Abstract
Objective
Prolonged air leak increases cost and worsens outcomes after pulmonary resection. We aimed to develop a clinical prediction tool for prolonged air leak using pretreatment and intraoperative variables.
Methods
Patients who underwent pulmonary resection for lung cancer/nodules (1/2009-6/2014) were stratified by prolonged parenchymal air leak (>5 days). Using backward stepwise logistic regression with bootstrap resampling for internal validation, candidate variables were identified and a nomogram risk calculator developed.
Results
A total of 2317 patients underwent pulmonary resection for lung cancer/nodules. Prolonged air leak (8.6%, n=200) was associated with significantly longer hospital stay (median 10 versus 4 days; p<0.001). Final model variables associated with increased risk included low percent forced expiratory volume in 1 second, smoking history, bilobectomy, higher annual surgeon caseload, prior chest surgery, Zubrod score>2, and interaction terms for right-sided thoracotomy and wedge-resection by thoracotomy. Wedge resection, higher body mass index, and unmeasured percent forced expiratory volume in 1 second were protective. Derived nomogram discriminatory accuracy was 76% (95% CI 0.72–0.79) and facilitated patient stratification into low, intermediate and high risk groups with monotonic increase in observed prolonged air leaks (2.0%; 8.9 %; and 19.2%, respectively; p-value<0.001). Intermediate and high risk patients were 4.80 (95% CI 2.86–8.07) and 11.86 times (95% CI 7.21–19.52) more likely to have prolonged air leak compared to low risk patients.
Conclusions
Using readily available candidate variables, our nomogram predicts increasing risk of prolonged air leak with good discriminatory ability. Risk stratification can support surgical decision-making, and help initiate proactive, patient-specific surgical management.
Keywords: Prolonged Air Leak, Persistent Air Leak, Air Leak, Pulmonary Resection, Lung Cancer, Multivariable, Risk Factors, Risk Stratification, Funnel Plot
Introduction
When risk factors for undesirable outcomes are identified and used to formulate validated, highly accurate risk stratification algorithms, allocation of effort and resources to individuals most likely to benefit can be optimized. For thoracic surgeons, prolonged air leak (PAL) following pulmonary resection is one such undesirable outcome. Defined in the Society of Thoracic Surgeons General Thoracic Surgery Database (STSGTSD) as a parenchymal air leak lasting >5 days, PAL complicates 6–18% of lung resections.1–5 PAL is associated with increased cost,6 hospital length of stay, 4,5,7 and incidence of empyema,6,8,9 among other complications.10 Often, persistent parenchymal air leak is the only reason for ongoing hospitalization, resulting in eventual discharge with indwelling chest tubes. If patients are unprepared for this eventuality or their care facilities are unable to accommodate patients with chest tubes, additional delays in discharge may be introduced. Therefore, identification of patients at-risk for PAL could facilitate proactive, patient-specific management.
Three centers, all outside the United States, have published PAL risk stratification models in an effort to improve targeted use of surgical adjuncts.1–3 Each propose different criteria for stratifying risk based on independent risk factors for PAL, including percent forced expiratory volume in 1 second (%FEV1), body mass index (BMI), and pleural adhesions, among other variables. Study design limitations, differences in how PAL was defined, how patients were selected, and lack of external prospective validation have inhibited wide clinical application. It is also unclear whether risk factors identified in these studies are generalizable and useful for predicting risk for PAL in patients in the United States.
Our study sought to develop a clinical prediction tool for prolonged air leak after pulmonary resection using pretreatment and intraoperative variables in a large patient dataset from a single center in the United States. We hypothesized that standard, readily available predictors could be used to stratify patients into risk classes associated with increasing prolonged air leak risk.
Methods
Patient Population and Data Definitions
Data for all patients were collected at our institution using variables defined by the Society of Thoracic Surgeons General Thoracic Surgery Database (STSGTSD; http://www.sts.org/national-database). Data variables were defined using versions 2.081 and 2.2; data were abstracted by trained data collectors within 4 to 6 weeks after operation for real-time quality monitoring and national benchmarking via bi-annual data submission to the STSGTSD National Data Center. Our Institutional Review Board gave approval for use of this data for the current study.
Pulmonary resection was performed (n=2522; 1/1/2009 – 6/30/2014) at eight hospital sites for malignant and benign lung tumors or nodules using International Classification of Diseases, Ninth Revision11 diagnosis codes (197.0, 212.3, 162.2, 162.3, 162.4, 162.5, 162.9, 518.89), excluding pneumonectomy, and extended chest wall/diaphragm resections. We did not include volume reduction, bullectomy, or lung biopsy for interstitial lung disease because of the significantly higher rate of PAL and differences in underlying risk factors in these populations, which would have heavily influenced the model and reduced discriminatory accuracy for PAL in lung nodule/cancer patients. Database variables included patient demographics, preoperative evaluation, surgical procedures, cancer staging, and post-operative events. Approach to operation was captured, but granular operative details were not available (e.g. intraoperative adhesiolysis, intraoperative sealant use, pleural tents, etc.).
We excluded 59 sleeve lobectomy patients, to minimize confounding parenchymal and anastomotic air leaks; 32 patients from two hospital sites who did not submit patients to the database over the entire study time frame; and 5 patients who died day 5 or before (prolonged air leak definition). Status of air leak for patients dying day 5 or before is not available in the dataset. Multiple lung resections were noted in 111 patients; data for the most recent surgery date was utilized and prior encounter(s) excluded. (Supplemental Figure 1)
Outcomes
Prolonged air leak (PAL), the dependent variable in our model, was defined as an air leak that persisted >5 days postoperatively. We compared in-hospital mortality, length of hospital stay, and cumulative incidence of hospital discharge by post-operative day (mortality treated as a competing risk),12 between groups.
Development of the Logistic Regression Model
We compared preoperative and intraoperative variables with chi-squared test or Fisher’s exact test for categorical variables expressed as frequencies, and Student’s t-test or Wilcoxon rank sum test for continuous variables expressed as mean ± 1 standard deviation or median/interquartile range using Stata/SE 14.1. (StataCorp, 2015; College Station, TX: StataCorp LP) Unless otherwise indicated, p-values were two-tailed; statistical significance defined by p<0.05.
Bivariable testing (p-value<0.15), clinical judgment and literature review guided variable selection for the initial multivariable model. We categorized percent forced expiratory volume in 1 second (%FEV1) based on clinical relevance: <60% considered moderate to severe obstruction, >=60 and <80% mild obstruction, >=80% borderline to normal range. We placed the 12.8% of patients who did not undergo preoperative pulmonary function testing, and were therefore missing on %FEV1, in a separate category as the majority had benign or secondary metastatic lung tumors (86.5%) removed with wedge resection (88.9%) suggesting that the data were systematically missing (i.e. pulmonary function testing was not performed intentionally). We excluded diffusing capacity of carbon monoxide (DLCO) due to high level of non-random missingness. We included sex and steroids variables despite p-values>0.15, given their significance in previous work. To assess collinearity, we assessed variance inflation factors (VIF) and ensured that all were <2 (indicating little (>1<2) to no (=1) correlation between variables).
