Abstract
Background
The purpose of the present investigation was to evaluate whether pre-treatment neural activation in response to rewards is a predictor of clinical response to Behavioral Activation Therapy for Depression (BATD), an empirically validated psychotherapy that decreases depressive symptoms by increasing engagement with rewarding stimuli and reducing avoidance behaviors.
Methods
Participants were 33 outpatients with major depressive disorder (MDD) and 20 matched controls. We examined group differences in activation, and the capacity to sustain activation, across task runs using functional magnetic resonance imaging (fMRI) and the monetary incentive delay (MID) task. Hierarchical linear modeling was used to investigate whether pre-treatment neural responses predicted change in depressive symptoms over the course of BATD treatment.
Result
MDD and Control groups differed in sustained activation during reward outcomes in the right nucleus accumbens, such that the MDD group experienced a significant decrease in activation in this region from the first to second task run relative to controls. Pretreatment anhedonia severity and pretreatment task-related reaction times were predictive of response to treatment. Furthermore, sustained activation in the anterior cingulate cortex during reward outcomes predicted response to psychotherapy; patients with greater sustained activation in this region were more responsive to BATD treatment.
Limitation
The current study only included a single treatment condition, thus it unknown whether these predictors of treatment response are specific to BATD or psychotherapy in general.
Conclusion
Findings add to the growing body of literature suggesting that the capacity to sustain neural responses to rewards may be a critical endophenotype of MDD.
Keywords: Major depressive disorder, Reward, Anhedonia, Functional magnetic resonance imaging, Nucleus accumbens, Psychotherapy
1. Introduction
A defining symptom of MDD is anhedonia, the loss of interest or pleasure in previously rewarding activities (American Psychiatric Association, 2013). Major depressive disorder is characterized by decreased responsiveness to rewarding stimuli, including decreased anticipation of forthcoming rewards, reduced pleasure derived from reward presentation, and impaired reward-based learning (Admon and Pizzagalli, 2015; Der-Avakian and Markou, 2012). Anhedonia may be more universally endorsed than other MDD symptoms (Hamilton, 1989) and is associated with risk for future depressive episodes (Wardenaar et al., 2012), a more chronic illness course (Moos and Cronkite, 1999; Spijker et al., 2001), and poorer treatment response to both pharmacologic (McMakin et al., 2012) and neurostimulation (Downar et al., 2014) interventions.
Functional neuroimaging studies have revealed that anhedonia is characterized by decreased responsiveness of mesocorticolimbic reward processing brain circuitry, including the dorsal and ventral striatum, and ventral lateral and midline prefrontal cortical areas (Dichter et al., 2012a; Stein, 2008; Zhang et al., 2013). This general pattern has been found in adolescent (Forbes et al., 2009; Gabbay et al., 2013) and adult populations (Epstein et al., 2006; Pizzagalli et al., 2009; Smoski et al., 2009) as well as in unipolar and bipolar presentations of MDD (Redlich et al., 2015), and is evident in remitted patients with a history of MDD (Dichter et al., 2012b; Schiller et al., 2013).
Altered functioning of the anterior cingulate cortex (ACC) in particular, which plays a central role in detecting the salience of external stimuli and in reward feedback monitoring (Seeley et al., 2007; Whitton et al., 2016), has been observed in patients with MDD during reward processing tasks (Diener et al., 2012; Knutson et al., 2008; Ubl et al., 2015; Yang et al., 2016). There is evidence of decreased functional connectivity between the ACC and the middle frontal gyrus (Wu et al. (2016), the caudate (Admon et al., 2015), and dorsolateral and ventrolateral prefrontal cortices (Alexopoulos et al., 2013) in MDD. Further, a meta-analysis by Fu et al. (2013) found that increased pretreatment ACC activation was associated with response to a range of pharmacologic and cognitive interventions for MDD, highlighting the relevance of ACC functioning in MDD to understanding not only MDD pathophysiology but also to developing predictive models of antidepressant treatment response.
Given the centrality of anhedonia and reward processing deficits to MDD, responses to rewards may be promising endophenotypes to understand not only the pathophysiology of MDD, but also biomarkers of response to antidepressant treatments (Dichter et al., 2009; Lammers et al., 2000; Vrieze et al., 2013). Therefore, the purpose of this study was to investigate whether pretreatment neural responses to rewards are predictive of response to Behavioral Activation Treatment for Depression (BATD) psychotherapy using functional magnetic resonance imaging (fMRI). This intervention was originally developed to ameliorate symptoms of MDD by promoting interactions with potentially positive reinforcers and inhibiting avoidance behaviors as well as supporting sustained interaction with potentially rewarding activities (Hopko et al., 2003; Jacobson et al., 2001).
When considering the literature addressing reward processing in MDD, it is important to note that not all neuroimaging studies have consistently reported decreased neural response to rewards in MDD (Harvey et al., 2007; Knutson et al., 2008; Mitterschiffthaler et al., 2003; Schaefer et al., 2006). One recent conceptualization of hedonic capacity in MDD that potentially addresses such inconsistencies is that MDD may be characterized by decreased capacity to sustain response to rewards over time (Pizzagalli et al., 2008). In support of this framework, a recent emotion regulation study reported that participants with MDD demonstrated decreased capacity to sustain nucleus accumbens (NAcc) activity during conscious upregulation of positive emotions across the scan session (Heller et al., 2009). Furthermore, the degree of decrease in NAcc activity predicted the magnitude of self-reported positive affect in the MDD sample. In a follow-up study, Heller et al. (2013) reported that the magnitude of change in positive affect following two months of treatment with fluoxetine or venlafaxine was associated with sustained activation of the NAcc during upregulation of positive emotions.
Given that the capacity to sustain response to rewards may be a critical endophenotype of MDD, the present investigation examined whether overall neural activation, as well as the capacity to sustain neural activation in response to rewards predicted clinical response to BATD. We used the monetary incentive delay task (MID) because this reward task reliably elicits mesocorticolimbic activation and allows for dissociation of responses during reward anticipation and outcomes (Keedwell et al., 2005; Mitterschiffthaler et al., 2003; Pizzagalli et al., 2005). By presenting two runs of the MID task, we were able to evaluate changes in neural activation from the first task run to the second task run as a potential predictor of response to BATD. Because previous investigations have shown linkages between anhedonia in MDD and decreased activation of the striatum (e.g., Pizzagalli et al., 2009; Stoy et al., 2012), we predicted that the capacity to sustain striatal activation would predict the magnitude of clinical response to BATD, with a particular emphasis on declines in symptoms of anhedonia. We are reporting results of connectivity analyses from this sample separately (Walsh et al. submitted for publication), and thus here we focus on analyses of task-based activation as a predictor of treatment response.
