Abstract
This study aims to evaluate the overall prognosis, prognostic factors, and efficacy of treatment in patients with mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS) who have access to third generation anti-epileptic drugs but not to epilepsy surgery. Eighty-five MTLE-HS patients were retrospectively placed into a seizure-free (seizure-free for >1 year) or drug-resistant group, and the two groups were compared on the basis of age, sex, age at onset of seizures, duration of epilepsy, side of lesion, handedness, EEG findings, history of CNS infection, history of febrile convulsions, history of head trauma, history of cognitive impairment, family history of seizures, number of current anti-epileptic drugs (AEDs), total number of AED trials, and presence of individual AEDs. Only 24.7% of MTLE-HS patients had achieved seizure freedom for >1 year. Poor prognosis and drug-resistance were associated with younger age at onset of seizures (p=0.002), longer duration of epilepsy (p=0.018), greater number of current AEDs (p<0.001), and greater total number of AED trials (p<0.001). In addition, regimens with newer AEDs had no greater efficacy than regimens with older AEDs. Most medically managed MTLE-HS patients do not achieve seizure freedom despite multiple AED trials, and treatment with third generation AEDs should not preclude evaluation for epilepsy surgery.
Search Terms: Anti-epileptic drugs, Epilepsy surgery, Hippocampal sclerosis, Natural history studies (prognosis), MRI
1. Introduction
Mesial temporal lobe epilepsy (MTLE) is the most prevalent form of focal epilepsy worldwide. In cases of MTLE refractory to medical therapy, the most commonly encountered pathology is hippocampal sclerosis (MTLE-HS) (Kim et al., 1999). The definitive treatment for refractory MTLE-HS is surgical, with approximately 70–80% of patients achieving seizure freedom post-operatively (Kim et al., 1999; Malmgren and Thom, 2012). Patients may be managed medically if not surgical candidates due to extrahippocampal pathology or if surgery is not an option due to financial constraints (Kurita et al., 2016).
Several studies have examined the prognostic factors associated with the success of medical therapy in MTLE-HS, finding that such treatment results in complete remission in only 5 to 42% of patients, a percentage much lower than that for other forms of epilepsy (Giussani et al., 2016; Kim et al., 1999; Kumlien et al., 2002; Kurita et al., 2016; Kuzmanovski et al., 2016; Semah F et al., 2002; Stephen et al., 2001). Additionally, patients with MTLE-HS may remit and later relapse despite a period of seemingly adequate seizure control (Coan et al., 2015). Negative prognostic factors include earlier age at onset of epilepsy, bilateral or left-sided lesions, head injury at a young age, EEG abnormalities, and a large number of previously tried anti-epileptic drugs (AEDs) (Gomez-Ibañez et al., 2013; Sànchez et al., 2014). Studies are mixed regarding the role of gender in determining prognosis (Kuzmanovski et al., 2016; Varoglu et al., 2009). Though some evidence suggests that adjunctive lacosamide specifically may have good success in treating MTLE-HS (Borzì et al., 2016), no significant evidence has yet emerged suggesting an increased relative effectiveness of third generation AEDs compared to first and second generation AEDs in achieving seizure freedom.
This retrospective, cross-sectional study was conducted on a population of MTLE-HS patients who lack access to health insurance, and thus surgical intervention, giving the unique opportunity to evaluate the efficacy of newer anti-epileptic drugs.
