Abstract
Fear of hypoglycemia is common in parents of young children with type 1 diabetes (T1D), but little is known about the specific fears that parents most often experience. Hypoglycemia fear has been associated with poorer glycemic control in older children, though not yet studied in a large cohort of very young children.
Parents of 549 children <7 years (mean 5.2±1.2 years [19% <3 years]) with a mean diabetes duration of 2.4±1.0 years (range 1–6 years) and mean HbA1c 8.2%±1.1% (66±12 mmol/mol) registered in the T1D Exchange completed the worry scale of the Hypoglycemia Fear Survey modified for parents (HFS-P).
Mean parental fear of hypoglycemia worry score was 36.1±23.1 (possible range 0 to 100), with most frequent worries related to the child having a low while asleep and the child not recognizing a low. The mean worry score was not associated with the child’s age, glycemic control, or recent severe hypoglycemic event. Parental worries about lows while sleeping were significantly higher in pump users than non-users (61% vs. 45%; p<0.001), and tended to be higher in CGM users than non-users (62% vs. 51%; p=0.02).
The greatest worries of parents of young children with T1D were related to hypoglycemia during sleep and other times/circumstances during which it would be difficult to detect hypoglycemia. Using advanced diabetes technologies may be an effort to temper fears about hypoglycemia during sleep, though the directionality of this relationship is undetermined. Additional studies can clarify this association and leverage use of diabetes technologies to improve glycemic control.
Keywords: type 1 diabetes, fear of hypoglycemia, young children, T1D Exchange clinic registry
Introduction
One of the ever-present and feared complications of type 1 diabetes (T1D) is severe hypoglycemia (SH), which can lead to seizure and loss of consciousness. Fear of hypoglycemia is especially common in families of young children with T1D due to the challenges parents face in striving to maintain optimal glycemic control during this unique developmental stage (1). In addition to unpredictable eating habits, irregular bouts of physical activity, and labile behavior, young children have limited ability to communicate symptoms of low blood glucose concentrations, and parents often cannot recognize that their children’s glucose levels are falling (2). Even in older children, parental detection of hypoglycemia is limited (3).
Parental fear of hypoglycemia may interfere with achievement of glycemic treatment targets (4), as some families may aim for higher blood glucose levels for their children in order to avoid lows, due to concerns about acute and chronic neurologic effects of hypoglycemia. Among parents with older children (mean ages 8–10 years) with T1D, greater parental worry has been associated with poorer glycemic control (5–7). In contrast, small single-center studies of parents with younger children (ages 2–8 years) have not identified a significant relationship (1, 8), and their smaller size limited generalizability to the broader national population of young children with T1D. A clearer understanding of the specific fears experienced by parents of young children related to hypoglycemia, and of the relationship between these fears and glycemic outcomes, is essential before diabetes clinicians and researchers can develop intervention strategies targeting parental fear of hypoglycemia.
Characterizing fear of hypoglycemia among parents of young children also is an important step towards achieving the more stringent targets for glycemic control that have recently been recommended in this young population. Data suggesting that hypoglycemia was especially damaging to the developing brain (9, 10) previously led the American Diabetes Association to recommend that parents of pre-school children with T1D aim to maintain HbA1c between 7.5–8.5% (58–69 mmol/mol) compared to the target HbA1c of <7.5% (<58 mmol/mol) for adolescents (11). However, recent reports from the Diabetes Research in Children Network (DirecNet) have shown that chronic hyperglycemia plays at least as important a role in altered brain growth and development as hypoglycemia (12, 13), and other studies have not confirmed elevated risk for cognitive dysfunction after SH in children (14, 15). Moreover, youth in the T1D Exchange clinic registry (T1D Exchange) with HbA1c levels well above the target range have reported the same frequency of hypoglycemia-induced seizures and loss of consciousness as those with HbA1c levels within the target range (16, 17). These data led the American Diabetes Association in 2014 to recommend a more stringent HbA1c goal of <7.5% (<58 mmol/mol), and even <7% (<53 mmol/mol) if this can be achieved without frequent hypoglycemia, across all age groups, including young children (18). This lower HbA1c target requires parents to aim for lower glucose levels, which may trigger more parental worries about hypoglycemia.
