Abstract
Aromatase inhibitors (AIs) have been commonly used as an effective adjuvant therapy in treatment of breast cancer, especially for menopausal women with estrogen receptor positive breast cancer. Due to the nature of aromatase, the key enzyme for endogenous estrogen synthesis, inhibitory of aromatase-induced side effects, such as cognitive impairment has been reported in both human and animal studies. While extensive evidences suggested that physical exercises can improve learning and memory activity and even prevent age-related cognitive decline, basic research revealed some common pathways between exercise and estrogen signaling that affected cognitive function. This review draws on clinical and basic studies to assess the potential impact of exercise in cognitive function from women treated with AIs for breast cancer and explore the potential mechanism and effects of exercise on estrogen-related cognition.
Keywords: breast cancer, aromatase inhibitors, exercise, cognition
Introduction
Aromatase is a key enzyme in estrogen synthesis and is widely expressed in many tissues, such as ovary, breasts and brain. Unlike ovary-synthesized estrogen, which is mainly released into the bloodstream, brain-synthesized estrogen mostly acts locally to maintain brain functions under normal conditions and plays neuroprotective roles in age-related cognitive decline and even Alzheimer’s disease (AD) [1,2]. Brain aromatase is important for maintaining local endogenous estrogen levels. Studies showed that reduction of brain aromatase is directly linked to decline of cognitive function and risk of AD in females [2–7].
Breast cancer is one of the top cancers that occur frequently in women and about 75% of all breast cancers are estrogen receptor-positive [8]. Estrogen receptor-positive breast cancer is directly associated with over-proliferation of mammary gland epithelial cell by the stimulation of estrogen. Therefore, inhibition of estrogen synthesis and reduction of estrogen level has been an effective treatment to decrease the incidence of estrogen receptor-positive breast cancer. During the past decade, aromatase inhibitors (AIs) have been widely used as a standard therapy for estrogen receptor-positive breast cancer, which accounts for majority of the invasive breast tumors in postmenopausal woman [8]. Clinically, AIs are generally well tolerated; however, increasing basic and clinical reports suggest that AIs therapy may be associated with long-term cognitive impairment in some breast cancer patients, especially in breast cancer survivors [9–11]. Other side effects of AIs are also reported which include osteoporosis, and bone fracture [12,13]. Increasing evidence showed that exercise can boost patients’ immunity, reduce inflammation, and relieve joint pain during treatment of breast cancers [14,15]. It is also well recognized that exercise can improve cognitive function and prevent age- and disease-related cognitive decline including estrogen deficiency-induced cognitive impairment [16]. However, it is unknown that whether exercise could prevent the risk of cognitive decline induced by AIs treatment. In this review, we will focus on the potential exercise intervention in cognitive decline caused by AIs treatment.
Protection of estrogen on cognitive
Estrogen not only plays an important role in reproduction but also modulate cognition process, especially learning and memory. Recently, a cognitive function study recruited 1884 women with 8 years annual following up and found that subjects with surgical menopause at early age had faster decline in global cognition, specifically episodic memory and semantic memory compared to age-matched women underwent natural menopause [17]. It is also suggested that women of surgical menopause were more likely engaged in cognitive impairment that primarily affected verbal episodic memory in later life compared with women of natural menopause [18]. In addition, the same study also reported that earlier onset of menopause was associated with increased AD neuropathology, especially neuritic plaques which had been proved that resulted from estrogen deficiency [17]. Furthermore, while some studies demonstrated controversial findings of a correlation between plasma estrogen levels and cognitive function in elderly women, more recent investigations found that the level of brain estrogen is directly associated with risk of AD in aged females [5–7]. Early and long-term hormone replacement therapy was associated with reduction of the age-related global cognition decline [17]. And these results were consistent with previous studies showing that women with unilateral or bilateral oophorectomy had higher risk of cognitive impairment or dementia compared to age-matched women without oophorectomy [19,20]. Estrogen replacement therapy after menopausal showed a neuroprotection effect on cognitive impairment induced by surgical menopause [21,22]. Clinical evidences had also proved further evidence on estrogen-induced cognitive improvement in aged women. For example, Wroolie and his colleagues compared verbal memory between post-menopausal women (>55 years old) with risk factors for AD received 17β-estradiol (E2) and conjugated equine estrogen (CEE). They found that women treated with E2 had better performance in verbal memory test compared to subjects received CEE [23]. A functional magnetic resonance imaging (fMRI) study revealed that estrogen treatment induced activation in the prefrontal cortex in young women (27–49 years old) with Lupron-induced ovarian suppression underwent estrogen replacement therapy (ERT) compared age-matched Lupron-treated women without ERT. In addition, studies have demonstrated that long-term use of estrogen would increase activation of hippocampal with better performances in verbal and figure memory tests [24,25]. Together, these studies suggested a brain functional improvement by estrogen. However, there were also studies showed limited or no effect of estrogen on cognitive function. For example, another fMRI study showed significant activation in the regions of prefrontal cortex in estradiol-treated women compared with placebo-treated controls during verbal, spatial and visual memory tasks, but only reduced errors of perseveration during verbal recall [26]. The inconsistent in the effects of estrogen on cognitive function might be related by the variation of ERT durations and the specific cognitive tasks. For instance, the verbal memory, a most sensitive variable for women, was not always included. Taken together, prefrontal cortex and hippocampus are the most important brain regions associated cognitive function related to estrogen therapy.
