Abstract
Objective
Newborns requiring hospitalisation frequently undergo painful procedures. Prevention of pain in infants is of prime concern because of adverse associations with physiological and neurological development. However, pain mitigation is currently guided by behavioural observation assessments that have not been validated against direct evidence of pain processing in the brain. The aim of this study was to determine whether cry presence or amplitude is a valid indicator of pain processing in newborns.
Design
Prospective observational cohort.
Setting
Newborn nursery.
Patients
Healthy infants born at >37 weeks and <42 weeks gestation.
Interventions
We prospectively studied newborn cortical responses to light touch, cold and heel stick, and the amplitude of associated infant vocalisations using our previously published paradigms of time-locked electroencephalogram (EEG) with simultaneous audio recordings.
Results
Latencies of cortical peak responses to each of the three stimuli type were significantly different from each other. Of 54 infants, 13 (24%), 19 (35%) and 35 (65%) had cries in response to light touch, cold and heel stick, respectively. Cry in response to non-painful stimuli did not predict cry in response to heel stick. All infants with EEG data had measurable pain responses to heel stick, whether they cried or not. There was no association between presence or amplitude of cries and cortical nociceptive amplitudes.
Conclusions
In newborns with distinct brain responses to light touch, cold and pain, cry presence or amplitude characteristics do not provide adequate behavioural markers of pain signalling in the brain. New bedside assessments of newborn pain may need to be developed using brain-based methodologies as benchmarks in order to provide optimal pain mitigation.
INTRODUCTION
Prevention of pain in newborns is essential because repeated painful exposures have short-term and long-term adverse effects on physiological and neurological development.1 Because the development of somatosensory processing after birth is dependent on early sensory experience, atypical exposures can have grave consequences for long-term sensory processing and pain perception.2,3 Procedures such as heel sticks, venipunctures or intubations are associated with lower cognitive and motor scores in early childhood and with later alterations in visual-perceptual ability and somatosensory sensitivity.4,5
Optimal neonatal pain management requires valid non-verbal pain assessment, yet clinical methodology to assess neonatal pain is rudimentary. Semiquantitative scales of behavioural observations are widely used, however their validation rarely involves comparison to non-painful stimuli. Similarly, cry acoustic measures are sometimes associated with behavioural scales but not with nociceptive (pain) processing6 in the brain.5 Amplitude of cortical nociceptive-evoked potentials accurately reflects the intensity of pain perception.7,8 Furthermore, functional MRI (fMRI) evidence in both adults and infants demonstrates activation of the anterior cingulate cortex (emotional processing) in response to painful stimuli, indicating that infants process pain even if they do not exhibit adult behavioural expressions of pain.7,8 Therefore, brain-based methodologies can objectively and quantitatively detect cortical responses to somatosensory stimuli, including pain, even in infants and preverbal children.9–13
Assessment of infant cries is a potential approach to quantitative bedside evaluation of pain, logistically less challenging than brain-based techniques. However, the association between presence or loudness of a cry (amplitude) and cortical intensity of nociceptive signals is not known; nor is the specificity of the cry response to a painful stimulus compared with other somatosensory inputs, such as pressure due to light touch or changes in temperature. We therefore performed a prospective study using time-locked EEG methodologies to test the hypothesis that presence of cry and acoustic amplitudes of cries during painful procedures in infants are associated with amplitude of the brain nociceptive responses, and are distinct from responses to light touch and cold.
METHODS
We included healthy term infants born at >37 weeks and <42 weeks gestation, between 24 hours and 72 hours of age, before blood sampling for their state-mandated metabolic screen. Infants had been examined by board-certified paediatricians and diagnosed as healthy term newborns with no major congenital abnormalities or illnesses since birth. We excluded infants with signs of neonatal abstinence syndrome, with mothers having a history of substance abuse, and who had skin punctures or circumcisions prior to data collection. We recorded demographic information, time of breast feeding or bottle feeding preceding the testing and total bilirubin level if indicated at testing time.
