Abstract
Aging is a main risk factor for intervertebral disc (IVD) degeneration, the main cause of low back pain. FOXO transcription factors are important regulators of tissue homeostasis and longevity. Here, we determined the expression pattern of FOXO in healthy and degenerated human IVD and the associations with IVD degeneration during mouse aging. FOXO expression was assessed by immunohistochemistry in normal and degenerated human IVD samples and in cervical and lumbar IVD from 6, 12, 24, and 36-month-old C57BL/6J mice. Mouse spines were graded for key histological features of disc degeneration in all the time points and expression of two key FOXO downstream targets, sestrin 3 (SESN3) and superoxide dismutase (SOD2), was assessed by immunohistochemistry. Histological analysis revealed that FOXO proteins are expressed in all compartments of human and mouse IVD. Expression of FOXO1 and FOXO3, but not FOXO4, was significantly deceased in human degenerated discs. In mice, degenerative changes in the lumbar spine were seen at 24 and 36 months of age whereas cervical IVD showed increased histopathological scores at 36 months. FOXO expression was significantly reduced in lumbar IVD at 12, 24 and 36-month-old mice and in cervical IVD at 36-month-old mice when compared with the 6-month-old group. The reduction of FOXO expression in lumbar IVD was concomitant with a decrease in the expression of SESN3 and SOD2. These findings suggest that reduced FOXO expression occurs in lumbar IVD during aging and precedes the major histopathological changes associated with lumbar IVD degeneration.
Keywords: intervertebral disc, aging, FOXO
INTRODUCTION
Low back pain is the leading cause of physical disability and imposes a great burden on global health (1–3). Intervertebral disc degeneration (IDD) is the most common underlying cause of back pain in the older adults (4–6). The pathophysiology of IDD is not well understood and there are no current treatments to prevent or ameliorate disease onset or progression (2). Given the progressive increase in life expectancy worldwide, with a projected 1.5 billion people 65 years and older by 2050 (3), it has become a priority to identify the causes and mechanisms of IDD to identify new therapeutic targets and approaches.
The intervertebral disc (IVD) is the largest avascular organ in the body. It is comprised of the nucleus pulposus (NP) that is rich in extracellular matrix (ECM) proteins such as aggrecan and type II collagen and confers the resistance to compressive strength; the annulus fibrosus (AF) that is primarily composed of type I collagen and provides the necessary tensile strength to contain the NP; and the cartilaginous endplates (EP) that have a structure similar to articular cartilage (7–10). Aging, a well-established risk factor for IDD (11–13), is caused in part by accumulation of damaged organelles and macromolecules, leading to cell senescence, cell death, tissue dysfunction and limited regenerative capacity of the tissues (14, 15). Unlike any other connective tissues, IDD begins early in life and involves a cascade of changes at the cellular and molecular level that results in structural and functional alterations, leading to biomechanical failure and degeneration (9, 16–19). These early changes include accumulation of molecular damage in the main components of the extracellular matrix (ECM) (8), as well as in DNA (20) and proteins (21), and are thought to be the consequence of defective homeostatic mechanisms that might trigger the onset of IDD (10). However, IDD pathophysiology is not well understood and the early molecular events that lead to disruption of cell homeostasis and result in accumulation of molecular damage remain to be discovered.
The FOXO proteins are an evolutionarily conserved family of transcription factors with important functions in development, aging and longevity (22). In mammals, the FOXO family has four members (FOXO1, FOXO3, FOXO4 and FOXO6) with distinct and overlapping functions (23). FOXO1, FOXO3 and FOXO4 are ubiquitously expressed whereas FOXO6 expression is restricted to neuronal tissues (24). FOXO control of cellular homeostasis is mediated by regulation of cellular responses to stress (24, 25). FOXO expression and activity are induced under oxidative stress conditions (24) and FOXO transcriptionally induce several antioxidant enzymes such as catalase and manganese superoxide dismutase (26, 27). FOXO proteins also regulate two major intracellular clearance mechanisms, autophagy and the ubiquitin-proteasome system, to eliminate damaged and aggregated proteins (25). Dysregulation of FOXO expression or activity contributes to the pathogenesis of age-related diseases in several different tissues, including bone (28, 29) and muscle (30, 31). Despite FOXO are key regulators of cell homeostasis and aging, their expression and function in IVD has not yet been studied.
Here, we examined for the first time the expression of FOXO in human and mouse IVD and compared the changes in FOXO expression with key histological and molecular features of IDD in mouse spines spanning the entire adult age spectrum.
METHODS
Human samples
Human lumbar IVD samples were collected from four cadaveric donors (age 40–45, mean 43±3) without any known spine pathology and from four donors (age 53–74, mean 62±10) undergoing spinal fusion surgery. Macroscopic assessment of disc degeneration in the cadaveric samples was performed according to Thompson grading (32). Additional details of the samples are listed in Table 1.
Table 1.