2. Materials and Methods
This cross-sectional study was approved by the Baylor College of Medicine Institutional Review Board (H-32620) as well as the Harris Health System Institutional Review Board (16-03-1365). In this retrospective study, we reviewed all patients with a diagnosis of MTLE-HS treated in the Smith Neurology Clinic of the Harris Health System in Houston, Texas, USA, between September 2012 and February 2017. This clinic is staffed by medicine, psychiatry, and neurology residents who use a standard template for evaluating patients, overseen by board-certified neurology faculty at Baylor College of Medicine. One hundred and thirty patients with evidence of hippocampal sclerosis on MRI (hippocampal atrophy or structural alteration on T1-weighted images and/or hyperintensity on T2-weighted or FLAIR imaging) were marked for further review. All MR imaging was performed on a 1.5-Tesla scanner and all studies were reviewed and interpreted by a board-certified neuroradiologist. Patients were excluded for the following reasons: patient-reported or laboratory-based evidence of persistent noncompliance, MRI evidence of a potential seizure focus outside the hippocampus (such as encephalomalacia, tumors, and cortical dysplasia), EEG results inconsistent with ipsilateral temporal lobe epilepsy, history of epilepsy surgery, not having undergone at least one six-month trial of anticonvulsant therapy, MRI performed only at an outside hospital, and suspicion of provoked seizures (Figure 1). Six months was deemed an appropriate minimum length of therapy to assess a change in seizure frequency since seizures frequently occurred less than three times per month. This was also a standard time for a follow up appointment for patients and, therefore, for many of our patients, the minimum length of any AED trial. EEGs were interpreted by neurologists at Baylor College of Medicine with board certification in clinical neurophysiology and/or epilepsy. Additionally, patients who were not seizure-free for >1 year were excluded if they had failed fewer than two AED trials – i.e., had only tried one AED, or were on one AED and had failed a previous AED trial due to side effects. These criteria were devised to isolate a group of patients that would be candidates for surgery under different economic circumstances.
Figure 1. Flowchart of inclusion and exclusion criteria.
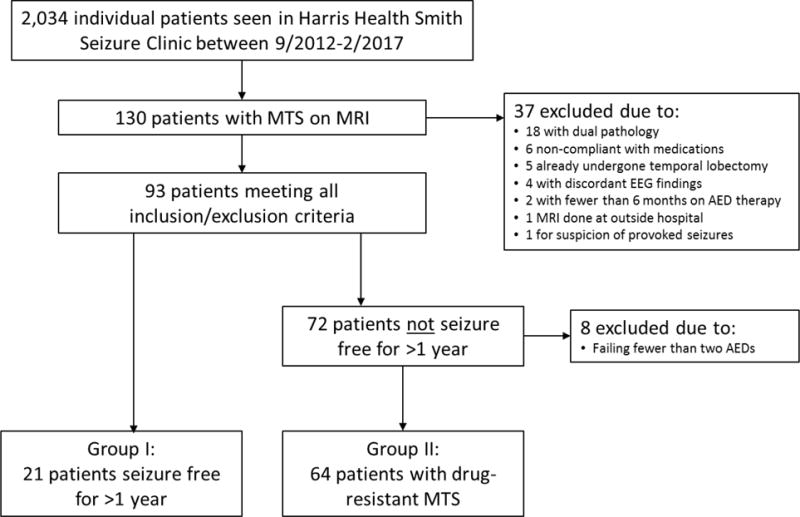
MTS, mesial temporal sclerosis. MRI, magnetic resonance imaging. EEG, electroencephalogram. AEDs, anti-epileptic drugs
Patients were placed into one of two groups, group I or group II, based on presence of seizure and aura within the twelve months prior to the most recent clinic visit. Group I, the seizure-free group, exhibited a total absence of seizure activity or only auras. Group II, the drug-resistant group, displayed focal dyscognitive (previously “complex partial”) or generalized seizures within the last year. Patients with seizures only within the setting of a brief, well-defined period of noncompliance were placed in Group I. Other clinical and demographic factors were also recorded, including age, sex, age at onset of seizures, duration of epilepsy, side of lesion, handedness, EEG findings, history of CNS infection, history of febrile convulsions, history of head trauma, history of cognitive impairment, family history of seizures, number of current AEDs, total number of AED trials, and presence of individual AEDs. Age referred to the patient age at last clinic visit, and age at onset referred to the age at which typical seizures began. A patient was considered to have a history of cognitive impairment if provider notes recorded a history of developmental delay or mental retardation for the patient. Absence of documentation for history of febrile convulsions, CNS infection, head trauma, cognitive impairment, and family history of seizures was considered to be a negative history. Older AEDs were defined as those approved by the United States Food and Drug Administration (FDA) before 1990, including phenytoin, carbamazepine, valproate, phenobarbital, and primidone, while the newer AEDs were defined as those FDA approved after 1990, including levetiracetam, lamotrigine, zonisamide, topiramate, oxcarbazepine, lacosamide, gabapentin, and clobazam.