To form a parsimonious model, we employed backward stepwise variable selection with likelihood ratio test p-value stopping rules of 0.15 (to enter model) and 0.10 (to remain in model); PAL was the dependent variable. Bootstrap resampling was used to assess how well variables predict occurrence of PAL outside the original sample.8,13 We repeated the stepwise algorithm on 1000 resamples drawn randomly with replacement and equal to 100% size of the original. We considered variables selected in >50% of 1000 repetitions for the reduced model and tested plausible two-way interactions.
Development of the PAL Nomogram
Using the Stata program nomolog,14 we assigned points to predictors using proportional rescaling of the logistic equation regression coefficients. We first forced positive coefficients with negative regression coefficients by subtracting all categories of the variable by the category with the most negative coefficient. We then multiplied all coefficients by the scaling factor, F (F=10/ αi, where αi is the largest model coefficient) to convert coefficients into point values. We calculated PAL probability based on total points (1/ (1+e−(F*α0+TP)/F), where αo is the regression equation constant (adjusted accordingly from variables forced positive), and TP is the sum of points across all predictors. We derived a three-level risk table based on the nomogram point scoring system (cutoffs chosen to maximize C-statistics), and classified each patient into a risk class using their total point score (rounded to nearest 0.5). We calculated observed and predicted frequencies, odds ratios, and 95% confidence intervals for each risk class.
Model Performance
We used calibration and discrimination to assess model predictive accuracy.15 For calibration, we plotted observed and predicted rates of PAL by deciles of risk, and assessed goodness-of-fit (Hosmer-Lemeshow test). We calculated discriminatory accuracy (C-statistic) for the regression equation, nomogram, and risk table. Using a classification probability cutoff of 0.11 (the average PAL rate in five large PAL studies1–5 combined with ours), we calculated correct classification rates, and positive and negative predictive values. We used bootstrap resampling to adjust the logistic C-statistic for over-optimism or overfitting.15,16 We repeated the original stepwise multivariable selection with the addition of identified interaction effects in 500 resamples. In each repetition, we calculated the difference between the C-statistic in the resample and the original sample for the selected model. To adjust for optimism, we subtracted the average difference from the C-statistic of the final model in the original sample.
Surgical Caseload Effect
We used a funnel plot to explore the relationship between total surgeon case load and PAL rate,17 including control limits (95% and 99% confidence intervals) around the average PAL rate in our study. Because unadjusted case volume does not account for variations in patient populations between surgeons, we developed risk-adjusted models for surgeon annual case load effect, adjusting for case mix and clustering. (See Appendix)
Results
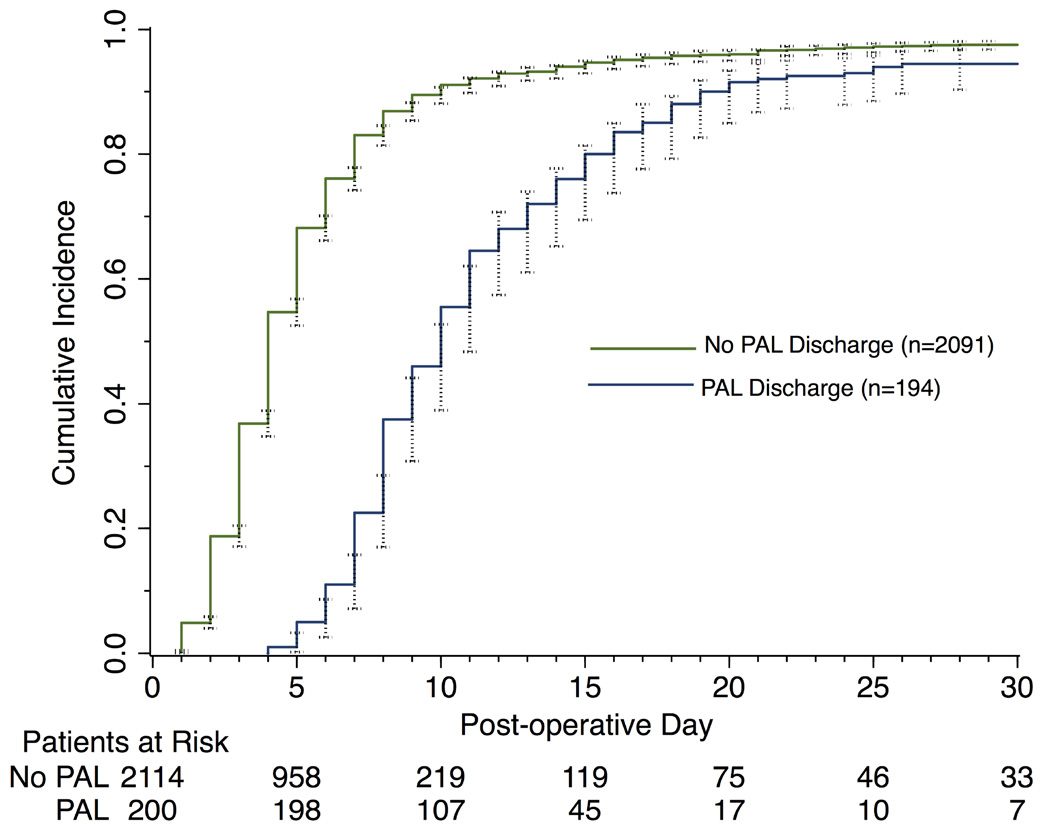
A total of 2317 patients met inclusion criteria for bivariable analysis, and 2273 patients for multivariable analysis. Incidence of prolonged air leak was 8.6% (200/2317). The majority of operations were video-assisted thoracoscopic (VATS) lobectomy/segmentectomy for primary lung cancer. (Table 1) PAL significantly prolonged median length of hospital stay (10 versus 4 days, p<0.001); by day 5 and 10, respectively, only 1% and 47% of patients with PAL had been discharged compared to 54% and 90% of patients without PAL. (Figure 1) Patients with PAL had a higher rate of in-hospital death (3.0% vs 1.1%, p=0.034; Table 1).
Table 1.
