Abstract
Purpose: The aim of this study is to observe the synergistic effect of two active compounds of secang, brazilin and brazilein, combined with cisplatin on WiDr colon cancer cells.
Methods: Cytotoxic activities of brazilin (Bi) and brazilein (Be) in single and in combination with cisplatin (Cisp) were examined by MTT assay. Synergistic effect was analyzed by combination index (CI) parameter. Apoptosis and cell cycle profiles were observed by using flow cytometry.
Results: The result of MTT assay showed that IC50 value of brazilin and brazilein on WiDr cancer cells were 41 µM and 52 µM respectively. The combination of ½ IC50 of Bi-Cisp reduced cells viability up to 64% and showed synergistic effect with CI value less than 1 (CI = 0.8). The combinations of ½ IC50 of Be-Cisp also reduced cells viability up to 78% and showed synergistic effect (CI=0.65). Combination of Bi-Cisp and Be-Cisp induced apoptosis higher than the single treatments. Further analysis on the cell cycle progression showed that single treatment of ½ IC50 of Be and Bi induced S-phase and G2/M-phase accumulation, while combination of Be-Cisp and Bi-Cisp enhanced S-phase accumulation.
Conclusion: Both combination of Bi-Cisp and Be-Cisp showed synergistic effect on WiDr cells through induction of apoptosis and halted the cell cycle progression, thus, WiDr cells growth were significantly reduced.
Keywords: Brazilin; Brazilein; Caesalpinia sappan, L.; Cisplatin; Synergistic effect; WiDr cells
Introduction
Some of colorectal cancer (CRC) cases are associated with poor survival because of p53 mutation.1 The p53 gene is a tumor suppressor and the key regulator of DNA damage responses. This gene plays a role on tumor suppression processes including cell cycle arrest and apoptosis.2 Mutation in p53 gene leading to the loss of wild-type p53 activity is frequently detected in many different tumor types.3 Enhancing of invasiveness, attenuating of apoptosis and increasing of genomic instability are generally occurred after mutation of p53.4 The p53-mutant cancer may not be treated with the same agent as p53 wild type cancer.
Surgery, chemotherapeutic drugs and radiation therapy are some of the cancer therapies that frequently be used to treat colon cancer patient.5 Cisplatin and its derivatives are the effective DNA-damaging anti-tumor agent for various human cancers, including colon cancer.6 The p53 protein is stabilized and its level increases in response to various DNA damaging agents, including cisplatin. However, side effects including resistance to cisplatin arise in p53-mutant cancer cells.7 Thus, we need to investigate potential anticancer compounds that work on p53-independent pathway. Currently, combination of chemotherapy regiments based on platinum-derived compounds with other drugs (co-chemotherapeutic agent) constitute the pharmacological therapy of choice for the treatment of colon cancer. The p53-mutant colon cancer may need to be treated with combination therapy.
The major compounds of Caesalpinia sappan L. (Caesalpiniaceae) i.e. brazilin and brazilein have been reported to have activities as antiinflammation, antioxidant, hepatoprotective and antiviral.8-11 Brazilin and brazilein induce apoptosis and inhibit cell growth on several cancer cells.12-14 Brazilein suppresses cancer cells migration and invasion.15 Thus, brazilin and brazilein have potential to be developed as co-chemotherapeutic agent. Nevertheless, synergistic effect of combination of brazilin and brazilein with cisplatin against p53-mutant WiDr cancer cells has never been reported. In this study, we observed the synergistic cytotoxic effect of combination of brazilin and brazilein with cisplatin on WiDr cancer cells.
Materials and Methods
Chemicals
Brazilin and brazilein were isolated from Caesalpinia sappan, L. using previously reported method.9 Cisplatin was purchased from Wako (Japan).
Cell culture
WiDr cell line was obtained Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta.
Cytotoxic assay
The 1×104 cells/well were seeded in 96-well plate. Cells were treated with various concentrations of brazilin, brazilein, and cisplatin in single or in combination then incubated in 37°C, 5% CO2 for 24 h. Cells were then washed with PBS (Sigma), were treated with 100 µL culture medium containing 0.5 mg/mL MTT (Sigma) and incubated in 37°C, 5% CO2 for 4 h. The reaction was stopped by adding to the cells with SDS reagent (10% sodium dodecyl sulphate (Merck) in HCl 0.01M (Merck)) then was incubated overnight in room temperature. The absorbance was measured with microplate reader (Bio-Rad) at λ 595 nm.