Human intervertebral discs used in the study.
| Disc Level | Morphologic Grade | Age | Gender |
|---|---|---|---|
| L3-L4 | IV | 53 | Male |
| L5-S1 | IV | 54 | Male |
| L4-L5 | IV | 66 | Male |
| L4-L5 | IV | 74 | Male |
| L4-L5 | II | 40 | Male |
| L4-L5 | II | 45 | Female |
| L4-L5 | II | 45 | Female |
| L4-L5 | II | 49 | Male |
Mouse samples
All animal experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee at our institution. Male C57BL/6J mice were housed in a temperature-controlled environment with a 12:12 hours light/dark cycle and free access to food and water. Eight mice per age group were euthanized at 6, 12, 24 and 36 months of age, and the cervical and lumbar regions of the spine were collected for histology or gene expression analysis.
Histological analysis
Human and mouse samples were washed in phosphate buffered saline (PBS) and fixed using zinc buffered formalin (ZFix, Anatech) for 48 hours. After fixing, samples were decalcified in a Shandon TBD-2 decalcifier (Thermo Scientific) for 24 hours on a shaker, thoroughly washed with PBS and embedded in paraffin. 4-μm-thick sections were cut and stained with Safranin O-fast green. In mouse spines, histological analysis of C3/C4 and L4/L5 IVD was performed as follows. Degenerative changes in NP and AF were graded following the system described by Masuda et al (33) whereas changes in EP were scored according to the grading system described by Boos et al (34). Samples were graded by two different observers blinded to the experimental conditions. Overall disc height, NP and AF cellularity, and the area of NP occupied by cells were measured in images obtained under 20× magnification using ImageJ software.
Immunohistochemistry (IHC)
IHC was performed in human and mouse samples as described previously (35). Briefly, 4-μm-thick sections were deparaffinized, rehydrated, and incubated with 2.5 mg/ml of hyaluronidase for 60 min at 37°C. The sections were blocked with 10% goat serum for 1 hour at room temperature followed by an overnight incubation with primary antibodies against FOXO1 (1:250, Abcam, ab70382), FOXO3 (1:500, Abcam, ab70315), FOXO4 (1:500, Abcam, ab63254), SOD2 (1:500, StressMarq Biosciences, SPC-118), SESN3 (1:100, Proteintech, 24532-1-AP) at 4°C. After washing, sections were incubated with ImmPRESS DAB Reagent (Vector Laboratories) for 30 minutes at room temperature followed by a 2-minute incubation with DAB Peroxidase (HRP) Substrate Kit (Vector Laboratories). Stained samples were mounted and visualized under optical microscopy and images of the C3/C4 and L4/L5 IVD were obtained under 20× magnification. Specificity of the FOXO1, FOXO3 and FOXO4 antibodies was assessed in mouse lumbar spine samples containing blood vessels, bone marrow and spinal cord, respectively (Supplementary Figure 1). For human samples, positive cells were quantified in three fields of 1cm2 in the NP and the AF. For mouse samples, one section per animal was obtained and positive cells were quantified in the NP, AF and EP of C3/C4 and L4/L5 IVD. Data was expressed as the percentage of positive cells relative to the total number of cells.
RNA isolation and gene expression analysis
Mouse lumbar spine samples were collected at 6 and 12 months of age, NP and AF from lumbar discs were resected separately and homogenized in Qiazol Lysis Reagent (Qiagen). RNA was isolated using Direct-zol RNA miniprep kit (Zymo Research). Gene expression was measured by real-time PCR using pre-designed TaqMan gene expression assays (Life Sciences) for mouse Foxo1, Foxo3, Sod2 and Sesn3. Gapdh was used as reference gene.
Statistical analysis
Data are reported as the mean ± 95% CI (95% confidence interval). Differences between groups were assessed by one-way analysis of variance (ANOVA) followed by a post-hoc Tukey test. Comparisons between two groups were assessed by an unpaired, two-tailed T-test after testing for equal variance using an F-test. All statistical analyses were performed using Prism 6 software (GraphPad Software). P-values less than 0.05 were considered significant.
RESULTS
FOXO expression in human IVD
To determine the expression pattern of FOXO transcription factors in human IVD, IHC analysis was performed in normal and degenerated IVD for FOXO1, FOXO3 and FOXO4 isoforms. Normal human IVD showed strong expression for all FOXO isoforms in the nucleus pulposus (NP), inner (IAF) and outer annulus fibrosus (OAF) (Figure 1). FOXO1 and FOXO3 immunostaining was present in more than 70% of cells whereas FOXO4 positive cells were less abundant at about 40%. Discs from donors undergoing spinal fusion surgery for IDD exhibited histological abnormalities including fibrotic NP with some cell clusters, loss of NP/AF demarcation, small ruptures in the AF and general disorganization of AF lamellar structure. In all degenerated discs, there was a significant decrease of FOXO1 and FOXO3 but not FOXO4 staining in the NP, IAF and OAF (Figures 1A–B).
Figure 1.