This study was undertaken using data from a large registry of individuals with T1D from diabetes clinics across the United States to characterize the extent of parental fear of hypoglycemia in parents of young children under age 7 with T1D, and to identify its associations with demographic and clinical characteristics, including HbA1c and use of diabetes management technology. The survey and clinical data were analyzed to test our hypotheses that greater parental fear of hypoglycemia would be associated with younger age of the child, HbA1c above target range, recent SH, and use of insulin pumps and continuous glucose monitoring (CGM). An exploratory aim was to identify the specific areas of greatest and least parental fear of hypoglycemia in this young age range. This knowledge is necessary to inform future interventions aimed at improving glycemic control in young children with T1D while mitigating parental fear of hypoglycemia.
Methods
The T1D Exchange includes 74 endocrinology practices based in the United States, including 58 centers serving youth with T1D (19). Each clinic received approval from a local institutional review board. Informed consent was obtained according to institutional review board requirements. Data on young children were collected for the registry’s central database from the participant’s medical record and by having the parent/guardian (hereto forward “parent”) of the participant complete a comprehensive questionnaire, as previously described (18). This report includes 549 registry participants from 41 sites enrolled from February 1, 2015 to May 2, 2016 who were less than age 7 years and had a clinical diagnosis of T1D for at least 1 year, whose parent completed a questionnaire including the Hypoglycemia Fear Survey (HFS) (7), as well as information on demographic and diabetes management characteristics at the time of registry enrollment. Insulin pump and CGM use were reported by the parent and confirmed by clinic. The most recent HbA1c measurement within 6 months prior to registry enrollment was obtained from the clinic medical record. HbA1c values were measured by point-of-care device or local laboratory. Occurrences of SH and diabetic ketoacidosis (DKA) during the 3 months prior to enrollment were reported by the parent. SH was defined as hypoglycemia resulting in seizure or loss of consciousness. The definition of DKA included diagnosis by a clinician and a visit to a hospital, emergency room, or other health care facility.
Hypoglycemia Fear Survey for use by parents (HFS-P)
The original HFS (20) was developed by Cox et al. to assess both behaviors and worries related to hypoglycemia in adults with T1D. This tool was modified for use by parents/guardians (HFS-P) of children with T1D (7), and has been shown to be a reliable and valid tool (21, 22). The questionnaire provided to parents of youth in this analysis included only the worry scale, in which the term “reaction” was modified to “low” and the item on “child losing control” was expanded to “child losing control due to low blood sugar”. Parents were asked to rate their agreement with 15 statements regarding worries related to hypoglycemia using a 0 (never) to 4 (almost always) Likert scale, which was scored for each participant as the mean of all non-missing responses multiplied by 25, for a maximum total score out of 100. Higher worry scores represent greater fear of hypoglycemia.
Statistical Analyses
The mean worry score was calculated overall and according to various demographic and clinical characteristics. Associations between mean HFS-P worry score and each demographic and clinical characteristic were assessed using T-tests or the Wilcoxon Rank-Sum procedure (or Kruskal Wallis if >2 levels) as appropriate. Adjusted linear regression models were performed to assess associations between HFS-P worry score and each glycemic outcome: HbA1c, occurrence of SH, and occurrence of DKA, separately. The demographic and clinical characteristics used to adjust the models were determined by a stepwise selection procedure, where any factor with a p-value <0.1 remained in the final adjusted model. The associations between worry score and glycemic outcomes were further stratified by insulin pump and CGM use.
Parental ratings for each individual item on the HFS-P worry scale were tabulated overall and according to age group (≤3 and 4–6 years). Additionally, associations of the most frequently reported worry with diabetes management and glycemic outcomes use were assessed using chi-square tests. Data analyses were performed using SAS version 9.4 (2011 SAS Institute Inc., Cary, NC). All p-values were two-sided. Due to multiple comparisons only p-values <0.01 were considered statistically significant.
Results
Participant Characteristics
Parents/guardians of 549 children with T1D under age 7 participated in the survey. The mean age of the child participants was 5.2±1.2 years (19% <3 years) and mean duration of diabetes was 2.4±1.0 years (range 1–6 years). Mean HbA1c was 8.2%±1.1% (66±12 mmol/mol). Approximately one-third (32%) of the children were current CGM users and more than half (58%) were using insulin pumps. Additional cohort characteristics are shown in Table 1.
Table 1.