Demographic and clinical factors were compared between groups I and II using an unpaired t-test or the Mann-Whitney U test (depending on normality of distributions) for numerical variables and the Fisher’s Exact t-test or chi-squared test for categorical variables. For the unpaired t-tests, Welch’s correction was added if the standard deviations were not equal. Normality for the numerical variables was determined by the D’Agostino-Pearson omnibus normality test. For the categorical variables, Fisher’s Exact t-test was performed whenever possible. The chi-squared test was performed if there were three or more categories. Linear regression analysis was performed to evaluate the correlation between duration of epilepsy and all other numerical variables. Statistical analyses were performed using GraphPad Prism version 7.02 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. p-values less than 0.05 were considered significant.
Additionally, percent success rates were calculated for individual AEDs. A successful trial was defined as the presence of an AED on the current regimen of a seizure-free (Group I) patient. An unsuccessful trial was defined as the presence of an AED in the current regimen of a drug-resistant (Group II) patient, or past usage of the AED by any patient that was terminated due to ineffectiveness or unknown reasons (but not due intolerable side effects). Percent success rate for an AED was defined as the number of successful trials divided by the number of total trials multiplied by 100.
3. Results
Of 2,034 individual patients seen at the Harris Health Smith Clinic between September 2012 and February 2017, 130 were found to have evidence of hippocampal or mesial temporal sclerosis (MTS) on MRI. After exclusions, a total of 85 patients remained and were included in the analysis (Figure 1).
Of those patients, 31 were male and 54 were female (Table 1). 37 had right-sided MTS, 42 had left-sided MTS, and 6 had bilateral MTS. Handedness information was not available for 9 patients, and EEG information was not available for 7 patients.
Table 1.
Baseline Characteristics
| Group I: Seizure-free | Group II: Drug-resistant | Total n | p-value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| All patients | 21 | 24.7 | 64 | 75.3 | 85 | ||
|
| |||||||
| Gender | M | 6 | 19.4 | 25 | 80.6 | 31 | 0.443 |
| F | 15 | 27.8 | 39 | 72.2 | 54 | ||
| Handedness | Right | 16 | 23.2 | 53 | 76.8 | 69 | 0.073 |
| Left | 4 | 57.1 | 3 | 42.9 | 7 | ||
| Lesion Laterality | Right | 7 | 18.9 | 30 | 81.1 | 37 | 0.416 |
| Left | 13 | 31.0 | 29 | 69.0 | 42 | ||
| Bilateral | 1 | 16.7 | 5 | 83.3 | 6 | ||
| EEG | Normal | 11 | 35.5 | 20 | 64.5 | 31 | 0.120 |
| Abnormal | 9 | 19.1 | 38 | 80.9 | 47 | ||
| History of Febrile Convulsions | Yes | 1 | 7.7 | 12 | 92.3 | 13 | 0.171 |
| No | 20 | 27.8 | 52 | 72.2 | 72 | ||
| History of CNS Infection | Yes | 4 | 50.0 | 4 | 50.0 | 8 | 0.099 |
| No | 17 | 22.1 | 60 | 77.9 | 77 | ||
| History of Head Trauma | Yes | 4 | 16.7 | 20 | 83.3 | 24 | 0.404 |
| No | 17 | 27.9 | 44 | 72.1 | 61 | ||
| Family History of Seizures | Yes | 2 | 14.3 | 12 | 85.7 | 14 | 0.501 |
| No | 19 | 26.8 | 52 | 73.2 | 71 | ||
| History of Cognitive Impairment | Yes | 2 | 20.0 | 8 | 80.0 | 10 | 1.000 |
| No | 19 | 25.3 | 56 | 74.7 | 75 | ||
Twenty-one patients were seizure-free for at least one year (Group I) and 64 were drug-resistant (Group II). There was no significant difference between the two groups in gender, handedness, lesion laterality, EEG findings, history of febrile convulsions, history of CNS infections, history of head trauma, history of cognitive impairment, and family history of seizures (Table 1). There was also no significant difference in age at last clinic visit between the two groups (p=0.451) (Table 2). However, there was a significant difference in age at onset, duration of epilepsy, number of AEDs in current regimen, and total number of AED trials between the two groups (Table 2). Average age at onset was 25.4 for the seizure-free population and 13.7 for the drug-resistant population (Table 2) (p=0.002). Average duration of epilepsy was 21.6 years for the seizure-free population and 31.1 years for the drug-resistant population (Table 2) (p=0.018). Average number of AEDs in current regimen was 1.40 for the seizure-free population and 2.10 for the drug-resistant population (Table 2) (p<0.001). Finally, average total number of AED trials was 2.52 for the seizure-free population and 4.50 for the drug-resistant population (Table 2) (p<0.001).