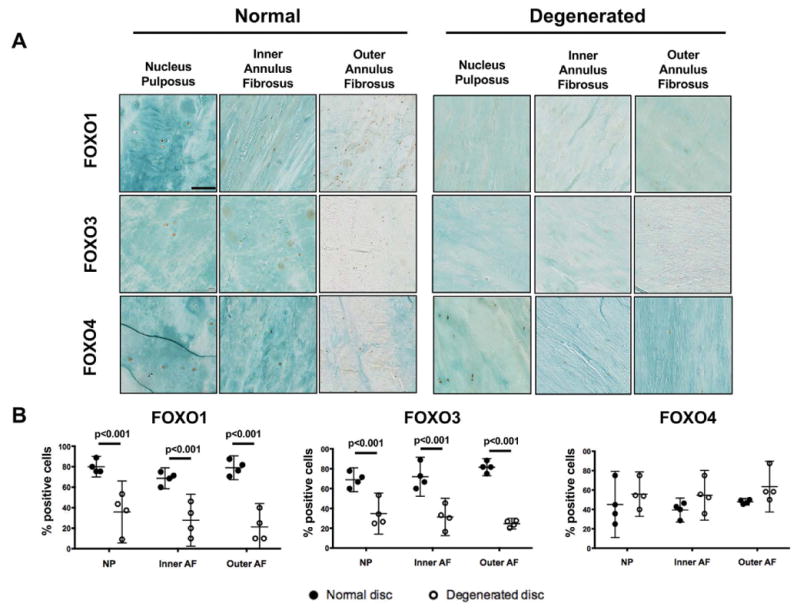
FOXO expression in human intervertebral discs (IVD). A) Representative images of FOXO1, −3, and −4 immunohistochemical staining in the nucleus pulposus and annulus fibrosus of lumbar IVD from normal donors and donors with disc degeneration (n=3 per group). Magnification bar = 100 μm. B) Quantification of FOXO1, −3, and −4 immunopositive cells in IVD from normal donors and donors with disc degeneration (n=3 per group). Data is presented as percentage of positive cells. All values are mean ± 95% CI.
Degenerative changes in the mouse spine with aging
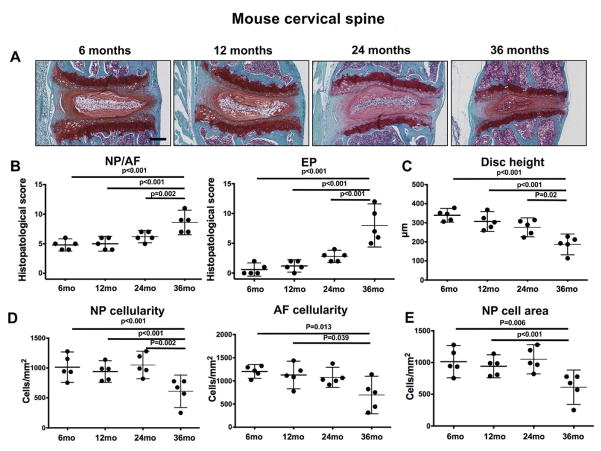
We collected mouse spines at several time points throughout the entire adult spectrum to allow a detailed temporal analysis of histological and molecular changes. To determine the aging-related pattern and tissue compartments in which changes occur, cervical and lumbar spine segments from 6, 12, 24, and 36-month-old mice were stained with safranin-O and analyzed histologically. As shown in Figure 2, cervical IVD from mice exhibited profound histological changes that are characteristic of severe IVD degeneration at 36 months and resulted in significantly higher histopathological scores. These changes included severely decreased cellularity, complete disruption of the NP/AF borders, an increase in NP fibrosis without an apparent loss of proteoglycan staining, ruptured fibers and general disorganization of the AF, thinning and ossification of the EP, and osteophyte formation. Histomorphometric analysis revealed a reduction in disc height (Figure 2C), cell density in NP and AF (Figure 2D), and area of NP occupied by cells (Figure 2E).
Figure 2.
Age-related changes in mouse cervical intervertebral discs (IVD). A) Representative images of cervical IVD from 6, 12, 24, and 36-month-old mice (n=6 per group) stained with safranin-O. Magnification bar = 100 μm. B) Histopathological scores in the nucleus pulposus/annulus fibrosus (NP/AF) and endplates (EP) of cervical IVD from 6, 12, 24, and 36-month-old mice (n=6 per group). C) Measurement of total disc height in cervical IVD from 6, 12, 24, and 36-month-old mice (n=6 per group). All values are mean ± 95CI. D) Quantification of cellularity in the NP and AF of cervical IVD from 6, 12, 24, and 36-month-old mice (n=6 per group). E) Quantification of NP area occupied by NP cells in cervical IVD from 6, 12, 24, and 36-month-old mice (n=6 per group). All values are mean ± 95% CI.