We recorded EEG using a previously described event-related potential (ERP) system.14,15 Infants were tested in a quiet room in an examiner’s lap, in drowsy or quiet alert states. No infants were tested in skin-to-skin holds. No sucrose or other oral solutions were used during the procedure, as per standard practice in the Newborn Nursery. A 128-channel EEG soft-sponge net (Geodesic Sensor Net, EGI, Eugene, Oregon, USA) soaked in warm saline was applied to the infant’s head. As per published protocols, the midline Cz electrode was used as the reference14 (figure 1), sampling was every 1 ms with filters set at 0.1–400 Hz, continuously recorded using Net Station V.4.3; (EGI, Eugene, Oregon, USA). We recorded vocalisations using a portable high-quality field recorder (TASCAM DR-100) with a unidirectional external microphone located 25 cm from the subject’s mouth. Cry samples were digitised using a 32-bit analogue to digital converter at 44.1 kHz sampling rate.
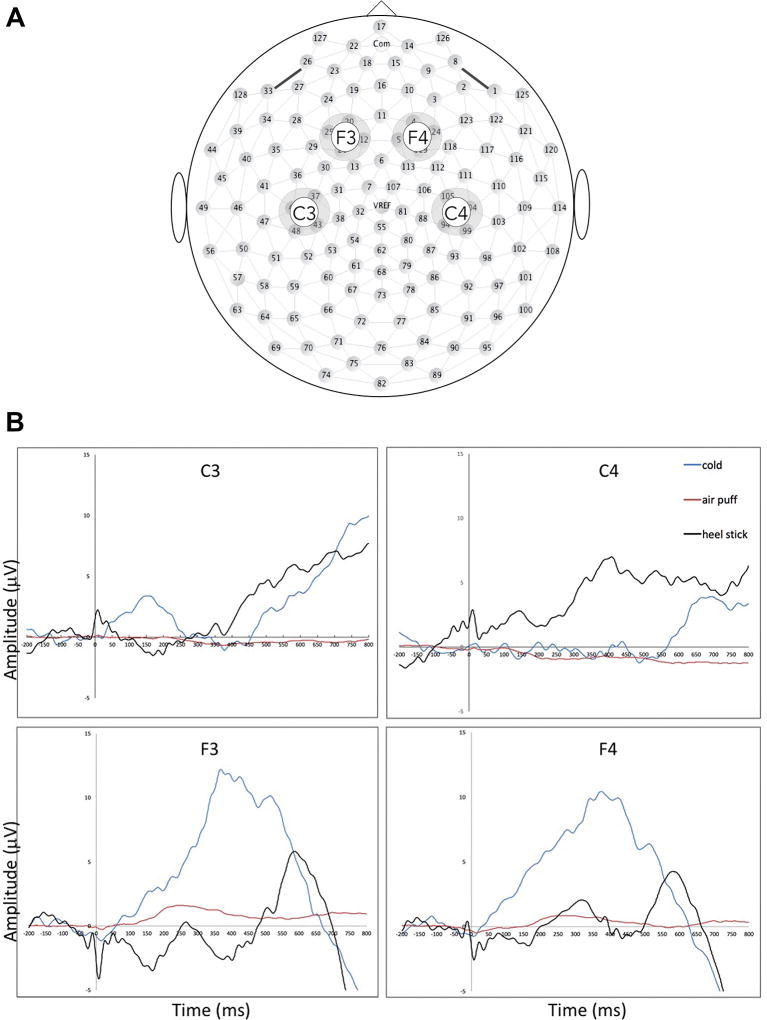
Figure 1.
(A) Distribution of electrodes and scalp locations of interest. Scalp locations for analysis and grand averaged waveform were as follows: F3, frontal left; F4, frontal right; C3, central left, C4, central right. (B) Cold, air puff and heel stick response amplitude across time. Comparison of cortical responses to air puff, cold, and heel stick stimulus. All tracings represent grand averaged waveforms of all patients with available data across all valid trials. Stimulus occurs at time 0 ms.