In consistent with human studies, the estrogen-induced cognitive improvement had also been demonstrated in various animal studies [27,28]. It is also reported that the estrogen-induced cognitive improvement has also a brain regional-dependent effect. E2 administration could improve prefrontal cortex and hippocampus associated learning activities in rats while a negative effect of estradiol on the striatum-dependent learning was observed [29,30].
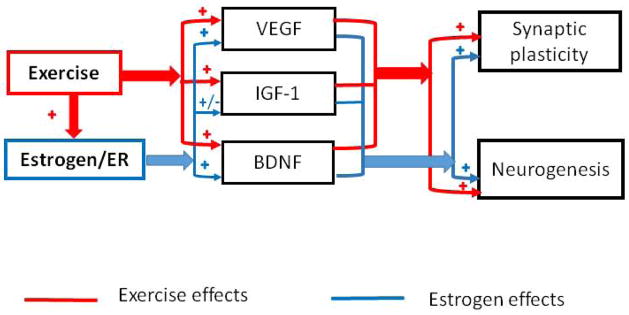
There are several hypotheses of underlying mechanism of estrogen-induced cognitive function improvement. One of the most popular mechanisms is the regulatory effect of estrogen on brain derived neurotrophic factor (BDNF). Many studies have showed that estrogen treatment can ameliorate memory by increasing spine density through elevation of BDNF level [31,32]. Some of the estrogen-induced BDNF neurotrophic effects are estrogen receptor-dependent. Tamoxifen, an estrogen receptor antagonist, decreased BDNF level in mice cerebellar, and BDNF administration increased Purkinje cell growth in these animals [33]. Estrogen receptors are also partially responsible for the estrogen-induced cognitive function improvement. For instance, estrogen receptor α was involved in estradiol enhanced object recognition memory in ovariectomized (OVX) mice [34]. Moreover, injection of ICI 182 780, an estrogen receptor antagonist, into rat hippocampus bilaterally reversed the systemic estrogen administration-induced enhancement in place learning, suggesting that the estrogen-induced enhancement on place learning was dependent on the activation of hippocampal estrogen receptor [35]. Furthermore, various neurotransmitters are also playing roles in estrogen-induced cognitive improvement, such as norepinephrine, dopamine, serotonin, and acetylcholine [36,37]. In addition, epigenetic regulation of estrogen is also an assignable factor in the amelioration of memory. A series of studies have showed that dorsal hippocampal H3 acetylation and DNA demethylation of genes necessary for memory formation including BDNF gene are essential for E2 to enhance object recognition memory consolidation in female mice [38]. There were some reports on estrogen regulating vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) in both human and animal studies. It is general accepted that estrogen promotes cognitive function partially through activation of VEGF and IGF-1 [39,40], although the effect of estrogen on IGF1 remains controversial which might be associated with the different estrogen administration routs [41] (figure 1).
Fig. 1. The relationship of mechanisms between exercise- and estrogen- induced neuroprotection.
Exercise can facilitate neuroplasticity and neurogenesis through improving the expression of VEGF, IGF-1, BDNF as indicated as red lines, while the effects of estrogen on cognitive function might be mediated through estrogen receptors and regulate VEGF, IGF-1 and BDNF to exert neuroprotective functions as shown in blue lines.
Cognitive impairment associated with AIs
AIs have been used as an adjuvant therapy for estrogen receptor-positive breast cancer patients by inducing an abrupt reduction of endogenous estrogen levels. As side effects of low endogenous estrogen, AIs can lead to risk of osteoporosis, fractures, cardiovascular disease and cognitive decline [9,11,42,43]. For example, the Intergroup Exemestane Study reported that patients switching to exemestane, one of AIs, had a higher incidence of fractures than those continuing receiving tamoxifen, a selective estrogen receptor modulator (SERM) as 7.0 vs 4.6%; P=0.003 [43]. Cardiovascular events were more common in patients receiving exemestane than those switched to tamoxifen as 20.8 vs 18.9%; P=0.09 [43].
Human studies
Although multiple studies demonstrated cognitive impairment associated with AIs treatment in breast cancer patients, the effect of aromatase on cognitive function by pharmacological inhibition remains controversial. For example, in humans, several studies showed that AIs treatment, a standard therapeutic strategy for women with estrogen receptor-positive breast cancer, showed a significant decline in cognition, particularly in cognitive processing speed and verbal memory [9–11]. One of the studies compared the outcome from cognitive tests performance and self-reported cognitive function in patients treated with AI and SERM, such as exemestane or tamoxifen and healthy controls. The results showed that all patients had significant decline in their objective cognition (two category fluency tests and one information processing speed tests) and subjective cognitive function compared with healthy controls [10]. Similarly, Collins and his colleagues used a longitudinal design to study the effect of adjuvant therapy on breast cancer patient’s cognition by conducting a baseline assessment (T1) and a second assessment (T2) after 5–6 months treatment in breast cancer patients and age-matched healthy controls. A significant decline of cognition from T1 to T2 was observed in patients under adjuvant therapy, such as tamoxifen and anastrozole, one of AIs, compared with healthy controls. Among the all cognitive performance tests, the most effected cognitive domains affected by adjuvant therapy were processing speed and verbal memory [9]. Since the plasma estrogen level was much lower in breast cancer patients treated with anastrozole than those received tamoxifen treatment, Bender and his colleagues compared cognitive function between patients received anastrozole and tamoxifen for 3 months, and found that women received anastrozole had poorer visual and verbal learning and memory than those received tamoxifen [44]. In addition, Lejbak et al. demonstrated additional estrogen sensitive cognitive domains, such as letter fluency, complex visuomotor attention and manual dexterity [45].
