Abstract
Background
Reduced lung function is associated with clinical outcomes like cardiovascular disease. However, little is known about its association with incident end-stage renal disease (ESRD) and chronic kidney disease (CKD).
Study Design
Prospective cohort study.
Setting & Participants
14,946 participants aged 45–64 years at baseline (1987–1989) in the Atherosclerosis Risk in Communities (ARIC) Study (45.0% male and 25.2% black), with follow-up through 2012.
Predictors
Race- and sex-specific quartiles of percent-predicted forced vital capacity (FVC) and the proportion of forced expiratory volume in 1 second of expiration to FVC (FEV1/FVC) at baseline determined with spirometry.
Outcomes
Incident ESRD (defined here as renal replacement therapy or death due to CKD) as the primary outcome and incident CKD (defined here as ESRD, ≥25% decline in estimated glomerular filtration rate to a level <60 mL/min/1.73 m2, or CKD-related hospitalizations/deaths) as the secondary outcome.
Results
During a median follow-up of 23.6 years, 526 (3.5%) participants developed ESRD. After adjusting for potential confounders, the cause-specific HR of incident ESRD for the lowest (versus highest) quartile was 1.72 (95% CI, 1.31–2.26) for percent-predicted FVC and 1.33 (95% CI, 1.03–1.73) for FEV1/FVC. Compared with high normal lung function pattern, mixed pattern (ie, percent-predicted FVC <80% and FEV1/FVC <70%; 3.4% of participants) demonstrated the highest adjusted cause-specific HR of ESRD at 2.28 (95% CI, 1.50–3.45), followed by the restrictive pattern (ie, percent-predicted FVC <80% and FEV1/FVC ≥70%; 4.8% of participants) at 2.03 (95% CI, 1.47–2.81), obstructive pattern (ie, percent-predicted FVC ≥80% and FEV1/FVC <70%; 18.9% of particpants) at 1.47 (95% CI, 1.09–1.99), and low-normal pattern (ie, (percent-predicted FVC 80–<100% and FEV1/FVC ≥70%, or percent-predicted FVC ≥80% and FEV1/FVC 70–<75%; 44.3% of participants) at 1.21 (95% CI, 0.94–1.55). Similar associations were seen with incident CKD.
Limitations
Limited number of participants with moderate/severe lung dysfunction and spirometry only at baseline.
Conclusions
Reduced lung function, particularly lower percent-predicted FVC, is independently associated with CKD progression. Our findings suggest potential pathophysiological contribution of reduced lung function to the development of CKD and need of monitoring kidney function in persons with reduced lung function.
INDEX WORDS: Lung function, restrictive lung function, obstructive lung function, spirometry, chronic kidney disease (CKD), end-stage renal disease (ESRD), estimated glomerular filtration rate (eGFR), Atherosclerosis Risk in Communities (ARIC) Study
The prevalence of impaired lung function, namely reduced forced expiratory volume in 1 second of expiration (FEV1) and/or forced vital capacity (FVC) values, is nearly 20% among adults in the United States.1,2 In recent years, a growing number of studies have shown the independent associations of reduced lung function with various adverse clinical outcomes such as mortality,3,4 coronary heart disease,5,6 heart failure,7 stroke,8,9 and cognitive impairment.10–12 Several causal mechanisms, such as hypoxia,13 right ventricular dysfunction,14 and chronic systemic inflammation,15,16 have been considered to account for these associations.
Hypoxia, right ventricular dysfunction, and chronic systemic inflammation are also thought to contribute to the development of chronic kidney disease (CKD). Specifically, nocturnal intermittent hypoxia due to sleep disordered breathing increases the risk of CKD progression partly through activation of hypoxia-inducible factor 1α,17 which in turn activates the sympathetic nervous system18 and the renin–angiotensin system,19 and also promotes vascular inflammation, calcification, and atherosclerosis.20,21 Right ventricular dysfunction, as a consequence of reduced lung function, can also contribute to CKD progression through reduced kidney perfusion due to elevated renal venous pressure.22
Despite these plausible mechanisms linking lung function to CKD, only a few cross-sectional studies have reported the association between reduced lung function and the prevalence of CKD,23–27 mostly in patients with chronic obstructive pulmonary disease.23–26 However, given the nature of cross-sectional design, it remains unknown whether the reduced lung function is prospectively associated with the development of CKD. Therefore, the objective of this study is to investigate the associations of reduced lung function with incident end-stage renal disease (ESRD) and CKD in a bi-racial community-based cohort, the Atherosclerosis Risk in Communities (ARIC) Study.
METHODS
Study Design and Study Participants
The ARIC Study is a population-based cohort of 15,792 adults aged 45–64 years at study visit 1 (1987–1989) from four US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). Participants attended subsequent visits at 3-year intervals until their fourth visit (1996–1998). Visit 5 occurred during 2011–2013. Details of the ARIC study cohort have been published previously.28 In the present study, we excluded participants who were neither white nor black as recorded at ARIC study visit 1 (n = 48); who had missing data for lung function (n = 144), kidney function (n = 133), covariates (n = 501), and incident ESRD (n = 14); or who had an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 (n = 6), resulting in a study population of 14,946 participants (Fig S1, available as online supplementary material). The institutional review board at each participating study center approved the study protocol, and written informed content was obtained from all participants.
Assessment of Lung Function
At baseline, lung function was assessed using a water-sealed Collins Survey II volume displacement spirometer (Collins Medical, Inc) and Pulmo-Screen II software (PDS Healthcare Products Inc). At least 3 acceptable spirograms were obtained from a minimum of 5 forced expirations. The best single spirogram was identified by a computer and confirmed by a technician. Quality control was carefully monitored throughout the study. All procedures were performed by trained and certified pulmonary technicians according to the American Thoracic Society guidelines.29 For the present study the main measures of lung function of interest were percent-predicted FVC and FEV1/FVC. Percent-predicted FVC was the maximal volume of gas exhaled after maximal inspiratory effort expressed as a percentage of the predicted value based on age, sex, height, and race according to recommendations from the Epidemiology Standardization Project.30 FEV1/FVC was the proportion of gas exhaled in the first second of expiration out of FVC.29 In addition, according to the percent-predicted FVC and FEV1/FVC, we categorized lung function into five patterns: high normal (percent-predicted FVC ≥100% and FEV1/FVC ≥75%), low normal (percent-predicted FVC 80%–<100% and FEV1/FVC ≥70%, or percent-predicted FVC ≥80% and FEV1/FVC 70%–<75%), obstructive (percent-predicted FVC ≥80% and FEV1/FVC <70%), restrictive (percent-predicted FVC <80% and FEV1/FVC ≥70%), and mixed (percent-predicted FVC <80% and FEV1/FVC <70%).2 The thresholds of FVC 100% and FEV1/FVC 75% within normal range largely corresponded to the threshold of Q2 and Q3 for each lung function parameter.
