ. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin J Am Soc Nephrol. 2008;3:1274–81. doi: 10.2215/CJN.01260308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 16.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 17.Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–95. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 19.Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. J Am Coll Cardiol Intv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Katholi RE, Taylor GJ, McCann WP, Woods WT, Jr, Womack KA, McCoy CD, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995;195:17–22. doi: 10.1148/radiology.195.1.7892462. [DOI] [PubMed] [Google Scholar]
- 21.Katholi RE, Taylor GJ, Woods WT, Womack KA, Katholi CR, McCann WP, et al. Nephrotoxicity of nonionic low-osmolality versus ionic high-osmolality contrast media: a prospective double-blind randomized comparison in human beings. Radiology. 1993;186:183–7. doi: 10.1148/radiology.186.1.8416561. [DOI] [PubMed] [Google Scholar]
- 22.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolarity iodinated contrast media. Radiology. 1993;188:171–8. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 23.Kini AS, Mitre CA, Kim M, Kamran M, Reich D, Sharma SK. A protocol for prevention of radiographic contrast nephropathy during percutaneous coronary intervention: effect of selective dopamine receptor agonist fenoldopam. Catheter Cardiovasc Interv. 2002;55:169–73. doi: 10.1002/ccd.10038. [DOI] [PubMed] [Google Scholar]
- 24.Kahn JK, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Shimshak TM, et al. High-dose contrast agent administration during complex coronary angioplasty. Am Heart J. 1990;120:533–6. doi: 10.1016/0002-8703(90)90006-j. [DOI] [PubMed] [Google Scholar]
- 25.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–20. doi: 10.1016/0002-9343(90)90180-l. [DOI] [PubMed] [Google Scholar]
- 26.Vlietstra RE, Nunn CM, Narvarte J, Browne KF. Contrast nephropathy after coronary angioplasty in chronic renal insufficiency. Am Heart J. 1996;132:1049–50. doi: 10.1016/s0002-8703(96)90021-6. [DOI] [PubMed] [Google Scholar]
- 27.Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–75. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–9. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 29.Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14–22. doi: 10.1111/j.1523-1755.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 30.Katzberg RW. Contrast medium-induced nephrotoxicity; which pathway? Radiology. 2005;235:752–5. doi: 10.1148/radiol.2353041865. [DOI] [PubMed] [Google Scholar]
- 31.Azzalini L, Spagnoli V, Ly HQ. Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can J Cardiol. 2016;32:247–55. doi: 10.1016/j.cjca.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Geenen RW, Kingma HJ, van der Molen AJ. Contrast-induced nephropathy: pharmacology, pathophysiology and prevention. Insights Imaging. 2013;4:811–20. doi: 10.1007/s13244-013-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol. 1994;5:125–37. doi: 10.1681/ASN.V52125. [DOI] [PubMed] [Google Scholar]
- 34.Detrenis S, Meschi M, Musini S, Savazzi G. Lights and shadows on the pathogenesis of contrast-induced nephropathy: state of the art. Nephrol Dial Transplant. 2005;20:1542–50. doi: 10.1093/ndt/gfh868. [DOI] [PubMed] [Google Scholar]
- 35.Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal haemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol Dial Transplant. 2005;20:i6–11. doi: 10.1093/ndt/gfh1069. [DOI] [PubMed] [Google Scholar]
- 36.Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3:288–96. doi: 10.2215/CJN.02600607. [DOI] [PubMed] [Google Scholar]
- 37.Hardiek K, Katholi RE, Ramkumar V, Deitrick C. Proximal tubule cell response to radiographic contrast media. Am J Physiol Renal Physiol. 2001;280:F61–70. doi: 10.1152/ajprenal.2001.280.1.F61. [DOI] [PubMed] [Google Scholar]
- 38.Katholi RE, Woods WT, Jr, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64–71. doi: 10.1053/ajkd.1998.v32.pm9669426. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Yang J, Lu G, Guo J, Dou Y. Limb remote ischemic post-conditioning reduces brain reperfusion injury by reversing eNOS uncoupling. Indian journal of experimental biology. 2014;52:597–605. [PubMed] [Google Scholar]
- 40.Peng B, Guo QL, He ZJ, Ye Z, Yuan YJ, Wang N, et al. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain research. 2012;1445:92–102. doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 41.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–41. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, et al. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–7. doi: 10.1038/ki.2011.156. [DOI] [PubMed] [Google Scholar]
- 43.Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, et al. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–54. doi: 10.1016/j.jtcvs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Lim SY, Hausenloy DJ. Remote Ischemic Conditioning: From Bench to Bedside. Frontiers in Physiology. 2012;3:27. doi: 10.3389/fphys.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarbock A, Kellum JA. Remote Ischemic Preconditioning and Protection of the Kidney--A Novel Therapeutic Option. Crit Care Med. 2016;44:607–16. doi: 10.1097/CCM.0000000000001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circulation research. 2014;114:1601–10. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 47.Murillo D, Kamga C, Mo L, Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide. 2011;25:70–80. doi: 10.1016/j.niox.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, et al. RIPHeart Study Collaborators. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med. 2015;373:1397–407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 51.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, et al. ERICCA Trial Investigators. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373:1408–17. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 52.Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial) Circulation. 2012;126:296–303. doi: 10.1161/CIRCULATIONAHA.112.096370. [DOI] [PubMed] [Google Scholar]
- 53.Igarashi G, Iino K, Watanabe H, Ito H. Remote Ischemic Pre-Conditioning Alleviates Contrast Induced Acute Kidney Injury in Patients With Moderate Chronic Kidney Disease. Circ J. 2013;77:3037–44. doi: 10.1253/circj.cj-13-0171. [DOI] [PubMed] [Google Scholar]
- 54.Pei H, Wu Y, Wei Y, Yang Y, Teng S, Zhang H. Remote Ischemic Preconditioning Reduces Perioperative Cardiac and Renal Events in Patients Undergoing Elective Coronary Intervention: A Meta-Analysis of 11 Randomized Trials. PLoS One. 2014;9:e115500. doi: 10.1371/journal.pone.0115500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamanaka T, Kawai Y, Miyoshi T, Mima T, Takagaki K, Tsukuda S, et al. Remote ischemic preconditioning reduces contrast-induced acute kidney injury in patients with ST-elevation myocardial infarction: a randomized controlled trial. Int J Cardiol. 2015;178:136–41. doi: 10.1016/j.ijcard.2014.10.135. [DOI] [PubMed] [Google Scholar]
- 56.Olafiranye O, Ladejobi A, Wayne M, Martin-Gill C, Althouse AD, Sharbaugh MS, et al. Renal Protection Using Remote Ischemic Peri-Conditioning During Inter-Facility Helicopter Transport of Patients With ST-Segment Elevation Myocardial Infarction: A Retrospective Study. J Interv Cardiol. 2016;29:603–11. doi: 10.1111/joic.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-Gill C, Wayne M, Guyette FX, Olafiranye O, Toma C. Feasibility of Remote Ischemic Peri-conditioning during Air Medical Transport of STEMI Patients. Prehosp Emerg Care. 2016;20:82–9. doi: 10.3109/10903127.2015.1056894. [DOI] [PubMed] [Google Scholar]
- 58.Ladejobi A, Wayne M, Martin-Gill C, Guyette FX, Althouse AD, Sharbaugh MS, et al. Association of remote ischemic peri-conditioning with reduced incidence of clinical heart failure after primary percutaneous coronary intervention. Cardiovasc Revasc Med. 2017;18:105–9. doi: 10.1016/j.carrev.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]