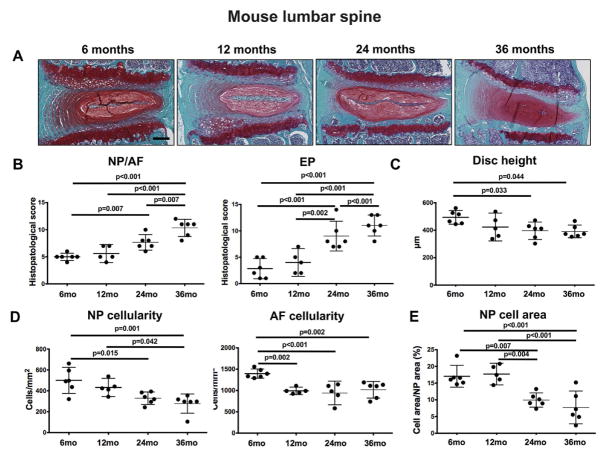
In lumbar discs, mild degenerative changes were seen in some samples as early as 12 months of age and included loss of AF cellularity, decrease in EP thickness, and loss of proteoglycan staining in the EP. The degree of disc degeneration markedly increased at 24 and 36 months of age, and histopathological scores were much higher than those found in the cervical IVD (Figure 3B). In addition, there was a significant decrease in disc height (Figure 3C), NP and AF cellularity (Figure 3D), and in the area of NP occupied by cells in 24 and 26-month-old mice when compared with 6-month-old controls (Figure 3E). These results indicate that there is an age-related degeneration of the mouse IVD that is more pronounced and has an earlier onset in the lumbar than in the cervical region of the spine.
Figure 3.
Age-related changes in mouse lumbar intervertebral discs (IVD). A) Representative images of lumbar IVD from 6, 12, 24, and 36-month-old mice (n=8 per group) stained with safranin-O. Magnification bar = 100 μm. B) Histopathological scores in the nucleus pulposus/annulus fibrosus (NP/AF) and endplates (EP) of lumbar IVD from 6, 12, 24, and 36-month-old mice (n=8 per group). C) Measurement of total disc height in lumbar IVD from 6, 12, 24, and 36-month-old mice (n=8 per group). D) Quantification of cellularity in the NP and AF of lumbar IVD from 6, 12, 24, and 36-month-old mice (n=6 per group). E) Quantification of NP area occupied by NP cells in lumbar IVD from 6, 12, 24, and 36-month-old mice (n=6 per group). All values are mean ± 95% CI.
Age-related decrease in FOXO expression in the mouse intervertebral disc
Next, we investigated the temporal changes in FOXO expression and the relationship with tissue degeneration. In the cervical spine of normal mature 6-month-old mice, FOXO1 and FOXO3 staining was detected in all the IVD structures (Figure 4A). No staining for FOXO4 was detected. The NP and AF, FOXO1 and FOXO3 had a similar percentage of positive cells (46±5 and 55±18, respectively), whereas FOXO3 was more abundantly expressed in EP cells (31±11) than FOXO1 (9±4). With aging, FOXO expression in the NP and AF remained stable up to 24 months of age and markedly decreased in 36-month-old mice when compared with younger mice (Figure 4C).
Figure 4.
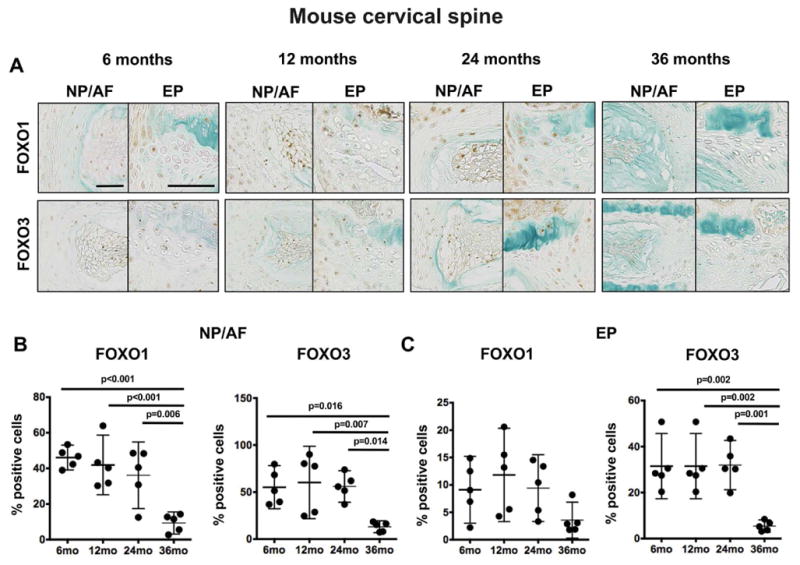
Changes in FOXO expression in mouse cervical intervertebral discs (IVD) during aging. A) Representative images of FOXO1 and FOXO3 immunohistochemical staining in cervical IVD from 6, 12, 24, and 36-month-old mice (n=5 per group). Magnification bar = 50 μm. B–C) Quantification of FOXO1 and FOXO3 immunopositive cells in the nucleus pulposus/annulus fibrosus (NP/AF) (B) and endplates (EP) (C) of cervical IVD from 6, 12, 24, and 36-month-old mice (n=5 per group). All values are mean ± 95% CI.