Table 2.
Differences between Group I and Group II.
| Group I: Seizure-free | Group II: Drug-resistant | p-value | |||
|---|---|---|---|---|---|
| mean (range) | SD | mean (range) | SD | ||
| Age (years) | 47.00 (30–70) | 10.92 | 44.84 (20–79) | 11.46 | 0.442 |
| Age at onset (years) | 25.38 (4–54) | 16.54 | 13.72 (0–54) | 13.43 | *0.002 |
| Duration of epilepsy (years) | 21.62 (1–55) | 15.25 | 31.13 (1–72) | 14.90 | *0.018 |
| Seizure frequency (per month) | 0.00 | 0.00 | 3.26 (0.1–30) | 6.20 | *<0.001 |
| Number of AEDs in current regimen | 1.40 (1–2) | 0.50 | 2.22 (1–4) | 0.70 | |
| Total number of appropriate AED trials | 2.52 (1–6) | 1.33 | 4.50 (2–10) | 2.08 | *<0.001 |
AED=anti-epileptic drug,
p<0.05
Regression analysis revealed a significant correlation between duration of epilepsy and number of current AEDs (r2=0.42, p<0.001) and total AED trials (r2=0.51, p=0.0014) in the seizure-free group, but not the drug-resistant group (Figure 2). There was also a significant correlation between duration of epilepsy and age of onset for Group I (r2=0.59, p<0.001) and Group II (r2=0.46, p<0.001). Duration of epilepsy also correlated with age at last visit for Group II (r2=0.26, p<0.001) but not for Group I (r2=0.05, p=0.31). There was no significant correlation with seizure frequency for either group.
Figure 2.
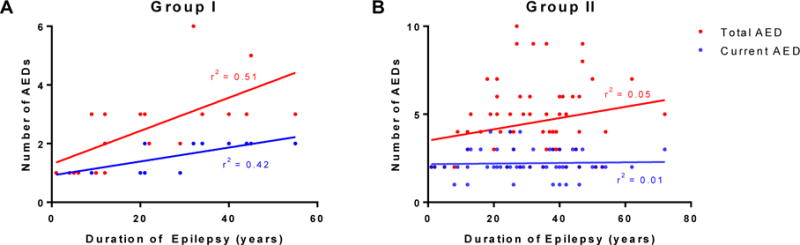
Significant correlation between duration of epilepsy and number of current AEDs (p=0.001) and total AEDs (p<0.001) for seizure-free (A) but not drug-resistant (B) patients.
Comparison of current drug regimens between the seizure-free and drug-resistant groups yielded no significant difference for the presence of any individual AED (Table 3). Additionally, comparing the presence of older and newer AEDs also yielded no significant difference between the two groups (Table 3). Calculation of percent success rate for individual AEDs revealed that the top two most “successful” AEDs were lamotrigine (23.8%) and levetiracetam (22.1%) (Supplemental Table 1).
Table 3.
