Abstract
Introduction
Acute ischemic injury leads to severe neuronal loss. One of the key mechanisms responsible for this effect is inflammation, which is characterized by the activation of myeloid cells, including resident microglia and infiltrating monocytes/macrophages. P2X4 receptors (P2X4Rs) present on these immune cells modulate the inflammatory response. For example, excessive release of adenosine triphosphate during acute ischemic stroke triggers stimulation of P2X4Rs, leading to myeloid cell activation and proliferation and further exacerbating post-ischemic inflammation. In contrast, during recovery P2X4Rs activation on microglia leads to the release of brain-derived neurotrophic factor (BDNF), which alleviate depression, maintain synaptic plasticity and hasten post-stroke behavioral recovery. Therefore, we hypothesized that deletion of the P2X4R specifically from myeloid cells would have differential effects on acute versus chronic recovery following stroke.
Methods
We subjected global or myeloid-specific (MS) P2X4R knock-out (KO) mice and wild-type littermates of both sexes to right middle cerebral artery occlusion (60 min). We performed histological, behavioral (sensorimotor and depressive), and biochemical (quantitative PCR and flow cytometry) analyses to determine the acute (three days after occlusion) and chronic (30 days after occlusion) effects of receptor deletion.
Results
Global P2X4R deletion led to reduced infarct size in both sexes. In MS P2X4R KO mice, only females showed reduced infarct size, an effect that did not change with ovariectomy. MS P2X4R KO mice of both sexes showed swift recovery from sensorimotor deficits during acute recovery but exhibited a more pronounced post-stroke depressive behavior phenotype that was independent of infarct size. Quantitative PCR analysis of whole cell lysate as well as flow-sorted myeloid cells from the perilesional cortex showed increased cellular interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) mRNA levels but reduced plasma levels of these cytokines in MS P2X4R KO mice after stroke. The expression levels of BDNF and other depression-associated genes were reduced in MS P2X4R KO mice after stroke.
Conclusions
P2X4R deletion protects against stroke acutely but predisposes to depression-like behavior chronically after stroke. Thus, a time-sensitive approach should be considered when targeting P2X4Rs after stroke.
Keywords: P2X4R, Stroke, Myeloid-specific, Depression, Neuroprotection
1. Introduction
Ischemic stroke causes acute release of excessive adenosine triphosphate (ATP) from both dying neuronal and non-neuronal cells, activating membrane-bound P2X4 receptors (P2XRs). These receptors are highly expressed in all cell types of the central nervous system (CNS) but are especially abundant on cells of myeloid origin (i.e., microglia and monocytes) (North and Jarvis, 2013; Cavaliere et al., 2003). Microglia, the resident immune cells of the CNS, respond to disruptions in homeostasis in brain function including sterile injuries such as ischemic stroke. After stroke, a notable shift in microglial morphology occurs, transitioning from thin and ramified to large amoeboid structures that produce inflammatory factors such as IL-1β, IL-6, and TNF-α (Ritzel et al., 2015; Lambertsen et al., 2012). These changes are accompanied by upregulation and activation of microglial P2X4Rs (Vazquez-Villoldo et al., 2014). Prior studies using global P2X4R knockout (KO) mice showed diminished inflammasome signaling following spinal cord injury or allergen-induced airway inflammation (de Rivero Vaccari et al., 2012; Zech et al., 2016). In these injury models, P2X4R-positive leukocytes (i.e., macrophages, dendritic cells, and T cells) are the main sources of pro-inflammatory cytokine release. These results suggest that P2X4R-positive microglia/-monocytes could be a significant source of pro-inflammatory cytokines, which are critical factors in early ischemic damage.
Compelling evidence suggest that pro-inflammatory cytokines (e.g., IL-6, IL-1β, and TNF-α) also play a significant role in chronic post-stroke recovery; in particular, they have been implicated in depressive behaviors by modulating the release and synthesis of neuropeptides and growth factors in the CNS (Felger and Lotrich, 2013). In addition, microglial activation has been linked to depression in recent studies that used lipopolysaccharide administration in animal models; these changes could be reversed with selective serotonin reuptake inhibitor treatment (Yirmiya et al., 2001). Finally, P2X4R activation drives the release of brain-derived neurotrophic factor (BDNF) from microglial cells (Trang et al., 2009). BDNF is necessary for the maintenance of synaptic plasticity, enhances post-stroke recovery, and has an anti-depressant effect (Verma et al., 2014, 2016). Thus, microglial-induced neuroinflammation may be responsible for the depressive phenotype observed after stroke; this especially could be the case in immunocompromised animals, in which virus can rapidly gain access to the brain, infect microglia (but not neurons), and induce their activation (Kaul et al., 2001). On the contrary, several lines of evidence suggest that depression induced by microglia activation might not be associated with inflammatory challenges (Yirmiya et al., 2015).
We hypothesized that P2X4R activation plays a diverse role at various time points of stroke recovery. In the present study, we examined the behavioral and biochemical effects of P2X4R ablation using both global and MS P2X4R KO mice both acutely and chronically after experimental ischemic stroke.
2. Materials and methods
2.1. Mice
Global and MS P2X4R KO mice were maintained and bred at the University of Connecticut Health Center (Farmington CT). Details of the generation of global and MS Cre mice are provided in Fig. S1. We confirmed deletion by Western blot analysis (Fig. S2). Weight-matched littermates were used as WT controls ~20–25 g (Felger and Lotrich, 2013; Yirmiya et al., 2001; Trang et al., 2009 weeks old) as detailed in the Supplementary file. A total of 63 global P2X4R KO and WT mice (both male and female) were randomly divided and subjected to stroke. In the final analysis, a total of 58 mice were included due to stroke-induced death of five male WT mice. Weused a total of 109 MS P2X4R KO and WT mice (both males and females) for infarct and quantitative PCR (qPCR) analyses (acute outcome at day 3), out of which we used 103 mice in the final analyses due to the death of six mice (4 male and 2 female) after stroke. We used a total of 116 MS P2X4R KO and WT mice (both males and females) for biochemical and chronic behavioral analyses, out of which total 103 mice were used in the final analyses due to the death of 13 mice (8 male and 5 female) after stroke. The details of the mice used and excluded for each experiment are given in specific figure legends and in Table S1. The study was approved by the institutional animal care and use committee and the procedures were in accordance with institutional, National Institutes of Health, STAIR, and RIGOR guidelines (Lapchak et al., 2013).
2.2. Middle cerebral artery occlusion and bilateral ovariectomy
We induced focal transient cerebral ischemia by a 60-min right middle cerebral artery occlusion (MCAo) under isoflurane anesthesia followed by reperfusion for either 3, 15, or 30 days. We selected the three-day outcome because the infarct matures completely and shows minimal variation in its volume by that time. The 15- and 30-day time points represent sub-acute and chronic recovery as discussed previously (Verma et al., 2014, 2016). Briefly, we performed a midline ventral neck incision and unilateral right MCAo by advancing a 6.0 silicone rubber-coated monofilament (Doccol Corporation, CA) 10–11 mm from the internal carotid artery bifurcation via an external carotid artery stump. We monitored rectal temperatures with a temperature control system (Fine Science Tools, Canada), maintaining the temperature at ~37 °C during surgery with an automatic heating pad. We used laser doppler flowmetry (DRT 4/Moor Instruments Ltd, Devon, UK) to measure cerebral blood flow and to confirm occlusion (reduction to 15% of baseline cerebral blood flow) and reperfusion. All animals were fed with wet mash for one week after surgery to ensure adequate nutrition for chronic endpoints, as animals have rearing deficits after stroke. In sham mice, we performed identical surgeries except the suture was not advanced into the internal carotid artery. For the gonadal hormone-mediated outcome study, we ovariectomized (ovxed) female mice two weeks prior to stroke surgery. Details of the ovariectomy procedure are provided in the Supplementary file.