When comparing the cervical and lumbar regions of the mouse spine, FOXO expression patterns showed substantial differences. The number of FOXO1 expressing cells in all IVD compartments of 6-month-old mice was higher in lumbar (NP/AF: 78±13, EP: 50±10) than in cervical (NP/AF: 46±5, EP: 9±4) discs (Figure 5). FOXO3 expression was similar in the NP and AF between the different spine regions studied (lumbar: 55±17, cervical: 55±18), but much higher in the EP of lumbar IVD (50±10) when compared with cervical discs (9±4). The pattern of FOXO expression with aging was also markedly different between cervical and lumbar discs. In lumbar IVD, there was already a significant reduction in FOXO1 and FOXO3 expression at 12 months of age when compared with 6-month-old controls (Figure 5B). This reduction in FOXO1 and FOXO3 expression by 12 months of age was corroborated at the RNA level by qPCR analysis (Supplementary Figure 2A). These findings indicate that FOXO expression is reduced with age in cervical and lumbar IVD, with a more pronounced and much earlier decline in lumbar than in cervical discs. In addition, the reduction in FOXO expression in lumbar spine precedes cell loss and degenerative changes in the IVD.
Figure 5.
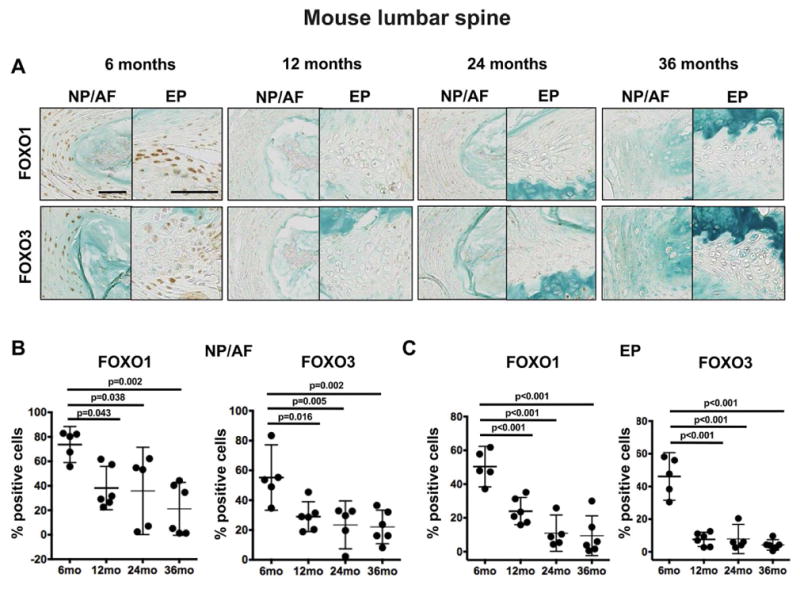
Changes in FOXO expression in mouse lumbar intervertebral discs (IVD) during aging. A) Representative images of FOXO1 and FOXO3 immunohistochemical staining in lumbar IVD from 6, 12, 24, and 36-month-old mice (n=5 per group). Magnification bar = 50 μm. B–C) Quantification of FOXO1 and FOXO3 immunopositive cells in the nucleus pulposus/annulus fibrosus (NP/AF) (B) and endplates (EP) (C) of lumbar IVD from 6, 12, 24, and 36-month-old mice (n=5 per group). All values are mean ± 95% CI.
Decreased expression of FOXO targets in mouse IVD with aging
Since FOXO expression was reduced in mouse IVD during aging, particularly in the lumbar discs, we determined whether this was associated with compromised FOXO function. For this purpose, we determined the expression of two canonical FOXO targets, SESN3 (36) and SOD2 (27), which are important in cellular stress responses. SESN3 and SOD2 expression was detected predominantly in the NP of 6-month-old mice (Figure 6A). There was a significant reduction of SESN3 and SOD2 positive cells in the NP of 12, 24 and 36-month-old mice when compared with 6-month-old controls (Figure 6B). Quantitative PCR analysis revealed that SOD2 and SESN3 expression was also reduced at the mRNA level in the NP of 12-month-old mice compared with younger controls (Supplementary Figure 2B). These results suggest that the age-related reduction in FOXO expression in lumbar spine is accompanied by a reduction in important effectors of FOXO signaling.
Figure 6.
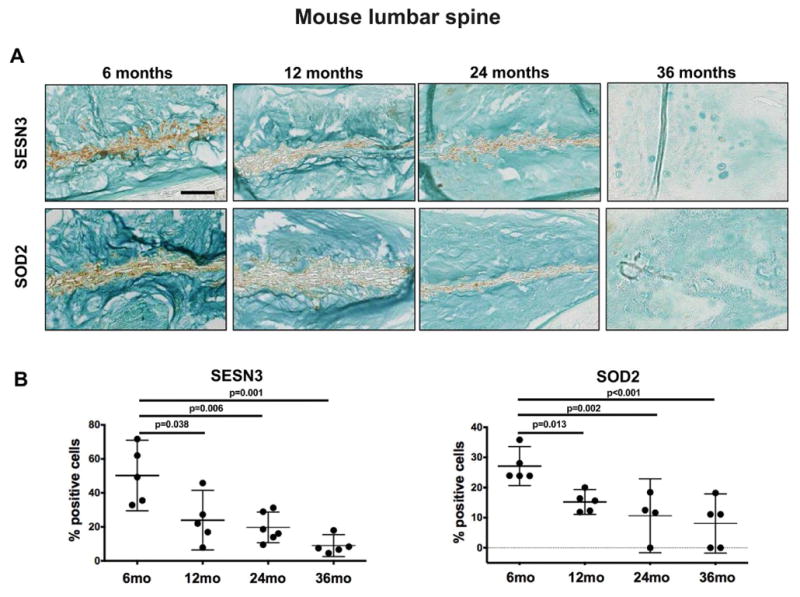
Changes in SESN3 and SOD2 expression in mouse intervertebral discs (IVD) during aging. A) Representative images of SESN3 and SOD2 immunohistochemical staining in lumbar IVD from 6, 12, 24, and 36-month-old mice (n=5 per group). Magnification bar = 50 μm. B) Quantification of SESN3 and SOD2 immunopositive cells in the nucleus pulposus of lumbar IVD from 6, 12, 24, and 36-month-old mice (n=5 per group). All values are mean ± 95% CI.