Covariates
Information on baseline demographics, lifestyle habits, medical history, and medication use was obtained using standardized questionnaires by trained interviewers at baseline. For smoking history, participants identified themselves as current, former, or never smokers. The reported average number of cigarettes per day and number of years of smoking were multiplied to derive cigarette-years of smoking. Ascertainment of bronchitis, emphysema, and asthma was based on a self-report of physician diagnosis. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). Blood samples were collected according to standardized procedures.31 Participants were classified as having diabetes if they had fasting serum glucose level ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, a self-reported history of physician diagnosis of diabetes, or were taking antidiabetic medications. Three seated measurements of systolic and diastolic blood pressure were performed by certified technicians using a random-zero sphygmomanometer after 5 minutes of rest, and the average of the second and third readings was used for analysis. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or self-reported use of antihypertensive medications. Medication use was verified by self-report of medication intake during the last two weeks and by reviewing medications brought by participants to their visit. Prevalent cardiovascular disease (CVD) included history of coronary heart disease, stroke, and heart failure. Coronary heart disease and stroke were defined as self-reported history before visit 1. Prevalent heart failure was identified as presence of heart failure according to Gothenburg criteria32 or self-reported heart failure medication use in the past two weeks. The eGFR was calculated using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.33 Plasma total and high-density lipoprotein cholesterol levels and blood inflammatory markers (i.e., white blood cell count, fibrinogen, and albumin) were measured in central laboratories using standardized and validated methods as previously described.34
Outcome Assessment
ESRD was treated as the primary outcome since it is a hard kidney outcome, with impact on patients and society.35 Incident ESRD was defined as initiation of dialysis therapy, transplantation, or death due to CKD,36 and dialysis therapy and transplantation were determined by linkage to the US Renal Data System (USRDS) data.37 Participants free of ESRD were censored at the time of loss to follow-up, death, or the end of follow-up for this study (December 31, 2012) as appropriate although death was treated as a competing event in sub-distribution hazards models (detailed subsequently).
Incident CKD was treated as the secondary outcome and defined as a composite of eGFR decline (defined as eGFR <60 mL/min/1.73 m2 and at least 25% decline in eGFR from baseline), CKD-related International Classification of Diseases, Ninth or Tenth Revisions, diagnostic codes for hospitalizations and deaths that occurred or USRDS-identified dialysis therapy and transplantation from baseline through December 31, 2012. This definition was previously validated in the ARIC study.38,39 Events based on eGFR decline were determined at study visits 2 and 4 (creatinine was not measured at visit 3), whereas CKD-related hospitalizations/deaths, dialysis therapy, or transplantation were determined at the date of relevant event during follow-up. As done for ESRD analysis, individuals free of CKD were censored at loss of follow-up, death (treated as a competing event in sub-distribution hazards models), or the end of study. Participants with eGFR <60 mL/min/1.73 m2 at baseline (n = 168) and with missing data for incident CKD (n = 1) were excluded from analyses of incident CKD (Fig S1).
Statistical Analysis
Percent-predicted FVC and FEV1/FVC vary widely by race and sex;40 hence, the independent associations of percent-predicted FVC and FEV1/FVC with kidney outcomes were first analyzed using their race- and sex-specific quartiles. Baseline characteristics were summarized according to race- and sex-specific quartiles of percent-predicted FVC and FEV1/FVC, and presented as number (percent) for categorical variables and mean ± standard deviation for continuous variables with a normal distribution or median (interquartile range) for those with a skewed distribution. Differences across quartiles were assessed using analysis of variance or Kruskal-Wallis test and chi-squared tests for continuous and categorical variables, as appropriate. A Spearman’s rank correlation coefficient was calculated for percent-predicted FVC and FEV1/FVC because the latter had left-skewed distribution.
As death is a competing event, we estimated cumulative incidence of kidney events over follow-up time according to the race- and sex-specific quartiles of percent-predicted FVC and FEV1/FVC using sub-distribution hazards models. Subsequently, to assess the impact of potential confounders, multivariable Cox proportional hazards regression models and sub-distribution hazards models in the presence of the competing event of death were used to estimate adjusted cause-specific and sub-distribution hazard ratios (HRs) and 95% confidence intervals (CIs) of incident ESRD and CKD by percent-predicted FVC and FEV1/FVC. Since both models demonstrated similar results, we only present data of cause-specific HRs for the secondary outcome of incident CKD. Models were incrementally adjusted for the following potential confounders based on theoretical considerations and their availability in the ARIC study: model 1 adjusted for age, gender, race, education levels, and height; model 2 additionally accounted for known risk factors of CKD: diabetes status, systolic blood pressure, anti-hypertensive drugs, smoking status, BMI, history of CVD, total cholesterol, high-density lipoprotein cholesterol, and baseline eGFR; and model 3 further included white blood cell count as a measure of inflammation. The proportionality assumption was inspected by plotting log cumulative hazard function against log (survival time) for both cause-specific and sub-distribution hazards models. Tests for linear trend across quartiles were conducted by applying the median value of each quartile to relevant participants and model that variable as a continuous variable in the models. Subsequently, we assessed the combined associations of percent-predicted FVC and FEV1/FVC with kidney outcomes, by simultaneously modeling percent-predicted FVC and FEV1/FVC and repeating the analysis with lung function patterns (high normal, low normal, obstructive, restrictive, and mixed).
We performed several sensitivity analyses to evaluate the robustness of our main findings. Stratified analysis was conducted by age, gender, race, smoking status, and presence/absence of diabetes mellitus. To avoid too few events in some quartiles in each subgroup, percent-predicted FVC and FEV1/FVC were modeled as continuous variables and HRs were expressed for their 10% decrement. Potential interactions were tested by comparing regression models with and without relevant interaction terms using likelihood ratio tests. Also, sub-distribution hazards models were performed with death as a competing event of incident ESRD. Although urine albumin-creatinine ratio was not assessed at baseline, we investigated whether accounting for urine albumin-creatinine ratio measured at visit 4 (1996–1998) attenuated the association between lung function and incident ESRD (10,973 participants who attended visit 4 were included in this analysis). All tests were two-sided and P <0.05 was considered statistically significant. Analyses were conducted using Stata/IC version 12.1 (Stata Corporation, College Station, TX).
RESULTS
Participant Characteristics
Overall, the mean age at baseline was 54.2 ± 5.8 (standard deviation) years; 25.2% were African American; and 45.0% were male. There were 83.6% with eGFR ≥90 mL/min/1.73 m2, 15.3% with eGFR 60 to <90 mL/min/1.73 m2, and 1.1% with eGFR 15 to <60 mL/min/1.73 m2. The mean values of percent-predicted FVC and FEV1/FVC were 100.7% ± 15.3% and 74.4% ± 8.1%, respectively. The thresholds of race-and sex-specific quartiles for percent-predicted FVC and FEV1/FVC are summarized in Table S1; for percent-predicted FVC, the upper limits of the lowest (Q1) quartile ranged from 85%–95%, and the lower limits of the highest (Q4) quartile ranged from 104%–114%. Corresponding values for FEV1/FVC were 69%–74% and 78%–82%. Compared to participants with higher percent-predicted FVC, those with lower percent-predicted FVC were more likely to be older, hypertensive, diabetic, dyslipidemic, current smokers, less educated, and have higher levels of BMI and inflammatory markers and higher prevalence of CVD (Table 1). As expected, participants with lower percent-predicted FVC also tended to have a higher prevalence of self-reported diagnoses of bronchitis, emphysema, and asthma. Similarly, compared to participants with higher FEV1/FVC, those with lower FEV1/FVC had poorer health status with some exceptions such as lower diastolic blood pressure, higher high-density lipoprotein cholesterol, and a lower prevalence of diabetes (Table S2). Lower percent-predicted FVC and FEV1/FVC were both associated with higher prevalence of eGFR <60 mL/min/1.73 m2 (Tables 1 and S2). Among 14,946 ARIC participants, there were 4,276 participants (28.6%) with high normal lung function pattern, 6,627 (44.3%) with low normal pattern, 2,822 (18.9%) with obstructive pattern, 716 (4.8%) with restrictive pattern, and 505 (3.4%) with mixed pattern. The correlation between percent-predicted FVC and FEV1/FVC was very weak (ρ = 0.03; P = 0.002).