Population characteristics of patients with or without prolonged air leak
| Prolonged air leak | |||||
|---|---|---|---|---|---|
| Variables | Total n=2317 |
No n=2117 |
Yes n=200 |
p-value | |
| Demographics | |||||
| Age (mean ± SD) | 65 ± 12 | 65± 12 | 67 ± 11 | 0.013 | |
| BMI, kg/m (mean ± SD) | 28 ± 7 | 28 ± 7 | 26 ± 5 | <0.001 | |
| Sex | Female | 1296 (56) | 1193 (56) | 103 (51) | 0.186 |
| Male | 1021 (44) | 924 (44) | 97 (49) | ||
| Race | White | 2168 (94) | 1975 (93) | 193 (96) | 0.288* |
| Black | 119 (5) | 113 (5) | 6 (3) | ||
| Other | 30 (1) | 29 (1) | 1 (1) | ||
|
| |||||
| Treatment Variables | |||||
| Surgery Year | 2009/10 | 857 (37) | 777 (37) | 80 (40) | 0.615 |
| 2011/12 | 886 (38) | 815 (38) | 71 (35) | ||
| 2013/14 | 574 (25) | 525 (25) | 49 (25) | ||
| Hospital Type | Academic | 1125 (49) | 1030 (49) | 95(48) | 0.755 |
| Community | 1192 (51) | 1087 (51) | 105 (52) | ||
| Annual Surgeon Caseload, median [IQR]a | 53 [34–74] | 53[34–74] | 64 [36–74] | <0.001† | |
| Disease Category | Malignant cancer | 1441 (62) | 1290 (61) | 151 (76) | <0.001 |
| Benign/infection | 367 (16) | 346 (16) | 21 (10) | ||
| Metastatic tumor | 509 (22) | 481 (23) | 28 (14) | ||
| Procedure Type | Lobe/Segment | 1500 (65) | 1339 (63) | 161 (81) | <0.001* |
| Wedge resection | 780 (34) | 752 (36) | 28 (14) | ||
| Bilobectomy | 37 (1) | 26 (1) | 11 (5) | ||
|
| |||||
| Comorbidities | |||||
| Smoking History | Never Smoker | 684 (30) | 655 (31) | 29 (14) | <0.001 |
| Past Smoker | 1180 (50) | 1058 (50) | 122 (61) | ||
| Current Smoker | 453 (20) | 404 (19) | 49 (25) | ||
| Zubrod Score | 0 | 163 (7) | 153 (7) | 10 (5) | 0.012 |
| 1 | 1756 (76) | 1615 (77) | 141 (71) | ||
| 2–5 | 390 (17) | 342 (16) | 48 (24) | ||
| ASA Classification | I/II | 277 (12) | 263 (13) | 14 (7) | 0.077 |
| III | 1781 (77) | 1618 (76) | 163 (82) | ||
| IV | 259 (11) | 236 (11) | 23 (11) | ||
| Weight Loss | ≥2kg | 1985 (86) | 1825 (86) | 160 (80) | 0.527 |
| >2kg | 113 (5) | 102 (5) | 11 (5) | ||
| Missing | 219 (9) | 190 (9) | 29 (15) | ||
| Hypertension | 1297 (56) | 1192 (56) | 105 (53) | 0.300 | |
| Congestive Heart Failure (24% missing) | 76 (4) | 67 (4) | 9 (6) | 0.234 | |
| Peripheral Vascular Disease | 225 (10) | 199 (10) | 26 (13) | 0.101 | |
| Interstitial Fibrosis | 27 (1) | 23 (1) | 4 (2) | 0.286* | |
| Diabetes | 419 (18) | 392 (19) | 27 (14) | 0.077 | |
| COPD | 771 (33) | 672 (32) | 99 (50) | <0.001 | |
| Cerebrovascular Disease | 141 (6) | 127 (6) | 14 (7) | 0.574 | |
| Preoperative Chemotherapy | 470 (20) | 433 (21) | 37 (19) | 0.505 | |
| Preoperative Radiation Therapy | 314 (14) | 282 (13) | 32 (16) | 0.294 | |
| Steroids | 127 (5) | 112 (5) | 15 (8) | 0.190 | |
| Prior Cardiothoracic Surgery | 512 (22) | 455 (22) | 57 (29) | 0.024 | |
| Reoperationb | 289 (13) | 249 (12) | 40 (20) | 0.001 | |
|
| |||||
| Laboratory | |||||
| FEV1, % predicted (12.8% missing; mean ± SD) |
83 ± 22 | 84 ± 21 | 76 ± 24 | <0.001 | |
| FEV1, % predicted | >80 | 1173 (51) | 1089 (52) | 84 (42) | <0.001 |
| <60 | 561 (24) | 496 (23) | 65 (33) | ||
| ≥60 and <80 | 285 (12) | 238 (11) | 47 (23) | ||
| Unmeasured | 297 (13) | 293 (14) | 4 (2) | ||
| DLCO, % predicted (28% missing; mean ± SD) |
71 ± 23 | 72 ± 23 | 66 ± 24 | 0.002 | |
| Last Hemoglobin (mean ± SD) | 12.1±1.7 | 12.2±1.7 | 12.0±1.7 | 0.152 | |
| Last Creatinine, median [IQR] | 0.85 [0.7–1] | 0.87 [0.7–1] | 0.80 [0.7–1] | 0.628† | |
|
| |||||
| Operative Details | |||||
| Status | Elective | 2214 (96) | 2018 (96) | 196 (98) | 0.145 |
| Urgent/Emergent | 90 (4) | 86 (4) | 4 (2) | ||
| Preoperative | <1 day | 2157 (93) | 1963 (93) | 194 (97) | 0.025 |
| Hospitalization | ≥1 day | 158 (7) | 152 (7) | 6 (3) | |
| Laterality | Left | 915 (40) | 854 (40) | 61 (30) | 0.004 |
| Right | 1377 (59) | 1238 (59) | 139 (70) | ||
| Missing | 25 (1) | 25 (1) | 0 (0) | ||
| Robot-Assisted Surgery | 82 (3) | 79 (4) | 3 (2) | 0.102 | |
| Surgical Approach | Thoracotomy | 603 (26) | 515 (24) | 88 (44) | <0.001 |
| VATS | 1714 (74) | 1602 (76) | 112 (56) | ||
| OR Blood Transfusion | 94 (4) | 84 (4) | 10 (5) | 0.487 | |
|
| |||||
| Outcomes | |||||
| Hospital Stay, median [IQR] | 4 [3–7] | 4 [3–6] | 10 [8–14] | <0.001† | |
| Hospital Death | 29 (1) | 23 (1) | 6 (3) | 0.034* | |
Values n (%), and <1% missing unless indicated otherwise.
average annual cases by patient’s operating surgeon in study time period
cardiac or thoracic re-operation that affects operative field
Fisher’s exact
Wilcoxon rank sum test
BMI, body mass index,%FEV1, percentage of predicted value of forced expiratory volume in 1 second; DLCO, diffusing capacity of carbon monoxide; ASA, American Association of Anesthesiology; COPD, chronic obstructive pulmonary disease; VATS, video-assisted thoracoscopic surgery; SD, standard deviation; OR, operating room; IQR, interquartile range
Figure 1. Stratified Cumulative Incidence of Hospital Discharge by Post-Operative Day.