Cell cycle distribution
Propidium iodide (PI) staining kit (Becton Dickinson) was used to analyze DNA content. 5×104 cells/well were seeded in 24-well plate. Cells were treated with various concentrations of samples in single or in combination then incubated in 37°C, 5% CO2 for 24 h. Cells were harvested and washed in PBS, fixed with 70% ethanol, labeled with PI (2 µg/mL), and incubated in room temperature, protected from light, for 10 min. The DNA content was analyzed using flow cytometry (Becton Dickinson) and followed by flowing software (version 2.5.1) and Excel MS Office 2013.
Apoptosis detection
Apoptotic cells population was determined using PI-Annexin V assay (Annexin V-FITC Apoptosis Detection Kit Roche). 5×104 cells/well were seeded in 24-well plate. Cells were treated with various concentrations of samples either single or in combination for 24 h. Cells were harvested, added with 1× binding buffer, labeled with PI-Annexin V, and incubated at room temperature in the dark for 5 min. The cells suspension was analyzed using a flow cytometry (Becton Dickinson) and followed by flowing software (version 2.5.1) and Excel MS Office 2013.
Statistical analysis
Statistical analysis was performed using Student t-test (Microsoft Excel 2013). P-values less than 0.05 were considered significant.
Effects of combinations on growth inhibition were analyzed using the Combination index (CI) equation developed by Reynolds and Maurer.16
Results and Discussion
Cytotoxic effect of brazilin and brazilein on WiDr cells.
First, we observed the cytotoxic activity to find out the potency of brazilin and brazilein in inhibiting of WiDr cells proliferation. The cytotoxic activity was performed by using MTT assay. The results showed that brazilin and brazilein inhibited WiDr cells growth in a dose-dependent manner with IC50 value were 41 µM and 52 µM respectively (Figure 1), while the IC50 value of cisplatin was 15 µM (data not shown).
Figure 1.
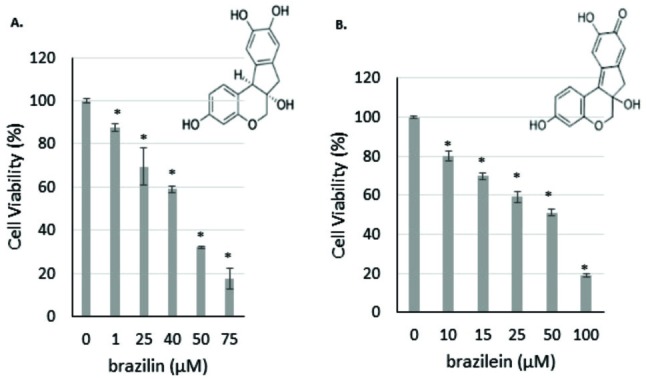
Cytotoxic effect of brazilin and brazilein on WiDr cells. A. brazilin (1-75 µM), B. brazilein (10-100 µM). Cells were treated with various concentrations of samples for 24 h before assessed by MTT assay. Data was collected from three replications (P<0.05).
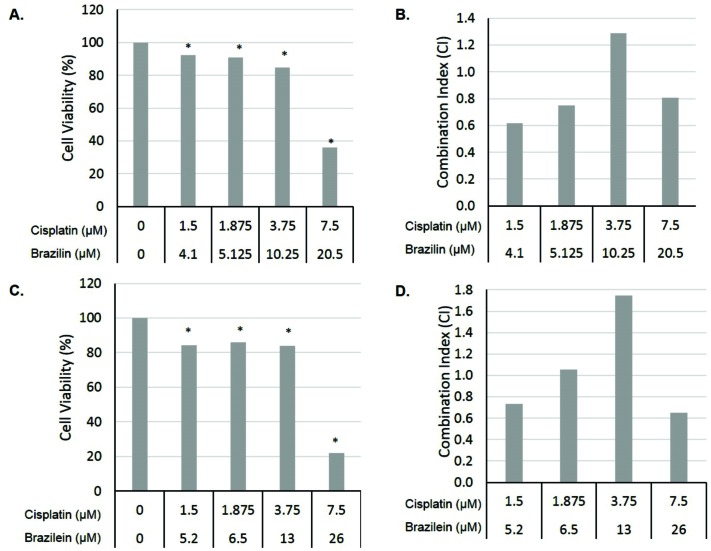
Synergistic effect of combination of Bi-Cisp and Be-Cisp on WiDr cells growth
Discovery of agents that reduce resistance of chemotherapeutic agent is urgently needed and become one main target of today research. We investigated the effect of brazilin and brazilein in combination with cisplatin, a standard chemotherapeutic agent, on WiDr colon cancer cells. As shown in Figure 2, the combination of 1/10, ⅛, ¼ and ½ IC50 of either Bi-Cisp or Be-Cisp showed synergistic effect on inhibition of WiDr cells growth (CI<1). Moreover, the combination of ½ IC50 of Bi-Cisp inhibited cell viability up to 64%. These data indicate that both compounds are potential to be developed as co-chemotherapeutic agent on colon cancer cells. To understand the mechanism underlies the synergistic effect, we observed the effect of the combinations on cell cycle modulation and on the induction of apoptosis.