The system14–16 delivered tactile stimuli supplied from a standard medical air outlet reduced by a regulator to a steady 5 pounds per square inch (psi) at the skin. Two valves produced either a stimulus at the foot or a sham (air puff directed away from foot), with 50 air puffs and 50 shams (20 ms duration) delivered at randomly varying intervals between 2000 ms and 2500 ms. E-prime software V. 2.0 (PST, Pittsburgh, Pennsylvania, USA) triggered the puffs. The apparatus included custom inputs that detected trigger releases on the cold spray and heel stick, converting them to distinct markings in the EEG, using an interface modified from Slater and colleagues.10 A single administration of a medical cold spray (Medi-First Topical Skin Cold Spray) generated a temperature of 4°C at the skin when triggered for 0.5 s at 10 cm from the foot. Due to spray diffusion, the stimulus generated <1 psi at the skin. A 2 min wait followed to rewarm the heel with a pad. Heel stick sampling required for routine state-mandated laboratory testing provided the pain stimulus. Heel sticks were performed by two physicians who underwent manualised training for heel stick collection (including written and standardised video material). A Newborn Nursery nurse trainer required 100% compliance with the protocol from both physicians. Because every heel stick was preceded by heel warming, no manual squeezing was necessary to obtain the initial data recordings or drops of blood for the newborn screen. When squeezing did occur, it was after >2 min, well beyond the EEG and audio recording times.
The sequence of recording events was standardised and maintained across all subjects as follows: ERP net placement (5 min), tactile paradigm (6 min), cold puff (2 min), rewarming (2 min), heel stick (1 min).
Preprocessing of audio recording performed using Adobe Audition CS6 removed identifying information and extraneous noise. The acoustic analysis approach was based on the source-filter model of speech production.17 Mean values were subtracted to compensate for variability during recording and remaining signals were divided into 25 ms frames comprising periods of voiced waveform for the signals to appear quasistationary and to contain at least one cycle of the desired frequency.18
ERP data were filtered using a 0.3–40 Hz bandpass filter and segmented.19,20 Segments contaminated by motor or ocular artefacts were excluded using standard algorithms included in NetStation and verified by manual review. Four scalp regions of interest were defined using clusters of electrodes identified in published studies of somatosensory stimuli (figure 1). Time windows after stimulus onset were 250–350 ms for air puff and 350–700 ms for heel stick.21 For cold, mean amplitudes were calculated across 100 ms intervals centred on the maximal amplitude of the grand averaged response (320–420 ms) as this stimulus was not previously described in infants. Latency to peak maxima were determined for each stimulus.
Sample Power: we initially powered the study to distinguish cry acoustics during painful versus non-painful stimuli. The effect size for the difference (Cohen’s d=0.81) was based on fundamental frequency (F0) values between infants with rated pain scores.22 Assuming a correlation between conditions of r=0.30 and a two-tailed p < 0.05, 26 subjects would allow a power of 0.90. Allowing for 15% data loss due to unanalysable recordings, we proposed a sample of n = 30 infants. We continued enrolment until obtaining the planned sample of subjects with cries.
Statistical analyses were performed using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA), with two-sided p-values <0.05 considered statistically significant. Normality assumption was confirmed for all models and Kenward-Roger adjustment accounted for unequal variances across groups.23 Paired-test comparisons studied latency differences to response maximum in individual subjects for each pair (puff-stick, puff-cold, cold-stick) and compared cry amplitude characteristics between stick versus cold, among infants who cried after both stimuli. Z-tests for differences in paired proportions determined whether infants who cried to puff, cold, or both puff and cold were more likely to also cry to pain. Repeated measures analysis of variance with scalp location as a within-subject repeated factor compared ERP amplitudes between subjects with cry versus none, and quantified associations between cry characteristics and ERP mean amplitudes among infants who cried. Where significant interactions indicated that estimated effects of stimulus, cry status or cry amplitude differed by scalp region, we assessed comparisons within each region, while taking the covariance structure of the repeated factors into account. When no significant interactions were noted, we computed marginal means and associations, averaged over scalp regions. The Bonferroni-Holm procedure adjusted for multiple comparisons.24 When analysing cry versus ERP amplitudes, we excluded those infants who had a grimace but no audible cry.