DISCUSSION
This is the first study that analyzed the expression pattern of the FOXO family of transcription factors in IVD. Our data demonstrates that FOXO are expressed in all structures of human IVD, with FOXO1 and FOXO3 being the most abundant isoforms, and that there is a significant decrease in the expression of FOXO1 and FOXO3 in human degenerated discs. In addition, using a mouse model to explore in detail the temporal relationship between FOXO expression in cervical and lumber IVD and degenerative changes that occur with aging, we demonstrated that FOXO expression is reduced with aging in IVD and that this event precedes major histological features of disc degeneration in the lumbar spine.
The first finding of our study is that FOXO are constitutively expressed in all structures of human and mouse IVD, particularly in NP and AF. Cells within the IVD reside in a unique biological niche since the avascular nature of disc tissue results in a hypoxic environment with low glucose concentrations (37). Under these circumstances, NP and inner AF cells have evolved to be exquisitely adapted to hypoxia, mainly mediated by a constitutive activation of HIF-1 (hypoxia-inducible factor 1) (37). However, it is likely that additional pathways contribute to the adaptation of NP and AF cells to the IVD niche. FOXO transcription factors are associated with a wide range of biological processes that promote tissue and organismal homeostasis, including DNA repair, anti-oxidative defense, cell cycle arrest, and maintenance of proteostasis (24, 38). Importantly, FOXO are key factors in the cellular response to hypoxia (39) and energy stress (40). Therefore, it is likely that a constitutive FOXO expression is an important mechanism to facilitate cell survival in the unique disc environment, and further supports the notion that loss of FOXO during aging may contribute to the pathophysiology of IDD.
Aging is considered to have a major role in IDD (41). IDD begins as early as the second decade of life and greatly increases in incidence with advancing age (34, 41). Over the last decades, there has been substantial progress in identifying age-related changes in the IVD and in characterizing the catabolic events that occur during disc degeneration (19, 34, 42, 43). However, as disc aging and disc degeneration can be viewed as two different processes, the challenge is to identify changes that are characteristic of disc aging and understand how they differ from pathologic degeneration. With this purpose, Vo and colleagues have recently organized the biochemical cascade of IVD aging into three distinct phases: accumulation of damage to biomolecules, aberrant cellular response to damage, and loss of biologic structure and function (11). Whereas the latter two phases have substantial overlap between physiological aging and disc degeneration, it is the first phase that has a unique component related to aging. This phase is characterized by an accumulation of damage to DNA and proteins that are likely a result from exposure to an oxidative and inflammatory environment (44–46). The rate and extent of this accumulation of biomolecular damage is likely to drive the early stages of IDD and can be influenced by defective homeostatic mechanisms, environmental factors, and genetics (11). This notion is in keeping with the general paradigm of aging that considers aging as a time-dependent accumulation of cellular damage (14, 15). In this regard, it is important to note that the ability of FOXO to promote longevity in model organisms has been linked to proteotoxic stress resistance, DNA damage repair, and enhancement of antioxidant defenses (15, 24, 38, 47).
A strength of the present study is the use of a mouse model spanning the entire adult age spectrum to characterize the age-related changes in cervical and lumbar IVD. Animal models of human diseases are vital for research aimed at identifying disease mechanisms and novel targets for therapeutic prevention and treatment. Most of the studies in disc degeneration utilize surgically manipulated animal models to create IDD-like changes (33, 48–51). A limitation of these approaches is that they do not recapitulate the changes that are characteristic of age-related disc degeneration. Here, we showed for the first time that lumbar and cervical discs in the mouse spine undergo degenerative changes with aging that resemble those present on human discs, including narrowing of the disc space, reduced cellularity, disappearance of the NP/AF interface, disorganization of the lamellar structure of the AF, EP ossification and osteophyte formation (34). Moreover, degenerative changes in mouse lumbar IVD were more severe and developed much earlier than those of the cervical spine. This observation is in keeping with the higher susceptibility for disc degeneration in human lumbar discs than other regions of the spine (41, 52). These striking similarities are despite the differences between both species, that include size, biomechanical properties (biped vs quadruped), nutrient diffusion due to disparity in disc size and the presence of notochordal cells in adult mouse discs (44). Our data thus validates the mouse as a spontaneous model of age-related disc degeneration and provides a temporal pattern of major histological changes that constitute a valuable reference for basic spine research.