However, there were also several studies showed no effects of AIs on cognitive function in breast cancer patients [46–49]. For example, Hermelink and his colleagues assessed cognitive function in 101 breast cancer patients (62 treated with tamoxifen, anastrozole or letrozole for average 19 weeks) at the beginning of treatments, the end of treatments and 1 year after the first assesses. They failed to identify any significant effects of anti-estrogen treatments on patient’s cognition tests [46]. This result might be caused by learning effect with the same test conducted twice. Cross-session studies from Breckenridge et al. and Kilickap et al. evaluated cognition among breast cancer patients (aged 33–81) and found the effects of anti-estrogen therapy were distinct for patients at different menopausal status [47,48]. In addition, Hurria and his colleagues examined the relationship between AIs and cognition in 32 breast cancer patients aged >60 years old who had positron emission tomography (PET) scans and found no significant decline in cognition in patients treated with AIs therapy for 6 months compared with age-matched healthy controls. However, there were significant changes in cerebral metabolic activity between the AIs treated patients and healthy controls and the primary effective brain regions involved the medial temporal lobes and Broca’s area which are associated with long-term memory and verbal exposition, respectively [50]. This result suggested that AIs therapy might affect cognitive function through dysregulating metabolic activities in associated brain regions. However, the association between PET and cognition in AIs treated patients need further confirmative investigations due to the modest sample size with only 32 patients [50]. Furthermore, studies showed that cognitive function of postmenopausal women with breast cancer improved one year after the anti-estrogen therapy [51]. However, they did not conduct baseline assessment before treatment to get whether patients cognitive function could back to original statement [51].
In contrast, the effect of anti-estrogen therapy on cognition by self-reported cognitive performance were relative consistent. Studies showed that AIs treatment in breast cancer patients caused decline of subjective cognitive function [10,47,52], and such a cognitive impairment persisted for long time after the treatments reported in the follow-up studies [53]. However, studies showed irrelevance between self-reported cognitive functioning and cognitive test performance [10,54] or positive correlation only in the area of verbal memory [55]. The most assessments of subjective cognitive functioning in self-reported studies were the Cognitive Failure Questionnaire which primarily focusing on perception, attention and motor function [10,54,56], while the most AIs effective domain of cognition were verbal memory and information processing speed [9–11]. Therefore, these self-reported studies on subjective cognitive function might reflect partially, but not targeted cognitive impairment in AIs treated breast cancer patients. Bender et al. used the Patient’s Assessment of Own Functioning to evaluated perceived cognitive function, and discussed the relevance of objective and subjective cognition in early-stage breast cancer patients receiving adjuvant hormonal therapy (tamoxifen or anastrozole) and found that poorer cognitive function was related to poorer verbal learning and memory [55]. Again, all suggested that the controversial findings of AIs-related cognitive function in human might well be related to the type of cognitive function tests as well as menopausal status and variation of treatment durations.
Animal studies
Inhibition of aromatase induced cognitive impairment has been found in animal studies. Using genetic approaches, studies demonstrated that mice with genetic knockout of aromatase gene (ArKO) performed significant worse in Y-maze test for short-term spatial reference memory than wildtype control mice [57]. It was reported that the number of spine synapses was significantly reduced in the hippocampus of mice treated with letrozole (one of AIs) compared to wildtype mice [56]. Furthermore, studies of AIs treatment and long-term potentiation (LTP) in the hippocampus demonstrated a reduction in the magnitude of LTP in brain slices from the letrozole-treated animals compared to that from the control mice. And the LTP impairment increased significantly with longer treatment duration in female mice [58]. AIs, particularly letrozole, also could down-regulate steroid receptor coactivator-1 in specific brain regions primarily related to memory and integration [4]. Instead of causing reduction of spine synapse and LTP, some studies found beneficial effects of AIs on cognition. For example, systemic injection of letrozole increased spatial learning and memory activities in rats [59], while other research found an increase in expression of N-methyl-D aspartate (NMDA) receptor in female ArKO mice (14–16 weeks old) with higher performance in water maze test [60]. Moreover, an increased level of catecholaminergic neurotransmitters like noradrenaline and dopamine in the prefrontal and hippocampus after high dose (1mg/kg) of letrozole treatment for 6 weeks in OVX rats, while the level of catecholaminergic neurotransmitters above was decreased in OVX control group and intact letrozole treatment group [59]. These results indicated a potential window of protective action of AIs in OVX mice, not in intact mice.