(95% confidence intervals indicated by black-capped spikes)
Development of the Prediction Model
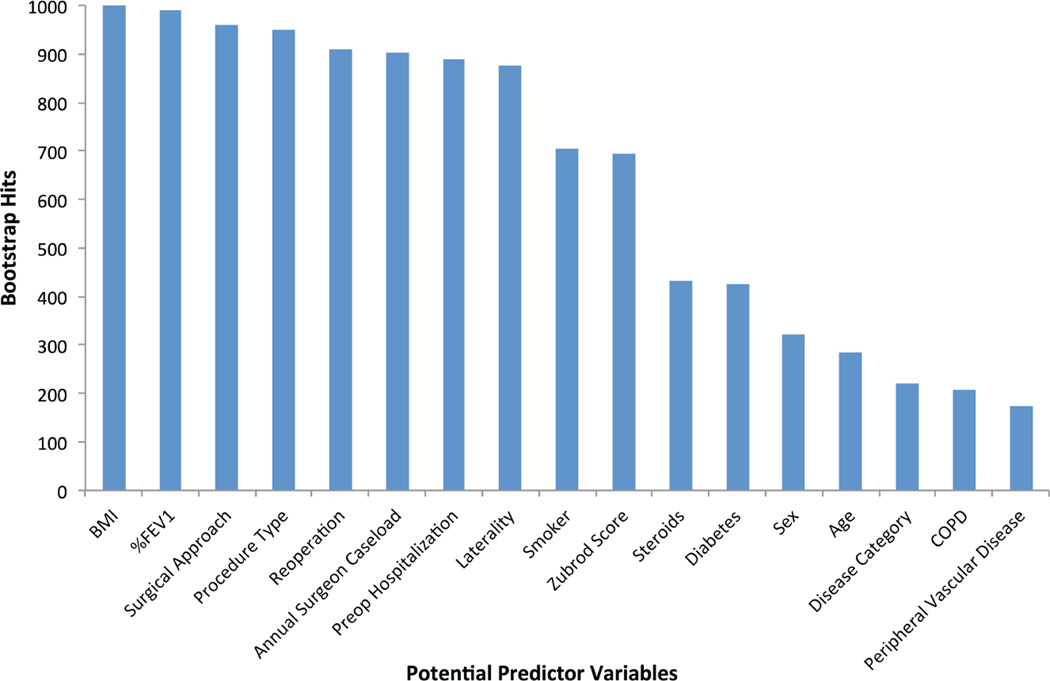
Patients with PAL were more likely to be older, male, have a lower BMI, prior smoking history, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), prior steroid use, higher Zubrod Score, and lower %FEV1. They were less likely to have diabetes or to be hospitalized prior to surgery. Patients with PAL were more likely to have primary lung cancer (versus benign or metastatic tumors), undergo lobectomy/segmentectomy or bilobectomy (versus wedge resection), have right-sided resection, undergo thoracotomy, and be operated on by a surgeon with higher annual caseloads. (Table 1) After backward stepwise logistic regression with bootstrap resampling for reliability, 10 variables comprising 13 separate coefficients were chosen in >50% of resamples and included in the final model. (Figure 2) Among all variables excluded in stepwise variable selection, or on bivariable testing (p-values between 0.15 and 0.30), none except hypertension were found to have adjusted p-values<0.10 or change regression coefficients by >10% with (re)inclusion in the final model. We incorporated the significant interaction effect ‘right-sided thoracotomy’ (between surgical approach and laterality) and ‘wedge resection by thoracotomy’ (between surgical approach and wedge resection) for their regression coefficient adjustment of the individual variables. The final regression model included BMI, %FEV1, annual surgeon caseload, smoking, Zubrod score, preoperative hospitalization, reoperation, procedure type, laterality, surgical approach, right-sided thoracotomy, and wedge resection by thoracotomy. (Table 2)
Figure 2. Bootstrap Reliability of Variables Associated with Prolonged Air Leak.
Variables selected >50% of the time were considered for forming the final model.
Table 2.
Analysis of potential predictors for prolonged air leak
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio |
P-value | Odds Ratio |
95% CI | Coefficient | P-value |
| Intercepta | 0.043 | 0.022–0.086 | −3.137 | |||
|
| ||||||
| Age (per 1 year increase) | 1.017 | 0.013 | ||||
| BMI (kg/m2) | 0.936 | <0.001 | 0.938 | 0.912–0.964 | −0.064 | <0.001 |
| Sex (Female) | 0.822 | 0.186 | ||||
|
| ||||||
| Smoker (vs non-smoker) | 2.642 | <0.001 | 1.560 | 1.013–2.402 | 0.445 | 0.043 |
| Zubrod Score 2–5 (vs 0 or 1) | 1.643 | 0.004 | 1.489 | 1.015–2.184 | 0.398 | 0.042 |
| Diabetes | 0.686 | 0.077 | ||||
| Steroids | 1.451 | 0.190 | ||||
| Malignant Cancer | 1.665 | 0.030 | ||||
| COPD | 2.106 | <0.001 | ||||
| Peripheral Vascular Disease | 1.431 | 0.101 | ||||
| Reoperationb | 1.877 | 0.001 | 1.771 | 1.183–2.651 | 0.572 | 0.005 |
|
| ||||||
| FEV1, % predicted, | <0.001 | |||||
| >80 | Ref. | Ref. | ||||
| ≥60 and <80 | 1.699 | 1.458 | 1.016–2.093 | 0.377 | 0.041 | |
| <60 | 2.560 | 2.228 | 1.467–3.384 | 0.801 | <0.001 | |
| Unmeasured | 0.177 | 0.380 | 0.132–1.096 | −0.967 | 0.073 | |
| Preop Hospitalization ≥1 day | 0.867 | 0.025 | 0.345 | 0.143–0.833 | −1.063 | 0.018 |