2.3. Neurological deficit score
The neurological deficit (ND) score is a crude assessment of post-stroke behavioral recovery. We recorded ND scores, ranging from 0 to 4, at several time points after stroke (Verma et al., 2014). Our standard scoring system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by the tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling.
2.4. Cresyl violet staining for infarct volume and tissue atrophy analysis
We measured tissue infarct (day 3) or tissue atrophy (day 30) after stroke as described previously (Verma et al., 2016). Briefly, we sacrificed the mice after stroke surgery with an overdose of Avertin (250 mg/kg intraperitoneally, i.p). After blood collection by cardiac puncture, we performed trans-cardiac perfusion on the mice using cold phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were then fixed overnight and placed in cryoprotectant (30% sucrose in PBS) for 72 h before processing. We then sliced the brains into 30-µm free-floating sections using a freezing microtome; every eighth slice was mounted and stained with cresyl violet. We then used these 30-µm sections for infarction, tissue atrophy calculations, and immunohistochemistry analysis as described previously (Verma et al., 2014). An investigator blinded to the experimental cohort performed the data analyses.
2.5. Sensorimotor and depressive behavioral tests
2.5.1. Sensory motor deficit test
We used the open field test (OFT) and rotarod test to measure spontaneous locomotor activity/anxiety-like behavior and motor balance coordination, respectively, at baseline and post-mcao days 2, 7, 14, 21, and 28 (Manwani et al., 2011; Truong et al., 2012). As the OFT is a non-stressful test, we performed the OFT prior to the rotarod test, on the same day with one hour difference between tests.
2.5.1.1. Open field test
The OFT is a common measure of exploratory behavior and general activity in rodents, which can be used both qualitatively and quantitatively. Briefly, we placed mice in a corner of a clear acrylic box (16″ × 16″) and allowed them to explore the box for ten minutes. We quantified locomotor activity as the total number of beam breaks by a computer-operated, open-field photobeam activity system (San Diego Instruments, San Diego, CA). We calculated the percentage of beam breaks in the center zone (16/3″ × 16/3″) compared to the total as a measure of anxiety-like behavior. Importantly, OFT tests can be administered at several time points to view trends without hindrance by habituation (Moy et al., 2012).
2.5.1.2. Rotarod test
The rotarod test examines motor coordination in mice. We placed mice on a rotating cylindrical rod accelerating from two to ten rotations per minute, over a span of five minutes. Each subject performed two trials with a 20-min break between the two trials. We recorded the latency to fall from the rotating rod for each trial (in seconds), and used the mean latency for comparison between groups (Truong et al., 2012).
2.5.1.3. Tail suspension test
We used the tail suspension test (TST) to assay depression-like phenotypes in mice, based on the premise that mice subjected to inescapable stress become immobile. We performed the TST as described previously (Harris et al., 2016). Briefly, we placed mice in the behavioral room for one hour prior to testing to allow acclimatization. We suspended mice individually by the tail on fixed rod using paper tape, 60 cm above the surface of the table. We recorded for six minutes using a digital video camera (JVC Everio, Victor Company, Japan). A trained observer who was blinded to the treatment conditions evaluated the duration of immobility. The mouse was considered immobile in the absence of initiated movement. Due to the potential stress induced by the TST, we performed this test only once prior to sacrifice, at day 29.
2.5.1.4. Sucrose consumption test
The sucrose consumption test (SCT) is a measure of anhedonia, a symptom of depressive behavior. We performed the SCT at pre-stroke day-3 and post-stroke days 15 and day 30 in separate cohorts as described previously (Verma et al., 2014) with a minor modification where we gave pre-weighed sugar pellets rather than sucrose solution. Briefly, we gave a pre-weighted (5 g) sucrose pellet to individually housed mice for overnight consumption in place of their regular food pellet. Twelve hours later, we recorded the remaining amount of sugar and calculated the amount of sugar consumed overnight. The mice were returned to normal conditions in the morning.
2.6. Immunohistochemistry and Western blot analysis
We sacrificed mice with an overdose of Avertin (250 mg/kg i.p) and collected blood from the right ventricle. After quick perfusion with 1xPBS, we rapidly removed the brain, and then separated and homogenized the frontal cortical region (perilesional cortex) of the right (ischemic) hemisphere as described previously (Verma et al., 2016). To determine the protein concentration, we used a bicinchoninic acid protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL) and performed Western blot analysis. We loaded a total of 20 mg of protein into each well of 4–15% sodium dodecyl sulfate electrophoresis gels and transferred to polyvinylidene difluoride membranes. We determined P2X4R expression in the ischemic or non-ischemic brain by both Western blot analysis using an anti-P2X4R antibody (1:500; Preoteintech Rosemont, IL) and immunolabeling with a different anti-P2X4R antibody (1:200 Alomone Labs, Jerusalem Israel). To determine cell-specific location, we co-labeled with target proteins using either NeuN (1:500, Abcam Cambridge, MA) or IbA-1 (1:500, Abcam, Cambridge, MA) antibodies as described (Verma et al., 2016).
2.7. Flow sorting of microglia and monocytes
We collected and prepared tissue for flow sorting of microglia and monocytes as described previously (Ritzel et al., 2015). We identified resident microglia as the CD45int CD11b+Ly6C− population and the bone marrow-derived monocytes as the CD45hiCD11b +Ly6C+ population. We used cell type-matched fluorescence minus one controls to determine the positivity of each antibody. To acquire data, we used an LSR II flow cytometer (BD Biosciences, Billerica, MA) with FACsDIVA 6.0 (BD Biosciences, Billerica, MA) and FlowJo (Treestar Inc. Ashland, OR) software. For each antibody, we determined gating based on fluorescence minus one controls. We collected sorted microglia or monocytes in trizol for qPCR analysis.
2.8. Quantitative PCR
We isolated total RNA from either perilesional cortex or flow-sorted microglia/monocytes of MS P2X4R KO and WT littermate mice using the triazole method. We performed reverse transcription and qPCR as per instructions of the TaqMan® RNA reverse transcription kit (Ambion, Life Technologies, Camarillo, CA) and Taqman universal master mix reagent (Ambion, Life Technologies, Camarillo, CA).
2.9. Enzyme-linked immunosorbent assay for IL-1β expression in plasma and tissue
Blood samples were collected prior to sacrifice the animal and spun at 6000g for ten minutes at 4 °C; collected plasma was stored at −80 °C until further use. We analyzed plasma samples (no dilution) and brain tissue homogenates (in HEPES lysis buffer) for IL-1β levels using an IL-1β (Mouse) enzyme-linked immunosorbent assay (ELISA) ready-set-go kit with a sensitivity: 8 pg/mL (eBio-science Thermofisher, Waltham, MA).
2.10. Statistics
Data from individual experiments are presented as mean ± SEM and statistically evaluated by Student’s t-test (for comparison between two experimental groups; WT vs. KO), by one-way or two-way with repeated measure (genotype and time as variables) analysis of variance (ANOVA) with a Bonferroni post hoc test to correct for multiple comparisons (GraphPad Prism Software Inc., San Diego, CA,). As the ND scores are ordinal in nature, we used the Mann-Whitney U test. We considered a probability value of p < 0.05 to be statistically significant. An investigator blinded to the experimental groups performed the data analyses.