Figure 2.
Combination of ½ IC50 of Bi-Cisp (20.5 µM- 7.5 µM) and Be-Cisp (26 µM- 7.5 µM) synergistically inhibited WiDr cells growth. A. Effect of combination of Bi-Cisp on WiDr cells growth. B. Combination Index (CI) of Bi-Cisp on WiDr cells. C. Combination of Be-Cisp on WiDr cells growth. D. CI of Be-Cisp on WiDr cells. Cells were treated with samples for 24 h before assessed by MTT assay. Data was collected from three replications (P<0.05).
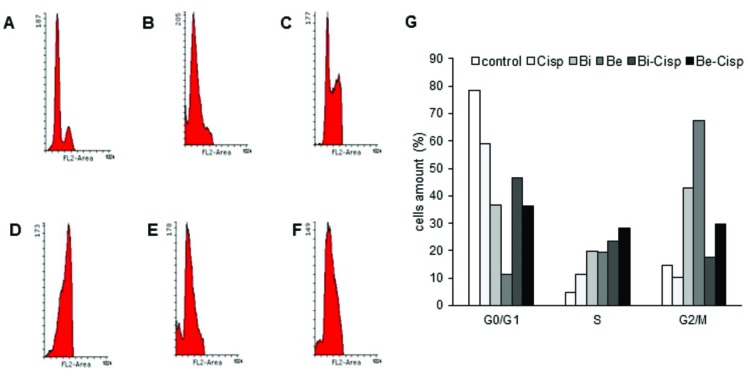
Cell cycle modulation of brazilin and brazilein in combination with cisplatin
We conducted cell cycle analysis to elucidate how brazilin, brazilein and their combination with cisplatin inhibited cells proliferation. The results of cell cycle analysis (Figure 3) showed that single treatment of either brazilin or brazilein caused S-phase and G2/M-phase accumulation. Treatment of ½ IC50 of brazilein (Figure 3D) induced higher G2/M accumulation compared to ½ IC50 of brazilin (Figure 3C). On the other hand, single treatment of ½ IC50 of cisplatin induced S-phase accumulation (Figure 3B). When brazilin or brazilein was combined with cisplatin, the combinations increased cells accumulation in S-phase compared to its single treatment, whereas both combination showed accumulation in G2/M-phase compared to control cells (Figure 3E-G).
Figure 3.
Combination of ½ IC50 of Bi-Cisp and Be-Cisp induced S-phase accumulation on WiDr cells. A. Control. B. Cisplatin (7.5 µM). C. Brazilin (20.5 µM). D. Brazilein (26 µM). E. Combination of Bi-Cisp (20.5 µM -7.5 µM). F. Combination of Be-Cisp (26 µM -7.5 µM). G. Cell cycle distribution of ½ IC50 of Bi and Be in single and combination with Cisp. Data was collected according to the description in Method.
Apoptosis induction of brazilin and brazilein in combination with cisplatin.
Inhibition of proliferation may be caused by modulation in cell cycle and by the induction of apoptosis. To investigate the effect of brazilin, brazilein and their combination with cisplatin on apoptosis, we did FACS analysis on WiDr cells which were treated with samples and were stained with fluorescein isothiocyanate-conjugated annexin V and fluorescent dye propidium iodide (PI). Single treatment of cisplatin, brazilin and brazilein exhibited 14%, 10% and 12% of total apoptotic cells respectively. Either combination of brazilin-cisplatin or brazilein-cisplatin increased total apoptotic cells up to 22% compared to control or 9% compared to cisplatin alone (Figure 4). Combination of brazilein-cisplatin treatment showed highest accumulation in late apoptotic and necrosis, while combination of brazilin-cisplatin exhibited highest accumulation in early apoptotic cells following 24 hours of incubation. These results suggested that brazilin and brazilein increased the induction of apoptosis of cisplatin on WIDr cells.