|
| ||||||
| Annual Surgeon Caseloadc | 1.014 | <0.001 | 1.010 | 1.003–1.018 | 0.010 | 0.009 |
|
| ||||||
| Resection Type | <0.001 | |||||
| Lobe/Segment | Ref. | Ref. | ||||
| Wedge Resection | 0.310 | 0.427 | 0.249–0.731 | −0.851 | 0.002 | |
| Bilobectomy | 3.519 | 1.778 | 0.793–3.985 | 0.575 | 0.162 | |
|
| ||||||
| Right-sided resection (vs left) | 1.571 | 0.004 | 1.045 | 0.697–1.566 | 0.044 | 0.832 |
| Thoracotomy (vs VATS) | 2.444 | <0.001 | 0.757 | 0.410–1.398 | −0.278 | 0.370 |
|
| ||||||
| Interaction terms added to final model | ||||||
|
| ||||||
| Right-Sided Thoracotomy | 2.910 | 1.430–5.924 | 1.068 | 0.003 | ||
| Wedge Resection by Thoracotomy |
2.006 | 0.783–5.140 | 0.696 | 0.147 | ||
intercept of final model used along with regression coefficients to derive nomogram scoring
average annual cases of patient’s operating surgeon in study time period
cardiac or thoracic re-operation that affects operative field
BMI, body mass index; %FEV1, percentage of predicted value of forced expiratory volume in 1 second; VATS, video-assisted thoracoscopic surgery; COPD, chronic obstructive pulmonary disease; CI, confidence interval
Categorizing Risk using the Nomogram
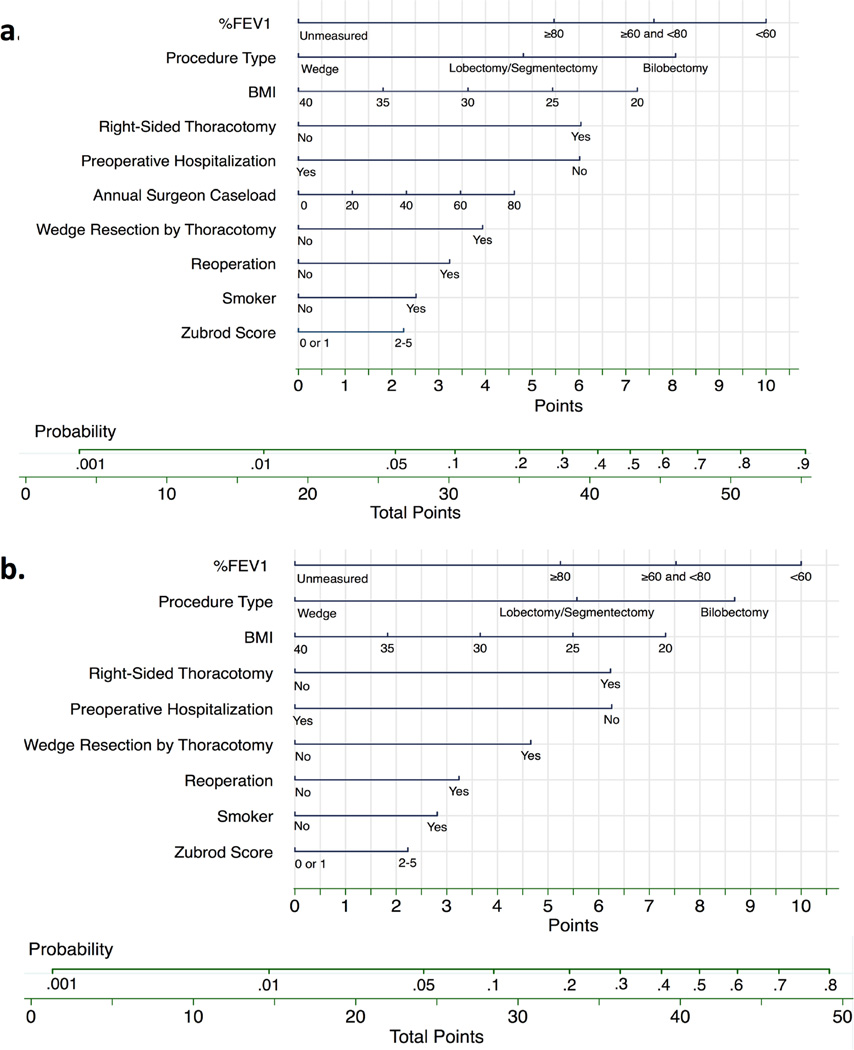
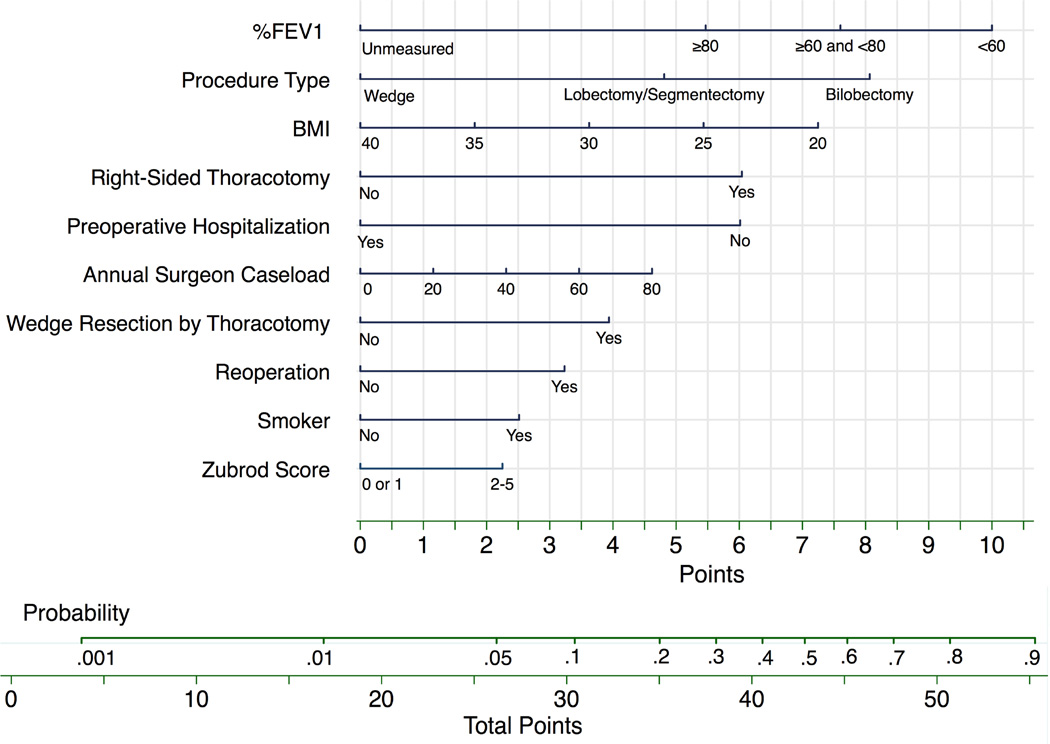
Using the regression coefficients and intercept from the final model, (Table 2) we created a nomogram to calculate the probability of PAL. (Figure 3a) For the interaction effects ‘right-sided thoracotomy’ and ‘wedge resection by thoracotomy,’ the main effect variables laterality (right-sided=0.2pts) and surgical approach (VATS=1.6pts) had small point values and are not depicted in the nomogram. We calculated a nomogram point score for each patient, and categorized patients into low, intermediate and high risk groups. (Table 3) Compared to the lowest risk group, patients in the intermediate risk group were >5 times more likely to have a PAL while the high risk group was >12 times more likely. (Table 3) The observed rates of PAL were 2.0%, 8.8% and 19.3% (non-parametric test of trend p-value <0.001), which matched closely with the predicted rates according to the model. A more generalizable nomogram identically derived except for removal of our institution specific annual surgeon caseload variable was also created. (Figure 3b)
Figure 3. Nomogram for Probability of Prolonged Air Leak.
a.) To calculate the probability of prolonged air leak, sum points over all variables to a total point score with its corresponding probability. Example: A smoker (2.5 pts) with a BMI=32 (3 pts), %FEV1=65 (7.5 pts), and Zubrod Score=1 (0 pts) without prior chest operation (0 pts) or preoperative hospitalization ≥1 (6 pts) is having a right-sided open (6 pts) lobectomy (5 pts) by a surgeon who has an annual caseload of 50 (3 pts). Total points=33. Probability of PAL is around 15%. b.) We removed our institution specific variable annual surgeon caseload to create a more generalizable model that had a C-statistic=0.755 (95% CI, 0.722 to 0.788).