3. Results
3.1. P2X4R expression increases after stroke
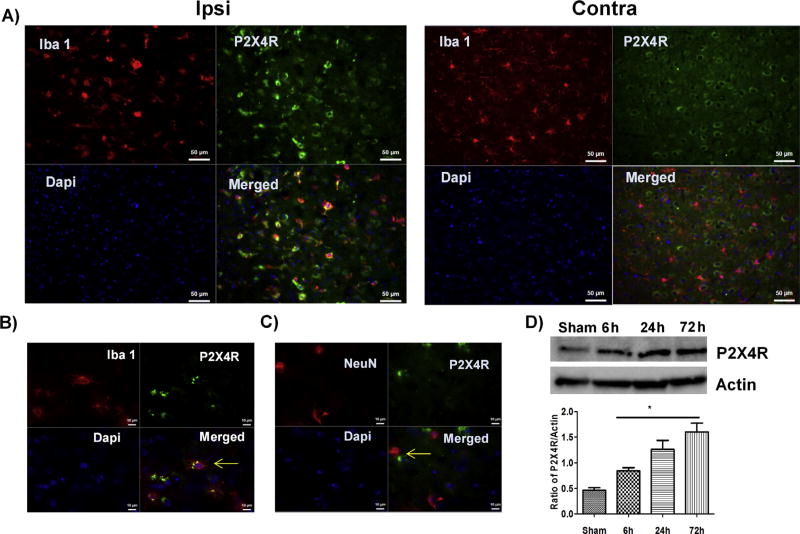
Three days after stroke, P2X4R expression increased in the perilesional cortical region of the ischemic hemisphere in male WT mice (Fig. 1A). Using co-localization studies, we found that the P2X4R expression was primarily on microglial cells (Iba1+; Fig. 1A, B) and to a lesser extent on neuronal cells (NeuN+, Fig. 1C). Confirming the immunohistochemistry data, Western blot analysis showed a progressive increase in P2X4R expression over the first three days after stroke as compared to sham (Fig. 1D).
Fig. 1.
Immunolabeling of P2X4R in male WT mice three days after stroke induction. (A) P2X4R expression (green) was increased and Iba 1+ cells (red) showed an activated phenotype (round cell bodies with low processes) in the ipsilateral (stroke) versus contralateral (non-stroke) hemisphere (20x; scale bar 50 µm). (B) Ipsilateral staining: P2X4R (green) co-localized with Iba+ cells (red) (63x; scale bar 10 µm). (C) Ipsilateral staining: Co-localization of P2X4R (green) with NeuN+ve neurons (red) and DAPI (blue; marking nuclei) showed qualitatively reduced expression of P2X4R in neurons (n = 3 M WT) (63x; scale bar 10 µm) (D). Stroke led to a significant time-dependent increase in P2X4R expression in whole cell lysate from the ipsilateral brain (*p < 0.05; stroke vs. sham, one-way-ANOVA; graphs show mean + S.E.M.; n = 12; 3/group/time point; no exclusion). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Global and MS P2X4R KO show changes in microglial morphology after stroke
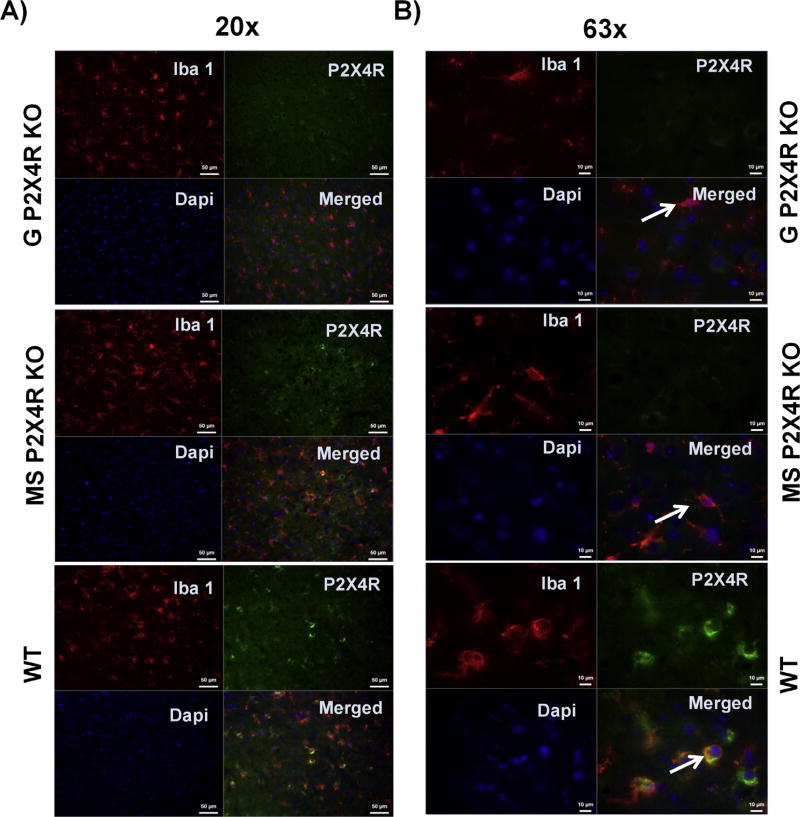
In addition to the high expression of P2X4R in microglial cells, co-labeling with Iba-1 revealed an increase in microglial activation (based on morphological changes) in WT male mice after three days of stroke in the perilesional cortex of the ipsilateral hemisphere. While the WT mice showed an amoeboid or round morphology characteristic of a highly activated state, both the global and MS P2X4R KO mice showed shorter processes, less arborization, and larger soma, indicating an intermediate activation state (Fig. 2A & B). These data suggest that P2X4Rs are involved in microglia/macrophage cell activation.
Fig. 2.
Qualitative microglia activation phenotype after three days of stroke in the perilesional cortex of the ipsilateral hemisphere on (A) lower magnification (20x; scale bar 50 µm) and (B) higher magnification (63x; scale bar 10 µm). We examined Iba-1 and P2X4R co-immunostained microglia. Global and MS P2X4R KO mice showed intermediate activation of microglia, based on shorter processes, less arborization, and larger soma. WT mice showed an amoeboid or round morphology characteristic of a highly activated state.
3.3. Global P2X4R KO mice show acute neuroprotection
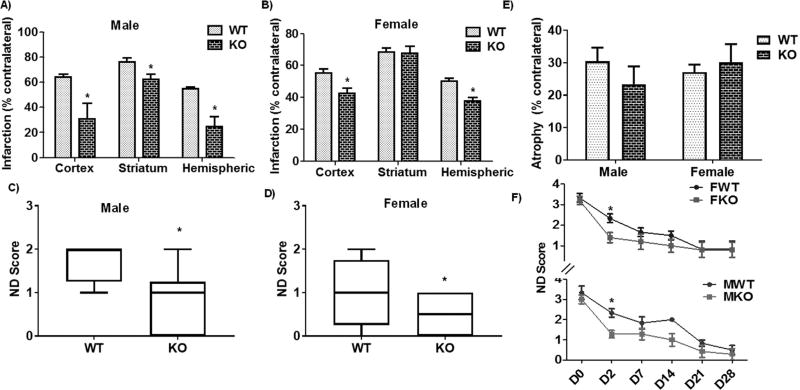
We next examined post-stroke recovery at an acute time point after stroke (day 3). We found that both male and female P2X4R global KO mice showed significant neuroprotection at this time point. Specifically, male mice showed a reduction in cortical, striatal, and total hemispheric infarct volume compared to WT littermates (Fig. 3A); female mice displayed a similar reduction in cortical and total hemispheric infarct volume but not in striatal infarct volume (Fig. 3B). Behaviorally, both sexes showed improvements in the ND score compared to control mice (Fig. 3C, D), indicating acute post-stroke behavioral benefits. In contrast to the acute time point, we did not see any changes in tissue atrophy (Fig. 3E) or ND scores (Fig. 3F) between male or female global KO and WT mice at a chronic stage of stroke (day 30), suggesting that the acute benefits in the KO mice were lost during progressive recovery.
Fig. 3.