Table 1.
Baseline characteristics according to race- and sex-specific quartiles of percent-predicted FVC in ARIC Study (1987–2012)
| Variable | Total | Quartile of percent-predicted FVCa | Pb | |||
|---|---|---|---|---|---|---|
| Q4 (highest) | Q3 | Q2 | Q1 (lowest) | |||
| No. of Participants | 14,946 | 3,736 | 3,737 | 3,737 | 3,736 | |
| Lung function | ||||||
| FVC, % of predicted | ||||||
| White men | 98.7±14.1 | 116.0±6.7 | 103.3±2.6 | 94.7±2.5 | 80.7±8.6 | |
| White women | 104.4±15.0 | 122.6±6.9 | 109.4±2.7 | 100.3±2.9 | 85.1±9.3 | |
| Black men | 94.7±15.0 | 113.5±7.9 | 99.1±2.6 | 90.2±2.6 | 75.9±9.1 | |
| Black women | 99.8±16.4 | 120.3±9.7 | 104.7±3.1 | 94.9±2.9 | 79.2±8.6 | |
| FEV1, % of predicted | ||||||
| White men | 90.3±17.1 | 106.5±10.8 | 95.6±9.4 | 87.7±35.7 | 71.5±12.7 | <0.001 |
| White women | 96.7±16.7 | 113.2±9.9 | 102.3±7.7 | 93.9±8.2 | 77.3±14.1 | <0.001 |
| Black men | 89.3±17.2 | 106.6±11.2 | 94.1±8.9 | 85.9±34.6 | 70.7±14.8 | <0.001 |
| Black women | 95.2±17.0 | 112.7±11.4 | 100.8±7.8 | 91.5±9.0 | 75.9±12.5 | <0.001 |
| FEV1/FVC, % | ||||||
| White men | 72.5±8.6 | 73.0±6.5 | 73.5±7.1 | 73.5±7.9 | 69.8±11.3 | <0.001 |
| White women | 74.8±7.1 | 74.8±5.5 | 75.7±5.6 | 75.7±6.3 | 72.9±9.9 | <0.001 |
| Black men | 75.0±8.8 | 74.9±6.9 | 75.6±7.2 | 75.7±7.8 | 73.7±12.1 | 0.009 |
| Black women | 77.3±7.7 | 76.1±7.0 | 78.0±5.8 | 77.9±7.4 | 77.3±10.0 | <0.001 |
| Self-reported diagnosis of bronchitis | 1,262 (8.4) | 204 (5.5) | 242 (6.5) | 327 (8.8) | 489 (13.1) | <0.001 |
| Self-reported diagnosis of emphysema | 253 (1.7) | 30 (0.8) | 26 (0.7) | 48 (1.3) | 149 (4.0) | <0.001 |
| Self-reported diagnosis of asthma | 882 (5.9) | 164 (4.4) | 161 (4.3) | 210 (5.6) | 347 (9.3) | <0.001 |
| Age (years) | 54.2±5.8 | 53.6±5.7 | 53.8±5.7 | 54.2±5.7 | 55.1±5.7 | <0.001 |
| African American | 3,763 (25.2) | 940 (25.2) | 942 (25.2) | 940 (25.2) | 941 (25.2) | |
| Male sex | 6,724 (45.0) | 1,681 (45.0) | 1,681 (45.0) | 1,681 (45.0) | 1,681 (45.0) | |
| Educational level completed | <0.001 | |||||
| <12 y | 3,451 (23.1) | 688 (18.4) | 795 (21.3) | 848 (22.7) | 1,120 (30.0) | |
| 12–16 y | 6,152 (41.2) | 1,534 (41.1) | 1,540 (41.2) | 1,566 (41.9) | 1,512 (40.5) | |
| >16 y | 5,343 (35.8) | 1,514 (40.5) | 1,402 (37.5) | 1,323 (35.4) | 1,104 (29.6) | |
| Height (cm) | 168.5±9.3 | 168.4±9.7 | 168.5±9.4 | 168.7±9.1 | 168.4±9.0 | 0.5 |
| BMI (kg/m2) | 27.7±5.3 | 26.7±4.5 | 27.3±5.0 | 27.9±5.5 | 28.8±6.1 | <0.001 |
| Systolic BP (mmHg) | 121.0±18.4 | 118.3±17.1 | 120.0±17.6 | 121.4±18.9 | 124.2±19.6 | <0.001 |
| Diastolic BP (mmHg) | 73.6±11.1 | 73.0±10.7 | 73.7±10.6 | 73.7±11.4 | 73.8±11.7 | 0.01 |
| Antihypertensive use | 4,535 (30.3) | 807 (21.6) | 1,043 (27.9) | 1,194 (32.0) | 1,491 (39.9) | <0.001 |
| Smoking status | <0.001 | |||||
| Current | 3,838 (25.7) | 681 (18.2) | 814 (21.8) | 956 (25.6) | 1,387 (37.1) | |
| Former | 4,792 (32.1) | 1,258 (33.7) | 1,226 (32.8) | 1,185 (31.7) | 1,123 (30.1) | |
| Never | 6,316 (42.3) | 1,797 (48.1) | 1,697 (45.4) | 1,596 (42.7) | 1,226 (32.8) | |
| Cigarette-yearsc | 481 [216–780] | 340 [147–620] | 420 [180–700] | 500 [230–780] | 630 [344–900] | <0.001 |
| Current drinker | 8,409 (56.3) | 2,231 (59.7) | 2,148 (57.5) | 2,095 (56.1) | 1,935 (51.8) | <0.001 |
| Diabetes mellitus | 1,738 (11.6) | 234 (6.3) | 360 (9.6) | 457 (12.2) | 687 (18.4) | <0.001 |
| Prevalent CVDd | 1,475 (9.9) | 189 (5.1) | 286 (7.7) | 366 (9.8) | 634 (17.0) | <0.001 |
| Total cholesterol (mg/dL) | 215.0±41.9 | 213.2±41.4 | 215.2±41.6 | 215.7±41.7 | 216.0±42.9 | 0.02 |
| HDL cholesterol (mg/dL) | 51.6±17.1 | 54.5±17.4 | 52.3±16.9 | 50.7±16.9 | 48.8±16.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 102.4±15.3 | 102.6±14.4 | 102.7±14.9 | 102.3±15.6 | 102.1±16.3 | 0.3 |
| eGFR category | <0.001 | |||||
| <60 mL/min/1.73 m2 | 168 (1.1) | 31 (0.8) | 31 (0.8) | 45 (1.2) | 61 (1.6) | |
| 60 – <90 mL/min/1.73 m2 | 2,288 (15.3) | 546 (14.6) | 554 (14.8) | 580 (15.5) | 608 (16.3) | |
| ≥90 mL/min/1.73 m2 | 12,490 (83.6) | 3,159 (84.6) | 3,152 (84.4) | 3,112 (83.3) | 3,067 (82.1) | |
| Markers of inflammation | ||||||
| WBC count (1,000/mm3) | 6.1±2.0 | 5.7±1.7 | 6.0±2.2 | 6.1±1.9 | 6.7±2.1 | <0.001 |
| Plasma fibrinogen level (mg/L) | 303.0±64.9 | 289.7±57.8 | 296.4±59.3 | 305.5±65.7 | 320.3±71.8 | <0.001 |
| Serum albumin level (g/dL) | 3.9±0.3 | 3.9±0.3 | 3.9±0.3 | 3.9±0.3 | 3.8±0.3 | <0.001 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factor for cholesterol in mg/dL to mmol/L, ×0.02586.