In conclusion, aromatase deficiency at early age may lead to cognitive impairment while a potential bi-phases effect of AIs has been observed in aged animals. The animal studies are in consistent with human studies that AIs-induced cognitive impairments mostly occur in surgical menopausal women [61]. The impairment of cognition induced by aromatase deficiency might be induced by loss of spine synapse followed by impaired LTP. In addition, NMDA receptor, catecholaminergic neurotransmitters and steroid receptor coactivator-1 may also involve in the aromatase-related cognitive changes.
The possibility of exercise on improving the cognitive impairment induced by AIs
Positive effects of exercise on cognition
Physical activity, as a non-pharmacological therapy to cognitive impairment, has gained more and more attention recently. Numerous studies have confirmed the positive effects of exercise on cognition, especially in elderly adults with moderate cognitive impairment [62–65]. It was reported that multimodal physical training for 16 weeks reduced pro-inflammatory cytokines and improved BDNF peripheral levels, which lead the improvement of cognition in subjects with mild cognitive impairment [66]. Another independent study revealed that healthy adults at age 62–89 years after 12-month coordination exercise had better executive function mediated by the increased volume of basal ganglia nuclei [67]. Physical activity has been associated with better memory function mediated by increasing cerebral gray matter volume in prefrontal and cingulate cortex in healthy elderly adults [68,69]. The beneficial effects of exercise on memory storage and consolidation were also found in preadolescents and young adults [70–72], and this effect might be mediated by enhancing levels of BDNF and catecholamine [70]. Regression analyses showed that the exercise-induced enhancement of cognitive inhibitory control function in healthy young adults might be related to the increased cerebral-blood-flow (CBF) regulation as well as cerebrovascular function [73,74]. In addition, the positive association between physical activity and cognition improvement were also reported in breast cancer survivors [75]. Because of both people with estrogen deficiency and breast cancer survivors often reported cognitive decline [47], it might be worth to investigate whether exercise could prevent or treat cognitive impairment in breast cancer patients treated with or without AIs. Indeed, Pradhan et al. showed that an exercise for 3 months has great likelihood to provide better attention function in young breast cancer survivors [75].
Animal studies provided further support on cognitive improvement induced by exercise. Studies observed that six weeks of treadmill exercise could protect short-term and spatial memory impairments in aged rats through increasing neurogenesis and suppressing apoptosis in hippocampus [76]. Exercise also can up-regulate proteins related to energy metabolism (i.e. Glycolysis, ATP synthesis, ATP transduction and glutamate turnover) and synaptic plasticity to enhance cognitive function [77]. Furthermore, changes in neurotrophic and growth factors, such as BDNF, IGF-1 and VEGF induced by exercise also contribute important roles the protection of cognition decline [16,78–80]. For example, treadmill exercise of 39 days in early age (21 days old) rats increased mossy fibers density and expression of BDNF and its receptor tropomyosin-related kinase B in hippocampus, which induced the improvement of spatial learning and memory. These exercised-induced enhanced spatial memories were maintained for long which were measured at 96 days old [81]. Blocking the action of BDNF abolished improvement in learning acquisition and increased expression of proteins related to energy metabolism and synaptic plasticity induced by 1-week wheel running [82,83]. IGF-1 can interact with BDNF to participate the action on learning and memory through synaptic plasticity, neurogenesis and energy metabolism [16,84]. Interesting enough, studies showed that the exercise-induced learning and memory improvement would be abolished after blocked of VEGF and IGF-1 respectively [84,85]. Moreover, exercise can ameliorate cognitive function through gene level. For instance, Kohman et al. found that 8 weeks wheel running reversed age-induced decreasing expression of genes involved in cell growth and increasing expression related to immune function [86]. Other evidence also demonstrated that exercise-induced enhancement of learning and memory is partially mediated through facilitating DNA demethylation and acetylation of histone H3 localized to the promoter IV of the BDNF gene, a region intimate related to neuronal activity [87].
In conclusion, exercise can improve cognition through numerous pathways including improving cerebrovascular function, CBF regulation, immune system, stimulating neurotrophic factors and growth factors. The effects of exercise on growth factors-mediated cognitive function are similar to the pathways of estrogen-induced cognitive improvement (Fig. 1).
The interaction of exercise and estrogen on cognition
Clinical observation demonstrated that women with estrogen deficiency often engaged in cognitive impairment. As mentioned above, some of breast cancer survivors who had received AIs therapy also developed cognitive decline compared with healthy controls [9–11]. Higher incidence of AD in post-menopausal women was also reported than that in age-matched men [88]. Women with surgical menopause at early age have higher risk of AD than healthy age-matched women [17]. While exercise has been widely used for better cognitive function in general, a meta-analysis revealed that the exercise-induced beneficial effect was more effective in females than males [64]. Furthermore, Jianqiang and his colleagues reported that 2-months treadmill increased serum levels of estrogen and level of BDNF in the rats hippocampus [89]. An independent study also found that 30 days of treadmill running in OVX rats could restore estrogen deficiency-induced down-regulation of creatine kinase activity, an important enzyme in energy metabolism [90]. To investigate the effect of exercise on estrogen deficiency-induced impairment of memory and cognition, Juliana Ben and his colleagues examined roles of running on female Wistar rats at 3 months old with or without ovariectomy. The exercise was conducted as running in a moderate intensity three times per week for one month. They found that OVX mice demonstrated a significant impairment in the inhibitory avoidance and Morris Water Maze test compared to the shamed mice, while exercise can restore the inhibitory avoidance behavior and spatial navigation memory in the OVX mice [91]. Another study showed that exercise reversed the estrogen deficiency-induced increased activation of Na+,, K+-ATPase and acetylcholinesterase in hippocampus and cerebral cortex in OVX mice [92]. All together suggested that exercise not only increase cognitive performance in general, but also protects the estrogen deficiency-induced cognitive decline in females.