RESULTS
We studied 54 full-term infants (table 1). Data for one participant were excluded due to an insufficient number of artefact-free ERP trials in all conditions. Of the remaining 53 subjects, all had measurable, artefact-free cortical responses for light touch, 32 for cold and 33 for heel stick response.
Table 1.
Demographics
| n=54 | % | |
|---|---|---|
| EGA in weeks, median (IQR) | 39 (38, 40) | |
| Male sex | 25 | 46.2 |
| Race | ||
| Caucasian | 43 | 79.6 |
| African-American | 8 | 14.8 |
| Other | 3 | 5.5 |
| Hispanic ethnicity | 4 | 7.4 |
| Hours between feeding and heel stick, median (IQR) | 1.8 (0.8, 3.1) | |
| Bilirubin drawn at time of heel stick | 8 | 14.8 |
| Total bilirubin level (median, IQR)* | 8.7 (7.8, 10.1) |
One subject’s total bilirubin level drawn again 75 min later was 17.9 mg/dL, phototherapy followed.
IQR: 25th, 75th.
EGA, estimated gestational age at birth.
All infants with motion-artefact-free waveforms displayed measurable responses to heel stick and cold. Grand averaged waveforms are shown in figure 1. Latencies of cortical peak responses to each stimulus type were significantly different from each other (table 2). Mean ERP amplitudes to all stimuli were normally distributed (table 3). Responses to puff were significantly different from sham at all locations and in all subjects (p < 0.001).
Table 2.
Latency to maximal stimulus response at selected electrode clusters
| Electrode location |
Stimulus type | n | ms to maximum response (SD) | p value |
|---|---|---|---|---|
| C3 | Light touch versus pain | 33 | 301 (40.8) vs 569 (122.2) | <0.001 |
| Pain versus cold | 22 | 570 (120.9) vs 382 (42.5) | <0.001 | |
| Cold versus light touch | 31 | 394 (42.4) vs 297 (43.3) | <0.001 | |
| C4 | Light touch versus pain | 33 | 308 (39.8) vs 523 (132.3) | <0.001 |
| Pain versus cold | 22 | 522 (134.3) vs 379 (46.6) | <0.001 | |
| Cold versus light touch | 31 | 382 (41.9) vs 301 (37.5) | <0.001 | |
| F3 | Light touch versus pain | 33 | 292 (40.1) vs 532 (124.7) | <0.001 |
| Pain versus cold | 22 | 512 (130.8) vs 387 (34.8) | <0.001 | |
| Cold versus light touch | 31 | 384 (37.0) vs 293 (41.6) | <0.001 | |
| F4 | Light touch versus pain | 33 | 292 (38.2) vs 506 (118.3) | <0.001 |
| Pain versus cold | 22 | 494 (120.5) vs 390 (43.4) | <0.001 | |
| Cold versus light touch | 31 | 376 (45.3) vs 293 (41.1) | <0.001 |
F3, frontal left; F4, frontal right; C3, central left, C4, central right; ms, milliseconds.
Table 3.
Estimated mean stimulus response at selected electrode clusters in stimulus-associated time windows (all results in microvolts)
| Location | Stimulus | Mean | SE | Lower 95% CL |
Upper 95% CL |
|---|---|---|---|---|---|
| F3 | Cold | 10.95 | 3.37 | 4.08 | 17.83 |
| F3 | Pain | 1.20 | 2.91 | −4.71 | 7.12 |
| F3 | Puff | 1.43 | 0.28 | 0.87 | 1.99 |
| F3 | Sham | 0.54 | 0.31 | −0.08 | 1.16 |
| F4 | Cold | 9.79 | 3.10 | 3.46 | 16.12 |
| F4 | Pain | 0.97 | 2.46 | −4.04 | 5.97 |
| F4 | Puff | 0.79 | 0.26 | 0.27 | 1.31 |
| F4 | Sham | 0.15 | 0.28 | −0.42 | 0.72 |
| C3 | Cold | −0.50 | 2.30 | −5.18 | 4.18 |
| C3 | Pain | 4.18 | 2.24 | −0.38 | 8.75 |
| C3 | Puff | −0.41 | 0.25 | −0.92 | 0.10 |
| C3 | Sham | −0.18 | 0.19 | −0.56 | 0.19 |
| C4 | Cold | −0.55 | 3.29 | −7.27 | 6.16 |
| C4 | Pain | 5.50 | 3.87 | −2.37 | 13.37 |
| C4 | Puff | −0.90 | 0.23 | −1.36 | −0.44 |
| C4 | Sham | −0.56 | 0.26 | −1.08 | −0.04 |
F3, frontal left; F4, frontal right; C3, central left, C4: central right.