The thresholds of quartiles (%) are as follows: white men, 90.1, 98.9, and 108.0; white women, 95.1, 105.0, and 114.4; black men, 85.3, 94.9, and 103.8; and black women, 89.1, 99.5, and 110.1.
ANOVA or Kruskal-Wallis test for continuous variables and chi-squared test for categorical variables.
Cigarette-years of smoking in ever-smokers only.
Includes coronary heart disease, stroke, and heart failure.
Abbreviations: ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 second of expiration; FVC, forced vital capacity; HDL, high-density lipoprotein; Q, quartile; WBC, white blood cell
Associations of Percent-Predicted FVC and FEV1/FVC With Incident Kidney Disease
Independent Associations
During a median follow-up of 23.6 years, 526 participants (3.5%) had developed ESRD (crude incidence rate, 1.68 [95% CI, 1.54–1.83] per 1000 patient-years). Participants in the lower quartiles of percent-predicted FVC and FEV1/FVC had a higher 25-year cumulative incidence of ESRD, with more evident separation for percent-predicted FVC (6.1% versus 2.2% in the lowest versus highest quartiles) than for FEV1/FVC (4.3% versus 3.2%, respectively; Figure 1). In the demographically adjusted model, participants with the lowest (Q1) and second lowest (Q2) quartiles of percent-predicted FVC and the lowest quartile (Q1) of FEV1/FVC showed significantly higher risk of incident ESRD than those in the highest quartiles (Table 2). Although the association with percent-predicted FVC was considerably attenuated after further adjustment for other potential confounders, the associations remained significant for both percent-predicted FVC and FEV1/FVC (adjusted cause-specific HRs for the lowest [versus the highest] quartile of percent-predicted FVC and FEV1/FVC were 1.72 [95% CI, 1.31–2.26] and 1.33 [95% CI, 1.03–1.73], respectively, in model 3; Table 2). Overall, the dose-response relationship was less evident for FEV1/FVC than for percent-predicted FVC. In general, similar patterns were observed for incident CKD (Fig S2), although the associations for FEV1/FVC were not significant in multivariable models (Table S3). Similar results were observed when we replaced white blood cell count with other inflammatory markers, plasma fibrinogen or serum albumin (data not shown).
Figure 1. Cumulative incidence curves for incident ESRD by (A) percent-predicted FVC and (B) FEV1/FVC.
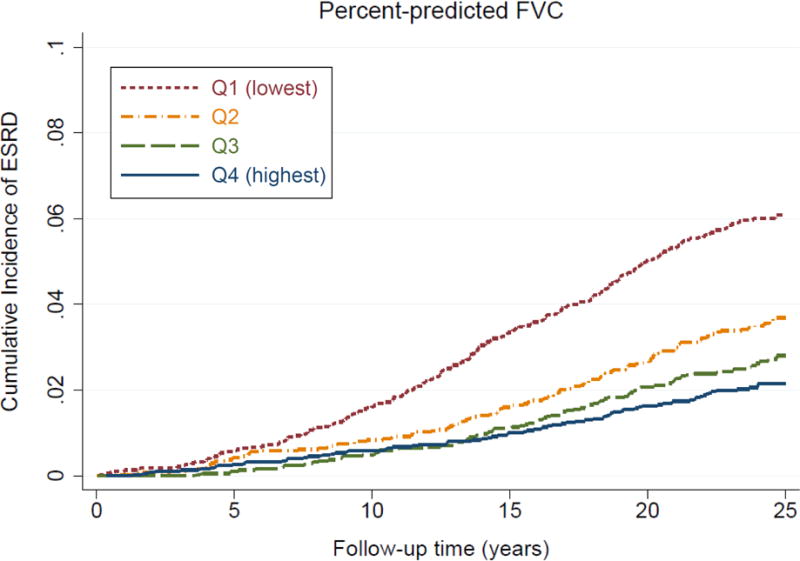
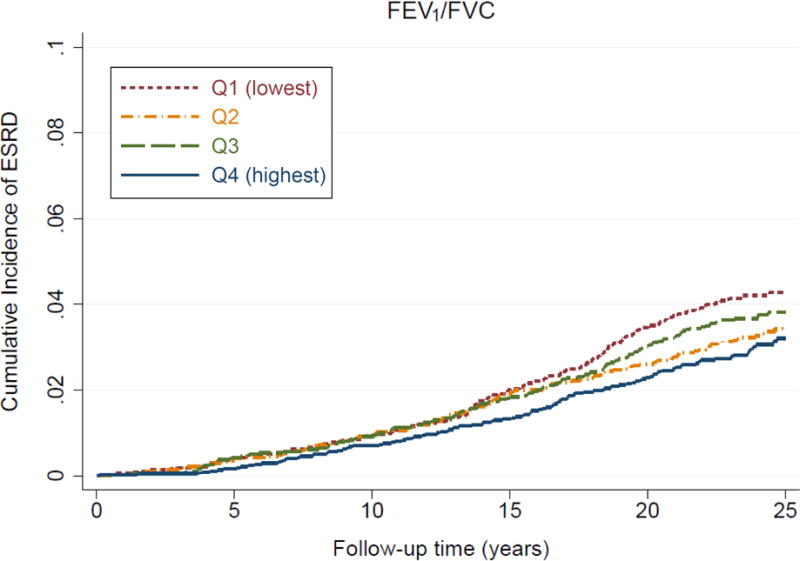
The thresholds of quartiles (%) are as follows: white men, 90.1, 98.9, and 108.0; white women, 95.1, 105.0, and 114.4; black men, 85.3, 94.9, and 103.8; and black women, 89.1, 99.5, and 110.1, for percent-predicted FVC; white men, 68.6, 74.0, and 78.2; white women, 71.5, 75.7, and 79.3; black men, 70.9, 76.5, and 80.7; and black women, 73.8, 78.5, and 82.0, for FEV1/FVC.
Abbreviations: ESRD, end-stage renal disease; FEV1, forced expiratory volume in 1 second of expiration; FVC, forced vital capacity
Table 2.