2. Materials and methods
2.1. Overview
The study protocol was approved by the Institutional Review Boards at Duke University Medical Center and the University of North Carolina at Chapel Hill, and all enrolled participants provided written informed consent. Participants with MDD were recruited via the Cognitive Behavioral Research and Treatment Program at Duke University Medical Center and nondepressed control participants were recruited via listservs at Duke University and UNC-Chapel Hill. Potential participants completed an initial brief phone screen, and those who passed the phone screen were clinically evaluated, including administration of the structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID; First et al., 2002) to assess for Axis I disorders, and completed the Hamilton Rating Scale for Depression (HAMD; Hamilton, 1960) and Beck Depression Inventory-II (BDI; Beck et al., 1996). If still eligible, they were invited to participate in the MRI scan session. Participants with MDD then began psychotherapy. HAMD scores were used to verify inclusion criteria, but only BDI scores are used in analyses. After their fMRI scans, MDD outpatients received an average of 11.67 (SD = 4.40; range: 2–15) weekly sessions of Brief Behavioral Activation Treatment for Depression (BATD). Up to 15 sessions of BATD were offered. Early responders were given the option to end therapy after eight sessions and non-responders received the maximum number of sessions before being referred to the community for additional treatment.
2.2. Participants
Participants in the MDD group met DSM-IV criteria for a current episode of MDD and scored 15 or above on the HAMD. Participants in the control group scored six or lower on the HAMD and did not meet criteria for a current Axis I disorder or lifetime episode of a mood disorder. Exclusion criteria included: 1) In the MDD group: current mood, anxiety, psychotic, or substance abuse disorder beyond unipolar MDD or dysthymia, 2) history of psychosis or mania; 3) active suicidal ideation, 4) evidence of organicity, 5) magnetic resonance imaging contraindication (e.g., metal in body), 7) history of neurological injury or disease, and 8) current pregnancy.
Participants were paid for participating in the clinical assessment and neuroimaging sessions. Thirty-eight outpatients with MDD (11 male; mean (SD) age = 33 (7.1)) and twenty matched controls (6 male; mean (SD) age = 31 (8.8)) enrolled in the study. Two MDD participants did not return for psychotherapy after the first imaging session and were therefore excluded from all analyses since the objective of this study was to predict treatment response. Additionally, three MDD subjects taking psychoactive medications were excluded from analyses. Thus, the final sample was 33 outpatients with MDD and 20 non-depressed control participants. Groups did not differ in age, estimated IQ (measured by the North American Adult Reading Test (Blair and Spreen, 1989; NAART), or gender distribution, p’s >.32 (see Table 1 for participant characteristics).
Table 1.
Participant characteristics.
| MDD N = 33 | Con N = 20 | ||||
|---|---|---|---|---|---|
|
|
|
|
|||
| Mean (SD) | Range | Mean (SD) | Range | p | |
| Sex (M/F) | 11/22 | 6/14 | .803 | ||
| Age (yrs) | 33.2 (6.5) | 21–45 | 31.1 (8.82) | 20–44 | .315 |
| NAART | 111.28 (4.83) | 99.2–117.3 | 112.03 (3.83) | 102.6–118.1 | .558 |
| Pre-treatment BDI | 25.27 (8.52) | 9–44 | 1.1 (1.65) | 0–5 | <.0001 |
| Previous Major Depressive Episodes | 2.97 (1.79) | 1–7 | 0 | 0 | _ |
| Duration Current Episode (months) | 36.03 (77.82) | 1–384 | _ | _ | _ |
2.3. Monetary incentive delay (MID) fMRI task
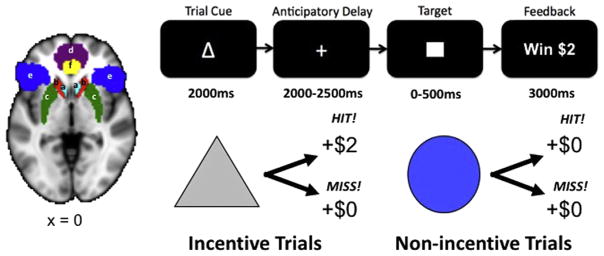
Participants practiced the fMRI task outside the scanner prior to the scan session. During this practice session, participant-specific average reaction times were recorded and used to adjust target reaction times during the scan sessions. Each trial consisted of: (1) a 2000 ms cue that indicated whether a fast enough response (a “hit”) to the forthcoming target bulls-eye could result in a “reward” (a triangle) or “no reward” (a circle); (2) a delay period during which a crosshair was presented for 2000–2500 ms; (3) a target bulls-eye that required a speeded button press presented for up to 500 ms; (4) 3000 ms of feedback that indicated whether that trial resulted in a “reward” or not; and (5) a variable length ITI crosshair presented such that the total duration of each trial was 12 s. Trial types (i.e., potential reward or not) were aperiodic and pseudorandomly ordered (Knutson et al., 2000). Participants could win $2 per trial, and feedback displayed the amount of money won on a given trial (e.g., “+$2”). Coincident with this feedback, a cumulative count of the number of dollars won within the run was presented. Participants were instructed to respond to all target bulls-eyes as quickly as possible, and outcomes were contingent on reaction times. The task was adaptive such that participants were successful on approximately two-thirds of trials, regardless of individual differences in reaction times. Each 8-min run contained 40 trials: 20 were potential reward trials, 20 were non-reward trials. The top of Fig. 1 illustrates the MID task condition.
Fig. 1.
Left: ROIs from Harvard-Oxford subcortical and cortical structural probabilistic atlases. a) Nucleus accumbens: light blue; b) caudate: red; c) putamen: green; d) frontal medial cortex: purple; e) orbitofrontal cortex: dark blue; f) anterior cingulate cortex: yellow. Right: The monetary incentive delay (MID) task presents a cue indicating whether money can be won, followed by an anticipatory phase, then a target, and feedback indicating whether or not money was won. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Each participant completed two functional imaging runs and reaction times were recorded as a behavioral index of motivation. Stimuli were presented using E-Prime presentation software v.1.1 (Psychology Software Tools Inc. Pittsburgh, PA) and displayed in the scanner through magnet-compatible goggles (Resonance Technology, Inc., Northridge, CA).