CL, confidence level.
Infants with and without audible cries
Of 54 infants, 13 (24%), 19 (35%) and 35 (65%) had cries in response to puff, cold and heel stick, respectively. There were no associations at group or individual levels between crying in response to the first two conditions and crying to heel stick. Specifically, the risk that an infant would cry after a heel stick was not associated with the risk of the same infant previously crying from an air puff with a paired proportion of 0.488 CI (0.348 to 0.641) no-cry-to-puff versus 0.615 CI (0.351 to 0.880) for cry-to-puff (p=0.422). Similarly, the risk that an infant would cry after a heel stick was not associated with the risk of the same infant previously crying from cold stimulus (0.486 CI (0.325 to 0.647) for no-cry-to-cold versus 0.588 CI (0.354 to 0.822) for cry-to-cold (p=0.487)). Similar results were found in infants who cried neither to cold nor puff (0.4706 CI (0.3028 to 0.6384) vs 0.6 CI (0.3853 to 0.8147), (p=0.358)).
Infants with ERP data
Thirty-two infants with cortical responses to light touch had analysable data after cold stimulation. Of these, 4 had an audible cry, 4 had a grimace but no cry and 26 had no audible cry or grimace. ERP amplitudes to cold stimulation at any location were not statistically different between infants who cried and those who did not (all p > 0.5).
Of 33 infants with cortical responses to both light touch and heel stick, 16 had an audible cry, 3 had a grimace but no cry and 14 had no audible cry or grimace. There was no statistically significant interaction between cry status and scalp location (p = 0.212). Marginal mean ERP pain responses for infants who did or did not cry were not statistically different (5.51 vs 0.91 µV, p = 0.193) (table 4).
Table 4.
Comparison of cortical responses to heel stick for infants with and without audible cries—estimated mean amplitudes in 500–700 ms time window
| Group | LS-mean | SE | Lower 95% CL |
Upper 95% CL |
p Vvalue |
|---|---|---|---|---|---|
| No cry | 0.91 | 1.95 | −3.07 | 4.88 | 0.1398 |
| Cry | 5.51 | 2.33 | 0.76 | 10.26 |
CL, confidence level; LS-mean, marginal mean.
The proportion of variance in ERP amplitude attributed to cry status was only 3%. Marginal means and 95% CIs for ERP amplitude among infants who cried versus didn’t cry showed considerable overlap, suggesting that even with a larger sample size there would be no significant difference.
Among infants who cried in response to pain, we found no significant association between ERP amplitude and cry amplitude characteristics (table 5) even in individual scalp locations (see online supplementary figure). We found no significant associations between time of breast/bottle feeding before testing or bilirubin levels with either cry presence or cry amplitude.
Table 5.
Lack of associations between cry amplitude characteristics and amplitudes of ERP responses
| Cry amplitude variable |
Estimated effect |
SE | Lower 95% CL |
Upper 95% CL |
p Value |
|---|---|---|---|---|---|
| Mean | −51.97 | 43.53 | −144.49 | 40.55 | 0.25 |
| Minimum | −45.20 | 26.60 | −101.72 | 11.33 | 0.11 |
| Maximum | 3.98 | 2.71 | −1.81 | 9.78 | 0.16 |
| Range | 4.16 | 2.56 | −1.32 | 9.64 | 0.13 |
CL, confidence level. ERP, event-related potential.
Estimated effect is interpreted as a 1 unit increase in cry amplitude characteristic corresponds to an increase/decrease in ERP amplitude.