No significant difference between efficacy of older and newer AEDs.
| Group I: Seizure-free | Group II: Drug resistant | Total n | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | n | % | ||||
| Older AEDs | Carbamazepine | 2 | 11.11 | 16 | 88.89 | 18 | 0.218 |
| Phenobarbital | 0 | 0.00 | 1 | 100.00 | 1 | 1.000 | |
| Phenytoin | 4 | 28.57 | 10 | 71.43 | 14 | 0.740 | |
| Primidone | 0 | 0.00 | 2 | 100.00 | 2 | 1.000 | |
| Valproate | 1 | 6.25 | 15 | 93.75 | 16 | 0.104 | |
|
| |||||||
| Newer AEDs | Clobazam | 0 | 0.00 | 2 | 100.00 | 2 | 1.000 |
| Gabapentin | 0 | 0.00 | 1 | 100.00 | 1 | 1.000 | |
| Lacosamide | 0 | 0.00 | 3 | 100.00 | 3 | 0.571 | |
| Lamotrigine | 5 | 23.81 | 16 | 76.19 | 21 | 1.000 | |
| Levetiracetam | 15 | 24.59 | 46 | 75.41 | 61 | 1.000 | |
| Oxcarbazepine | 1 | 7.69 | 12 | 92.31 | 13 | 0.171 | |
| Topiramate | 0 | 0.00 | 9 | 100.00 | 9 | 0.104 | |
| Zonisamide | 2 | 16.67 | 10 | 83.33 | 12 | 0.722 | |
|
| |||||||
| Older AEDs | 7 | 13.73 | 44 | 86.27 | 51 | 0.512 | |
| Newer AEDs | 23 | 18.85 | 99 | 81.15 | 122 | ||
4. Discussion
Mesial temporal lobe epilepsy is the most common cause of surgical resection for treatment of medication-refractory seizures, and surgical intervention remains the gold standard of treatment (Wiebe et al., 2001). Unfortunately, for many patients, epilepsy surgery is not a viable option. This may be a result of additional foci outside the temporal lobe, psychiatric or medical comorbid conditions, aversion to neurosurgery, a lack of access to a comprehensive epilepsy center, or financial considerations. In our study population, most patients were not surgical candidates due to a lack of health insurance. While surgical outcomes in MTLE-HS have been assessed by numerous studies, less attention has been given to outcomes of medical therapy, especially with respect to the effectiveness of newer agents.
In our study, 24.7% of patients exhibited seizure freedom of at least one year at most recent follow-up, in line with the 5% to 42% described in previous studies (Sànchez et al., 2014). There was no significant difference in prognosis associated with gender, laterality of lesion, history of CNS infections, history of head trauma, history of febrile seizures, history of cognitive impairment, family history of seizures, or current age. However, pharmacoresistant MTS was associated with an earlier age of onset (13.72 vs 25.38), longer duration of epilepsy (31.13 years vs 21.62 years), greater number of current AEDs (2.1 vs 1.4), and greater number of total AED trials (4.50 vs 2.52). Previous studies have mixed results over the significance of these clinical and demographic factors in determining prognosis, but support has been shown for the association between poor outcome and earlier age of onset, longer duration of epilepsy, and greater number of failed AED trials (Kuzmanovski et al., 2016; Pittau et al., 2009; Sànchez et al., 2014; Varoglu et al., 2009). Importantly, the association of poor outcome with greater number of failed AED trials is in line with previous studies showing a poor response to therapy after the first or second AED trial (Dlugos et al., 2001; Kwan and Brodie, 2000).
Interestingly, there was a significant association of duration of epilepsy with both current number of AEDs and total AED trials, but only in the seizure-free group. This finding suggests that once diagnosed with MTS, additional AEDs may need to be added over time to achieve or maintain seizure control, giving further evidence for a “silent period” after starting an AED which has been posited to be a part of the natural history of MTLE-HS (Wieser and ILAE Commission on Neurosurgery of Epilepsy, 2004).