2.4. Brief Behavioral Activation Treatment for Depression (BATD)
As described previously, behavioral activation treatments have gained increasing interest since Jacobson et al. (1996) critical study of cognitive behavioral therapy in which behavioral activation proved equally effective as cognitive therapy in relieving symptoms of depression. At follow-up, behavioral activation appeared as effective as cognitive therapy in preventing relapse (Gortner et al., 1998), and a subsequent large-scale randomized trial found that behavioral activation psychotherapy was equivalent to paroxetine in reducing symptoms in moderately to severely depressed individuals (Dimidjian et al., 2006). In parallel, Lejuez and Hopko developed Brief Behavioral Activation Treatment for Depression (BATD) (Lejuez et al., 2001). Although similar to previous behavioral activation approaches, BATD is unique in that it is shorter than traditional treatments (only 8–15 sessions) and does not require as extensive skills on the part of the therapist or the patient (Hopko et al., 2003). Treatment proceeds through a series of structured units that a) educate subjects about MDD and provide a rationale for the treatment approach; b) assess and monitor baseline activity levels; c) develop individualized goals according to subjects’ values and initiate a multi-layered plan to achieve these goals; and d) monitor, support, and encourage accomplishing behavioral goals. BATD effectively reduces MDD symptoms and is well-tolerated in both outpatient (Hopko et al., 2005; Lejuez et al., 2001) and inpatient (Hopko et al., 2003) settings.
2.5. Treatment outcome measures
Treatment outcomes in the MDD group were evaluated by the Beck Depression Inventory-II (BDI; Beck et al., 1996), which was collected at the scan session, every two weeks during treatment, and at the last psychotherapy session. BDI scores of 0–13 indicate minimal MDD severity, 14–19 indicates mild MDD severity, 20–28 indicates moderate MDD severity, and 29–63 indicates severe MDD severity (Beck et al., 1996). The BDI provides an overall measure of MDD severity and includes items that tap multiple MDD symptom dimensions. We examined BDI total scores as well as BDI anhedonia subscale scores, derived from items 4, 12, 15, and 21 (Joiner et al., 2003).
2.6. Imaging methods
Functional images were acquired at the Duke-UNC Brain Imaging and Analysis Center (BIAC) on a General Electric (Waukesha, WI, USA) MR750 3.0 T scanner equipped with 50 mT/m gradients (200 T/m/s slew rate) and an 8-channel head coil for parallel imaging. High resolution T1-weighted anatomical images were acquired with 162 axial slices using a FSPGR pulse sequence (TR = 7.584 ms; TE = 2.936 ms; FOV = 256 mm; image matrix = 256 × 256; voxel size = 1 × 1 × 1 mm; flip angle = 12°) and used for normalization and coregistration with the functional data. This structural image was aligned in a near axial plane defined by the anterior and posterior commissures. Whole-brain functional images were acquired using a spiral-in SENSE sequence (TR = 1500 ms; TE = 30 ms; FOV = 240 mm; image matrix, 64 × 64; flip angle = 60°; voxel size, 3.75 × 3.75 × 4.0 mm; 34 axial slices) to reduce susceptibility artifacts and recover signal in orbital frontal regions (Pruessmann et al., 2001; Truong and Song, 2008). A semi-automated high-order shimming program ensured global field homogeneity.
2.7. Imaging data preprocessing
The first four volumes of each functional imaging dataset were discarded to allow for magnetic field stabilization. Data were preprocessed using FSL version 5.0.1 (Oxford Center for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, U. K.). Timing files were converted to FSL compatible format and NIFTI image data files were generated. Preprocessing was applied in the following steps: (i) brain extraction for non-brain removal (Smith et al., 2004), (ii) motion correction using MCFLIRT (Smith, 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (iv) mean-based intensity normalization of all volumes by the same factor, and (v) high-pass filtering (Jenkinson et al., 2002). Functional images of each participant were co-registered to structural images in native space, and structural images were normalized into a standard stereotaxic space (Montreal Neurological Institute) for intersubject comparison. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using an intermodal registration tool (Jenkinson et al., 2002; Smith et al., 2004). Voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (FILM; (Jenkinson and Smith, 2001)).
2.8. Regions of interest
Activation analyses used a region-of-interest (ROI) approach to target canonical reward processing regions. ROI’s were the NAcc, caudate nucleus, putamen, frontal medial cortex, orbitofrontal cortex, and anterior cingulate cortex (including both rostral and dorsal subdivisions). These ROIs were defined using the Harvard-Oxford subcortical and cortical structural probabilistic atlases. Frontal medial cortex and anterior cingulate cortex were divided into left and right hemispheric regions using a custom MATLAB script. Additionally, a striatum ROI was constructed by combining the caudate nucleus, putamen, and NAcc masks. Results of whole-brain analyses are provided as Supplementary Materials.
2.9. fMRI data analysis
For each ROI and participant, condition- and run-specific mean parameter estimates reflecting activation were calculated and extracted using the Featquery tool within FSL separately for anticipation and outcome phases of the MID task. For the anticipation phase, the contrast of interest was potential win versus non-win trials; for the outcome phase, the contrast of interest was wins versus non-wins. Parameter estimates (reflecting activation intensity) were then analyzed via Group (MDD, Control) × Run (run 1, run 2) repeated measures ANOVAs conducted for each ROI (main effects of Group derived from these models were used to evaluate whether groups differed in the overall level of activation, regardless of differences between runs). ROI’s with significant Group × Run interaction effects as well as significant decrease in activation between runs 1 and 2 were then queried to evaluate weather decreases in activation from run 1 to run 2 predicted response to BATD, measured as both BDI total scores and BDI anhedonia sub-scale scores.
2.10. Analysis of treatment outcomes
Data were analyzed in two-level hierarchical linear models, with people at level 2 and treatment weeks (i.e., assessments with the BDI) at level 1. Treatment week was utilized as a continuous time variable, and was uncentered. Study hypotheses were tested using models in which the current week’s BDI score were predicted from: (1) current treatment week, (coefficient interpreted as the simple effect of treatment on BDI scores over time), (2) brain activation during run 1 (coefficient interpreted as the impact of run 1 activation on baseline BDI scores) (3) change in brain activation from run 1 to run 2 (calculated as run 1 minus run 2; coefficient interpreted as the impact of changes in activation on baseline BDI scores), (4) the interaction of brain activation in run 1 and treatment week (coefficient interpreted as the impact of baseline brain activation on the slope of a participant’s trajectory of BDI change over time during treatment), and (5) the interaction of change in brain activation and treatment week (coefficient interpreted as the impact of changes in brain activation across runs on the slope of a participant’s trajectory of BDI change over time during treatment). Inclusion of the run 1 functional connectivity predictors gives a specific meaning to the change predictors; that is, the change predictors represent only the degree of reduction in brain activation from run 1 to run 2. Preliminary unconditional growth models indicated significant between-person differences in both baseline BDI scores and in the slope of treatment week on BDI scores. Therefore, random effects were specified for both the intercept and the slope of treatment week on BDI scores. It is this variance in the slope of treatment week on BDI scores that will be explained using baseline fMRI activation magnitudes.