Effect of stroke on infarct volume and ND score in global P2X4R KO mice. (A) Volume was significantly reduced (p < 0.05 vs. WT littermate, two-tailed Student’s t-test, n, KO = 6 WT = 9) in cortical, striatal, and total hemispheric infarct in global KO male mice. (B) Female global KO mice displayed a similar reduction in the volume of cortical and total hemispheric infarct (p < 0.05; KO vs. WT littermates, two-tailed Student’s t-test; KO = 6,WT = 8) but not in the striatum. (C-D) ND scores confirmed acute behavioral benefits in both sexes as compared to WT controls (*p < 0.05; KO vs. WT; Mann-Whitney U test). We did not observe a change in (E) tissue atrophy or (F) ND score between global KO and WT mice for males (M) or females (F) at a chronic stage of stroke (day 30). However, a two-way ANOVA (genotype vs time) analysis in male [F (1, 66) = 16.07; p = 0.002)] as well as female [F (1, 54) = 3.927; p = 0.05] suggested a main effect of genotype (KO = 6 and WT = 8). Further, a multiple comparison analysis at individual time point between KO and WT of both sexes showed a significant difference in ND score at day 2 after stroke (*p < 0.05 vs. WT; Mann-Whitney-U test;) (FWT-male WT, FKO-Female KO; MWT-male WT, MKO-male WT).
3.4. MS P2X4R KO mice show acute neuroprotection in female mice after stroke
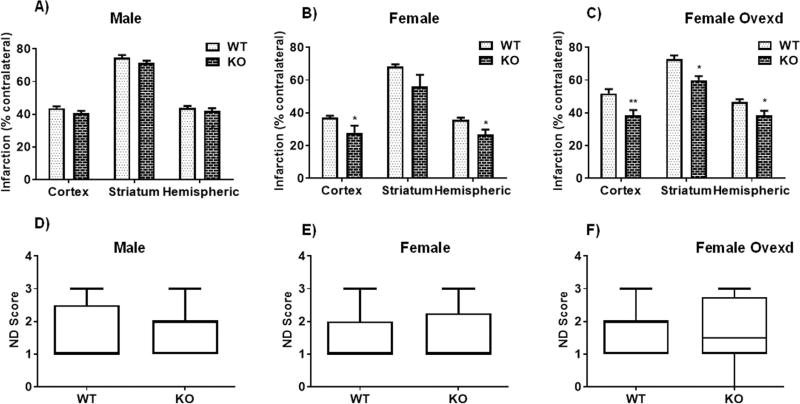
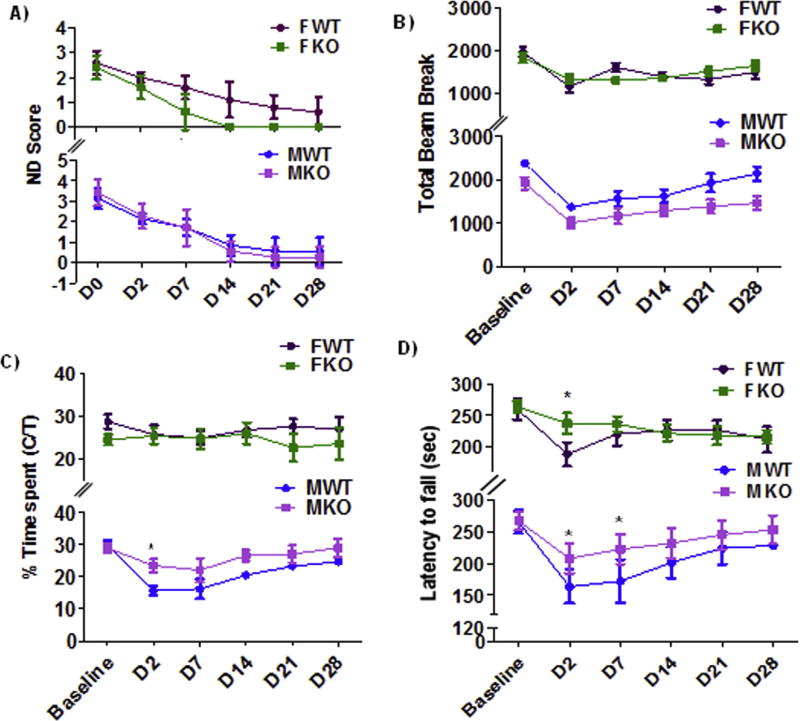
Given that the majority of P2X4Rs are expressed in the brain on myeloid cells (microglia, monocytes, and macrophages) (Cavaliere et al., 2003), we next examined the effects of stroke in mice lacking P2X4R specifically in myeloid cells. Three days after stroke, MS P2X4R KO and WT male mice showed no change in hemispheric, striatal, or cortical infarct volume (4 A). However, female KO mice showed a significant reduction in cortical and total hemispheric infarct volume and a trend for a reduction in striatal infarct volume (68.23 ± 1.5 vs. 56.3 ± 7.0, p = 0.1 vs WT) (Fig. 4B). To probe the role of the ovarian sex hormone estrogen in this effect, we ovxed MS P2X4R KO female mice two weeks prior to stroke surgery; these mice showed the same level of neuroprotection in terms of striatal, cortical and hemispheric infarct volume change as non-ovxed mice (Fig. 4C). Thus, acute estrogen does not appear to contribute to the P2X4R effect. At a chronic time point (day 30), we did not observe differences in tissue atrophy between male or female MS P2X4R KO versus WT mice (data not shown). We next assessed behavioral recovery in the MS P2X4R KO mice, and observed no change in ND score in male or female (ovxed or non-ovxed) KO mice three days after stroke compared to WT littermates (Fig. 4D,–F). However, female MS P2X4R KO mice showed complete recovery by day 14 in ND score (Fig. 5A), unlike WT controls or male P2X4R KO mice.
Fig. 4.
Effect of stroke on infarct volume and ND score in MS P2X4R KO mice. (A) Male mice did not show any differences in infarct volume or ND score between KO (n = 7) and WT (n = 9) mice after three days of stroke. (B) Female mice showed a significant reduction in cortical and total hemispheric (but not striatal) infarct volume between KO (n = 6) and WT (n = 10) mice at an equivalent time point (*p < 0.05; KO vs. WT littermates; two-tailed Student’s t-test; graphs showed mean ± S.E.M.) but did not show any change in ND score (one mouse was excluded due to death). (C) The same magnitude of difference in infarct volume was maintained in ovxed female KO (n = 18) and WT (n = 11) mice (**p < 0.01, *p < 0.05 vs. WT littermates, two-tailed Student’s t-test, graphs showed mean ± S.E.M), suggesting no effect of ovarian hormone estrogen for neuroprotection in female KO mice after stroke. (D-F) Moreover, similar to non-ovxed mice, ovxed KO and WT females did not show any difference in ND score (Mann-Whitney U test).
Fig. 5.
Effect of stroke on sensorimotor deficits in male (M) and female (F) MS P2X4R KO mice. (A) We did not observe a difference in ND Score between KO (n = 7) and WT (n = 10) male mice [F (1, 66) = 0.3 p = 0.5858; two-way ANOVA). Female KO (n = 8) versus WT (n = 9) mice recovered completely (based on ND score) within two weeks. A two-way ANOVA suggested a significant main effect of genotype in female [F (1, 78) = 47.76; p < 0.0001 WT vs KO]. (B) We observed no differences between genotype in total exploratory activity measured by the OFT after baseline correction in either male or female (C) Male KO mice showed as a swift reversal of anxiety-like behavior during the first week of stroke where a two-way ANOVA suggested a significant main effect of genotype (F (1, 66) = 14.48; p = 0.0003] and multiple comparison analysis at different time points suggested a significant difference at day 2 (*p < 0.05; KO vs. WT); we observed no differences in females. (D) In the rotarod test, both male and female KO mice showed reduced impact of ischemic injury on motor balance and coordination during the acute recovery period (days 2–7 post stroke). In male mice a two-way ANOVA suggested a significant main effect of genotype [(F (1, 66) = 4.51; p = 0.034)]. However in female a two-way ANOVA did not show a significant main effect of genotype [F (1, 78) = 1.19 p = 0.2786). However, multiple time point comparison test suggested a significant effect at day 2 in both sexes (*p < 0.05; KO vs. WT) A total of six mice died before the completion of experiments and were not included in the analysis.