Figure 4.
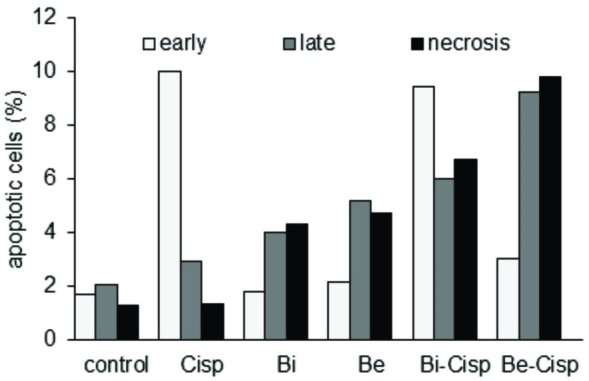
Combination of ½ IC50 of Bi-Cisp and Be-Cisp synergistically induced apoptosis on WiDr cells. Cells were treated with Bi, Be and Cisp (20.5 µM, 26 µM, and 7.5 µM respectively) as indicated in the graph for 24 h. Data was collected according to the description in Method.
Cisplatin is a platinum-based drug that is used for the treatment of a wide-variety of primary human cancers. However, the efficacy of cisplatin is often limited by the rise of drug resistance.6,7 Brazilin and brazilein are the active compounds from Caesalpinia sappan L. The structures of brazilin and brazilein are almost similar, whereas brazilein has C=O group and brazilin does not have C=O group in its structure.9 In this study, brazilin showed the better cytotoxic effect against WiDr cancer cells than brazilein (Figure 1). Nevertheless, both of compounds performed synergistic effect while were combined with cisplatin.
Cell cycle analysis showed that single treatment of either brazilin or brazilein induced S-phase and G2/M-phase accumulation, while single treatment of cisplatin only accumulated cells in S-phase (Figure 4). Interestingly, when brazilin and brazilein were given as combination with cisplatin, both compounds seemed to work effectively in two fields. Both brazilin and brazilein enhance S-phase accumulation of cell cycle up to 23% and 28% respectively compared to single treatment of cisplatin. The rest of the cells, which were lead to G2/M phase were also blocked by brazilin and brazilein (Figure 4). The mechanism underlies the S-phase and G2/M-phase arrest-induced by brazilin and brazilein needs further investigation. Previous study reported that brazilin induces G2/M arrest through inactivation of histone deacetylase and followed by the activation of CDK inhibitor p27.14 The G2/M accumulation on brazilein treatment on colon cancer cells may be related with the ability of brazilein to bind with COX2 receptor (data not shown). Brazilein may induce G2/M arrest through inhibition of COX2, which is followed with the activation of p21. The p21 inhibits the CDK1/cyclin B complex and maintains the G2 arrest.17 Since WiDr cell expresses p53 mutant, p21 may be induced by p53-independent pathway.18,19 The S phase arrest-induced by the combination of brazilin-cisplatin and brazilein-cisplatin are possibly due to the action of cisplatin which as reported by Yuan et al,20 single treatment of cisplatin induces S-phase arrest via activation of CHK1 and CHK2 checkpoint kinase. The proteins induce cell death through DNA damage and cell cycle arrest during S phase. Hence, S-phase arrest induced by cisplatin through CHK1 activation on p53-mutant cells has been reported.21 Hence, we need to investigate that S-phase arrest induced by both of the combinations underwent apoptosis instead of DNA repair.