However, the effect of exercise on estrogen deficiency-induced cognitive decline may be dependent on the length of exercise as well as the duration of estrogen deprivation. Studies showed that levels of BDNF mRNA in the hippocampus were lower in rats with ovariectomy for long term (7 weeks) than short term (3 weeks). Five days wheel running restored BDNF gene expression in short-term estrogen-deprivation rats only, suggesting that long term absence of estrogen might change the threshold to trigger a BNDF gene response, which leading a limitation for exercise in OVX rats with long term estrogen deficiency [93]. Evidence from Moreno-Piovano confirmed this speculation. Moreno-Piovano and his colleagues found higher methylation levels of regulatory sequences of BDNF in long-term OVX mice compared with short-term ones. In addition, estradiol failed to increase BDNF expression after long-term estrogen deficiency, while estradiol exerted an ideal effect on increasing BDNF and synaptophysin protein expression [94]. Similarly, Marosi and his colleagues compared the effects of E2 and long term moderate exercise on cognitive function and related intracellular molecular signaling pathways and found while E2 and exercise alone both enhanced attention and memory and activation of PKA/Akt/CREB and MAPK/CREB pathways in female rats at 15 months old, exercise alone failed to ameliorate behavior and molecular mechanism in female rats at 27 months old. In addition, E2+exercise improved cognitive function with enhanced activation of relevance pathways both in rats at 15- and 27-month of age [95]. Therefore, these data suggested that exercise may share similar beneficial effects on cognition and combination of exercise with estrogen treatment might play provide more power in improving cognitive function, particularly in aged females.
Conclusions
Estrogen is very important in the regulation of cognitive function. Lack of estrogen like aromatase inhibitor treatment among breast cancer patients might cause cognitive impairment. Exercise is an effective intervention to improve cognitive function. This improvement is more significant among persons with cognitive disease. Numerous evidences revealed that exercise and estrogen have many common ways in the regulation of cognitive function. Therefore, it is provided an opportunity for exercise to ameliorate cognitive decline induced by AIs treatment in breast cancer patients.
Acknowledgments
This present work was supported by grants from the National Natural Science Foundation of China (NO.31171004), the Shanghai Science and Technology Commission (NO.13490503600), Shanghai University Top Discipline Construction Plans and the Graduate Students Visit Abroad Program of Shanghai University of Sport (NO.stfx20140214).
Abbreviations
- AIs
Aromatase inhibitors
- AD
Alzheimer’s disease
- E2
17β-estradiol
- CEE
Conjugated equine estrogen
- fMRI
Functional magnetic resonance imaging
- ERT
Estrogen replacement therapy
- BDNF
Brain derived neurotrophic factor
- OVX
Ovariectomized
- SERM
Selective estrogen receptor modulator
- PET
Positron emission tomography
- ArKO
Knockout of aromatase gene
- LTP
Long-term potentiation
- NMDA
N-methyl-D aspartate
- CBF
Cerebral-blood-flow
- IGF-1
Insulin-like growth factor-1
- VEGF
Vascular endothelial growth factor
References
- 1.Boon WC, Chow JDY, Simpson ER. The Multiple Roles of Estrogens and the Enzyme Aromatase. Progress in brain research. 2010;181:209–232. doi: 10.1016/s0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 2.Janicki SC, Park N, Cheng R, Schupf N, Clark LN, Lee JH. Aromatase variants modify risk for Alzheimer’s disease in a multiethnic female cohort. Dementia and geriatric cognitive disorders. 2013;35(5–6):340–346. doi: 10.1159/000343074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medway C, Combarros O, Cortina-Borja M, Butler HT, Ibrahim-Verbaas CA, de Bruijn RF, Koudstaal PJ, van Duijn CM, Ikram MA, Mateo I, Sanchez-Juan P, Lehmann MG, Heun R, Kolsch H, Deloukas P, Hammond N, Coto E, Alvarez V, Kehoe PG, Barber R, Wilcock GK, Brown K, Belbin O, Warden DR, Smith AD, Morgan K, Lehmann DJ. The sex-specific associations of the aromatase gene with Alzheimer’s disease and its interaction with IL10 in the Epistasis Project. European journal of human genetics: EJHG. 2014;22(2):216–220. doi: 10.1038/ejhg.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian C, Zhao Y, Guo Q, Xiong Y, Cai W, Zhang J. Aromatase inhibitor letrozole downregulates steroid receptor coactivator-1 in specific brain regions that primarily related to memory, neuroendocrine and integration. The Journal of steroid biochemistry and molecular biology. 2014;141:37–43. doi: 10.1016/j.jsbmb.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(52):19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Cui J, Shen Y. Brain sex matters: estrogen in cognition and Alzheimer’s disease. Molecular and cellular endocrinology. 2014;389(1–2):13–21. doi: 10.1016/j.mce.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y. Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Molecular neurobiology. 2013;47(3):857–867. doi: 10.1007/s12035-012-8377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales AR, Ganjei-Azar P, Gomez-Fernandez C, Nadji M. Immunohistochemistry of Estrogen and Progesterone Receptors Reconsidered. American Journal of Clinical Pathology. 2005;123(1):21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 9.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psycho-oncology. 2009;18(8):811–821. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 10.Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, Van Dam FS, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta oncologica. 2009;48(1):76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho-oncology. 2004;13(1):61–66. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 12.Cheung AM, Heisey R, Srighanthan J. Breast cancer and osteoporosis. Current opinion in endocrinology, diabetes, and obesity. 2013;20(6):532–538. doi: 10.1097/01.med.0000436195.10599.dd. [DOI] [PubMed] [Google Scholar]