Adjusted cause-specific hazard ratios for incident ESRD by quartiles of percent-predicted FVC and FEV1/FVC
| Q4 (highest) | Q3 | Q2 | Q1 (lowest) | P for trendc | |
|---|---|---|---|---|---|
| Percent-Predicted FVCa | |||||
| No. of Participants | 3,736 | 3,737 | 3,737 | 3,736 | |
| Events | 77 (2.1%) | 98 (2.6%) | 130 (3.5%) | 221 (5.9%) | |
| Incidence rated | 0.93 (0.74–1.16)d | 1.20 (0.99–1.47)d | 1.66 (1.40–1.98)d | 3.10 (2.72–3.54)d | |
| Model 1 | 1.00 (reference) | 1.28 (0.95–1.73) | 1.77 (1.33–2.34) | 3.20 (2.46–4.15) | <0.001 |
| Model 2 | 1.00 (reference) | 1.04 (0.77–1.40) | 1.24 (0.93–1.65) | 1.76 (1.34–2.31) | <0.001 |
| Model 3 | 1.00 (reference) | 1.03 (0.76–1.39) | 1.23 (0.92–1.64) | 1.72 (1.31–2.26) | <0.001 |
| FEV1/FVCb | |||||
| No. of Participants | 3,736 | 3,736 | 3,737 | 3,737 | |
| Events | 112 (3.0%) | 137 (3.7%) | 122 (3.3%) | 155 (4.1%) | |
| Incidence rated | 1.37 (1.14–1.65)d | 1.70 (1.44–2.01)d | 1.54 (1.29–1.84)d | 2.14 (1.83–2.51)d | |
| Model 1 | 1.00 (reference) | 1.19 (0.92–1.52) | 1.00 (0.77–1.29) | 1.33 (1.03–1.70) | 0.05 |
| Model 2 | 1.00 (reference) | 1.21 (0.94–1.56) | 1.05 (0.81–1.36) | 1.35 (1.04–1.76) | 0.04 |
| Model 3 | 1.00 (reference) | 1.20 (0.93–1.54) | 1.04 (0.80–1.35) | 1.33 (1.03–1.73) | 0.06 |
Note: n=14,946. Unless otherwise indicated, values are given as number (percentage) or hazard ratio (95% confidence interval). Model 1 is adjusted for age, sex, race, education levels, and height; model 2 is adjusted for the variables in model 1 plus known cardiovascular and kidney risk factors (i.e., diabetes, systolic blood pressure, antihypertensive medication, history of cardiovascular disease, smoking status, cigarette-years of smoking, body mass index, estimated glomerular filtration rate, and total and high-density lipoprotein cholesterol; and model 3 is adjusted for the variables in model 2 plus inflammatory marker (i.e., white blood cell count). Models accounted for the competing risk of death using cause-specific hazards models by treating death as censoring.
The thresholds of quartiles (%) are as follows: white men, 90.1, 98.9, and 108.0; white women, 95.1, 105.0, and 114.4; black men, 85.3, 94.9, and 103.8; and black women, 89.1, 99.5, and 110.1.
The thresholds of quartiles (%) are as follows: white men, 68.6, 74.0, and 78.2; white women, 71.5, 75.7, and 79.3; black men, 70.9, 76.5, and 80.7; and black women, 73.8, 78.5, and 82.0.
Linear trend across the quartiles of percent-predicted FVC and FEV1/FVC.
Unadjusted incidence rate (95% confidence interval) per 1000 person-years.
Abbreviations: ESRD, end-stage renal disease; FEV1, forced expiratory volume in 1 second of expiration; FVC, forced vital capacity; Q. quartile
In the subgroup analyses, lower percent-predicted FVC was associated with increased risk of incident ESRD in all examined subgroups, except in the subgroup with eGFR <60 mL/min/1.73 m2 (Figure 2A). Nonetheless, there was no significant interaction between percent-predicted FVC and baseline eGFR (P for interaction = 0.4). In contrast, lower FEV1/FVC was significantly associated with incident ESRD only in a handful of subgroups (Figure 2B). Diabetes significantly modified the associations of ESRD risk with both percent-predicted FVC and FEV1/FVC, (Figure 2), with greater contributions of lung function to ESRD risk in non-diabetic individuals than in diabetic participants.
Figure 2. Adjusted cause-specific hazard ratios of ESRD associated with 10% decrease of (A) percent-predicted FVC and (B) FEV1/FVC in predefined subgroups.
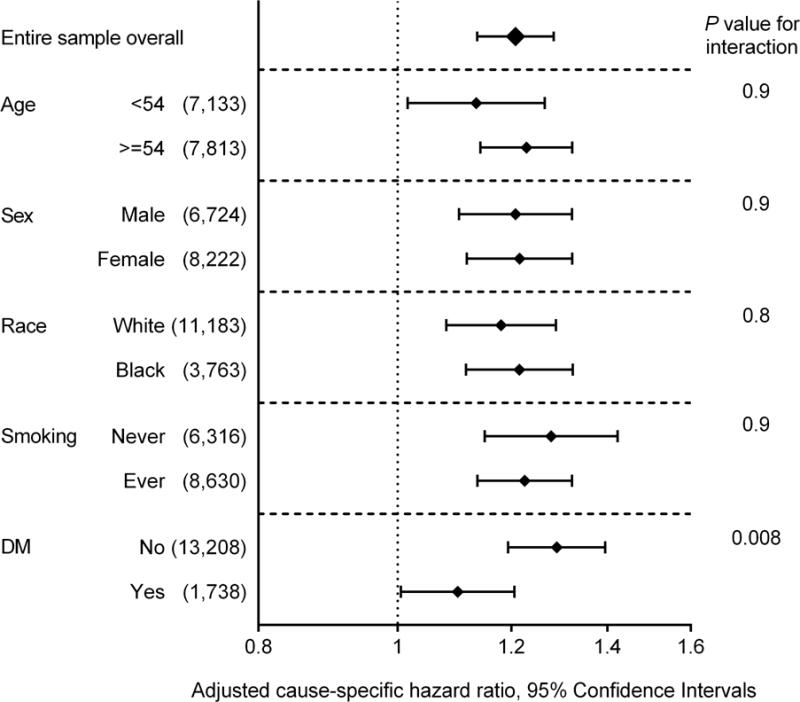
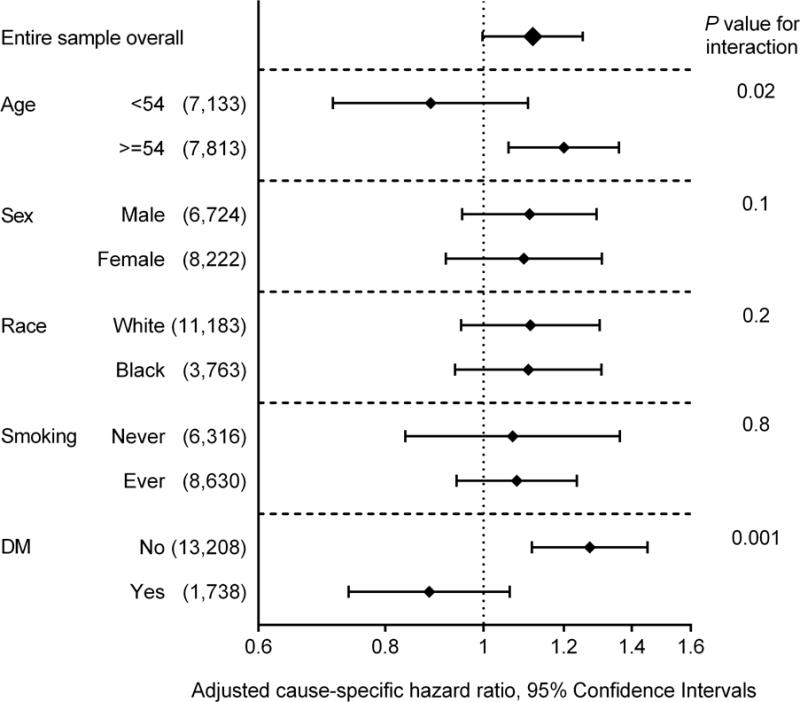
Data are adjusted for age, sex, race, education levels, height, known cardiovascular and kidney risk factors (i.e., diabetes, systolic blood pressure, antihypertensive medication, history of cardiovascular disease, smoking status, cigarette-years of smoking, body mass index, eGFR, and total and HDL cholesterol), and inflammatory marker (i.e., white blood cell count).