Mean HFS-P Worry Score According to Demographic and Clinical Characteristics
| Mean± SD or N(%) | Mean HFS-P Worry Score (mean ± SD) |
p-value | |
|---|---|---|---|
| Child Age in years | 0.47 | ||
| (mean ± SD) | 5.2 ± 1.2 | ||
| ≤3 years | 102 (19%) | 36.7 ± 21.4 | |
| 4–6 years | 447 (81%) | 36.0 ± 23.5 | |
|
| |||
| Child Gender | 0.04 | ||
| Female | 254 (46%) | 33.7 ± 21.6 | |
| Male | 295 (54%) | 38.2 ± 24.2 | |
|
| |||
| Child Race/Ethnicity | 0.21 | ||
| White Non-Hispanic | 415 (77%) | 36.8 ± 22.8 | |
| Black Non-Hispanic | 34 (6%) | 32.8 ± 25.7 | |
| Hispanic or Latino | 55 (10%) | 32.5 ± 23.6 | |
| Other | 32 (6%) | 39.8 ± 26.0 | |
|
| |||
| Annual Household Income | 0.39 | ||
| <$75,000 | 230 (48%) | 38.5 ± 25.6 | |
| ≥$75,000 | 254 (52%) | 35.1 ± 20.6 | |
|
| |||
| Highest Level of Parent Education | 0.13 | ||
| High school/GED or less | 219 (41%) | 35.7 ± 25.7 | |
| Associate or Bachelor degree | 183 (34%) | 38.7 ± 22.7 | |
| Master, Professional, or Doctorate degree | 137 (25%) | 34.1 ± 19.2 | |
|
| |||
| Duration of Diabetes in years | 0.61 | ||
| (mean ± SD) | 2.4 ± 1.0 | ||
| 1–<3 years | 409 (74%) | 35.8 ± 23.0 | |
| 3–<7 years | 140 (26%) | 37.1 ± 23.4 | |
|
| |||
| Reported Frequency of Blood Glucose Monitoring | 0.004 | ||
| (mean ± SD) | 7.1 ± 2.5 | ||
| <6 times per day | 145 (26%) | 31.8 ± 22.3 | |
| ≥6 times per day | 404 (74%) | 37.7 ± 23.2 | |
|
| |||
| Current Pump Use | 0.06 | ||
| Yes | 307 (58%) | 36.9 ± 21.8 | |
| No | 223 (42%) | 34.5 ± 24.8 | |
|
| |||
| Current Continuous Glucose Monitor (CGM) Use | 0.86 | ||
| Yes | 173 (32%) | 36.1 ± 22.3 | |
| No | 376 (68%) | 36.2 ± 23.5 | |
|
| |||
| Most Recent HbA1c* | 0.89 | ||
| (mean ± SD) | 8.2% ±1.1% (66±12 mmol/mol) |
||
| <7.5% (<58 mmol/mol) | 133 (25%) | 35.0 ± 21.5 | |
| ≥7.5% (≥58 mmol/mol) | 405 (75%) | 36.5 ± 23.8 | |
|
| |||
| Occurrence of ≥1 SH event in past 3 months*,† | 0.95 | ||
| Yes | 38 (7%) | 35.3 ± 25.8 | |
| No | 511 (93%) | 36.2 ± 22.9 | |
|
| |||
| Occurrence of ≥1 DKA event in past 3 months*,† | 0.71 | ||
| Yes | 27 (5%) | 34.8 ± 23.7 | |
| No | 522 (95%) | 36.2 ± 23.1 | |
P-value from the adjusted model. All models were adjusted for gender and frequency of self-monitoring blood glucose per day
Occurrence of at least one severe hypoglycemia (SH) event in the past 3 months resulting in seizure or loss of consciousness, and occurrence of at least one diabetic ketoacidosis (DKA) event in the past 3 months
Mean Worry Score
The mean HFS-P worry score was 36.1±23.1. Parents of children ≤3 years did not report greater worry than parents of children aged 4–6 years (p=0.47, Table 1). Parental worry was slightly higher for children using an insulin pump vs. injections, though this difference was not significant (p=0.06, Table 1). No difference in parental worry was observed for children using CGM vs. no CGM (p=0.86, Table 1). The mean worry score among parents who reported performing blood glucose monitoring ≥6 times per day was higher than those who reported checking blood glucose <6 times per day (37.7±23.2 vs. 31.8±22.3; p=0.004).