3. Results
3.1. Treatment response
Treatment resulted in significant reductions in BDI total scores (pretreatment mean (SD) = 25.27 (8.52), post-treatment mean (SD) = 14.73 (9.96), p<.001) and BDI anhedonia subscale scores (pretreatment mean (SD) = 4.91 (2.26), post-treatment mean (SD) = 2.87 (2.00), p<.001).
3.2. Pretreatment anhedonia as a predictor of treatment response
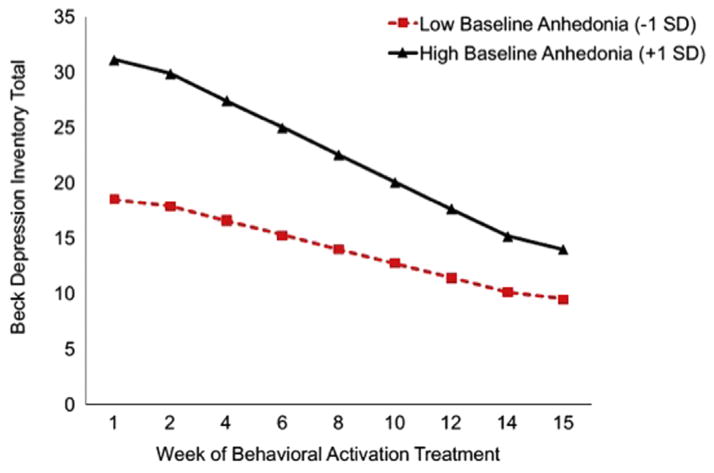
Because BATD targets motivation and reward-oriented behaviors, we examined whether severity of pre-treatment anhedonia predicted response to BATD. We observed a significant interactive effect of pre-treatment BDI anhedonia subscale scores and treatment week in predicting change in BDI total scores (γANHEDONIA*TREATMENTWEEK = −.16, SE = .035, t(173) = −4.60, p = <.0001) such that patients with higher pre-treatment BDI anhedonia subscale scores showed greater reductions in BDI total scores over time, though total BDI scores remained higher after treatment in patients with greater BDI anhedonia subscale scores. This pattern is illustrated in Fig. 2. Pseudo R2 calculations (Raudenbush and Bryk, 2002, p. 85) indicated that baseline BDI anhedonia scores accounted for 43% of the between-person differences in the effect of treatment week on BDI total scores.
Fig. 2.
Within the MDD group, higher levels anhedonia, measured by the BDI anhedonia subscale, at pretreatment predicted greater improvement in BDI total scores over the course of BATD treatment. The plot is a graphical illustration of the significant interaction between pre-treatment anhedonia and time predicting change in BDI scores from the HLM models. The lines represent the expectation for change in an individual who is one SD below the mean and one SD above the mean. Note the lines are model-based estimates and do not represent averages but rather ranges of anhedonia variability.
3.3. Pretreatment behavior as a predictor of treatment response
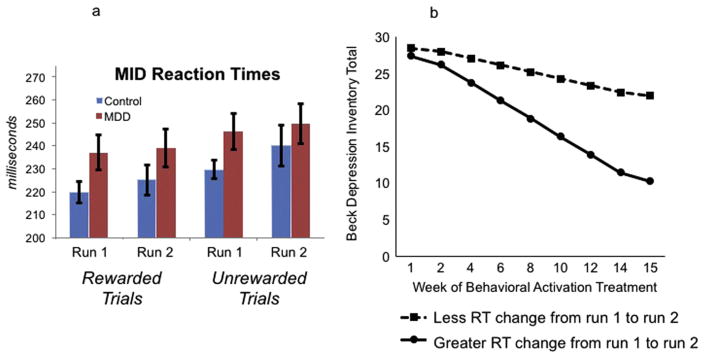
Fig. 3(a) illustrates MID reaction times from MDD and control groups separated by task run as well as trial type (reaction time data were unavailable for one MDD participant). These data were analyzed via a Group (MDD, Control) × Trial (Rewarded, Unrewarded) × Run (Run 1, Run 2) ANOVA with Group as a between subjects factor and Trial and Run as within subjects factors. The omnibus Group × Trial × Run interaction was not significant, multivariate F(1,50) = 1.66, p>.20. Additionally, the Trial × Run interaction was not significant, multivariate F(1,50) = 0.44, p>.50, the Group × Trial interaction was not significant, multivariate F (1,50) = 0.33, p>.50, and the Group × Run interaction was not significant, multivariate F(1,50) = 1.20, p>.25. There was a main effect of run, multivariate F(1,51) = 5.00, p<.03, reflecting that across groups and trial types, RTs were faster during run 1 than run 2, and a main effect of trial type, multivariate F(1,50) = 54.56 p<.0001, reflecting that across groups and runs, RTs were faster for rewarded than unrewarded trials, but no main effect of Group, F(1,50) = 1.66, p>.20. Finally, t-tests revealed that within the MDD and Control groups separately, there were no significant reaction time differences between run 1 and run 2 (for both rewarded and unrewarded trials), p’s>.40, and that Groups did not differ on reaction times to either trial type during run 1 or run 2, p’s>.05. Fig. 4.
Fig. 3.
3a: Average reaction times during the MID task, separated by group (MDD, Control), run (run 1, run 2), and trial type (rewarded, unrewarded). 3b: Within the MDD group, greater change in reaction times during reward trials (i.e., faster response at run 2) at pretreatment predicted greater reductions in BDI total scores and anhedonia scores (not shown) over the course of BATD treatment. The plot is a graphical illustration of the significant interaction between pre-treatment depression and time predicting change in BDI scores from the HLM models. The lines represent the expectation for change in an individual who is one SD below the mean and one SD above the mean. Note the lines are model-based estimates and do not represent averages but rather ranges of RT variability.