Abbreviations: DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; FEV1, forced expiratory volume in 1 second of expiration; FVC, forced vital capacity
In the sub-distribution hazards analyses, lower percent-predicted FVC, but not FEV1/FVC, was significantly associated with higher risk of incident ESRD (Table S4). Further adjustment for the urine albumin-creatinine ratio measured at study visit 4 (1996–1998) did not fundamentally affect the associations (Table S5).
Combined Associations
When both percent-predicted FVC and FEV1/FVC were included in the fully adjusted model, the adjusted cause-specific HRs of ESRD for the lowest (versus the highest) quartile of percent-predicted FVC and FEV1/FVC were 1.76 (95% CI, 1.33–2.31) and 1.36 (95% CI, 1.05–1.77), respectively (P for interaction = 0.1; Table S6). The 25-year crude cumulative incidence of ESRD was highest for the restrictive (9.8%) lung function pattern followed by mixed (7.0%), obstructive (3.7%), low normal (3.7%), and high normal patterns (2.3%; Figure 3). After adjustment for potential confounders, the mixed pattern demonstrated the highest adjusted cause-specific HR of ESRD at 2.28 (95% CI, 1.50–3.45), followed by restrictive at 2.03 (95% CI, 1.47–2.81), obstructive at 1.47 (95% CI, 1.09–1.99), and then low normal at 1.21 (95% CI, 0.94–1.55), compared with the high normal pattern (Table 3).
Figure 3. Cumulative incidence curves for incident ESRD by lung function patterns.
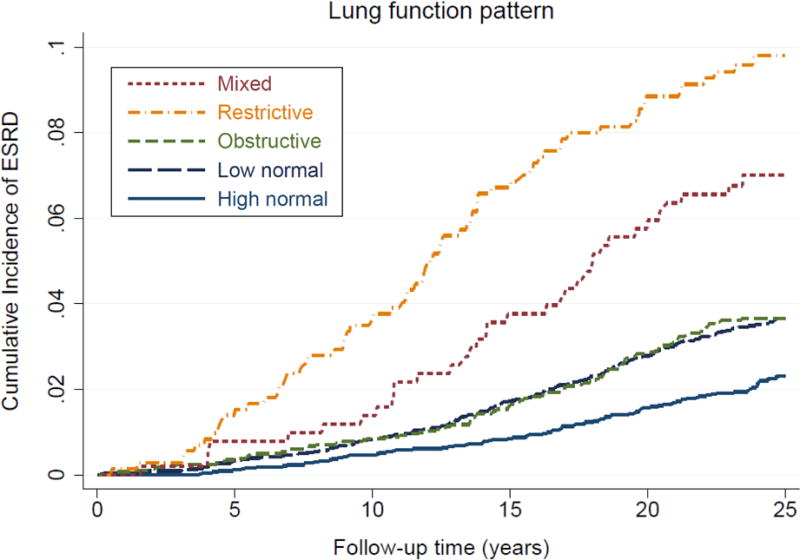
Abbreviations: ESRD, end-stage renal disease
Table 3.
Adjusted cause-specific hazard ratios for incident ESRD by lung function patterns
| Lung function pattern | |||||
|---|---|---|---|---|---|
| High normal | Low normal | Obstructive | Restrictive | Mixed | |
| No. of Participants | 4,276 | 6,627 | 2,822 | 716 | 505 |
| Events | 91 (2.1%) | 230 (3.5%) | 101 (3.6%) | 69 (9.6%) | 35 (6.9%) |
| Incidence ratea | 0.94 (0.77–1.16)a | 1.63 (1.43–1.85)a | 1.82 (1.49–2.21)a | 5.39 (4.25–6.82)a | 4.57 (3.28–6.36)a |
| Model 1 | 1.00 (reference) | 1.52 (1.19–1.94) | 1.72 (1.28–2.30) | 3.91 (2.84–5.38) | 3.91 (2.63–5.83) |
| Model 2 | 1.00 (reference) | 1.23 (0.96–1.57) | 1.50 (1.11–2.03) | 2.09 (1.51–2.89) | 2.33 (1.54–3.53) |
| Model 3 | 1.00 (reference) | 1.21 (0.94–1.55) | 1.47 (1.09–1.99) | 2.03 (1.47–2.81) | 2.28 (1.50–3.45) |
Note: n=14,946. Unless otherwise indicated, values are given as number (percentage) or hazard ratio (95% confidence interval). M model 1 is adjusted for age, sex, race, education levels, and height; model 2 is adjusted for the variables in model 1 plus known cardiovascular and kidney risk factors (i.e., diabetes, systolic blood pressure, antihypertensive medication, history of cardiovascular disease, smoking status, cigarette-years of smoking, body mass index, estimated glomerular filtration rate, and total and high-density lipoprotein cholesterol; and model 3 is adjusted for the variables in model 2 plus inflammatory marker (i.e., white blood cell count). Models accounted for the competing risk of death using cause-specific hazards models by treating death as censoring.
Unadjusted incidence rate (95% confidence interval) per 1000 person-years.
Abbreviation: ESRD, end-stage renal disease
The analyses using incident CKD as an outcome yielded similar associations (Fig S3 and Table S7). The highest adjusted HR of CKD was also seen in the mixed pattern at 1.53 (95% CI, 1.27–1.85), followed by restrictive at 1.42 (95% CI, 1.23–1.65), obstructive at 1.15 (95% CI, 1.03–1.27), and low normal at 1.08 (95% CI, 1.00–1.17) patterns, compared with the high normal pattern (Table S7). Results were largely consistent in various subgroups (Fig S4), but again the associations with ESRD were less evident in those with diabetes than in those without. The associations were much the same after accounting for the competing risk of death (Table S8) or adjusting for albuminuria (Table S9).