Table 3.
Prolonged Air Leak Risk Classification
| Risk Class (Score) | PAL Incidence (n) |
Observed Frequency (%) |
Predicted Frequency Logistic Model (%) |
Odds Ratio (95% CI) |
|---|---|---|---|---|
| Low (≤25) | 938 | 2.0 | 2.5 | Ref |
| Intermediate (26–29) | 747 | 8.8 | 7.7 | 4.69 (2.79–7.88) |
| High (≥30) | 588 | 19.2 | 19.9 | 11.51 (6.99–18.94) |
Predictive Performance of the Model and Nomogram
Discriminatory accuracy of the final regression model was 75.9% (C-statistic=0.759; 95% CI, 0.725 to 0.792). The Hosmer-Lemeshow goodness-of-fit test was nonsignificant (p=0.735), indicating good model fit for the data, and the calibration slope was 1.000 with an intercept of 0.000 (showing perfect model calibration). After adjusting for optimism using bootstrap resampling, discriminatory accuracy was 73.8%. The model correctly classifies 74.9% of patients with a positive predictive value of 19.4% and a negative predictive value of 95.2% when calculated using a classification probability cutoff of 0.11. The discriminatory accuracy was 75.7% for the nomogram (C-statistic=0.762; 95% CI, 0.724 to 0.790), and 72.9% for the risk table after rounding to the nearest 0.5 for point calculation (C-statistic=0.729; 95% CI, 0.698 to 0.760), respectively.
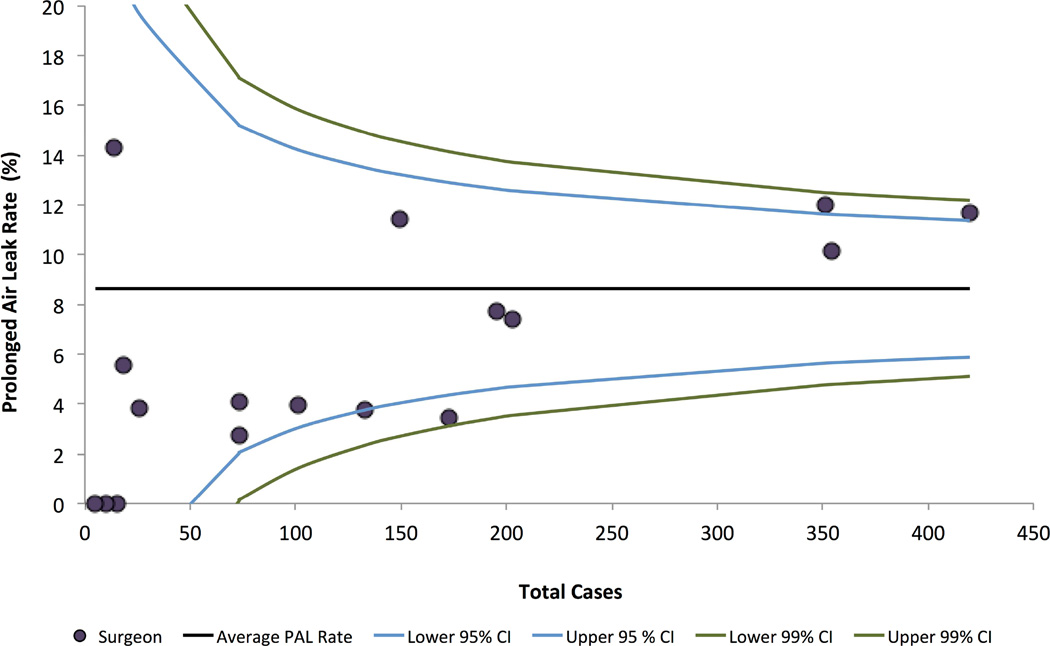
Surgical Volume Effect on PAL Incidence
To determine the relationship between surgeon volume and PAL incidence, we assessed funnel plots with calculated control limits. (Figure 4) Surgeons with higher annual caseload, on average, have higher PAL incidence (linear correlation 0.38). Data points falling inside control limits are consistent with common cause variation or expected variability in patient population or surgical management. All surgeons lie within the 99% control limit, essentially excluding special cause or unexpected variation, such as inferior (or superior) surgical technique or management practices. After risk-adjustment for case-mix (using available STS data) and clustering of PAL among surgeon, annual surgeon caseload remained a significant predictor. (Appendix Table A1/Figure A1)
Figure 4. Funnel Plot of Prolonged Air Leak Rate of Operating Surgeons by Case Volume.
95% and 99% control limits derived as follows: (θ± z*Sqrt(θ(1- θ)/p), where z is the z-score, θ the study’s average PAL rate, and p the total cases of individual surgeons.
Discussion
Using data from a large, United States-based patient dataset, our study tested the hypothesis that standard, readily available predictors could be used to stratify patients into risk classes associated with increasing risk of prolonged air leak. Our analysis identified 10 risk factors for PAL comprising a total of 13 categories with two interaction effects and resulted in a parsimonious, reliable, and accurate clinical prediction model for prolonged air leak (PAL) after pulmonary resection. The model coefficients were used to produce a nomogram which generated probability estimates for PAL and risk classification of patients into low, intermediate, and high risk groups. Importantly, these risk groups showed monotonic increase in the rate of observed PAL, indicating clear differences in risk between categories. As expected, we found that prolonged air leak was associated with delayed hospital discharge, an effect that persists nearly three weeks after surgery.
An accurate and generalizable PAL risk stratification tool could facilitate surgical decision-making and patient-specific care. Some authors have advocated a fast-track discharge pathway for pulmonary resection patients to increase patient satisfaction and reduce hospital costs.8,18–21 Given the need for carefully developed standardized protocols to effectively manage and monitor patients, the ability to predict PAL preoperatively would be valuable. In addition, both intraoperative (e.g. pleural tenting, sealants, buttressed staple lines) and post-operative (e.g. pneumoperitoneum, water seal, flutter valve) methods for PAL management exist, but their efficacy remains unclear.10,22–27 For example, Cerfolio showed that digital chest drainage tubes compared to analog systems reduce hospital stay and lead to quicker chest tube removal; they can also be used for remote electronic monitoring.18 A generalizable risk prediction tool could be used to facilitate randomized controlled trials of air leak reduction methods and help guide cost-effective use of these adjuncts.