In the present study, we showed a reduction in FOXO expression in mouse lumbar IVD during early aging, before macroscopic degenerative changes are observed. These findings suggest that a reduction in FOXO expression and activity may have a deleterious impact on cell homeostasis during early stages of disc aging, and might result in an accelerated rate of biomolecular damage accumulation. Further supporting this rationale, our data shows an age-related reduction in the expression of SESN3 and SOD2 of mouse lumbar discs. SESN are a family of highly conserved stress-responsive proteins that protect cells from oxidative stress and have been shown to delay aging-associated pathologies in model organisms (53). On the other hand, SOD2 is the major mitochondrial antioxidant protein and plays a key role in regulating oxidative stress resistance (54). Therefore, a reduction in the expression of these important proteins would compromise the ability of IVD cells to neutralize reactive oxygen species, potentially resulting in increased oxidative damage.
A limitation of our study is that the analysis of FOXO expression in human IVD compared samples from young healthy individuals and old donors with disc degeneration. In this regard, the reduction in FOXO expression observed in our human samples could be either a consequence of not being produced by the aging cells and/or to loss of cellularity. Further studies comparing healthy and degenerated discs from different age groups are needed to elucidate the precise expression patterns of FOXO in IVD during aging and degeneration. In addition, while the present findings are the first to demonstrate a correlation between age-related decrease in FOXO expression and disc structural damage in the mouse, a causal relationship needs to be established. This limitation should be addressed in future studies using mouse models with tissue-specific FOXO deletion or overexpression to fully define FOXO function in IVD homeostasis, aging and degeneration.
Therapies aimed at preserving healthy joints during aging have become an appealing strategy to prevent musculoskeletal diseases on the elderly population, including IDD. To achieve this goal, it is of crucial importance to identify the precise pathways or mechanisms that lead to the initiation of the biochemical cascade that progressively leads to IDD. In the present study, we showed that the FOXO family of transcription factors is expressed in human and mouse IVD. Importantly, we showed a reduction in FOXO expression with aging in mouse lumbar IVD, preceding major histopathological changes associated with IDD. The aging related decline in FOXO is potentially an early initiating event in IDD and may constitute a novel biomarker and a future therapeutic target for prevention and treatment of the disease.
Supplementary Material
Assessment of antibody specificity. A) Representative images of immunohistochemical staining using FOXO1, FOXO3, FOXO4 antibodies in mouse lumbar spine samples isolated from 6-month-old mice showing strong staining for FOXO1, 3 and 4 in blood vessels, bone marrow, and spinal cord, respectively. B) Representative images of immunohistochemical staining using rabbit IgG antibody in lumbar spine samples isolated from 6-month-old mice showing negative staining in intervertebral disc tissues. Panels on the right show high magnification detail of nucleus pulposus (NP), annulus fibrosus (AF) and endplate (EP). Magnification bar = 100 μm
Changes in gene expression in mouse lumbar intervertebral discs (IVD) during aging. A) Real-time PCR analysis of Foxo1 and Foxo3 expression in the nucleus pulposus (NP) and annulus fibrosus (AF) of lumbar IVD isolated from 6 and 12-month-old mice (n=4 per group). A) Real-time PCR analysis of Sesn3 and Sod2 expression in the nucleus pulposus (NP) of lumbar IVD isolated from 6 and 12-month-old mice (n=4 per group). GAPDH was used as reference gene. All values are mean ± 95% CI.
Acknowledgments
This study was supported by NIH grants AG053747 and AG007996. We thank Josan Chung, Stuart Duffy, and Lilo Creighton for their technical assistance.
Footnotes
Author contributions: OA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. OA, KM, and MKL, designed research; OA, TM, and MO performed research; OA, KM, and MKL analyzed data; and OA and MKL wrote the paper. All authors read and approved the final version of the manuscript.
Contributor Information
Oscar Alvarez-Garcia, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA, USA.
Tokio Matsuzaki, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA, USA.
Merissa Olmer, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA, USA.
Koichi Masuda, Department of Orthopedic Surgery, University of California-San Diego, San Diego, CA, USA.
Martin K. Lotz, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA, USA.
References
- 1.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation. 2014;17(Suppl 2):3–10. doi: 10.1111/ner.12018. [DOI] [PubMed] [Google Scholar]
- 3.The Department of Economic and Social Affairs of the United Nations. World Population Prospects. The 2015 Revision. http://esa.un.org/unpd/wpp/Publications/FIles/Key_FIndings_WPP_2015.pdf2015.