Worry score and HbA1c were not significantly associated (unadjusted p=0.79, p=0.89 after adjusting for gender of the child and frequency of blood glucose monitoring; Table 1). Significant associations of the worry scale with HbA1c also were not observed in separate analyses of pump users compared with non-users and of CGM users compared with non-users.
The mean HFS-P worry score was not significantly greater among parents/guardians of children that had at least one SH event (7% of sample) in the past three months compared with those who had no SH events in the past three months (adjusted p=0.95; Table 1). Similarly, no association was found between worry score and occurrence of a DKA event (5% had ≥1 event in the past 3 months, adjusted p=0.71; Table 1). Stratifying these analyses by pump use and CGM use did not demonstrate any significant relationship between occurrences of SH and DKA and total worry score.
Frequency of Reported Worries
Parents reported most frequently worrying about their child having a low blood glucose while asleep and their child not recognizing a low glucose, with 54% and 47% respectively reporting feeling worried ‘often’ or ‘almost always’ about these experiences (Table 2). The least frequently reported worries involved the child embarrassing themselves, friends, or family in social situations and appearing ‘stupid’ or clumsy (Table 2).
Table 2.
Frequent and Infrequent Parental-Reported Worries about their Child and Hypoglycemia
| Concern | Percent often or almost always worried |
|---|---|
| Having a low while asleep | 54% |
| Not recognizing/realizing he/she is having a low | 47% |
| Having a low | 42% |
| Developing long-term complications from frequent low blood sugar | 31% |
| Having a low while alone | 30% |
| No one being around to help during a low | 30% |
| Feeling light-headed or faint | 23% |
| Having seizures or convulsions | 22% |
| Not having food, fruit, or juice | 22% |
| Losing control of behavior due to low blood sugar | 19% |
| Feeling dizzy or passing out in public | 17% |
| Getting a bad evaluation at school because of something that happens when sugar is low | 14% |
| Making a mistake or having an accident at school | 10% |
| Embarrassing self or friends/family in social situation | 8% |
| Appearing “stupid” or clumsy | 7% |
Since parents most frequently worried about hypoglycemia during sleep, this most common worry was further evaluated as shown in Figure 1. There was not a statistically significant difference in frequency of worry about the child having a low while asleep among parents of children ≤3 years of age compared with parents of children age 4–6 (p=0.09, Figure 1). Parents whose children used an insulin pump worried significantly more often about their child having a low while asleep than parents whose children did not use an insulin pump (61% of parents of pump users vs. 45% of parents of non-users; p<0.001, Figure 1). There were trends towards higher parental worry about lows during sleep among CGM users compared with non-users (62% of parents of CGM users vs. 51% of parents of non-users; p=0.02) and among parents whose children had no SH event in the past 3 months compared with those with at least one event (56% with no event vs. 34% with event; p=0.01, Figure 1). No difference in frequency of worry about lows during sleep was seen among parents of children with HbA1c below the target versus above the target range, or for those with children who had no DKA event versus at least one DKA event in the past three months.
Figure 1. Endorsed Worry about Child having Hypoglycemia while Asleep.
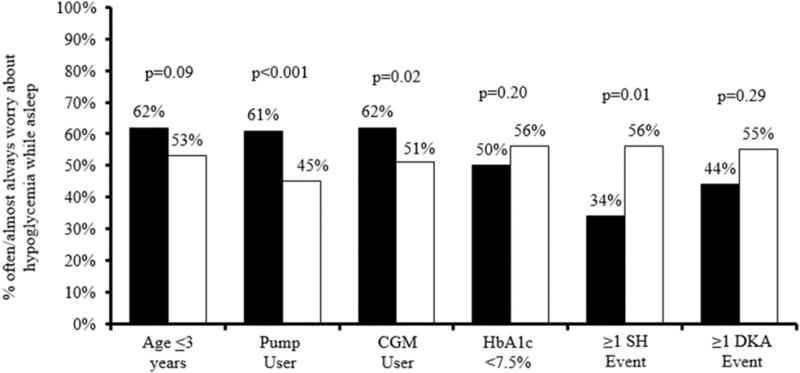
Solid black bar represents yes.
Sold white bar represents no.