Fig. 4.
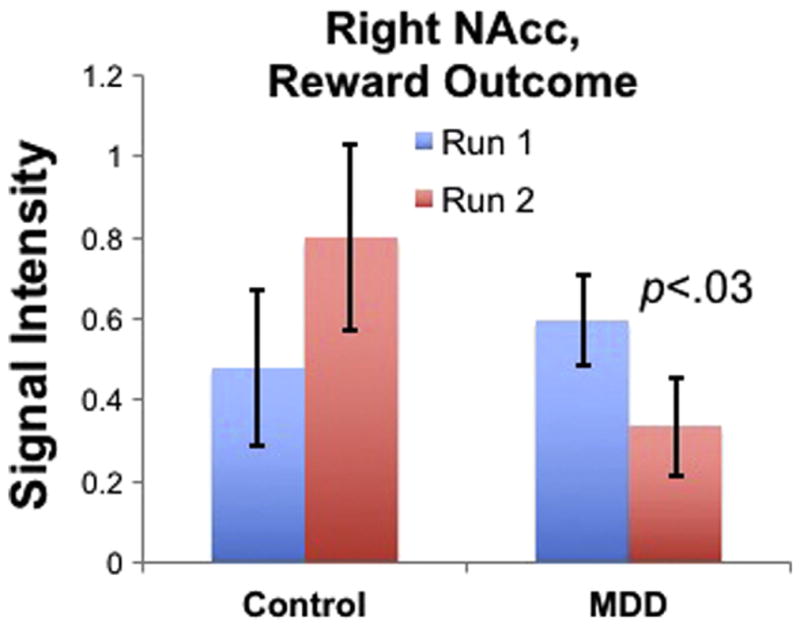
Signal intensity in the right nucleus accumbens (NAcc) during reward outcomes averaged by groups and task runs. The Group (MDD, Control) × Run (run 1, run 2) interaction term in this region was significant, p<.04, as was the effect of Run in the MDD group alone, p<.03.
Change in RTs during scanning was, however, a significant predictor of response to treatment in the MDD group, as illustrated in Fig. 3(b). Greater change in RTs from run 1 to run 2 during reward trials was associated with greater declines in BDI total and BDI anhedonia subscale scores during treatment (BDI total score: γRT CHANGE* TREATMENTWEEK = −.02, SE = .001, t(185) = −2.41, p = .02; BDI anhedonia subscale score: γRT CHANGE*TREATMENTWEEK = −.004, SE = .001, t(182) = −2.24, p = .03). That is, MDD participants who exhibited increased speed (i.e., greater decline in RT) from run 1 to run 2 during reward trials showed greater reductions in symptoms following BATD. Pseudo-R2 calculations indicated that change in RTs from run 1 to run 2 accounted for 15% of the between-person variance in the within-person effect of treatment week on BDI total scores, and 17% of the between-person variance in the within-person effect of treatment week on BDI anhedonia subscale scores.
3.4. Predicting treatment response from fMRI results
As described earlier, task-related activations were analyzed by deriving parameter estimates for each ROI that were analyzed via Group (MDD, Control) × Run (run 1, run 2) repeated measures ANOVAs separately during the anticipation and outcome phases of the MID task. Table 2 presents main effects and interactions between Group and Run as well as t-tests comparing groups and runs for each ROI. As can be seen from Table 2, during both phases of the task, there were no main effects of Run, of Group, or Group × Run interaction for any ROI. There were a number of regions that showed a reduced capacity to sustain activation (i.e., greater decreases from run 1 to run 2) in the MDD group: in the anticipation phase, this was evident in left ACC, right ACC, left putamen, left striatum, and right striatum, and in the outcome phase this was evident in the same regions as well as left NAcc, right NAcc, right caudate, right FMC, left OFC, and right putamen. However, the only region in which these declines in sustained activation were moderated by Group status was in the right NAcc during reward outcomes. We then used hierarchical linear modeling (HLM) to examine whether group differences in sustained activation of the NAcc predicted response to BATD. HLM revealed that changes in right NAcc activation from run 1 to run 2 during reward outcomes were not predictive of treatment response.
Table 2.
Group × Run MANOVAs and t-tests within groups and runs. NAcc: nucleus accumbens, ACC: anterior cingulate cortex, FMC: frontal medial cortex, OFC: orbitofrontal cortex.
| Anticipation phase | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ROI | Main Effect of Run (F (p)) | Main Effect of Group (F (p)) | Group × Run (F (p)) | Runs t-test in MDD group (t (p)) | Runs t-test in Control Group (t (p)) | Group t-test during Run 1 (t (p)) | Group t-test during Run 2 (t (p)) |
| Left NAcc | .61 (0.44) | .05 (0.82) | 1.22 (0.27) | 1.78 (0.08) | −.17 (0.86) | −.25 (0.80) | .65 (0.51) |
| Right NAcc | .05 (0.83) | .20 (0.66) | 2.34 (0.13) | 1.73 (0.09) | −.67 (0.51) | −.22 (0.82) | 1.00 (0.32) |
| Left ACC | 1.30 (0.26) | .08 (0.78) | 1.63 (0.21) | 2.07 (0.05) | −.08 (0.94) | −.89 (0.38) | .34 (0.74) |
| Right ACC | 1.51 (0.22) | .01 (0.93) | 1.40 (0.24) | 2.19 (0.04) | .03 (0.98) | −.64 (0.52) | .43 (0.67) |
| Left caudate | .17 (0.68) | .23 (0.63) | 1.38 (0.25) | 1.54 (0.13) | −.39 (0.70) | −.05 (0.96) | .93 (0.36) |
| Right caudate | .26 (0.61) | .03 (0.86) | 1.70 (0.20) | 1.77 (0.09) | −.41 (0.69) | −.39 (0.70) | .80 (0.43) |
| Left FMC | .89 (0.35) | .28 (0.60) | 1.38 (0.25) | .19 (0.85) | −1.33 (0.20) | −.43 (0.67) | 1.05 (0.30) |
| Right FMC | .73 (0.40) | .08 (0.78) | 1.88 (0.18) | .46 (0.65) | −1.26 (0.22) | −.79 (0.43) | .98 (0.34) |
| Left OFC | .52 (0.47) | .14 (0.71) | 1.35 (0.25) | 1.48 (0.15) | −.30 (0.77) | −.35 (0.73) | .83 (0.41) |
| Right OFC | .21 (0.65) | .40 (0.53) | 1.05 (0.31) | 1.34 (0.19) | −.31 (0.76) | .05 (0.96) | .96 (0.34) |
| Left putamen | 1.99 (0.16) | .41 (0.52) | 1.03 (0.32) | 2.06 (0.05) | .24 (0.82) | −1.14 (0.26) | −.05 (0.96) |
| Right putamen | 1.33 (0.25) | .16 (0.70) | 1.16 (0.29) | 1.90 (0.07) | .05 (0.96) | −.91 (0.37) | .18 (0.86) |