DISCUSSION
In this large community-based bi-racial cohort with up to 25 years of follow-up, we found that reduced lung function, particularly lower percent-predicted FVC, was independently associated with higher risk of incident ESRD. Participants in the lowest quartile of percent-predicted FVC (<85%–95% depending on race and sex) had a ~1.7-fold higher risk of ESRD compared to those in the highest quartile (≥103%–114%), after adjusting for sociodemographic characteristics and known kidney disease risk factors (the higher risk was ~3.2-fold in crude analyses). Findings were generally consistent in several subgroups, after accounting for the competing risk of death, and with the secondary outcome of incident CKD. Compared to participants with high normal lung function pattern, those with mixed pattern had the highest risk of ESRD (~2.3-fold), followed by restrictive (~2.0-fold), obstructive (~1.5-fold), and then low normal (~1.2-fold) patterns, in the multivariable adjusted models.
A few observational studies have reported the association of reduced lung function with a higher prevalence of CKD,23–27 mostly in patients with chronic obstructive pulmonary disease.23–26 Among the general population, a recent population-based study demonstrated a significant association between reduced lung function, particularly restrictive pattern, and elevated level of albuminuria,27 a key marker of CKD definition and staging.38,41 Most importantly, all of these studies were cross-sectional and unable to determine the temporality of the association. In this context, our prospective investigation would be of value, with a long follow-up of 25 years and a hard kidney end point of ESRD.
Although our observational study cannot conclude a causal relationship, there are several plausible explanations for the association between reduced lung function and the risk of adverse kidney outcomes. Reduced lung function and CKD may share risk factors, such as older age, smoking,42 and higher levels of inflammatory markers.43 However, the association of reduced lung function with adverse kidney outcomes still remained statistically significant even after accounting for various potential confounders. Recently, an emerging body of evidence has indicated that renal hypoxia plays a key role in the pathogenesis of CKD progression, by inducing renal tubular epithelial cell damage and subsequent fibroblast proliferation and inflammatory reactions, which collectively lead to tubulointerstitial fibrosis.44 Therefore, systemic and local hypoxia due to reduced lung function may contribute to the CKD progression through tubulointerstitial injury.45 Additionally, venous congestion associated with right ventricular dysfunction secondary to pulmonary hypertension can elevate kidney interstitial and tubular hydrostatic pressures and thus also promote CKD progression.22 Other possible mechanisms would include higher incidence of acute kidney injury46 as well as higher risk of receiving nephrotoxic antibiotics47,48 in patients with lung diseases compared to those without.
It is unclear why lower percent-predicted FVC (i.e., restrictive pattern) was a stronger predictor of incident ESRD and CKD than a lower FEV1/FVC (i.e., or obstructive pattern) in our study. Individuals with reduced lung function, particularly those with restrictive pattern, are more likely to have exercise-induced oxygen desaturation and pulmonary hypertension than those with obstructive pattern,49,50 which may be an explanation of our observation. Nonetheless, it is important that this pattern (i.e., percent-predicted FVC as a stronger predictor than FEV1/FVC) has been consistent for other adverse clinical phenotypes such as incident heart failure,51 arterial hypertension,52 left ventricular hypertrophy,53 and type 2 diabetes.54
In the subgroup analysis, we observed significant interactions between lung function and diabetes. The contributions of lung function to incident ESRD were more evident in non-diabetic individuals than in diabetic participants. Although it is unclear whether this statistical interaction reflects any pathophysiological interactions, those with diabetes are at higher risk of ESRD regardless of lung function, and, thus, lung function may not add prognostic information in this clinical population.
There are a few clinical and research implications from our study. The prevalence of chronic lung diseases is high, affecting approximately 210–300 million people worldwide.55,56 However, to our knowledge, CKD has not been listed as a clinically important consequence in clinical guidelines of lung diseases.57,58 Our results suggest that clinical attention should be given to the trajectory of kidney function among those with reduced lung function, particularly when restrictive and mixed patterns are present. Also, it seems important to acknowledge the risk gradient within the normal range of FVC as such a finding has been reported in the context of other outcomes as well.5,9,12,51–53 The pathophysiological link between lung disease and kidney disease may deserve further investigation, particularly if our findings are confirmed in other settings.
The study results must be interpreted along with several limitations. First, there were a relatively small number of participants with moderately/severely reduced lung function. Similarly, the number of participants with CKD was limited. Therefore, to generalize our results to more advanced lung disease or CKD would require caution. Second, albuminuria, a strong risk factor for kidney disease progression, was not measured at study visit 1. However, results were robust even after adjustment for a measure of albuminuria assessed at study visit 4. Third, echocardiography was not available at baseline in the ARIC Study, and thus we could not explore whether right ventricular function attenuates observed associations. Fourth, spirometry was performed at baseline and may be subject to change over time. Finally, we are not able to eliminate the possibility of residual confounding.
There are also a few key strengths of our study. First, although smaller studies would need to rely on changes in eGFR or albuminuria as a kidney end point, the availability of incident ESRD as the primary outcome in this study is a notable strength. Second, a sizable number of participants with long-term follow-up and high retention rates enabled precise risk estimates with multivariable adjustment and in key subgroups. Third, the substantial number of people with normal lung function allowed the assessment of risk gradient within the normal group. Fourth, a standardized protocol, with rigorous quality control procedures, was used to measure lung function.
In conclusion, in this large bi-racial population-based cohort study, we found that reduced lung function, particularly lower percent-predicted FVC, was associated with subsequent risk of incident ESRD and CKD independently of known kidney disease risk factors. Our findings suggest a potential pathophysiologic contribution of reduced lung function to the development of CKD and the need for careful monitoring of kidney function in persons with reduced lung function.
Supplementary Material
Table S1: Thresholds of race- and sex-specific quartiles of percent-predicted FVC and FEV1/FVC.
Table S2: Baseline characteristics according to race- and sex-specific quartiles of FEV1/FVC.
Table S3: Adjusted cause-specific HRs for incident CKD by quartiles of percent-predicted FVC and FEV1/FVC.
Table S4: Adjusted subhazard ratios of incident ESRD according to quartiles of percent-predicted FVC and FEV1/FVC in subdistribution hazards models.
Table S5: Adjusted cause-specific HRs for incident ESRD according to quartiles of percent-predicted FVC and FEV1/FVC.
Table S6: Combined associations of percent-predicted FVC and FEV1/FVC with incident ESRD.
Table S7: Adjusted cause-specific HRs for incident CKD by lung function patterns.
Table S8: Adjusted subhazard ratios of incident ESRD according to lung function patterns in subdistribution hazards models.
Table S9: Adjusted cause-specific HRs for incident ESRD according to lung function patterns.
Figure S1: Flow diagram.
Figure S2: Cumulative incidence curves for incident CKD by percent-predicted FVC and FEV1/FVC.
Figure S3: Cumulative incidence curves for incident CKD by lung function patterns.
Figure S4: Adjusted cause-specific HRs of ESRD associated with lung function patterns in predefined subgroups.
Acknowledgments
The authors thank the staff and participants of the ARIC Study for important contributions. Some of the data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Support: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N SECTION:
In line with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal policies, Health Equity Editor Carmen A. Peralta, MD, served as Acting Editor-in-Chief and handled the peer-review and decision-making processes.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: KS, KM; data acquisition: KS, KM; data analysis/interpretation: KS, LK, MEG, KY, NP, CPK, JC, KM; statistical analysis: KS, KM; supervision or mentorship: KY, CPK, KM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by two external peer reviewers, a Statistics/Methods Editor, and an Acting Editor-in-Chief.