- 4.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25(4):487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–70. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 6.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976) 1988;13(2):173–8. [PubMed] [Google Scholar]
- 7.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–4. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 8.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29(23):2691–9. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 9.Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–70. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Wang SZ, Rui YF, Lu J, Wang C. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014;47(5):381–90. doi: 10.1111/cpr.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo NV, Hartman RA, Patil PR, Risbud MV, Kletsas D, Iatridis JC, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34(8):1289–306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowa G, Vadala G, Studer R, Kompel J, Iucu C, Georgescu H, et al. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33(17):1821–8. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 13.Videman T, Battie MC, Gibbons LE, Gill K. Aging changes in lumbar discs and vertebrae and their interaction: a 15-year follow-up study. Spine J. 2014;14(3):469–78. doi: 10.1016/j.spinee.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13(3):318–30. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Sivan SS, Hayes AJ, Wachtel E, Caterson B, Merkher Y, Maroudas A, et al. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur Spine J. 2014;23(Suppl 3):S344–53. doi: 10.1007/s00586-013-2767-8. [DOI] [PubMed] [Google Scholar]
- 18.Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta. 2014;1840(10):3181–9. doi: 10.1016/j.bbagen.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5(6 Suppl):260S–6S. doi: 10.1016/j.spinee.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Nasto LA, Ngo K, Leme AS, Robinson AR, Dong Q, Roughley P, et al. Investigating the role of DNA damage in tobacco smoking-induced spine degeneration. Spine J. 2014;14(3):416–23. doi: 10.1016/j.spinee.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharf B, Clement CC, Yodmuang S, Urbanska AM, Suadicani SO, Aphkhazava D, et al. Age-related carbonylation of fibrocartilage structural proteins drives tissue degenerative modification. Chem Biol. 2013;20(7):922–34. doi: 10.1016/j.chembiol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn AJ. FOXO3 and related transcription factors in development, aging, and exceptional longevity. J Gerontol A Biol Sci Med Sci. 2015;70(4):421–5. doi: 10.1093/gerona/glu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20(2):126–36. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 25.Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci. 2014;39(4):159–69. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295(5564):2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 27.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419(6904):316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 28.Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11(2):136–46. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147–60. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6(6):472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15(5):411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 34.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27(23):2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 35.Akasaki Y, Hasegawa A, Saito M, Asahara H, Iwamoto Y, Lotz MK. Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage. 2014;22(1):162–70. doi: 10.1016/j.joca.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18(4):592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176(4):1577–83. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daitoku H, Fukamizu A. FOXO transcription factors in the regulatory networks of longevity. J Biochem. 2007;141(6):769–74. doi: 10.1093/jb/mvm104. [DOI] [PubMed] [Google Scholar]
- 39.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28(6):941–53. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Otin C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell. 2016;166(4):802–21. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20(11):1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Gopal D, Ho AL, Shah A, Chi JH. Molecular basis of intervertebral disc degeneration. Adv Exp Med Biol. 2012;760:114–33. doi: 10.1007/978-1-4614-4090-1_8. [DOI] [PubMed] [Google Scholar]
- 43.Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473(6):1903–12. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vo N, Niedernhofer LJ, Nasto LA, Jacobs L, Robbins PD, Kang J, et al. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31(6):831–7. doi: 10.1002/jor.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasto LA, Robinson AR, Ngo K, Clauson CL, Dong Q, St Croix C, et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res. 2013;31(7):1150–7. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 48.Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013;31(8):1276–82. doi: 10.1002/jor.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keorochana G, Johnson JS, Taghavi CE, Liao JC, Lee KB, Yoo JH, et al. The effect of needle size inducing degeneration in the rat caudal disc: evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. Spine J. 2010;10(11):1014–23. doi: 10.1016/j.spinee.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1(4):239–45. doi: 10.1016/s1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 51.Sakai D, Nishimura K, Tanaka M, Nakajima D, Grad S, Alini M, et al. Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study. Spine J. 2015;15(6):1356–65. doi: 10.1016/j.spinee.2013.07.491. [DOI] [PubMed] [Google Scholar]
- 52.Weiler C, Schietzsch M, Kirchner T, Nerlich AG, Boos N, Wuertz K. Age-related changes in human cervical, thoracal and lumbar intervertebral disc exhibit a strong intra-individual correlation. Eur Spine J. 2012;21(Suppl 6):S810–8. doi: 10.1007/s00586-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budanov AV, Lee JH, Karin M. Stressin’ Sestrins take an aging fight. EMBO Mol Med. 2010;2(10):388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126(3):365–79. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of antibody specificity. A) Representative images of immunohistochemical staining using FOXO1, FOXO3, FOXO4 antibodies in mouse lumbar spine samples isolated from 6-month-old mice showing strong staining for FOXO1, 3 and 4 in blood vessels, bone marrow, and spinal cord, respectively. B) Representative images of immunohistochemical staining using rabbit IgG antibody in lumbar spine samples isolated from 6-month-old mice showing negative staining in intervertebral disc tissues. Panels on the right show high magnification detail of nucleus pulposus (NP), annulus fibrosus (AF) and endplate (EP). Magnification bar = 100 μm
Changes in gene expression in mouse lumbar intervertebral discs (IVD) during aging. A) Real-time PCR analysis of Foxo1 and Foxo3 expression in the nucleus pulposus (NP) and annulus fibrosus (AF) of lumbar IVD isolated from 6 and 12-month-old mice (n=4 per group). A) Real-time PCR analysis of Sesn3 and Sod2 expression in the nucleus pulposus (NP) of lumbar IVD isolated from 6 and 12-month-old mice (n=4 per group). GAPDH was used as reference gene. All values are mean ± 95% CI.