3.5. Effect of MS P2X4R deletion on sensorimotor function during chronic recovery after stroke
Next, we examined the role of MS P2X4R in more complex behavioral outcomes after stroke. At baseline, we found no differences between the genotypes for either of the behavioral tests we examined (i.e., OFT and rotarod test; data not shown). After ischemic stroke, MS P2X4R deletion did not affect total exploratory behavior in the OFT at any time point for either gender (Fig. 5B). However, male (but not female) MS P2X4R KO mice showed swift recovery of an anxiety-like behavior in the OFT at an acute time point (Fig. 5C). In the rotarod test, both male and female MS P2X4R KO mice were more resistant to the loss of grip strength during the acute period of ischemia/reperfusion injury (Fig. 5D). However, these differences diminished within two weeks of recovery.
3.6. MS P2X4R KO mice show a depressive-like behavioral phenotype
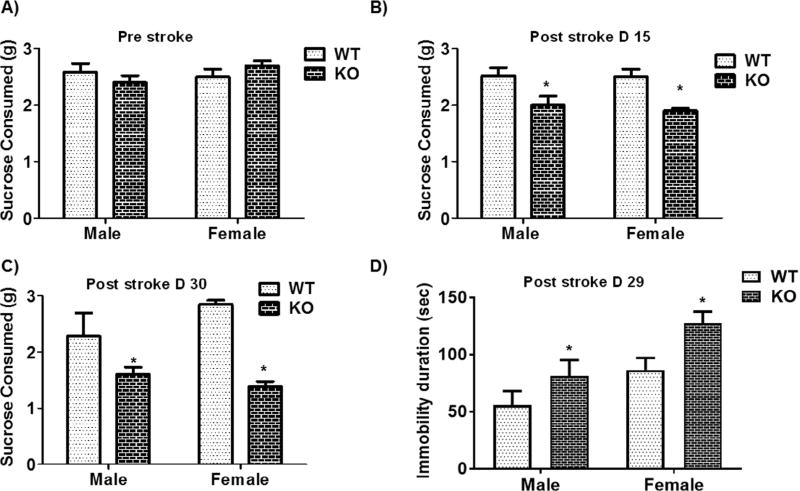
Given that loss of P2X4R leads to a reduction in BDNF, which is linked to depression (Harris et al., 2016), we performed tests for depressive-like behaviors (anhedonia and lack of motivation). At baseline, male and female MS P2X4R KO mice showed no difference in anhedonia (based on the SCT) compared to WT littermates (Fig. 6A); however, after stroke both genders showed reduced consumption of sucrose at a sub-acute (day 15) and chronic (day 30) time point (Fig. 6B,C). Consistent with the SCT results, MS P2X4R KO mice of both genders showed an increased duration of immobility (i.e., loss of escape behavior/lack of motivation to rescue itself) in the TST measured at day 29 after stroke (Fig. 6D). These data suggest that the absence of P2X4Rs on microglia/macrophages induces depression-like behavior after stroke.
Fig. 6.
Effect of stroke on depressive-like behaviors in MS P2X4R KO mice. (A) At baseline, both male and female mice showed no difference in the consumption of sucrose. However, MS P2X4R KO mice showed reduced consumption of sucrose pellet at post-stroke (B) day 15 [male (KO = 10, WT = 7) and female (KO = 8, WT = 9)] and (C) day 30 [males (KO = 5, WT = 4) and females (KO = 3, WT = 4)]. (D) Consistent with the SCT data, these mice (both sexes) [Female KO = 8 WT = 9; Male KO = 7, WT = 10)] showed a significant increase in depressive behavior (immobility duration) measured by the TST after 29 days of stroke (*p < 0.05; KO vs. WT; two-tailed Student’s t-test).
3.7. MS P2X4R KO mice show increased tissue mRNA levels of proinflammatory cytokines and decreased depression-related gene expression after stroke
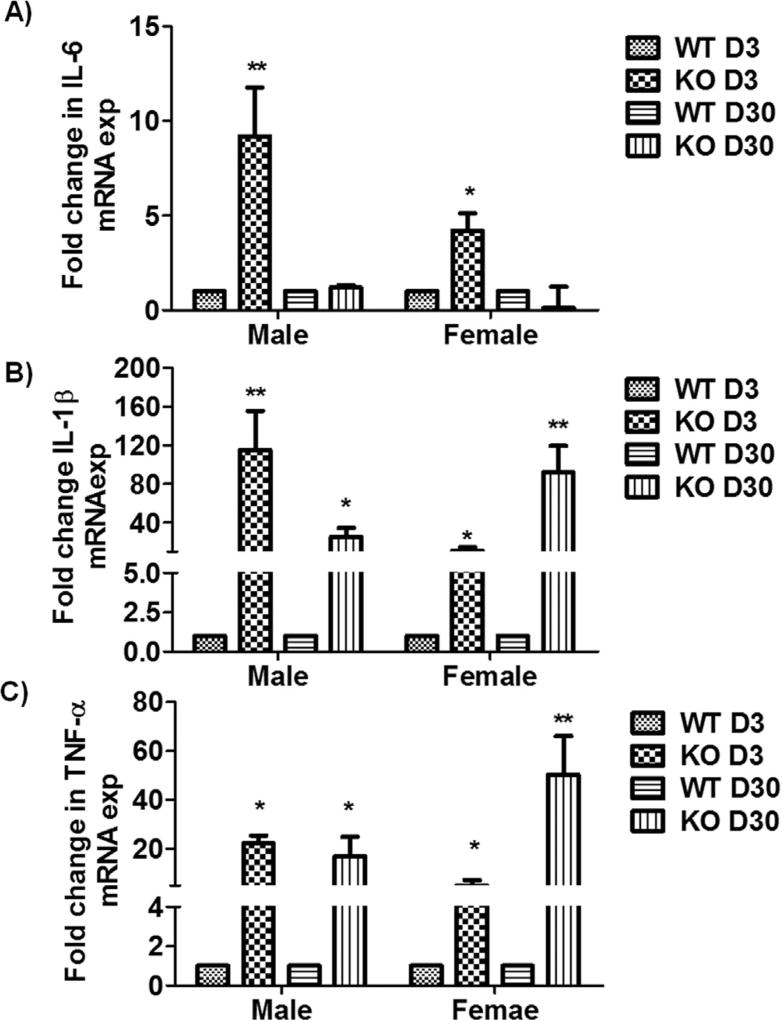
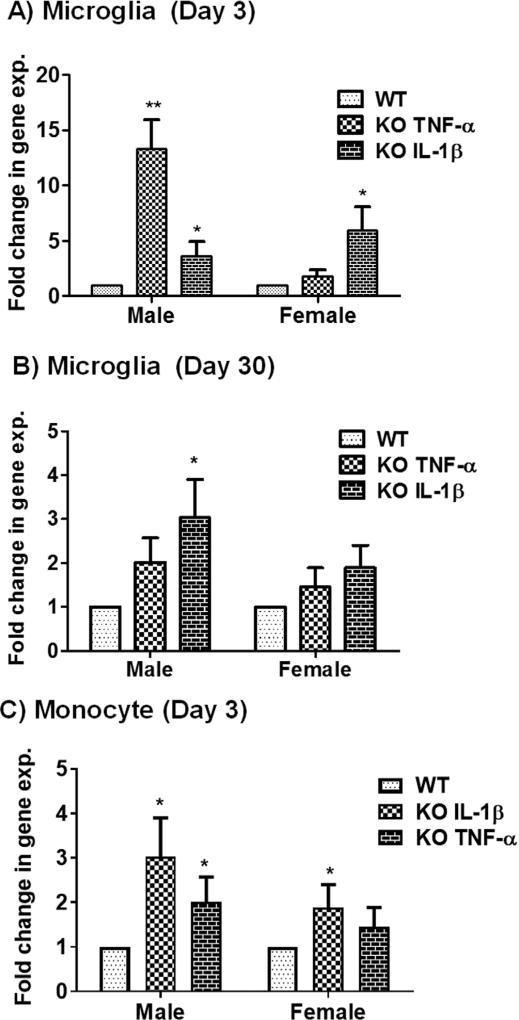
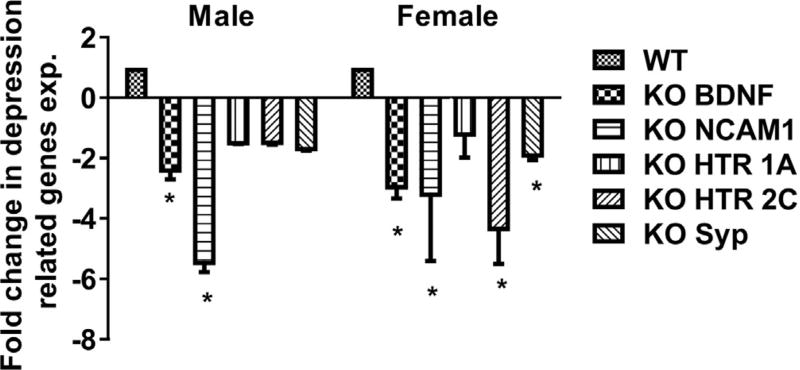
Pro-inflammatory cytokines, neurotrophins (e.g., BDNF), neuronal cell adhesion molecules, and serotonergic pathway genes have a well-established role in depression. Thus, we analyzed the expression levels of these genes using qPCR analysis on brain tissue from MS P2X4R KO mice at 3 and 30 days after stroke. Male and female KO mice showed a significant increase in the mRNAs for the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α compared to WT (Fig. 7A–C) except for IL-6 levels at day 30. We hypothesized that these elevated levels of cytokine transcripts were contributed primarily by myeloid cells. To test this, we sorted microglia and monocytes by flow cytometry and analyzed mRNA isolated from these cells. As anticipated, MS P2X4R KO mice showed elevated levels of intracellular cytokine mRNA in both microglia (days 3 and 30; Fig. 8A,B) and monocytes (day 3; Fig. 8C) isolated from KO mice as compared to WT mice. We could not detect these cytokines in monocytes collected at 30 days of stroke due the very low numbers of monocytes (few hundreds of cells/mouse tissue; data not shown). These observations suggest that MS P2X4Rs might be involved either in the release or maturation of inflammatory cytokines after stroke. Expression analysis of several important depression-related genes such as BDNF, synaptophysin (syp), neural cell adhesion molecule (NCAM) 1, and 5-hydroxytryptamine (serotonin) receptor 2C (HTR2c) and HTR1a revealed a trend or a significantly reduced levels in KO mice brain tissue of both male and female animals (Fig. 9). We did not observe any change in depressive gene at the acute time point (data not shown). This observation suggests a putative interaction between P2X4R and genes pertinent to depressive behaviors.