| Left striatum | 1.36 (0.25) | .03 (0.87) | 1.53 (0.22) | 2.18 (0.04) | −.04 (0.97) | −.73 (0.47) | .39 (0.70) |
| Right striatum | .88 (0.35) | .02 (0.89) | 1.77 (0.19) | 2.10 (0.04) | −.21 (0.84) | −.71 (0.48) | .48 (0.64) |
| Outcome phase | |||||||
|
| |||||||
| ROI | Main Effect of Run (F (p)) | Main Effect of Group (F (p)) | Group × Run (F (p)) | Runs t-test in MDD group (t (p)) | Runs t-test in Control Group (t (p)) | Group t-test during Run 1 (t (p)) | Group t-test during Run 2 (t (p)) |
|
| |||||||
| Left NAcc | .58 (0.45) | .20 (0.66) | 2.20 (0.14) | 2.00 (0.05) | −.40 (0.69) | −.37 (0.72) | 1.23 (0.22) |
| Right NAcc | .02 (0.88) | .23 (0.64) | 4.14 (0.04) | 2.24 (0.03) | −.94 (0.36) | −.72 (0.48) | 1.55 (0.13) |
| Left ACC | 6.82 (0.01) | 1.02 (0.32) | 1.10 (0.30) | 3.06 (0.005) | .95 (0.36) | −1.41 (0.17) | −.30 (0.76) |
| Right ACC | 4.26 (0.04) | .04 (0.83) | 1.25 (0.27) | 2.64 (0.01) | .58 (0.57) | −.77 (0.45) | .40 (0.69) |
| Left caudate | .02 (0.89) | .48 (0.49) | 3.05 (0.09) | 1.45 (0.16) | −1.04 (0.31) | −.32 (0.75) | 1.59 (0.12) |
| Right caudate | .81 (0.37) | .24 (0.63) | 3.78 (0.06) | 2.72 (0.01) | −.55 (0.59) | −.54 (0.59) | 1.56 (0.13) |
| Left FMC | .36 (0.55) | .33 (0.57) | 1.58 (0.21) | 1.67 (0.10) | −.37 (0.72) | −1.25 (0.22) | .47 (0.64) |
| Right FMC | .42 (0.52) | .25 (0.62) | 3.71 (0.06) | 2.33 (0.03) | −.71 (0.49) | −1.66 (0.10) | 1.01 (0.32) |
| Left OFC | 1.33 (0.25) | .02 (0.90) | 2.49 (0.12) | 2.50 (0.02) | −.23 (0.82) | −.89 (0.38) | 1.00 (0.33) |
| Right OFC | .87 (0.35) | .13 (0.72) | .93 (0.34) | 1.70 (0.10) | −.02 (0.99) | −.23 (0.82) | .82 (0.42) |
| Left putamen | 2.79 (0.10) | .74 (0.39) | 1.29 (0.26) | 2.36 (0.02) | .32 (0.75) | −1.16 (0.26) | .08 (0.93) |
| Right putamen | 3.85 (0.06) | .30 (0.59) | 1.10 (0.30) | 2.50 (0.02) | .556 (0.58) | −.94 (0.35) | .23 (0.82) |
| Left striatum | 1.36 (0.25) | .03 (0.87) | 2.52 (0.12) | 2.37 (0.02) | −.25 (0.81) | −.91 (0.37) | .88 (0.39) |
| Right striatum | 2.70 (0.11) | .00 (0.98) | 2.69 (0.11) | 2.91 (0.01) | .00 (0.99) | −.81 (0.42) | .98 (0.33) |
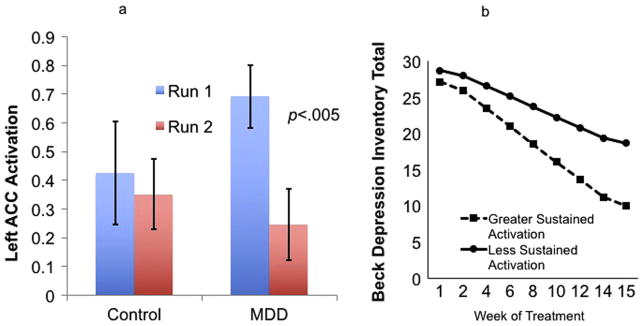
To more fully explore potential neural predictors of treatment response, we also ran HLM models predicting treatment response from overall activation (averaged across runs) and sustained activation (changes from run 1 to run 2) even if the Group × Run interaction term was not significant. These models revealed that ACC activation during reward outcomes predicted treatment response, as illustrated in Fig. 5(b). The significant model reflected that the capacity to sustain activation (i.e., less decrease) from run 1 to run 2 was associated with greater reductions in BDI total scores during treatment (γCHANGE IN ACTIVATION * TREATMENTWEEK = .46, SE = .20, t(200) = 2.38, p = .02. Pseudo-R2 calculations indicated that change in ACC activation from run 1 to run 2 accounted for 5.6% of the between-person variance in the within-person effect of treatment week on BDI total scores.
Fig. 5.
a: Signal intensity in the left ACC during reward outcomes averaged by groups and task runs. The Group (MDD, Control) × Run (run 1, run 2) interaction term in this region was not significant, but the effect of Run in the MDD group alone was significant, p<.005. b: Within the MDD group, decreased left ACC activation from run 1 to run 2 during reward outcomes predicted change in BDI total scores.
4. Discussion
The purpose of this study was to investigate whether neural responses to rewards were predictive of response to BATD, a treatment developed to ameliorate symptoms of MDD by promoting interactions with potentially positive reinforcers and inhibiting avoidance behaviors as well as supporting sustained interaction with potentially rewarding activities (Hopko et al., 2003; Jacobson et al., 2001). Specifically, we examined whether differences between MDD and control groups in the magnitude of activation in ROIs reflecting key reward processing brain regions predicted reductions in overall MDD symptoms and symptoms of anhedonia. We addressed this question by comparing groups’ magnitude of activation during the first and second runs of the fMRI task. This analytic plan was developed on the basis of prior data indicating that positive affect in MDD is characterized by decreased capacity to sustain nucleus accumbens activity during positive emotion regulation (Heller et al., 2009) and that the endurance of NAcc activation and connectivity during positive emotion regulation predicted clinical response to antidepressant treatment (Heller et al., 2013).