Discussion
Parents of young children with T1D in this large sample reported that they most frequently experience fear of hypoglycemia in contexts which may make it difficult for the parent to detect hypoglycemia – during sleep and when the child does not recognize he or she is having a low. Maintaining higher glucose levels to avoid hypoglycemia is a strategy reportedly used by some families in clinical practice to mitigate hypoglycemia fear; however our results indicate that fear of hypoglycemia persists regardless of glycemic control.
There are a limited number of studies evaluating fear of hypoglycemia in parents of young children with T1D. Patton et al studied parents of 81 children aged 2–8 years diagnosed with T1D and found that fear of hypoglycemia was common in parents, and greatest in mothers. Similar to our findings, there was no relationship between HbA1c and hypoglycemia fear (1, 8). While fear of hypoglycemia has been reported to be associated with worse glycemic control in older children and adolescents (5–7), unique characteristics of young children and their care may contribute to the lack of association, including the intense monitoring practiced by many parents. Thus, while main goals in young children with T1D are improving HbA1c and reducing glycemic variability, parental fear of hypoglycemia does not seem to be a primary barrier to meeting these goals.
Despite this finding, addressing fear of hypoglycemia is important in the youngest age group, especially in relation to parental quality of life. It is not surprising that the most frequent hypoglycemia worry in this young age group is related to hypoglycemia during sleep (8). Parents have reported that caring for a young child with T1D demands “constant vigilance” (23). The overnight time period presents challenges to this concept as constant vigilance is more difficult when children are asleep, and attempts to achieve it have detrimental effects on parents’ sleep quality (24) and, hence, on quality of life, which may lead parents to compensate by targeting a higher blood glucose overnight.
Advancements in diabetes technology may help address some of the difficulties managing young children’s diabetes during sleep. Preschool children require higher basal rates of insulin in the evening and lower doses after midnight, which can be challenging to achieve using insulin injections (25), while insulin pumps can provide small varying doses of basal insulin overnight and are associated with a reduced risk of SH in children (26). Insulin pump use may also contribute to the marked decrease in SH events in adolescents (27, 28), and SH events are no longer associated with achieving HbA1c targets (28). Additionally, CGM systems provide real-time as well as retrospective information about glucose levels, alerts for low glucose levels, and integrated, sensor-augmented pump systems can automatically suspend basal rates if sensor glucose levels reach a pre-determined low threshold. Use of these technologies has the potential to alleviate some parental burden such as worry of missing nocturnal hypoglycemia and waking to perform blood glucose checks, as well as provide data for dose adjustments to optimize overnight control. Indeed, CGM detects hypoglycemia more effectively than frequent blood glucose checks in young children (29).
Despite the presumed benefits of insulin pumps and CGM, worries about hypoglycemia during sleep were more frequent in parents of children using insulin pumps and tended to be higher in those using CGM. A potential explanation is that fear of hypoglycemia may motivate some families to use advanced diabetes technologies such as pumps and CGMs. On the other hand, heightened awareness of previously unknown glucose excursions, due to use of these technologies may also increase parental concerns. A prospective, longitudinal study will be necessary to understand the directionality and potential bidirectionality of this relationship. Such information would be valuable to inform families of the impact of technology use and assist parents in balancing use of diabetes technologies that allow for optimal diabetes management while preserving emotional well-being and quality of life.
Addressing fear of hypoglycemia is an important part of clinical care in families of young children with T1D. Given consistent high HFS-P worry ratings across groups in this large sample, parents’ common concerns about hypoglycemia risks should be addressed at clinical visits with young children regardless of the child’s age, glycemic control, treatment regimen, and family’s socioeconomic status. Additionally, the finding of less frequent fear of hypoglycemia during sleep in those who report recently experiencing a SH episode is surprising and may reflect having surmounted that hurdle, lack of understanding of the condition, or may simply be related to the small sample of events during the brief (3 month) time frame for SH reporting. Health care providers should understand that this fear is common. Empowering families to identify and effectively treat hypoglycemia as well as manage their worries with available devices, tools, and strategies has the potential to mitigate worry related to hypoglycemia.