We also investigated the specific AEDs used in the successful and failed treatment regimens. Though sample size precludes any definitive statements regarding their efficacy, lamotrigine and levetiracetam had the highest rate of use in successful treatment regimens (23.8% and 22.1%, respectively). However, these numbers may be inflated due to an increased frequency of use in many initial treatment regimens as well as generally positive side effect profiles. Importantly, analysis of individual AEDs showed no association between presence of any one AED and seizure freedom, and we found no association between newer AEDs collectively and seizure freedom. These data suggest that no currently approved AED is inherently more effective than another for achieving seizure freedom in patients with MTLE-HS and support the standard practice of defining pharmacoresistance as failure of at least two anti-epileptic drugs, regardless of the specific drugs (Kwan et al., 2010).
The major barrier to obtaining epilepsy surgery in this study was lack of access to health insurance. Many of these are undocumented immigrants and therefore lack access to Medicaid and Medicare. The decision by the state of Texas to not expand Medicaid under the Affordable Care Act has also significantly limited the availability of insurance to legal residents as well (Gong et al., 2017). Additionally, patients may also have low levels of employment, income, educational attainment, access to personal transportation, and English proficiency, making navigation of the healthcare system and acquisition of insurance more challenging.
This study had several limitations. First, due to its retrospective nature and the long duration of epilepsy in most patients, the dataset was incomplete with respect to patient history, and little was known about the frequency of seizures before anticonvulsant therapy. Additionally, some patients may have undergone other antiepileptic drug trials at outside clinics that they did not recall. This study also may not have captured the patients with MTLE-HS well-controlled on an initial AED without the need for a referral to a neurologist for full work-up and frequent follow up.
5. Conclusions
The findings from this study are applicable to any population with access to anti-epileptic drugs but not to epilepsy surgery, including patients who are indigent or uninsured, with limited access to a Comprehensive Epilepsy Center, or living in developing countries. After failing two appropriate AEDs, patients with MTLE-HS should continue to be referred for epilepsy surgery to maximize the chances of achieving seizure freedom, regardless of whether they have been taking older or newer AEDs.
Supplementary Material
Highlights.
The majority (75.3%) of patients with MTLE-HS who are treated with anti-epileptic drugs remain refractory to 2 or more appropriate AED trials.
Newer AEDs are not more likely to control seizures in MTLE-HS than older AEDs.
Duration of epilepsy significantly correlates with number of AED trials in those patients who are seizure free, but not those who are pharmacoresistant.
Acknowledgments
This study was supported by NIH K08 NS096029 (AM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Michael S. Pohlen, study design, acquisition of data, analysis and interpretation of data Jingxiao Jin, acquisition of data, analysis and interpretation of data
Ronnie S. Tobias, acquisition of data
Atul Maheshwari, study concept and design, critical revision of manuscript for intellectual content, study supervision
Author Disclosures:
Michael S. Pohlen – reports no disclosures
Jingxiao Jin – reports no disclosures
Ronnie S. Tobias – reports no disclosures
Atul Maheshwari – reports no disclosures
Contributor Information
Michael S. Pohlen, Baylor College of Medicine.
Jingxiao Jin, Baylor College of Medicine.
Ronnie S. Tobias, Department of Neurology, Baylor College of Medicine
Atul Maheshwari, Department of Neurology, Baylor College of Medicine.