- 13.Kalder M, Ziller V, Kyvernitakis I, Knöll D, Hars O, Hadji P. Influence of compliance on bone mineral density changes in postmenopausal women with early breast cancer on Anastrozole. Journal of Cancer Research and Clinical Oncology. 2013;139(6):915–923. doi: 10.1007/s00432-013-1402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeNysschen CA, Burton H, Ademuyiwa F, Levine E, Tetewsky S, O’Connor T. Exercise intervention in breast cancer patients with aromatase inhibitor-associated arthralgia: a pilot study. European journal of cancer care. 2014;23(4):493–501. doi: 10.1111/ecc.12155. [DOI] [PubMed] [Google Scholar]
- 15.Galantino ML, Greene L, Archetto B, Baumgartner M, Hassall P, Murphy JK, Umstetter J, Desai K. A qualitative exploration of the impact of yoga on breast cancer survivors with aromatase inhibitor-associated arthralgias. Explore. 2012;8(1):40–47. doi: 10.1016/j.explore.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Comprehensive Physiology. 2013;3(1):403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82(3):222–229. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson VW. Cognitive changes after menopause: influence of estrogen. Clinical obstetrics and gynecology. 2008;51(3):618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neuro-degenerative diseases. 2008;5(3–4):257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey DA. Alzheimer’s disease, dementia, mild cognitive impairment and the menopause: a ‘window of opportunity’. Women’s health. 2013;9(3):279–290. doi: 10.2217/whe.13.22. [DOI] [PubMed] [Google Scholar]
- 21.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause international. 2008;14(3):111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151(8):3795–3804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wroolie TE, Kenna HA, Williams KE, Powers BN, Holcomb M, Khaylis A, Rasgon NL. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17beta-estradiol versus conjugated equine estrogens. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2011;19(9):792–802. doi: 10.1097/JGP.0b013e3181ff678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick MPMASM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiology of aging. 2000;21(2):373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 25.Maki PM, Dennerstein L, Clark M, Guthrie J, LaMontagne P, Fornelli D, Little D, Henderson VW, Resnick SM. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain research. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 27.Lacreuse AMEW, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiology of aging. 2002;23(4):589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 28.Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology. 2013;154(2):842–852. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- 29.Buwalda B, Schagen SB. Is basic research providing answers if adjuvant anti-estrogen treatment of breast cancer can induce cognitive impairment? Life sciences. 2013;93(17):581–588. doi: 10.1016/j.lfs.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of learning and memory. 2005;84(2):132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luine VaMF. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. Neuroscience. 2013;239:34–45. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haraguchi S, Sasahara K, Shikimi H, Honda S, Harada N, Tsutsui K. Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. Cerebellum. 2012;11(2):416–417. doi: 10.1007/s12311-011-0342-6. [DOI] [PubMed] [Google Scholar]
- 34.Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor alpha. Neurobiology of learning and memory. 2014;114C:1–9. doi: 10.1016/j.nlm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiology of learning and memory. 2006;86(3):336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol Enhances Learning and Memory in a Spatial Memory Task and Effects Levels of Monoaminergic Neurotransmitters. Hormones and behavior. 1998;34(2):149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 37.Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29(3):423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Frontiers in neuroendocrinology. 2014 doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouk S, Hintz T, Li P, Duffy AM, MacLusky NJ, Scharfman HE. 17beta-estradiol increases astrocytic vascular endothelial growth factor (VEGF) in adult female rat hippocampus. Endocrinology. 2011;152(5):1745–1751. doi: 10.1210/en.2010-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Human brain mapping. 2014;35(3):847–865. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isotton AL, Wender MC, Casagrande A, Rollin G, Czepielewski MA. Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study. European journal of endocrinology/European Federation of Endocrine Societies. 2012;166(2):207–213. doi: 10.1530/EJE-11-0560. [DOI] [PubMed] [Google Scholar]