Fig. 7.
qPCR analysis of mRNA isolated from the perilesional ipsilateral cortex of MS P2X4R KO and WT mice. KO mice (n = 3–4 mice/sex/group/time point; total of 26 mice) showed significant upregulation of the pro-inflammatory cytokines (A) IL-6, (B) IL-1β, and (C) TNF-α at both acute and chronic time points, with the exception of IL-6 levels, which did not change for either sex at the chronic time point of recovery. Data are expressed as mean ± SEM and values on the Y axis are presented as fold change in gene expression of KO mice against their respective WT control, whose value were kept constant at 1 in determining fold change in KO (**p < 0.01, *p < 0.05; KO vs. WT, two-tailed Student’s t-test).
Fig. 8.
mRNA expression analysis of the pro-inflammatory cytokines TNF-α and IL-1β in flow-sorted microglia and monocyte after stroke in MSP2X4R KO and WT mice. (A) Microglia sorted at day 3 [males (KO = 5, WT = 4) and females (KO = 4, WT = 5); total of 18 mice] showed significantly higher levels of TNF-α in males (**p < 0.01; KO vs. WT, two-tailed Student’s t-test) and a trend of elevation in females. IL-1β levels were elevated in both sexes (*p < 0.05; KO vs. WT two-tailed Student’s t-test). (B) mRNA analysis from microglia sorted after 30 days showed elevated levels of IL-1β in males (*p < 0.01; KO vs. WT, two-tailed Student’s t-test) and a trend of elevation in females. No change in TNF-α was found for either of sex (n = 3 mice/group/sex; total 12). (C) Monocytes sorted at day 3 after stroke showed elevated levels of both IL-1β in both sexes (*p < 0.05; KO vs. WT; two-tailed Student’s t-test). TNF-α increased in male (*p < 0.05; KO vs. WT; two-tailed Student’s t-test) and showed a trend for increase in female KO. Very few monocytes were detected in the brain after 30 days, so no detectable expression of either gene was found. Similarly, no IL-6 levels exceeded the detection limit in both sorted microglia and monocytes. Data were expressed as mean ± SEM and values on the Y axis were plotted as fold change in gene expression of KO mice against their respective WT control.
Fig. 9.
qPCR analysis of depression-related genes. Several genes (BDNF, NCAM1, HTR 1a, HTR 2c and Syp) implicated in depression were downregulated in MS P2X4R KO in both male and female mice chronically after stroke (day 30). Data are expressed as mean ± SEM and values on the Y axis are presented as fold change in gene expression of KO mice against their respective WT control (*p < 0.05; KO vs. WT; two-tailed Student’s t-test).
3.8. MS P2X4R KO mice show reduced pro-inflammatory cytokine IL-1β in plasma
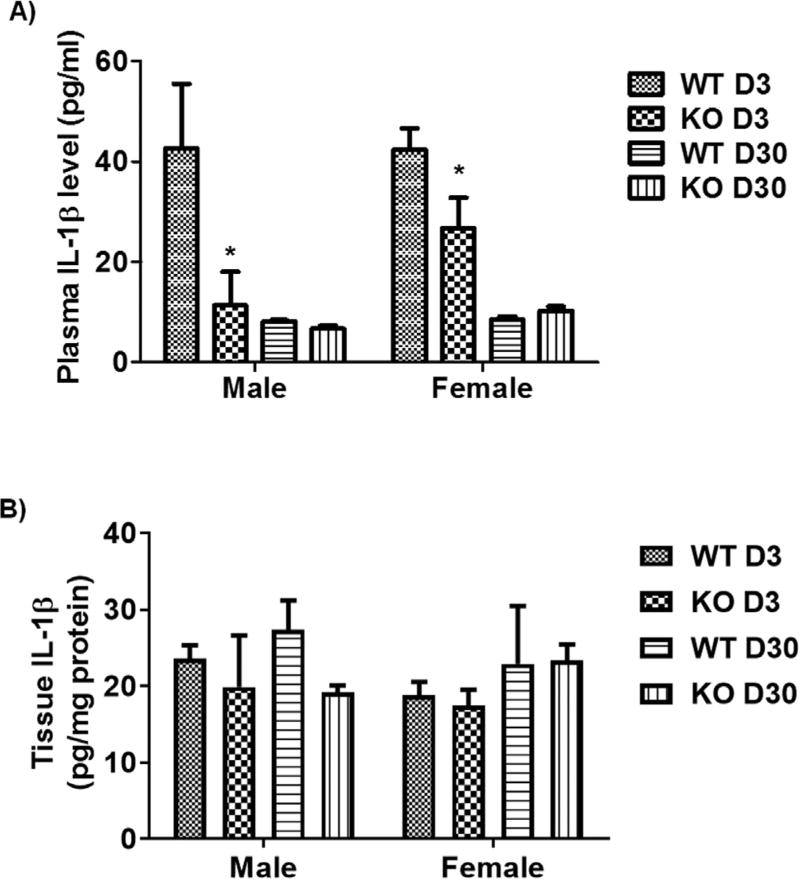
To further confirm our hypothesis that KO mice show reduced release of matured cytokines, we performed an ELISA on a highly modulated cytokine, IL-1β. At the acute time point, total plasma levels of IL-1β cytokine were significantly lower in KO mice as compared to WT mice; however, we did not observe any change at day 30 (Fig. 10A). We did not observe a difference in brain tissue protein levels of IL-1β between WT and KO mice at any time point (Fig. 10B). The microglial protein level of IL-1β may be different in KO vs. WT mice; however, this potential difference could not be measured due to the low amount of proteins available from isolated microglia. Nevertheless, the overall data are consistent with the hypothesis that the P2X4R might plays an important role in stimulating the release of IL-1β from myeloid cells during acute ischemic insults.