We developed the model from patients at our institution who underwent resection for benign and malignant lung tumors operated through lobectomy/segmentectomy, wedge resection, and bilobectomy by either a thoracotomy or VATS surgical approach. Previous risk models from Brunelli3 and Lee1 excluded wedge resection and Rivera2 included volume reduction and bullectomy. Though PAL incidence is lower after VATS wedge, inclusion of resection type and surgical approach as variables in our model allows PAL prediction for these previously excluded, but large patient populations. Similar to Brunelli and Lee, we excluded lung volume reduction patients because their high rate of air leak (approaching 50% in most studies), coupled with significant differences in baseline patient characteristics (e.g. higher rates of bullous emphysema) and surgical management (greater routine use of surgical adjuncts),25,28 would dominate the model and obscure the relationship between PAL and various predictors for patients with lung cancer and nodules.
The nomogram approach permitted inclusion of more risk factors plus interactions effects compared to previous work, with almost full preservation of predictive capability compared to the regression equation. A larger number of risk factors provides much greater discrimination between patients than would a simpler model that includes only a few binary variables, particularly when the groupings are common (e.g. male patients with low %FEV1). Consistent with prior risk factor studies, our model includes three commonly identified risk factors for PAL: low BMI,2,3 low %FEV1,1,3–5,9,29,30, and lobectomy.2,4,5,29 In addition, our population size allowed for sufficient number of PAL cases to includes prior smoking4,9 and right-sided resection2,31 variables that have previously been significant on bivariable but not multivariable analysis. Annual surgeon caseload, preoperative hospitalization, reoperation, Zubrod score and the interaction effects, right-sided thoracotomy and wedge resection by thoracotomy, have not been previously analyzed. Interaction variables allow proper interpretation of risk factors; inclusion of these interactions improved our model discriminatory accuracy. Others have reported age,3,31 and sex2,31 as independent predictors of PAL, but we found them to be non-significant after adjusting for the included variables. Emphysema,4 bronchitis,4 pleural adhesions,1–3,30 DLCO1, and upper lobe resection2,5,30 are other previously identified PAL risk factors, but were not available for analysis in our dataset.
Prolonged parenchymal air leaks result from impaired healing of disrupted alveoli, often associated with poor apposition of lung with parietal pleura. It is likely that surgeon- and institution-specific technical factors like method of fissure dissection, buttressing staple lines, sealants and glues, and pleural tenting are important.32 In addition, however, reduced wound healing, increased pulmonary compliance, and inflammation could all be influential factors.33 Indeed, our finding that increasing BMI was protective against PAL has been observed by others, with underweight patients (BMI<18.5 kg/m2) experiencing significantly higher PAL incidence (p<0.001) in one study.34 One hypothesis is that lower BMI is a marker of lower nutritional status and poor wound healing.3,5,32 However, this does not explain the continued decrease of PAL into the overweight and obese weight classes in our study. An alternative explanation may derive from consistent findings of higher respiratory rates, lower tidal volume, reduced total respiratory system compliance, and decreased expiratory reserve volume with preserved spirometry, gas exchange and airway resistance in obese patients,35 which may produce an intrathoracic milieu that favors sealing of parenchymal defects.
It is not surprising that PAL rates differed across surgeons, as prior studies have shown this to be an important risk factor.5 We observed higher PAL incidence with increasing case load and surgeon caseload remains a significant predictor after risk-adjustment for case mix and patient clustering among surgeon. This finding cannot be explained by unexpected variation from differences in baseline risk, treatment, or surgical management within the measured variables, as all surgeons fell with the 99% control limits. More likely, higher volume surgeons have different case-mix in which higher volume surgeons operate on subsets of patients with unmeasured variables contributing to higher baseline PAL risk. These unmeasured variables, not currently abstracted for the STSGTSD, could reveal modifiable technical factors to reduce PAL incidence and require further study. Given this, we include a more generalizable nomogram model excluding annual surgeon caseload.
Strengths and Limitations
Model development followed a step-by-step statistical analysis based on published guidelines.15 Further development requires external validation in a multicenter setting, prospective validation, and inclusion of important independent risk factors for PAL not captured in the STSGTSD (e.g. pleural adhesions and segmental lung resection). We chose to keep patients with unmeasured %FEV1, because the majority of these patients underwent wedge resection for a benign or secondary metastatic tumors. The unmeasured %FEV1 category could apply to patients at other centers who similarly did not undergo preoperative lung function testing, but the finding requires external validation.
Conclusion
We have developed a clinical prediction model for PAL with good discriminatory accuracy. It has the potential to improve patient care through fast track discharge pathways, better informed preoperative patient counseling, and selective use of surgical adjuncts. Discharge planning could be proactive, with patients prepared to be sent home on the second or third day with an indwelling chest tube, rather than waiting 5 or more days for the air leak to resolve. The model supports previous work analyzing risk factors for PAL while presenting a novel perspective on previously and newly identified risk factors. Prospective and external validation of our model is required to realize future clinical application.
Supplementary Material
Video legend: Narrated PowerPoint summary of key study findings
Central Message.
Prolonged air leak increases mortality and length of stay after pulmonary resection. Preoperative variables can be combined to predict risk, which may be useful to guide patient-specific management.
Perspective Statement.
We have identified known and new risk factors for prolonged air leak in a large dataset from the United States and developed a prediction nomogram with good discriminatory accuracy. Because prolonged air leak worsens perioperative outcomes, this type of highly accurate risk prediction tool, which uses pre- and intra-operative variables, has the potential to improve patient management and outcomes.
Central Picture.
Points for all risk factors are summed to a total score and probability estimate.
Acknowledgments
We thank Li Wang at the Clinical and Translational Science Institute for statistical support, and Connie Timko and Karan Moore for assistance with data acquisition.
Funding: The American Association of Thoracic Surgery Summer Intern Scholarship, and Award Numbers K07CA151613 (to K.S.N.) and UL1TR000005 from the National Cancer Institute and National Institutes of Health supported the study. The content is solely the responsibility of the authors, and does not represent the official views of the funding source.