- 42.Mincey BA, Duh MS, Thomas SK, Moyneur E, Marynchencko M, Boyce SP, Mallett D, Perez EA. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clinical breast cancer. 2006;7(2):127–132. doi: 10.3816/CBC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 43.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJH, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. The Lancet. 2007;369(9561):559–570. doi: 10.1016/s0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 44.Bender CM, Sereika SM, Brufsky AM, Ryan CM, Vogel VG, Rastogi P, Cohen SM, Casillo FE, Berga SL. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause. 2007;14(6):995–998. doi: 10.1097/gme.0b013e318148b28b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lejbak L, Vrbancic M, Crossley M. Endocrine therapy is associated with low performance on some estrogen-sensitive cognitive tasks in postmenopausal women with breast cancer. Journal of clinical and experimental neuropsychology. 2010;32(8):836–846. doi: 10.1080/13803391003596389. [DOI] [PubMed] [Google Scholar]
- 46.Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer. 2008;113(9):2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- 47.Breckenridge LM, Bruns GL, Todd BL, Feuerstein M. Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psycho-oncology. 2012;21(1):43–53. doi: 10.1002/pon.1860. [DOI] [PubMed] [Google Scholar]
- 48.Kilickap S, Hayran M, Cakir B, Cilingiroglu N, Erman M, Buyukdamgaci G, Ozisik Y. Effect of endocrine therapy on quality of life and cognitive functions in patients with breast cancer. Breast care. 2013;8(2):128–132. doi: 10.1159/000350780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(8):1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 50.Hurria A, Patel SK, Mortimer J, Luu T, Somlo G, Katheria V, Ramani R, Hansen K, Feng T, Chuang C, Geist CL, Silverman DH. The effect of aromatase inhibition on the cognitive function of older patients with breast cancer. Clinical breast cancer. 2014;14(2):132–140. doi: 10.1016/j.clbc.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips KA, Aldridge J, Ribi K, Sun Z, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast cancer research and treatment. 2011;126(1):221–226. doi: 10.1007/s10549-010-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seliktar N, Polek C, Brooks A, Hardie T. Cognition in breast cancer survivors: hormones versus depression. Psycho-oncology. 2014 doi: 10.1002/pon.3602. [DOI] [PubMed] [Google Scholar]
- 53.Ribi K, Aldridge J, Phillips KA, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J, Group BIGC International Breast Cancer Study G. Subjective cognitive complaints one year after ceasing adjuvant endocrine treatment for early-stage breast cancer. British journal of cancer. 2012;106(10):1618–1625. doi: 10.1038/bjc.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schilder CMSC, Linn SC, Boogerd W, Beex LV, Gundy CM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psycho-oncology. 2012;21(5):479–487. doi: 10.1002/pon. [DOI] [PubMed] [Google Scholar]
- 55.Bender CMPM, Sereika SM, Brufsky AM, Vogel VG, Rastogi P, Casillo FE, Richey SM, Ryan CM. What Do Perceived Cognitive Problems Reflect. J Support Oncol. 2008;6(5):238–242. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151(3):1153–1160. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]
- 57.Martin S, Jones M, Simpson E, van den Buuse M. Impaired spatial reference memory in aromatase-deficient (ArKO) mice. Neuroreport. 2003;14(15):1979–1982. doi: 10.1097/01.wnr.0000089571.45990.eb. [DOI] [PubMed] [Google Scholar]
- 58.Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(24):8116–8126. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aydin M, Yilmaz B, Alcin E, Nedzvetsky VS, Sahin Z, Tuzcu M. Effects of letrozole on hippocampal and cortical catecholaminergic neurotransmitter levels, neural cell adhesion molecule expression and spatial learning and memory in female rats. Neuroscience. 2008;151(1):186–194. doi: 10.1016/j.neuroscience.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Boon WC, Diepstraten J, van der Burg J, Jones ME, Simpson ER, van den Buuse M. Hippocampal NMDA receptor subunit expression and watermaze learning in estrogen deficient female mice. Brain research Molecular brain research. 2005;140(1–2):127–132. doi: 10.1016/j.molbrainres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Pines A. Surgical menopause and cognitive decline. Climacteric: the journal of the International Menopause Society. 2014;17(5):580–582. doi: 10.3109/13697137.2014.883244. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Yu JT, Wang HF, Tan CC, Meng XF, Tan L. Non-pharmacological interventions for patients with mild cognitive impairment: a meta-analysis of randomized controlled trials of cognition-based and exercise interventions. Journal of Alzheimer’s disease: JAD. 2014;42(2):663–678. doi: 10.3233/JAD-140660. [DOI] [PubMed] [Google Scholar]
- 63.Ohman H, Savikko N, Strandberg TE, Pitkala KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dementia and geriatric cognitive disorders. 2014;38(5–6):347–365. doi: 10.1159/000365388. [DOI] [PubMed] [Google Scholar]
- 64.Kramer SCaAF. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychological science. 2003;14(2):123–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 65.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of physical medicine and rehabilitation. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Nascimento CMPJ, de Andrade LP, Garuffi M, Talib LL, Forlenza OV, Cancela JM, Cominetti MR, Stella F. Physical Exercise in MCI Elderly Promotes Reduction of Pro-Inflammatory Cytokines and Improvements on Cognition and BDNF Peripheral Levels. Curr Alzheimer Res. 2014;11(8):799–805. doi: 10.2174/156720501108140910122849. [DOI] [PubMed] [Google Scholar]