Fig. 10.
IL-1β plasma and brain tissue protein levels examined using ELISA. (A) IL-1β plasma levels were significantly reduced in KO mice at an acute time point (day 3) following stroke in both sexes (*p < 0.05; KO vs. WT; two-tailed Student’s t-test), but no change was seen at a chronic time point (day 30). B) Tissue protein levels were not different between KO and WT mice at any time point after stroke in both sexes. Data were expressed as mean ± SEM and were plotted as IL-1β pg/ml plasma (A) or mg/tissue protein.
4. Discussion
In this study, we performed both acute and chronic survival experiments to explore the therapeutic potential of P2X4R loss. We first found that P2X4Rs are expressed robustly on microglial cells following stroke. Consistent with this finding, microglial cells show increased proliferation 2–3 days following stroke (Ritzel et al., 2015; Wixey et al., 2009; Tsuda et al., 2003), which coincides with the peak upregulation of microglial P2X4R expression (Wixey et al., 2009). Microglia and peripheral macrophages/monocytes (i.e., myeloid cells) are the principal producers of inflammatory cytokines after ischemic stroke (Clausen et al., 2008). Moreover, P2X4R-mediated activation of myeloid cells has been shown to contribute to neuroinflammation by releasing pro-inflammatory cytokines such as TNF-α and IL-1β (Lai and Todd, 2006; Weinstein et al., 2010). However, these studies either were performed in primary microglial cell culture, which is not physiologically relevant to stroke pathology in vivo, or used a pharmacological antagonist of P2X4R that can non-specifically block other P2XRs or block P2X4Rs on multiple cell types in the brain. Therefore, in this study, we took advantage of the Cre/LoxP system to generate MS P2X4R KO mice to investigate the role of myeloid P2X4R in stroke injury.
We saw an equal degree of neuroprotection in male and female global P2X4R KO mice compared to WT controls, likely due to an inhibition of release of pro-inflammatory cytokines like IL-1β and TNF-α and a subsequent blockade of inflammasome activation in the absence of P2X4R (de Rivero Vaccari et al., 2012). The lack of a difference in tissue atrophy after 30 days suggests a possible reversal of the early benefits of loss of P2X4R during chronic recovery due to the absence of physiological functions of P2X4R in KO mice. However, when we assessed MS P2X4R KO mice, only females showed neuroprotection, suggesting a sex difference in the P2X4R response after stroke. This finding is consistent with recent findings that microglia responses may be sexually dimorphic (Lehnardt et al., 2003; Sorge et al., 2011; Sorge et al., 2015). In these studies, the divergence between the male and female signaling pathways seemed to occur at the level of P2X4R with its upregulation in male mice only. However, other genes associated with microglial reactivity were shown to be upregulated in both sexes (Sorge et al., 2015); therefore, perhaps P2X4R present on brain cells other than microglia neutralized the P2X4R-mediated acute neuroprotection in the males. Sex differences in stroke have been largely attributed to neuroprotection due to the activational effects of gonadal hormones such as estrogen (Manwani et al., 2015). However, we observed a similar degree of neuroprotection in both ovxed and intact MS P2X4R KO mice, suggesting that the protection we observed was not related to the acute activational effects of estrogen. It is possible that sex chromosomes or organizational effects of steroids play a role early in development in this sexually dimorphic response (Manwani et al., 2015) which remains to be explored. The absence of chronic effects of global or MS P2X4R on tissue atrophy suggest that the acute benefits may have been counterbalanced by the chronic loss of the beneficial effect of P2X4R during recovery.
We next conducted mRNA gene expression profiles on several genes that are central to the neuroinflammatory response in cerebral ischemia (i.e., IL-1β, TNFα, and IL-6) both at day 3 and day 30 after stroke. We found consistently elevated levels of IL-6, IL-1β, and TNF-α mRNA in the brain of post-stroke MS P2X4R KO mice of both sexes as compared to WT mice, irrespective of infarct size. Moreover, we found a reciprocal relationship in expression between these cytokines and other depression-related gene levels in MS KO mice. The increased cytokine gene expression levels in the KO mice appears to be in conflict with prior work, where P2X4R activation has been shown to increase inflammation (Sakaki et al., 2013; Burnstock, 2016). However, these paradoxical findings may be explained by the notion that an activated microglia signal for increased pro-inflammatory cytokine mRNA expression is not associated with release of cytokines from cells due to an absence of P2X4R (Aonurm-Helm et al., 2008) in KO mice. Our cytokine expression data from flow-sorted microglia revealed elevated mRNA levels of IL-1β and TNF-α both at early and delayed time points after ischemic stroke are consistent with this concept. Thus, these KO mice may show protection from damage as a result of reduced release of pro-inflammatory cytokines at an early time point. The fact that the IL-1β protein level was not changed in the brain tissues but was reduced in plasma of KO vs. WT mice at day 3 after stroke point to reduced extracellular cytokines as a potential protective mechanism.
Despite the detrimental effect of early activation of P2X4Rs, these receptors are involved in the release of BDNF (Lai and Todd, 2006); thus they may modulate chronic recovery after stroke. We and several other laboratories have correlated the chronic loss of BDNF with poor behavioral recovery and depressive behaviors at chronic time points after ischemic injury (Verma et al., 2016; Harris et al., 2016; Chen et al., 2015; Qin et al., 2014). Furthermore, infarct size measurements alone can be misleading (Verma et al., 2016; Bouet et al., 2007; Mergenthaler and Meisel, 2012) and P2X4Rs may also modulate social behavioral responses (Wyatt et al., 2013). Therefore, we extended our study to ascertain the chronic behavioral effects of loss of MS P2X4R after stroke. We did not observe a change in tissue atrophy measured 30 days after stroke in MS P2X4R KO mice in either sexes, mirroring our finding with global KO mice. However, we found that MS ablation of P2X4Rs resulted in a range of abnormal behavioral phenotypes in both male and female mice. The acute recovery seen in the rotarod test and in anxiety-like behaviors that we observed in MS P2X4R KO might correspond to a reduced surge in inflammatory cytokines activity as a result of impaired release; however, during chronic survival these benefits were lost either due to an overall reduction in post-stroke inflammation or an absence of a beneficial effect of P2X4R such as the lack of BDNF. Interestingly, both male and female displayed depression-like behavior as measured by the TST and SCT; these effects were independent of infarct size difference. The depression-like behaviors in MS P2X4R KO mice suggest an important role of microglial P2X4R in chronic post-stroke recovery, likely mediated by BDNF release (Trang et al., 2009).