DISCUSSION
In contrast to our hypothesis, we found that cortical responses to pain following a heel stick are often not associated with either presence or amplitude of cries in term infants who have intact processing of light touch and cold. Nearly half of infants with measurable cortical responses to pain had no audible cry or grimace, behaviours traditionally associated with infants’ expression of physical pain and often used clinically to identify pain in infants. Infants cried to both painful and non-painful stimuli, and more infants overall cried in response to pain than to light touch or cold stimuli. However, in individual infants, we found no association between crying in response to one type of stimulus versus another.
The three types of somatosensory stimuli elicited signal processes in the brain that were distinct from each other, consistent with published literature on pain and touch. No other study has reported the cold response in infants. In adults, light touch, a non-nociceptive stimulus, activates low-pressure mechanoreceptors, resulting in neural signals conducted through Aβ myelinated fibres through the dorsal column-medial lemniscal system to the thalamus and eventually the somatosensory cortex.25 Cold activates thermosensitive excitatory transient receptors, Aδ and pain activates polymodal receptors; both are conducted through the anterolateral system.26 Polymodal nociceptors use slow conducting unmyelinated C-fibres and displayed a late response on ERP, as in previous infant work.6,10 Our ERP results are also consistent with published findings for touch and pain,27,28 indicating that our selected measures were appropriate and sensitive, even in newborns. Furthermore, the three stimulus types (touch, cold and pain) generated distinct neural responses, demonstrating that the infants we studied process noxious and benign somatosensory stimuli differently. Distinct cortical responses to pain in combination with a lack of stimulus-related differences in crying, further supports the contention that crying may not be an informative marker of pain experience in newborns.
In those infants who cried, we measured cry amplitude as a possible behavioural marker of pain response. While infant cry is presumed to be a common, possibly automatic, response to distress,29 a large proportion of infants in our study did not generate vocalisations in response to heel stick even though they processed the stimulation appropriately. Newborns’ cries are usually innate vocal reactions dependent on limbic vocal pathways rather than more complex laryngeal cortical pathways30 and it is possible that the connectivity necessary for these pathways remains immature at birth. In addition, laryngeal structures and control in response to stress are still immature in newborn infants.31 The use of cries for vocal communication, beyond brainstem-controlled cries, has also been demonstrated to involve motor learning.32 Because our study population was 48–72 hours old, systems responsible for vocal production may be too immature to deliver an audible cry reliably and in close temporal proximity to a painful stimulus. The current findings are consistent with reports suggesting that processing of pain responses, including associated behavioural manifestations, continues to develop several months after term birth.9,10
Our initial sample size was estimated to be representative of the term infant population. However, only 60% of the infants with cortical response to light touch had useable data for cold and pain stimuli. This discrepancy was due to the limitation of administering only one trial of cold and pain conditions, stimuli that could elicit motion artefact through reflex withdrawal. Although single-trial time-locked EEG studies are not optimal to study mechanistic processes, seminal studies on infant pain processing have resulted from this approach6,10 and used similar population sizes. Furthermore, while infant ERP data in our study were normally distributed and most likely representative of term newborns, we used a limited behavioural data set (cry and grimace). In addition, cry amplitude is only one acoustic property, and onset time, power in specific frequencies, and duration may be more informative.
Although brain-based measures appear more sensitive to pain than infant cries, it is not feasible to monitor brain activity during each painful experience in the clinical setting, especially for infants with prolonged hospitalisations. Human and animal evidence suggests that long-term outcomes may be related to changes in neural connections established between the thalamus and sensory cortex after exposure to painful stimuli in critical windows of development,2,33,34 often coincident with hospitalisations for ill or preterm newborns. Thus, continued examination of brain-behaviour connections for potentially accurate behavioural markers of pain remains critical to improving the outcomes of high-risk infants. Because maturation contributes to brain and behavioural responses, future studies should account for developmental differences in response to pain between full-term and preterm infants and changes in sensitivity or overt reactions to noxious stimuli over time.