- 67.Niemann C, Godde B, Staudinger UM, Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience. 2014;281C:147–163. doi: 10.1016/j.neuroscience.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 68.Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: an interventional study. Neurobiology of aging. 2011;32(7):1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein C, Knecht S. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010;49(3):2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 70.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiology of learning and memory. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 71.McNerney MW, Radvansky GA. Mind racing: The influence of exercise on long-term memory consolidation. Memory. 2014:1–12. doi: 10.1080/09658211.2014.962545. [DOI] [PubMed] [Google Scholar]
- 72.Pesce C, Crova C, Cereatti L, Casella R, Bellucci M. Physical activity and mental performance in preadolescents: Effects of acute exercise on free-recall memory. Mental Health and Physical Activity. 2009;2(1):16–22. doi: 10.1016/j.mhpa.2009.02.001. [DOI] [Google Scholar]
- 73.Guiney H, Lucas SJ, Cotter JD, Machado L. Evidence Cerebral Blood-Flow Regulation Mediates Exercise-Cognition Links in Healthy Young Adults. Neuropsychology. 2014 doi: 10.1037/neu0000124. [DOI] [PubMed] [Google Scholar]
- 74.Kitamura N, Araya R, Kudoh M, Kishida H, Kimura T, Murayama M, Takashima A, Sakamaki Y, Hashikawa T, Ito S, Ohtsuki S, Terasaki T, Wess J, Yamada M. Beneficial effects of estrogen in a mouse model of cerebrovascular insufficiency. PloS one. 2009;4(4):e5159. doi: 10.1371/journal.pone.0005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pradhan KR, Stump TE, Monahan P, Champion V. Relationships among attention function, exercise, and body mass index: a comparison between young breast cancer survivors and acquaintance controls. Psycho-oncology. 2014 doi: 10.1002/pon.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Experimental gerontology. 2010;45(5):357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. The European journal of neuroscience. 2006;24(5):1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 78.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiology of disease. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 79.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of neurophysiology. 2002;88(5):2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 80.Alomari MA, Khabour OF, Alzoubi KH, Alzubi MA. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behavioural brain research. 2013;247:34–39. doi: 10.1016/j.bbr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Gomes da Silva S, Unsain N, Masco DH, Toscano-Silva M, de Amorim HA, Silva Araujo BH, Simoes PS, da Naffah-Mazzacoratti MG, Mortara RA, Scorza FA, Cavalheiro EA, Arida RM. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus. 2012;22(2):347–358. doi: 10.1002/hipo.20903. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. The European journal of neuroscience. 2008;28(11):2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European journal of neuroscience. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 84.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 85.Fabel KFK, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. <VEGF is necessary for exercise-induced adult hippocampal neurogenesis..pdf>. The European journal of neuroscience. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 86.Amédée T, Kohman RA, Rodriguez-Zas SL, Southey BR, Kelley KW, Dantzer R, Rhodes JS. Voluntary Wheel Running Reverses Age-Induced Changes in Hippocampal Gene Expression. PloS one. 2011;6(8):e22654. doi: 10.1371/journal.pone.0022654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. The European journal of neuroscience. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zandi PPBJ, Anthony JC. Is pharmacological prevention of Alzheimer’s a realistic goal. Expert Opin Pharmacother. 2002;3(4):365–380. doi: 10.1517/14656566.3.4.365. [DOI] [PubMed] [Google Scholar]
- 89.Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H, Liu Y. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neuroscience letters. 2014;573:13–18. doi: 10.1016/j.neulet.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 90.Siebert C, Kolling J, Scherer EB, Schmitz F, da Cunha MJ, Mackedanz V, de Andrade RB, Wannmacher CM, Wyse AT. Effect of physical exercise on changes in activities of creatine kinase, cytochrome c oxidase and ATP levels caused by ovariectomy. Metabolic brain disease. 2014;29(3):825–835. doi: 10.1007/s11011-014-9564-x. [DOI] [PubMed] [Google Scholar]
- 91.Ben J, Soares FM, Scherer EB, Cechetti F, Netto CA, Wyse AT. Running exercise effects on spatial and avoidance tasks in ovariectomized rats. Neurobiology of learning and memory. 2010;94(3):312–317. doi: 10.1016/j.nlm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Ben J, Soares FM, Cechetti F, Vuaden FC, Bonan CD, Netto CA, Wyse AT. Exercise effects on activities of Na(+),K(+)-ATPase, acetylcholinesterase and adenine nucleotides hydrolysis in ovariectomized rats. Brain research. 2009;1302:248–255. doi: 10.1016/j.brainres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 93.Berchtold NCKJ, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. The European journal of neuroscience. 2001;14(12):1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 94.Moreno-Piovano GS, Varayoud J, Luque EH, Ramos JG. Long-term ovariectomy increases BDNF gene methylation status in mouse hippocampus. The Journal of steroid biochemistry and molecular biology. 2014;144(Pt B):243–252. doi: 10.1016/j.jsbmb.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Marosi K, Felszeghy K, Mehra RD, Radak Z, Nyakas C. Are the neuroprotective effects of estradiol and physical exercise comparable during ageing in female rats? Biogerontology. 2012;13(4):413–427. doi: 10.1007/s10522-012-9386-3. [DOI] [PubMed] [Google Scholar]