The clinical effectiveness of BATD in the current study was consistent with prior trials (Dichter et al., 2009; Hopko et al., 2003): average BDI scores declined 10.54 points, a clinically meaningful response (Jacobson and Truax, 1991). However, there was considerable variability in response, highlighting the need to develop predictors of treatment response to maximize the efficacy of empirically validated treatments (Kapur et al., 2012).
We found symptomatic, behavioral, and neural predictors of response to treatment. First, patients with more severe pretreatment anhedonia responded better to BATD. Because anhedonia is associated with poorer treatment response to pharmacologic (McMakin et al., 2012) and neurostimulation (Downar et al., 2014) interventions, it may be that BATD is particularly well-suited to address the anhedonic symptoms of depression through targeting approach and avoidance behaviors. Second, patients who exhibited decreased reaction times (i.e., faster responses) while making a speeded button press to receive a reward also fared better after treatment. Faster reaction to reward trials over time may reflect increased motivated responding (Pizzagalli et al., 2009), particularly when cues are provided (Mir et al., 2011). This finding suggests that BATD may also be an effective treatment for patients with greater capacity to anticipate incentives and/or relatively preserved hedonic responsiveness.
We had three central fMRI findings. First, contrary to previous reports, we found no group differences in brain activation responses using the MID task. This stands in contrast to previous reports of difference in MDD using the same task (Knutson et al., 2008; Pizzagalli et al., 2009). Although the precise reasons for these disparities are not clear, it may be that our region of interest analytic approach is one source of this difference. However, we did detect group differences in task responses when we considered differences in activation magnitudes between runs 1 and 2 of the task. Specifically, the MDD group showed decreased capacity to sustain activation in the right NAcc during reward outcomes relative to controls, highlighting the potential importance of hedonic endurance to understanding neural endophenotypes related to reward processing in MDD. The finding of altered NAcc activation during the outcome phase, but not the anticipation phase, is consistent with previous studies showing more robust effects in the caudate and NAcc during reward outcomes than reward anticipation (Pizzagalli et al., 2009) or reward predictions (Elliott et al., 2000; Ernst et al., 2005).
Finally, exploratory analyses revealed that sustained activation of the ACC (i.e., less change from run 1 to run 2) predicted better response to BATD, as evidenced by greater declines in total symptoms of depression. This finding is consistent with a prior report that MDD is characterized by reduced capacity to maintain frontostriatal activation and connectivity in the context of an emotion regulation task (Heller et al., 2009). The frontal medial cortex, including aspects of the ACC, is critically involved in controlling social approach–avoidance behaviors (Challis and Berton, 2015). Additionally, frontal medial regions of the prefrontal cortex are key components of neural circuits involved in detecting the motivational significance of external stimuli (Phan et al., 2005). Capacity to sustain ACC activation in the context of a reward may be a key predictor of response to a psychotherapy modality that specifically targets responding to motivationally salient aspects of the environment.
The current study only included a single treatment condition, and it will be important in future research to evaluate the capacity for neural responses to rewards to predict differential clinical responses to different antidepressant treatment modalities. It will also be important to examine post-treatment neuroimaging to evaluate whether the brain regions that are predictive of treatment response are those that show recovery of functioning after treatment. Despite these limitations, the current study found symptomatic, behavioral, and neural predictors of response to BATD. Critically, the only neural predictor to emerge as significant was from a model that evaluated changes in neural activations from the first half to the second half of the reward task. This finding extends the emerging framework of MDD that emphasizes capacity to sustain neural responses to hedonic stimuli in the pathophysiology of the disorder (Heller et al., 2013; Heller et al., 2009) to the domain of treatment prediction. More generally, combined with our prior examination of resting state predictors of BATD response (Crowther et al., 2015), and emerging evidence addressing neural predictors of response to other antidepressant treatment modalities (see Dichter et al., 2014 for a review), these findings contribute to the growing body of literature addressing pre-treatment neuroimaging endophenotypes as predictors of antidepressant treatment response (McGrath et al., 2013). Given this suboptimal response rate to available MDD treatments, the identification of methods to match specific patients with the most appropriate, personalized treatment option is an important way to help relieve the societal burden of MDD (Kapur et al., 2012).
Supplementary Material
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jad.2016.06.005.