References
- Borzì G, Di Gennaro G, Schmitt FC, D’Aniello A, Mumoli L, Zummo L, Daniele O, Russo E, Gambardella A, Labate A. Lacosamide in patients with temporal lobe epilepsy: An observational multicentric open-label study. Epilepsy Behav EB. 2016;58:111–114. doi: 10.1016/j.yebeh.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Coan AC, Campos BM, Bergo FPG, Kubota BY, Yasuda CL, Morita ME, Guerreiro CAM, Cendes F. Patterns of seizure control in patients with mesial temporal lobe epilepsy with and without hippocampus sclerosis. Arq Neuropsiquiatr. 2015;73:79–82. doi: 10.1590/0004-282X20140199. [DOI] [PubMed] [Google Scholar]
- Dlugos DJ, Sammel MD, Strom BL, Farrar JT. Response to first drug trial predicts outcome in childhood temporal lobe epilepsy. Neurology. 2001;57:2259–2264. doi: 10.1212/wnl.57.12.2259. [DOI] [PubMed] [Google Scholar]
- Giussani G, Canelli V, Bianchi E, Erba G, Franchi C, Nobili A, Sander JW, Beghi E, EPIRES Group Long-term prognosis of epilepsy, prognostic patterns and drug resistance: a population-based study. Eur J Neurol. 2016;23:1218–1227. doi: 10.1111/ene.13005. [DOI] [PubMed] [Google Scholar]
- Gomez-Ibañez A, Gasca-Salas C, Urrestarazu E, Viteri C. Clinical phenotypes within non-surgical patients with mesial temporal lobe epilepsy caused by hippocampal sclerosis based on response to antiepileptic drugs. Seizure. 2013;22:20–23. doi: 10.1016/j.seizure.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Gong G, Huey CC, Johnson C, Curti D, Philips BU. The Health Insurance Gap After Implementation of the Affordable Care Act in Texas. Tex Med. 2017;113:e1. [PubMed] [Google Scholar]
- Kim WJ, Park SC, Lee SJ, Lee JH, Kim JY, Lee BI, Kim DI. The prognosis for control of seizures with medications in patients with MRI evidence for mesial temporal sclerosis. Epilepsia. 1999;40:290–293. doi: 10.1111/j.1528-1157.1999.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Kumlien E, Doss RC, Gates JR. Treatment outcome in patients with mesial temporal sclerosis. Seizure. 2002;11:413–417. doi: 10.1053/seiz.2001.0614. [DOI] [PubMed] [Google Scholar]
- Kurita T, Sakurai K, Takeda Y, Horinouchi T, Kusumi I. Very Long-Term Outcome of Non-Surgically Treated Patients with Temporal Lobe Epilepsy with Hippocampal Sclerosis: A Retrospective Study. PloS One. 2016;11:e0159464. doi: 10.1371/journal.pone.0159464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmanovski I, Cvetkovska E, Babunovska M, Kiteva Trencevska G, Kuzmanovska B, Boshkovski B, Isjanovska R. Seizure outcome following medical treatment of mesial temporal lobe epilepsy: Clinical phenotypes and prognostic factors. Clin Neurol Neurosurg. 2016;144:91–95. doi: 10.1016/j.clineuro.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Malmgren K, Thom M. Hippocampal sclerosis–origins and imaging. Epilepsia. 2012;53(Suppl 4):19–33. doi: 10.1111/j.1528-1167.2012.03610.x. [DOI] [PubMed] [Google Scholar]
- Pittau F, Bisulli F, Mai R, Fares JE, Vignatelli L, Labate A, Naldi I, Avoni P, Parmeggiani A, Santucci M, Capannelli D, Di Vito L, Gambardella A, Baruzzi A, Tinuper P. Prognostic factors in patients with mesial temporal lobe epilepsy. Epilepsia. 2009;50(Suppl 1):41–44. doi: 10.1111/j.1528-1167.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- Sànchez J, Centanaro M, Solís J, Delgado F, Yépez L. Factors predicting the outcome following medical treatment of mesial temporal epilepsy with hippocampal sclerosis. Seizure - Eur J Epilepsy. 2014;23:448–453. doi: 10.1016/j.seizure.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Semah F, Lamy C, Demeret S. HIppocampal sclerosis and other hippocampal abnormalities in the early identification of candidates for epilepsy surgery. Arch Neurol. 2002;59:1042–1043. doi: 10.1001/archneur.59.6.1042-a. [DOI] [PubMed] [Google Scholar]
- Stephen LJ, Kwan P, Brodie MJ. Does the cause of localisation-related epilepsy influence the response to antiepileptic drug treatment? Epilepsia. 2001;42:357–362. doi: 10.1046/j.1528-1157.2001.29000.x. [DOI] [PubMed] [Google Scholar]
- Varoglu AO, Saygi S, Acemoglu H, Ciger A. Prognosis of patients with mesial temporal lobe epilepsy due to hippocampal sclerosis. Epilepsy Res. 2009;85:206–211. doi: 10.1016/j.eplepsyres.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wieser HG, ILAE Commission on Neurosurgery of Epilepsy ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