Data for this study were collected from the largest published national (US) sample of young children under age 7 from multiple diabetes treatment centers in geographically diverse areas, which contribute to both the validity and the generalizability of the results. However, the T1D exchange clinic registry is not a population based registry. A few limitations must be noted when interpreting the results of this study. Given that this study is cross-sectional, we cannot draw conclusions about the direction of associations between fear of hypoglycemia and the behavioral or clinical variables reported. Thus, we cannot determine how or if diabetes technology contributes to or ameliorates the commonly reported fears of hypoglycemia or determine whether these fears impact clinical outcomes such as glycemic control. Additional limitations include the generalizability to all young populations with T1D, given the relatively high average parental educational attainment and income, and relatively low racial/ethnic diversity. Gender of the survey respondent (parent) would have provided additional insight into interpreting the score, as greater hypoglycemia fear scores have been reported in mothers compared with fathers (1, 6).
It is noteworthy that 32% of families of young children in this study were using CGM, which is 2–3 fold higher than current CGM use in adolescents and young adults enrolled in the T1D Exchange Registry (30). This sharp increase in CGM utilization likely reflects an increase in parental and clinician perceptions of the benefits and increased ease of use of these devices, alone and in combination with insulin pump therapy. Thus, this may be an optimal time to assess how more widespread use of advances in diabetes technology impact fear of hypoglycemia in parents of young children with T1D.
Supplementary Material
Acknowledgments
MAVN contributed to data interpretation and wrote/edited the manuscript. MEH contributed to data interpretation and wrote/edited the manuscript. CTB performed statistical analyses and wrote/edited the manuscript. KMM, DJD, BJA, LML, SEW, LAD, and WVT contributed to data interpretation and reviewed/edited the manuscript.
Funding was provided by the Leona M. and Harry B. Helmsley Charitable Trust.
MAV received support from the National Institutes of Health grant K12-DK094714.
MEH and BJA also received support from the National Institute of Diabetes and Digestive and Kidney Disease, K12097696.
Abbreviations
- T1D
type 1 diabetes
- HbA1c
hemoglobin A1c
- T1D Exchange
T1D Exchange clinic registry
- CGM
continuous glucose monitoring
- HFS
Hypoglycemia Fear Survey
- DKA
diabetic ketoacidosis
Footnotes
Financial Disclosures
The authors do not have any financial disclosures to report.
References
- 1.Patton SR, Dolan LM, Henry R, Powers SW. Fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. J Clin Psychol Med Settings. 2008;15(3):252–9. doi: 10.1007/s10880-008-9123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Research in Children Network Study G. Tsalikian E, Tamborlane W, Xing D, Becker DM, Mauras N, et al. Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care. 2009;32(11):1954–9. doi: 10.2337/dc08-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graveling AJ, Noyes KJ, Allerhand MH, Wright RJ, Bath LE, Deary IJ, et al. Prevalence of impaired awareness of hypoglycemia and identification of predictive symptoms in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2014;15(3):206–13. doi: 10.1111/pedi.12077. [DOI] [PubMed] [Google Scholar]
- 4.Ly TT, Maahs DM, Rewers A, Dunger D, Oduwole A, Jones TW, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(Suppl 20):180–92. doi: 10.1111/pedi.12174. [DOI] [PubMed] [Google Scholar]
- 5.Freckleton E, Sharpe L, Mullan B. The relationship between maternal fear of hypoglycaemia and adherence in children with type-1 diabetes. Int J Behav Med. 2014;21(5):804–10. doi: 10.1007/s12529-013-9360-8. [DOI] [PubMed] [Google Scholar]
- 6.Haugstvedt A, Wentzel-Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with Type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med. 2010;27(1):72–8. doi: 10.1111/j.1464-5491.2009.02867.x. [DOI] [PubMed] [Google Scholar]
- 7.Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11(Suppl 1):189–94. doi: 10.1515/jpem.1998.11.s1.189. [DOI] [PubMed] [Google Scholar]
- 8.Patton SR, Dolan LM, Henry R, Powers SW. Parental fear of hypoglycemia: young children treated with continuous subcutaneous insulin infusion. Pediatr Diabetes. 2007;8(6):362–8. doi: 10.1111/j.1399-5448.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 9.Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45(4):262–8. doi: 10.1017/s0012162203000501. [DOI] [PubMed] [Google Scholar]