Abbreviations
- PAL
prolonged air leak
- %FEV1
percentage of predicted value of forced expiratory volume in 1 second
- STSGTSD
Society of Thoracic Surgeons General Thoracic Surgery Database
- DLCO
diffusing capacity of carbon monoxide
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Lee L, Hanley SC, Robineau C, Sirois C, Mulder DS, Ferri LE. Estimating the risk of prolonged air leak after pulmonary resection using a simple scoring system. Journal of the American College of Surgeons. 2011;212:1027–1032. doi: 10.1016/j.jamcollsurg.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: A nationwide study setting up the index of prolonged air leak. Annals of Thoracic Surgery. 2011;92:1062–1068. doi: 10.1016/j.athoracsur.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli A, Varela G, Refai M, et al. A Scoring System to Predict the Risk of Prolonged Air Leak After Lobectomy. Annals of Thoracic Surgery. 2010;90:204–209. doi: 10.1016/j.athoracsur.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 4.Liang S, Ivanovic J, Gilbert S, et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. Journal of Thoracic and Cardiovascular Surgery. 2013;145:948–954. doi: 10.1016/j.jtcvs.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: Is it a patient or surgeon related problem? Annals of the Royal College of Surgeons of England. 2012;94:422–427. doi: 10.1308/003588412X13171221592258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varela G, Jimenez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005;27:329–333. doi: 10.1016/j.ejcts.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Okereke I, Murthy SC, Alster JM, Blackstone EH, Rice TW. Characterization and importance of air leak after lobectomy. Annals of Thoracic Surgery. 2005;79:1167–1173. doi: 10.1016/j.athoracsur.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 8.Brunelli A, Rocco G. Internal validation of risk models in lung resection surgery: bootstrap versus training-and-test sampling. The Journal of thoracic and cardiovascular Surgery. 2006;131:1243–1247. doi: 10.1016/j.jtcvs.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Liberman M, Muzikansky A, Wright CD, et al. Incidence and Risk Factors of Persistent Air Leak After Major Pulmonary Resection and Use of Chemical Pleurodesis. Annals of Thoracic Surgery. 2010;89:891–898. doi: 10.1016/j.athoracsur.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. Journal of thoracic disease. 2014;6:271–284. doi: 10.3978/j.issn.2072-1439.2013.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United States. Public Health Service., United States. Health Care Financing Administration., National Center for Health Statistics (U.S.). ICD-9-CM international classification of diseases, ninth revision, clinical modification. NCHS CD-ROM 1996, no 1. Official ed. sixth. Washington, D.C: U.S. Dept. of Health and Human Services, Public Health Service, Health Care Financing Administration; 1996. p. 1. computer laser optical disc 4 3/4 in. + 1 booklet. [Google Scholar]
- 12.Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC medical research methodology. 2011;11:144. doi: 10.1186/1471-2288-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC, Tu JV. Bootstrap methods for developing predictive models. The American Statistician. 2004;58:131–137. [Google Scholar]
- 14.Zlotnik A, Abraira V. A general-purpose nomogram generator for predictive logistic regression models. Stata Journal. 2015;15:537–546. [Google Scholar]
- 15.Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. Springer Science & Business Media; 2008. [Google Scholar]
- 16.Harrell FE, Lee KL, Mark DB. Tutorial in biostatistics multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Mayer EK, Bottle A, Rao C, Darzi AW, Athanasiou T. Funnel plots and their emerging application in surgery. Annals of Surgery. 2009;249:376–383. doi: 10.1097/SLA.0b013e31819a47b1. [DOI] [PubMed] [Google Scholar]
- 18.Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. The Annals of thoracic Surgery. 2008;86:396–401. doi: 10.1016/j.athoracsur.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 19.McKenna RJ, Jr, Mahtabifard A, Pickens A, Kusuanco D, Fuller CB. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. The Annals of thoracic Surgery. 2007;84:1663–1667. doi: 10.1016/j.athoracsur.2007.05.058. discussion 1667–1668. [DOI] [PubMed] [Google Scholar]
- 20.Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2008;34:174–180. doi: 10.1016/j.ejcts.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Bryant AS, Cerfolio RJ. The influence of preoperative risk stratification on fast-tracking patients after pulmonary resection. Thoracic surgery clinics. 2008;18:113–118. doi: 10.1016/j.thorsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Belda-Sanchis J, Serra-Mitjans M, Iglesias Sentis M, Rami R. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev. 2010:CD003051. doi: 10.1002/14651858.CD003051.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Leyn P, Muller MR, Oosterhuis JW, et al. Prospective European multicenter randomized trial of PleuraSeal for control of air leaks after elective pulmonary resection. The Journal of thoracic and cardiovascular Surgery. 2011;141:881–887. doi: 10.1016/j.jtcvs.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert S, McGuire AL, Maghera S, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. The Journal of thoracic and cardiovascular Surgery. 2015;150:1243–1249. doi: 10.1016/j.jtcvs.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan AK, Doeing DC, Hogarth DK. Isolation of persistent air leaks and placement of intrabronchial valves. The Journal of thoracic and cardiovascular Surgery. 2013;145:626–630. doi: 10.1016/j.jtcvs.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng T, Ryder BA, Machan JT, Cioffi WG. Decreasing the incidence of prolonged air leak after right upper lobectomy with the anterior fissureless technique. The Journal of thoracic and cardiovascular Surgery. 2010;139:1007–1011. doi: 10.1016/j.jtcvs.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Schuchert MJ, Abbas G, Landreneau JP, Luketich JD, Landreneau RJ. Use of energy-based coagulative fusion technology and lung sealants during anatomic lung resection. The Journal of thoracic and cardiovascular Surgery. 2012;144:S48–S51. doi: 10.1016/j.jtcvs.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and Surgical Factors Influencing Air Leak After Lung Volume Reduction Surgery: Lessons Learned From the National Emphysema Treatment Trial. The Annals of thoracic Surgery. 2006;82:197–207. doi: 10.1016/j.athoracsur.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Cerfolio RJ, Bass CS, Pask AH, Katholi CR. Predictors and treatment of persistent air leaks. Annals of Thoracic Surgery. 2002;73:1727–1731. doi: 10.1016/s0003-4975(02)03531-2. [DOI] [PubMed] [Google Scholar]
- 30.Brunelli A, Monteverde M, Borri A, Salati M, Marasco RD, Fianchini A. Predictors of prolonged air leak after pulmonary lobectomy. Annals of Thoracic Surgery. 2004;77:1205–1210. doi: 10.1016/j.athoracsur.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 31.Petrella F, Rizzo S, Radice D, et al. Predicting prolonged air leak after standard pulmonary lobectomy: Computed tomography assessment and risk factors stratification. Surgeon. 2011;9:72–77. doi: 10.1016/j.surge.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Hunt BM, Aye RW. Prolonged Air Leak After Lung Resection. Current Respiratory Medicine Review. 2012;8:280–284. [Google Scholar]
- 33.Arslantas MK, Kara HV, Tuncer BB, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. The Journal of thoracic and cardiovascular Surgery. 2015;149:314–320. doi: 10.1016/j.jtcvs.2014.08.071. 321 e311. [DOI] [PubMed] [Google Scholar]
- 34.Thomas PA, Berbis J, Falcoz PE, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2014;45:652–659. doi: 10.1093/ejcts/ezt452. discussion 659. [DOI] [PubMed] [Google Scholar]
- 35.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17:43–49. doi: 10.1111/j.1440-1843.2011.02096.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video legend: Narrated PowerPoint summary of key study findings