Prior studies suggest that cytokines such as IL-1β, IL-6, and TNFα are associated with depressive behaviors both pre-clinically and clinically (Karelina et al., 2009; Miller and Raison, 2016). However, other reports contradict this notion by suggesting that depressive behaviors might be unrelated to the pro-inflammatory actions of these cytokines (Yirmiya et al., 2015). These cytokines also reduce BDNF release or interrupt its binding to the trk-B receptor and thus might further potentiate depressive behaviors (Felger and Lotrich, 2013). In addition to reduced BDNF in MS P2X4R KO mice, other possible causes of the depression-like behaviors in the KO mice may be related to reduced expression of NCAM1 and syp in mice, as genetic deletion of NCAM leads to depressive-like behaviors (Yirmiya et al., 2015) and reduced expression of syp was reversed by antidepressant drugs (Xu et al., 2004). The serotonergic system has also been widely implicated in major depressive disorder (MDD) in both clinical and preclinical research. The role of the serotonin transporter in MDD has been highlighted in genes by environment association studies (Morrissette and Stahl, 2014). In addition, the serotonin transporter is a critical player in the mechanism of most effective antidepressant treatments, selective serotonin reuptake inhibitors (Serretti et al., 2007). While the majority of the 15 known receptors for serotonin have been implicated in depression or depressive-like behaviors, the serotonin HTR 1A, 1B, and 2C receptors are amongst the most important and studied (Nautiyal and Hen, 2017). Our data provide preliminary evidence that P2X4R is somehow involved in microglial-mediated depressive behavior after stroke. However, a detailed study is needed to dissect the direct downstream targets of P2X4R that lead to depressive behavior.
5. Summary and conclusions
In summary, our data support that global absence of P2X4R provides early neuroprotection following stroke. However, when we deleted P2X4R specifically in myeloid cells, only female mice showed acute neuroprotection, independent of estrogen levels. This concept deserves further investigation in order to elucidate the sex difference in these mice after stroke. Interestingly, both male and female MS P2X4R KO mice showed increased mRNA levels for cellular pro-inflammatory cytokines but reduced levels of corresponding mature cytokines in plasma. Furthermore, both male and female MS P2X4R KO mice showed depressive-like behaviors, which is, to our knowledge, the first link between depression and P2X4R in myeloid cells. The depressive-like behavior in the MS P2X4R KO mice might be due to a P2X4R-mediated microglial response. Indeed, our preliminary findings suggest an interference in cytokine release/signaling and a reduction in BDNF levels and serotonergic signaling in these KO animals. In sum, our study indicates that P2X4R-based pharmacotherapy should be undertaken in a time-sensitive manner after stroke. Acute inhibition of the receptor might provide a benefit whereas chronic blockade might further exacerbate depressive behaviors.
Supplementary Material
Acknowledgments
We thank Dr. Rodney Ritzel and Dr. Min Jung Kim for their technical help in flow cytometry experiments and statistical analyses, respectively.
Sources of Funding
This work was supported by an institutional start-up grant, American Heart Association grants (14POST20380612 to R. Verma; 15SDG23250025 to V.R. Venna), a National Institute of Neurological Disorders and Stroke grant (NSO55215 to L.D. McCullough), a National Institutes of Health grant (HL48225) and the endowed Ray Neag Distinguished Professorship to (B. Liang).
Footnotes
Conflict(s)-of-Interest/Disclosure(s)
None declared.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2017.07.155.
References
- Aonurm-Helm A, Jurgenson M, Zharkovsky T, Sonn K, Berezin V, Bock E, Zharkovsky A. Depression-like behaviour in neural cell adhesion molecule (NCAM)-deficient mice and its reversal by an NCAM-derived peptide, FGL. Eur. J. Neurosci. 2008;28:1618–1628. doi: 10.1111/j.1460-9568.2008.06471.x. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp. Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Burnstock G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016;12:59–67. doi: 10.1007/s11302-015-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D’Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volonte C. Upregulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Chen HH, Zhang N, Li WY, Fang MR, Zhang H, Fang YS, Ding MX, Fu XY. Overexpression of brain-derived neurotrophic factor in the hippocampus protects against post-stroke depression. Neural Regen. Res. 2015;10:1427–1432. doi: 10.4103/1673-5374.165510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflam. 2008;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S. P2X4 receptors influence inflammasome activation after spinal cord injury. J. Neurosci. 2012;32:3058–3066. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NM, Ritzel R, Mancini N, Jiang Y, Yi X, Manickam DS, Banks WA, Kabanov AV, McCullough LD, Verma R. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol. Biochem. Behav. 2016;150–151:48–56. doi: 10.1016/j.pbb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc. Natl. Acad. Sci. U.S. A. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can. J. Physiol. Pharmacol. 2006;84:49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Trans. Stroke Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J. Cereb. Blood Flow Metab. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav. Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler P, Meisel A. Do stroke models model stroke? Dis. Model Mech. 2012;5:718–725. doi: 10.1242/dmm.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette DA, Stahl SM. Modulating the serotonin system in the treatment of major depressive disorder. CNS spectrums. 2014;19(Suppl 1):57–67. doi: 10.1017/S1092852914000613. quiz 54–57, 68. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nikolova VD, Riddick NV, Baker LK, Koller BH. Preweaning sensorimotor deficits and adolescent hypersociability in Grin1 knockdown mice. Dev. Neurosci. 2012;34:159–173. doi: 10.1159/000337984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Research. 2017;6:123. doi: 10.12688/f1000research.9736.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Jarvis MF. P2X receptors as drug targets. Mol. Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Jing D, Parauda S, Carmel J, Ratan RR, Lee FS, Cho S. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. J. Neurosci. 2014;34:2493–2502. doi: 10.1523/JNEUROSCI.4140-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflam. 2015;12:106. doi: 10.1186/s12974-015-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki H, Fujiwaki T, Tsukimoto M, Kawano A, Harada H, Kojima S. P2X4 receptor regulates P2X7 receptor-dependent IL-1beta and IL-18 release in mouse bone marrow-derived dendritic cells. Biochem. Biophys. Res. Commun. 2013;432:406–411. doi: 10.1016/j.bbrc.2013.01.135. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol. psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DT, Venna VR, McCullough LD, Fitch RH. Deficits in auditory, cognitive, and motor processing following reversible middle cerebral artery occlusion in mice. Exp. Neurol. 2012;238:114–121. doi: 10.1016/j.expneurol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Vazquez-Villoldo N, Domercq M, Martin A, Llop J, Gomez-Vallejo V, Matute C. P2X4 receptors control the fate and survival of activated microglia. Glia. 2014;62:171–184. doi: 10.1002/glia.22596. [DOI] [PubMed] [Google Scholar]
- Verma R, Friedler BD, Harris NM, McCullough LD. Pair housing reverses post-stroke depressive behavior in mice. Behav. Brain Res. 2014;269:155–163. doi: 10.1016/j.bbr.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Harris NM, Friedler BD, Crapser J, Patel AR, Venna V, McCullough LD. Reversal of the detrimental effects of post-stroke social isolation by pair-housing is mediated by activation of BDNF-MAPK/ERK in aged mice. Sci. Rep. 2016;6:25176. doi: 10.1038/srep25176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Koerner IP, Moller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixey JA, Reinebrant HE, Carty ML, Buller KM. Delayed P2X4R expression after hypoxia-ischemia is associated with microglia in the immature rat brain. J. Neuroimmunol. 2009;212:35–43. doi: 10.1016/j.jneuroim.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Wyatt LR, Godar SC, Khoja S, Jakowec MW, Alkana RL, Bortolato M, Davies DL. Sociocommunicative and sensorimotor impairments in male P2X4-deficient mice. Neuropsychopharmacology. 2013;38:1993–2002. doi: 10.1038/npp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, He J, Richardson JS, Li XM. The response of synaptophysin and microtubule-associated protein 1 to restraint stress in rat hippocampus and its modulation by venlafaxine. J. Neurochem. 2004;91:1380–1388. doi: 10.1111/j.1471-4159.2004.02827.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38(10):637–658. doi: 10.1016/j.tins.2015.08.001. Epub 2015/10/08. [DOI] [PubMed] [Google Scholar]
- Zech A, Wiesler B, Ayata CK, Schlaich T, Durk T, Hossfeld M, Ehrat N, Cicko S, Idzko M. P2rx4 deficiency in mice alleviates allergen-induced airway inflammation. Oncotarget. 2016 doi: 10.18632/oncotarget.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.