- 10.Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9(2):87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes A. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 12.Marzelli MJ, Mazaika PK, Barnea-Goraly N, Hershey T, Tsalikian E, Tamborlane W, et al. Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes. 2014;63(1):343–53. doi: 10.2337/db13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauras N, Mazaika P, Buckingham B, Weinzimer S, White NH, Tsalikian E, et al. Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes. 2015;64(5):1770–9. doi: 10.2337/db14-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blasetti A, Chiuri RM, Tocco AM, Di Giulio C, Mattei PA, Ballone E, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol. 2011;26(11):1383–91. doi: 10.1177/0883073811406730. [DOI] [PubMed] [Google Scholar]
- 15.Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, et al. Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care. 2003;26(4):1100–5. doi: 10.2337/diacare.26.4.1100. [DOI] [PubMed] [Google Scholar]
- 16.Cengiz E, Xing D, Wong JC, Wolfsdorf JI, Haymond MW, Rewers A, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14(6):447–54. doi: 10.1111/pedi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maahs DM, Hermann JM, DuBose SN, Miller KM, Heidtmann B, DiMeglio LA, et al. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia. 2014;57(8):1578–85. doi: 10.1007/s00125-014-3272-2. [DOI] [PubMed] [Google Scholar]
- 18.Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook A Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–54. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA, et al. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–9. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 20.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617–21. doi: 10.2337/diacare.10.5.617. [DOI] [PubMed] [Google Scholar]
- 21.Gonder-Frederick LA, Fisher CD, Ritterband LM, Cox DJ, Hou L, DasGupta AA, et al. Predictors of fear of hypoglycemia in adolescents with type 1 diabetes and their parents. Pediatr Diabetes. 2006;7(4):215–22. doi: 10.1111/j.1399-5448.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with Type 1 diabetes and their parents. Diabetes Manag (Lond) 2011;1(6):627–39. doi: 10.2217/DMT.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Constant vigilance: mothers’ work parenting young children with type 1 diabetes. J Pediatr Nurs. 2003;18(1):21–9. doi: 10.1053/jpdn.2003.4. [DOI] [PubMed] [Google Scholar]
- 24.Landau Z, Rachmiel M, Pinhas-Hamiel O, Boaz M, Bar-Dayan Y, Wainstein J, et al. Parental sleep quality and continuous glucose monitoring system use in children with type 1 diabetes. Acta Diabetol. 2014;51(3):499–503. doi: 10.1007/s00592-013-0545-z. [DOI] [PubMed] [Google Scholar]
- 25.DiMeglio LA, Boyd SR, Pottorff TM, Cleveland JL, Fineberg N, Eugster EA. Preschoolers are not miniature adolescents: a comparison of insulin pump doses in two groups of children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17(6):865–70. doi: 10.1515/jpem.2004.17.6.865. [DOI] [PubMed] [Google Scholar]
- 26.Johansen A, Kanijo B, Fredheim S, Olsen B, Hertz B, Lauridsen MH, et al. Prevalence and predictors of severe hypoglycemia in Danish children and adolescents with diabetes. Pediatr Diabetes. 2015;16(5):354–60. doi: 10.1111/pedi.12171. [DOI] [PubMed] [Google Scholar]
- 27.Abraham MB, Gallego PH, Brownlee WM, Smith GJ, Davis EA, Jones TW. Reduced prevalence of impaired awareness of hypoglycemia in a population-based clinic sample of youth with type 1 diabetes. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12460. [ePub ahead of Print] [DOI] [PubMed] [Google Scholar]
- 28.Karges B, Kapellen T, Wagner VM, Steigleder-Schweiger C, Karges W, Holl RW, et al. Glycated hemoglobin A1c as a risk factor for severe hypoglycemia in pediatric type 1 diabetes. Pediatr Diabetes. 2017;18(1):51–8. doi: 10.1111/pedi.12348. [DOI] [PubMed] [Google Scholar]
- 29.Sundberg F, Forsander G. Detection and treatment efficacy of hypoglycemic events in the everyday life of children younger than 7 yr. Pediatr Diabetes. 2014;15(1):34–40. doi: 10.1111/pedi.12057. [DOI] [PubMed] [Google Scholar]
- 30.Miller KM, Foster NC, DeSalvo D, DiMeglio LA, Laffel L, Tamborlane W, et al. Continuous Glucose Monitoring (CGM) Use in Type 1 Diabetes: An Update from the T1D Exchange Clinic Registry. Presented at the International Society for Pediatric and Adolescent Diabetes (ISPAD) Meeting; 2016; Valencia, Spain. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