References
- Admon R, Nickerson LD, Dillon DG, Holmes AJ, Bogdan R, Kumar P, Pizzagalli DA. Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol Med. 2015;45(1):121–131. doi: 10.1017/S0033291714001123. http://dx.doi.org/10.1017/S0033291714001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr Opin Psychol. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. http://dx.doi.org/10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, Gunning FM. Functional connectivity in apathy of late-life depression: a preliminary study. J Affect Disord. 2013;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023. http://dx.doi.org/10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V. 4. Washington, DC: 2013. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. http://dx.doi.org/10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- Challis C, Berton O. Top-down control of serotonin systems by the pre-frontal cortex: a path toward restored socioemotional function in depression. ACS Chem Neurosci. 2015 doi: 10.1021/acschemneuro.5b00007. http://dx.doi.org/10.1021/acschemneuro.5b00007. [DOI] [PMC free article] [PubMed]
- Crowther A, Smoski MJ, Minkel J, Moore T, Gibbs D, Petty C, Dichter GS. Resting-State Connectivity Predictors of Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.12. http://dx.doi.org/10.1038/npp.2015.12. [DOI] [PMC free article] [PubMed]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012a;4(1):19. doi: 10.1186/1866-1955-4-19. http://dx.doi.org/10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatr. 2009;66(9):886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2014;172C:8–17. doi: 10.1016/j.jad.2014.09.028. http://dx.doi.org/10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012b;136(3):1126–1134. doi: 10.1016/j.jad.2011.09.048. http://dx.doi.org/10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61(3):677–685. doi: 10.1016/j.neuroimage.2012.04.005. http://dx.doi.org/10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, Giacobbe P. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76(3):176–185. doi: 10.1016/j.biopsych.2013.10.026. http://dx.doi.org/10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20(16):6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163(10):1784–1790. doi: 10.1176/ajp.2006.163.10.1784. http://dx.doi.org/10.1176/appi.ajp.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. http://dx.doi.org/10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institut; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. http://dx.doi.org/10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. http://dx.doi.org/10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52(6):628–641. doi: 10.1016/j.jaac.2013.04.003. http://dx.doi.org/10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortner ET, Gollan JK, Dobson KS, Jacobson NS. Cognitive-behavioral treatment for depression: relapse prevention. J Consult Clin Psychol. 1998;66(2):377–384. doi: 10.1037//0022-006x.66.2.377. [DOI] [PubMed] [Google Scholar]
- Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MA. Frequency of symptoms in melancholia (depressive illness) Br J Psychiatry. 1989;154:201–206. doi: 10.1192/bjp.154.2.201. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12(8):767–775. doi: 10.1038/sj.mp.4002021. http://dx.doi.org/10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from anti-depressant treatment. Am J Psychiatry. 2013;170(2):197–206. doi: 10.1176/appi.ajp.2012.12010014. http://dx.doi.org/10.1176/appi.ajp.2012.12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. http://dx.doi.org/10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko DR, Bell JL, Armento MEA, Hunt MK, Lejuez CW. Behavior therapy for depressed cancer patients in primary care. Psychotherapy: Theory, Res, Pract, Train. 2005;42:236–243. [Google Scholar]
- Hopko DR, Lejuez CW, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev. 2003;23:699–717. doi: 10.1016/s0272-7358(03)00070-9. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, Prince SE. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64(2):295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: returning to contextual roots. Clin Psychology: Sci Pract. 2001;8:255–270. [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res. 2003;119(3):243–250. doi: 10.1016/s0165-1781(03)00131-8. http://dx.doi.org/10.1016/S0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105. http://dx.doi.org/10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. http://dx.doi.org/10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. http://dx.doi.org/10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. http://dx.doi.org/10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Lammers CH, Diaz J, Schwartz JC, Sokoloff P. Dopamine D3 receptor gene expression in the shell of nucleus accumbens is increased by chronic anti-depressant treatment. Mol Psychiatry. 2000;5(3):229. doi: 10.1038/sj.mp.4000756. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, Hopko SD. A brief behavioral activation treatment for depression. Treatment manual Behav Modif. 2001;25(2):255–286. doi: 10.1177/0145445501252005. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Mayberg HS. Toward a neuroimaging treatment selection bio-marker for major depressive disorder. JAMA Psychiatry. 2013;70(8):821–829. doi: 10.1001/jamapsychiatry.2013.143. http://dx.doi.org/10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Brent DA. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51(4):404–411. doi: 10.1016/j.jaac.2012.01.011. http://dx.doi.org/10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir P, Trender-Gerhard I, Edwards MJ, Schneider SA, Bhatia KP, Jahanshahi M. Motivation and movement: the effect of monetary incentive on performance speed. Exp Brain Res. 2011;209(4):551–559. doi: 10.1007/s00221-011-2583-5. http://dx.doi.org/10.1007/s00221-011-2583-5. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Sharma T. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14(2):177–182. doi: 10.1097/00001756-200302100-00003. http://dx.doi.org/10.1097/01.wnr.0000053758.76853.cc. [DOI] [PubMed] [Google Scholar]
- Moos RH, Cronkite RC. Symptom-based predictors of a 10-year chronic course of treated depression. J Nerv Ment Dis. 1999;187(6):360–368. doi: 10.1097/00005053-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. http://dx.doi.org/10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. http://dx.doi.org/10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. http://dx.doi.org/10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. http://dx.doi.org/10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46(4):638–651. doi: 10.1002/mrm.1241. http://dx.doi.org/10.1002/mrm.1241 (pii) [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- Redlich R, Dohm K, Grotegerd D, Opel N, Zwitserlood P, Heindel W, Dannlowski U. Reward processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.110. http://dx.doi.org/10.1038/npp.2015.110. [DOI] [PMC free article] [PubMed]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry. 2006;60(9):974–986. doi: 10.1016/j.biopsych.2006.03.024. http://dx.doi.org/10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Minkel J, Smoski MJ, Dichter GS. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord. 2013;151(2):756–762. doi: 10.1016/j.jad.2013.06.016. http://dx.doi.org/10.1016/j.jad.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. http://dx.doi.org/10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. http://dx.doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. http://dx.doi.org/10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103(2):122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr. 2008;13(7):561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C, Suchotzki K, Strohle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26(5):677–688. doi: 10.1177/0269881111416686. http://dx.doi.org/10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Truong TK, Song AW. Single-shot dual-z-shimmed sensitivity-encoded spiral-in/out imaging for functional MRI with reduced susceptibility artifacts. Magn Reson Med. 2008;59(1):221–227. doi: 10.1002/mrm.21473. http://dx.doi.org/10.1002/mrm.21473. [DOI] [PubMed] [Google Scholar]
- Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, Flor H. Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci. 2015;10(8):1102–1112. doi: 10.1093/scan/nsu158. http://dx.doi.org/10.1093/scan/nsu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73(7):639–645. doi: 10.1016/j.biopsych.2012.10.014. http://dx.doi.org/10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Dichter GS. Frontostriatal connectivity during reward processing predicts response to psychotherapy in major depressive disorder. doi: 10.1038/npp.2016.179. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardenaar KJ, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Symptom dimensions as predictors of the two-year course of depressive and anxiety disorders. J Affect Disord. 2012;136(3):1198–1203. doi: 10.1016/j.jad.2011.11.037. http://dx.doi.org/10.1016/j.jad.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Kakani P, Foti D, Veer AV, Haile A, Crowley DJ, Pizzagalli DA. Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(1):87–95. doi: 10.1016/j.bpsc.2015.09.007. http://dx.doi.org/10.1016/j.bpsc.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Lin P, Yang J, Song H, Yang R, Yang J. Dysfunction of the cingulo-opercular network in first-episode medication-naive patients with major depressive disorder. J Affect Disord. 2016;200:275–283. doi: 10.1016/j.jad.2016.04.046. http://dx.doi.org/10.1016/j.jad.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhong N, Imamura K, Lu S, Li M, Zhou H, Li K. Task and resting-state fMRI reveal altered salience responses to positive stimuli in patients with major depressive disorder. PLoS One. 2016;11(5):e0155092. doi: 10.1371/journal.pone.0155092. http://dx.doi.org/10.1371/journal.pone.0155092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151(2):531–539. doi: 10.1016/j.jad.2013.06.039. http://dx.doi.org/10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.