CONCLUSION
In term newborns with distinct cortical responses to light touch, cold and pain, presence of cry or cry amplitude characteristics do not provide adequate behavioural markers of pain signals in the brain. These findings have important implications for clinical care, procedural support and pain assessment methods in newborns. Clinicians should be aware that newborns experience pain in response to skin-breaking procedures whether or not overt behavioural manifestations are present.
Supplementary Material
What is already known on this topic?
-
►
Due to potential short-term and long-term developmental consequences, mitigation of procedural pain in newborns is a priority.
-
►
It is unclear at this time whether behavioural assessment of pain using newborn crying reflects actual pain experience.
What this study adds?
-
►
Newborns have distinct brain responses to light touch, cold and pain.
-
►
However, crying is not specific to pain nor does it reflect pain experience in the brain.
-
►
Cry amplitude characteristics also do not provide adequate behavioural markers of pain signalling in the brain.
Acknowledgments
Funding This work was supported by the Hobbs Discovery Grant from the Vanderbilt Kennedy Center, NICHD grants [P30HD015052], [U54HD083211], [K23H-D074736(01A1], [1R01HD081120(01A1], NCATS/NIH [UL1 TR000445].
Footnotes
Contributors NLM: designed the study in its final form, participated in data collection, carried out preliminary analyses, wrote, reviewed and revised all drafts the manuscript and approved the final manuscript as submitted. ARS: contributed to study interpretation, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted. CCMM: participated in the study design and in data collection, drafted the initial manuscript, and approved the final manuscript as submitted. ODC: participated in data collection, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted. DJF: designed the study in its final form, carried out preliminary analyses, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted. AFK: participated in data collection, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted. KW: designed the touch apparatus, carried out preliminary analyses, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted. MMC: designed and performed the final statistical analyses and approved the final manuscript as submitted. DMW: carried out preliminary analyses, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted. SB: designed the study in its final form, carried out preliminary analyses, reviewed and revised all drafts of the manuscript and approved the final manuscript as submitted.
Competing interests None declared.
Ethics approval Vanderbilt University Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Keels E, Sethna N, Watterberg KL, et al. Committee on fetus and newborn and section on anesthesiology and pain medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016;137:e20154271. doi: 10.1542/peds.2015-4271. [DOI] [PubMed] [Google Scholar]
- 2.Walker SM, Beggs S, Baccei ML. Persistent changes in peripheral and spinal nociceptive processing after early tissue injury. Exp Neurol. 2016;275:253–60. doi: 10.1016/j.expneurol.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmelzle-Lubiecki BM, Campbell KA, Howard RH, et al. Long-term consequences of early infant injury and trauma upon somatosensory processing. Eur J Pain. 2007;11:799–809. doi: 10.1016/j.ejpain.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–46. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller B. Meanings of discomfort and fussy-irritable in infant pain assessment. J Pediatr Health Care. 1996;10:255–63. doi: 10.1016/s0891-5245(96)90051-6. [DOI] [PubMed] [Google Scholar]
- 6.Fabrizi L, Slater R, Worley A, et al. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr Biol. 2011;21:1552–8. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MC, Mouraux A, Iannetti GD. Characterizing the cortical activity through which pain emerges from nociception. J Neurosci. 2009;29:7909–16. doi: 10.1523/JNEUROSCI.0014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts K, Papadaki A, Gonçalves C, et al. Contact heat evoked potentials using simultaneous EEG and fMRI and their correlation with evoked pain. BMC Anesthesiol. 2008;8:8. doi: 10.1186/1471-2253-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slater R, Cantarella A, Franck L, et al. How well do clinical pain assessment tools reflect pain in infants? PLoS Med. 2008;5:e129. doi: 10.1371/journal.pmed.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slater R, Worley A, Fabrizi L, et al. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;14:321–6. doi: 10.1016/j.ejpain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Williams G, Fabrizi L, Meek J, et al. Functional magnetic resonance imaging can be used to explore tactile and nociceptive processing in the infant brain. Acta Paediatr. 2015;104:158–66. doi: 10.1111/apa.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoshnejad M, Piché M, Saleh S, et al. Serial processing in primary and secondary somatosensory cortex: a DCM analysis of human fMRI data in response to innocuous and noxious electrical stimulation. Neurosci Lett. 2014;577:83–8. doi: 10.1016/j.neulet.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Schweizer R, Voit D, Frahm J. Finger representations in human primary somatosensory cortex as revealed by high-resolution functional MRI of tactile stimulation. Neuroimage. 2008;42:28–35. doi: 10.1016/j.neuroimage.2008.04.184. [DOI] [PubMed] [Google Scholar]
- 14.Maitre NL, Barnett ZP, Key AP, Apf K. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol. 2012;27:1276–83. doi: 10.1177/0883073811435682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitre NL, Key AP. Quantitative assessment of cortical auditory-tactile processing in children with disabilities. J Vis Exp. 2014:e51054–4. doi: 10.3791/51054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitre NL, Henderson G, Gogliotti S, et al. Feasibility of event-related potential methodology to evaluate changes in cortical processing after rehabilitation in children with cerebral palsy: a pilot study. J Clin Exp Neuropsychol. 2014;36:669–79. doi: 10.1080/13803395.2014.925094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent RD, Kim Y, et al. Acoustic analysis of speech. In: Ball MJ, Perkins MR, Mller N, Sara H, editors. The handbook of clinical linguistics. Oxford, UK: Blackwell Publishing Ltd; 2008. pp. 360–80. [Google Scholar]
- 18.France DJ, Shiavi RG, Silverman S, et al. Acoustical properties of speech as indicators of depression and suicidal risk. IEEE Trans Biomed Eng. 2000;47:829–37. doi: 10.1109/10.846676. [DOI] [PubMed] [Google Scholar]
- 19.Michail G, Dresel C, Witkovský V, et al. Neuronal oscillations in various frequency bands differ between pain and touch. Front Hum Neurosci. 2016;10:300. doi: 10.3389/fnhum.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz E, Tiemann L, Schuster T, et al. Neurophysiological coding of traits and states in the perception of pain. Cereb Cortex. 2011;21:2408–14. doi: 10.1093/cercor/bhr027. [DOI] [PubMed] [Google Scholar]
- 21.Worley A, Fabrizi L, Boyd S, et al. Multi-modal pain measurements in infants. J Neurosci Methods. 2012;205:252–7. doi: 10.1016/j.jneumeth.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellieni CV, Sisto R, Cordelli DM, et al. Cry features reflect pain intensity in term newborns: an alarm threshold. Pediatr Res. 2004;55:142–6. doi: 10.1203/01.PDR.0000099793.99608.CB. [DOI] [PubMed] [Google Scholar]
- 23.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97. [PubMed] [Google Scholar]
- 24.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 25.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–39. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björnsdotter M, Löken L, Olausson H, et al. Somatotopic organization of gentle touch processing in the posterior insular cortex. J Neurosci. 2009;29:9314–20. doi: 10.1523/JNEUROSCI.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slater R, Cornelissen L, Fabrizi L, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet. 2010;376:1225–32. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verriotis M, Fabrizi L, Lee A, et al. Cortical activity evoked by inoculation needle prick in infants up to one-year old. Pain. 2015;156:222–30. doi: 10.1097/01.j.pain.0000460302.56325.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahola Kohut S, Pillai Riddell R, Flora DB, Kohut SA, Riddell RP, et al. A longitudinal analysis of the development of infant facial expressions in response to acute pain: immediate and regulatory expressions. Pain. 2012;153:2458–65. doi: 10.1016/j.pain.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. Neuroscientist. 2011;17:197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva M, Mijovic B, Van den Bergh BR, et al. Decoupling between fundamental frequency and energy envelope of neonate cries. Early Hum Dev. 2010;86:35–40. doi: 10.1016/j.earlhumdev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Schneider DM, Mooney R. Motor-related signals in the auditory system for listening and learning. Curr Opin Neurobiol. 2015;33:78–84. doi: 10.1016/j.conb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014;4:57–67. doi: 10.2217/pmt.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunau RE. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med J. 2013;4:e0025. doi: 10.5041/RMMJ.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.