Although the total apoptosis event of single treatment of cisplatin, brazilin and brazilein were similar (Figure 2), the three compounds have different pattern. Cisplatin exhibited the highest distribution in early apoptosis. On the other hand, brazilin and brazilein exhibited the highest distribution in late apoptosis. Combination of both brazilin-cisplatin and brazilein-cisplatin enhanced induction of apoptosis compared to cisplatin single treatment. The results support our suggestion that S-phase accumulation induced by the combinations lead to apoptosis instead of DNA repair. Interestingly, even though the total apoptotic events of both of the combinations were similar, combination of brazilin-cisplatin exhibited the highest accumulation in the early apoptotic cells, while combination of brazilein-cisplatin treatment exhibited the highest accumulation in the late apoptotic cells and necrosis. This difference may be due to its different mechanism. Previous study reported that cisplatin induces apoptosis through FAS receptor pathway.22 FAS/FADD as well as TNF activate procaspase-8 to form caspase-8 and lead to increasing of proapoptotic protein Bax via intrinsic pathway.23 Caspase-8 also directly activated caspase-3 and 7 and lead to apoptosis via extrinsic pathway.24 Furthermore, brazilin induces apoptosis by increasing of cleavage caspase 3, cleavage caspase 7 and cleavage PARP,12 while brazilein directly inhibits anti apoptotic protein surviving.25 The p53 is a transcription factor for pro-apoptotic protein, while NFκB is a transcription factor for anti-apoptotic protein such as Bcl-2 and surviving.26 Hsieh reported that brazilein inhibits activation of NFκB.15 On p53-mutated cells with loss most of pro-apoptotic protein expression, inhibition of NFκB activation which down-regulates anti-apoptotic protein and followed by apoptosis induction is an important mechanism to suppress cancer cells growth. Downregulation of of anti-apoptotic protein induces activation of caspase-9 and caspase-3 and followed with apoptosis phenomena.25,27,28 Those support our finding that the combination of brazilin-cisplatin and brazilein-cisplatin work synergistically to induce apoptosis on WiDr cells.
Combination of several therapies facilitates the blockade of several survival mechanisms in cancer cells and their microenvironment, achieving a synergistic therapeutic effect.29 On combination therapy, reducing of concentration without reducing the effect may be feasible. It overcomes side effects of chemotherapeutic agent. Brazilin and brazilein are potential to be developed as a co-chemotherapeutic agent with cisplatin for treating colon cancer cells. However, further investigation need to be done to elucidate the molecular mechanism of brazilin, brazilein and its combination with cisplatin in inducing apoptosis and modulating S-phase and G2/M arrest on WiDr colon cancer cells.
Conclusion
Based on these results, we propose that brazilin and brazilein work synergistically with cisplatin in inhibiting colon cancer WiDr cells growth through a different target in cell cycle modulation and apoptosis induction.
Acknowledgments
This work was supported by the grant of Penelitian Unggulan Perguruan Tinggi (PUPT) 2015 from Indonesian Ministry of Research and Technology and High Education.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Sarasqueta AF, Forte G, Corver WE, de Miranda NF, Ruano D, van Eijk R. et al. Integral analysis of p53 and its value as prognostic factor in sporadic colon cancer. BMC Cancer. 2013;13:277. doi: 10.1186/1471-2407-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359–70. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller PA, Vousden KH. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell. 2014;25(3):304–17. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S. et al. Mutant p53 prolongs nf-kappab activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23(5):634–46. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM. et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (mrc clasicc trial): Multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–26. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 6.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64(3):706–21. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadhikar MA, Sciuto MR, Alves MV, Pickering CR, Osman AA, Neskey DM. et al. Chk1/2 inhibition overcomes the cisplatin resistance of head and neck cancer cells secondary to the loss of functional p53. Mol Cancer Ther. 2013;12(9):1860–73. doi: 10.1158/1535-7163.MCT-13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu AL, Shu SH, Qin HL, Lee SM, Wang YT, Du GH. In vitro anti-influenza viral activities of constituents from caesalpinia sappan. Planta Med. 2009;75(4):337–9. doi: 10.1055/s-0028-1112208. [DOI] [PubMed] [Google Scholar]
- 9.Nirmal NP, Rajput MS, Prasad RG, Ahmad M. Brazilin from caesalpinia sappan heartwood and its pharmacological activities: A review. Asian Pac J Trop Med. 2015;8(6):421–30. doi: 10.1016/j.apjtm.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Cuong TD, Hung TM, Kim JC, Kim EH, Woo MH, Choi JS. et al. Phenolic compounds from caesalpinia sappan heartwood and their anti-inflammatory activity. J Nat Prod. 2012;75(12):2069–75. doi: 10.1021/np3003673. [DOI] [PubMed] [Google Scholar]
- 11.Liang CH, Chan LP, Chou TH, Chiang FY, Yen CM, Chen PJ. et al. Brazilein from caesalpinia sappan l. Antioxidant inhibits adipocyte differentiation and induces apoptosis through caspase-3 activity and anthelmintic activities against hymenolepis nana and anisakis simplex. Evid Based Complement Alternat Med. 2013;2013:864892. doi: 10.1155/2013/864892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DY, Lee MK, Kim GS, Noh HJ, Lee MH. Brazilin inhibits growth and induces apoptosis in human glioblastoma cells. Molecules. 2013;18(2):2449–57. doi: 10.3390/molecules18022449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao LY, Li JY, Zhang JY. Brazilein, a compound isolated from caesalpinia sappan linn., induced growth inhibition in breast cancer cells via involvement of gsk-3beta/beta-catenin/cyclin d1 pathway. Chem Biol Interact. 2013;206(1):1–5. doi: 10.1016/j.cbi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Kim B, Kim SH, Jeong SJ, Sohn EJ, Jung JH, Lee MH. et al. Brazilin induces apoptosis and g2/m arrest via inactivation of histone deacetylase in multiple myeloma u266 cells. J Agric Food Chem. 2012;60(39):9882–9. doi: 10.1021/jf302527p. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh CY, Tsai PC, Chu CL, Chang FR, Chang LS, Wu YC. et al. Brazilein suppresses migration and invasion of mda-mb-231 breast cancer cells. Chem Biol Interact. 2013;204(2):105–15. doi: 10.1016/j.cbi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med. 2005;110:173–83. doi: 10.1385/1-59259-869-2:173. [DOI] [PubMed] [Google Scholar]
- 17.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M. et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park C, Kim GY, Kim GD, Choi BT, Park YM, Choi YH. Induction of g2/m arrest and inhibition of cyclooxygenase-2 activity by curcumin in human bladder cancer t24 cells. Oncol Rep. 2006;15(5):1225–31. [PubMed] [Google Scholar]
- 20.Yuan Z, Guo W, Yang J, Li L, Wang M, Lei Y. et al. Pnas-4, an early DNA damage response gene, induces s phase arrest and apoptosis by activating checkpoint kinases in lung cancer cells. J Biol Chem. 2015;290(24):14927–44. doi: 10.1074/jbc.M115.658419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, Perez RE, Davaadelger B, Maki CG. Two 4N Cell-Cycle Arrests Contribute to Cisplatin-Resistance. PLoS ONE. 2013;8(4):e59848. doi: 10.1371/journal.pone.0059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebillard A, Jouan-Lanhouet S, Jouan E, Legembre P, Pizon M, Sergent O. et al. Cisplatin-induced apoptosis involves a fas-rock-ezrin-dependent actin remodelling in human colon cancer cells. Eur J Cancer. 2010;46(8):1445–55. doi: 10.1016/j.ejca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Huang K, Zhang J, O'Neill KL, Gurumurthy CB, Quadros RM, Tu Y. et al. Cleavage by caspase 8 and mitochondrial membrane association activate the bh3-only protein bid during trail-induced apoptosis. J Biol Chem. 2016;291(22):11843–51. doi: 10.1074/jbc.M115.711051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3(1):1351–71. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong X, Wu B, Pan YJ, Zheng S. Brazilein inhibits survivin protein and mrna expression and induces apoptosis in hepatocellular carcinoma hepg2 cells. Neoplasma. 2009;56(5):387–92. doi: 10.4149/neo_2009_05_387. [DOI] [PubMed] [Google Scholar]
- 26.Zubair A, Frieri M. Role of nuclear factor-ĸB in breast and colorectal cancer. Curr Allergy Asthma Rep. 2013;13(1):44–9. doi: 10.1007/s11882-012-0300-5. [DOI] [PubMed] [Google Scholar]
- 27.Miyake N, Chikumi H, Takata M, Nakamoto M, Igishi T, Shimizu E. Rapamycin induces p53-independent apoptosis through the mitochondrial pathway in non-small cell lung cancer cells. Oncol Rep. 2012;28(3):848–54. doi: 10.3892/or.2012.1855. [DOI] [PubMed] [Google Scholar]
- 28.Rathore R, McCallum JE, Varghese E, Florea AM, Busselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (iaps) Apoptosis. 2017;22(7):898–919. doi: 10.1007/s10495-017-1375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eldar-Boock A, Polyak D, Scomparin A, Satchi-Fainaro R. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr Opin Biotechnol. 2013;24(4):682–9. doi: 10.1016/j.copbio.2013.04.014. [DOI] [PubMed] [Google Scholar]