Supplementary Material
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Thresholds of race- and sex-specific quartiles of percent-predicted FVC and FEV1/FVC.
Supplementary Table S2 (PDF). Baseline characteristics according to race- and sex-specific quartiles of FEV1/FVC.
Supplementary Table S3 (PDF). Adjusted cause-specific HRs for incident CKD by quartiles of percent-predicted FVC and FEV1/FVC.
Supplementary Table S4 (PDF). Adjusted subhazard ratios of incident ESRD according to quartiles of percent-predicted FVC and FEV1/FVC in subdistribution hazards models.
Supplementary Table S5 (PDF). Adjusted cause-specific HRs for incident ESRD according to quartiles of percent-predicted FVC and FEV1/FVC.
Supplementary Table S6 (PDF). Combined associations of percent-predicted FVC and FEV1/FVC with incident ESRD.
Supplementary Table S7 (PDF). Adjusted cause-specific HRs for incident CKD by lung function patterns.
Supplementary Table S8 (PDF). Adjusted subhazard ratios of incident ESRD according to lung function patterns in subdistribution hazards models.
Supplementary Table S9 (PDF). Adjusted cause-specific HRs for incident ESRD according to lung function patterns.
Supplementary Figure S1 (PDF). Flow diagram.
Supplementary Figure S2 (PDF). Cumulative incidence curves for incident CKD by percent-predicted FVC and FEV1/FVC.
Supplementary Figure S3 (PDF). Cumulative incidence curves for incident CKD by lung function patterns.
Supplementary Figure S4 (PDF). Adjusted cause-specific HRs of ESRD associated with lung function patterns in predefined subgroups.
References
- 1.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114(9):758–762. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest. 2013;143(5):1395–1406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. discussion 715–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schunemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118(3):656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung Function and Incident Coronary Heart Disease: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158(12):1171–1181. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14(4):414–422. doi: 10.1093/eurjhf/hfs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30(1):145–151. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Hozawa A, Billings JL, Shahar E, Ohira T, Rosamond WD, Folsom AR. Lung function and ischemic stroke incidence: the Atherosclerosis Risk in Communities study. Chest. 2006;130(6):1642–1649. doi: 10.1378/chest.130.6.1642. [DOI] [PubMed] [Google Scholar]
- 10.Chyou PH, White LR, Yano K, et al. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. Am J Epidemiol. 1996;143(8):750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- 11.Richards M, Strachan D, Hardy R, Kuh D, Wadsworth M. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom Med. 2005;67(4):602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- 12.Pathan SS, Gottesman RF, Mosley TH, Knopman DS, Sharrett AR, Alonso A. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol. 2011;18(6):888–898. doi: 10.1111/j.1468-1331.2010.03340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peers C, Dallas ML, Boycott HE, Scragg JL, Pearson HA, Boyle JP. Hypoxia and neurodegeneration. Ann N Y Acad Sci. 2009;1177:169–177. doi: 10.1111/j.1749-6632.2009.05026.x. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117(11):1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 15.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53. doi: 10.1186/1465-9921-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pialoux V, Hanly PJ, Foster GE, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med. 2009;180(10):1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 18.Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299(3):H925–931. doi: 10.1152/ajpheart.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maser RE, Lenhard MJ, Rizzo AA, Vasile AA. Continuous positive airway pressure therapy improves cardiovascular autonomic function for persons with sleep-disordered breathing. Chest. 2008;133(1):86–91. doi: 10.1378/chest.07-1580. [DOI] [PubMed] [Google Scholar]
- 20.Jung HH, Han H, Lee JH. Sleep Apnea, Coronary Artery Disease, and Antioxidant Status in Hemodialysis Patients. Am J Kidney Dis. 2005;45(5):875–882. doi: 10.1053/j.ajkd.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121(8):1014–1021. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(7):582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 23.Incalzi RA, Corsonello A, Pedone C, et al. Chronic renal failure: a neglected comorbidity of COPD. Chest. 2010;137(4):831–837. doi: 10.1378/chest.09-1710. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizawa T, Okada K, Furuichi S, et al. Prevalence of chronic kidney diseases in patients with chronic obstructive pulmonary disease: assessment based on glomerular filtration rate estimated from creatinine and cystatin C levels. Int J Chron Obstruct Pulmon Dis. 2015;10:1283–1289. doi: 10.2147/COPD.S80673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casanova C, de Torres JP, Navarro J, et al. Microalbuminuria and hypoxemia in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(8):1004–1010. doi: 10.1164/rccm.201003-0360OC. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel YR, Chonchol M, Hoeks SE, et al. Association between chronic obstructive pulmonary disease and chronic kidney disease in vascular surgery patients. Nephrol Dial Transplant. 2009;24(9):2763–2767. doi: 10.1093/ndt/gfp171. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Won JU, Ahn YS, Roh J. Poor lung function has inverse relationship with microalbuminuria, an early surrogate marker of kidney damage and atherosclerosis: the 5th Korea National Health and Nutrition Examination Survey. PLoS One. 2014;9(4):e94125. doi: 10.1371/journal.pone.0094125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 29.ATS statement–Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119(5):831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 30.Ferris BG, Jr, Speizer FE, Bishop Y, Prang G, Weener J. Spirometry for an epidemiologic study: deriving optimum summary statistics for each subject. Bull Eur Physiopathol Respir. 1978;14(2):145–166. [PubMed] [Google Scholar]
- 31.The ARIC Investigators. National Heart Lung and Blood Institute Atherosclerosis Risk in Communities (ARIC) Study. Bethesda, MD: National Heart, Lung and Blood Institute; 1987. Operations Manual 7: Blood Collection and Processing. [Google Scholar]
- 32.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnea–validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith BM, Kawut SM, Bluemke DA, et al. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127(14):1503–1511. 1511e1501–1506. doi: 10.1161/CIRCULATIONAHA.113.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2014. United States Renal Data System, 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 38.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 39.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Agarwal SK, Alonso A, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129(9):971–980. doi: 10.1161/CIRCULATIONAHA.113.004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 43.Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;60(2):225–232. doi: 10.1053/j.ajkd.2012.02.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol. 2014;307(11):F1187–1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 45.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 46.Barakat MF, McDonald HI, Collier TJ, Smeeth L, Nitsch D, Quint JK. Acute kidney injury in stable COPD and at exacerbation. Int J Chron Obstruct Pulmon Dis. 2015;10:2067–2077. doi: 10.2147/COPD.S88759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mapel DW, Marton JP. Prevalence of renal and hepatobiliary disease, laboratory abnormalities, and potentially toxic medication exposures among persons with COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:127–134. doi: 10.2147/COPD.S40123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venning V, Bartlett J, Jayaram L. Patients hospitalized with an infective exacerbation of bronchiectasis unrelated to cystic fibrosis: Clinical, physiological and sputum characteristics. Respirology. 2017 doi: 10.1111/resp.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadeli KO, Siegel EM, Sherrill DL, Beck KC, Enright PL. Predictors of oxygen desaturation during submaximal exercise in 8,000 patients. Chest. 2001;120(1):88–92. doi: 10.1378/chest.120.1.88. [DOI] [PubMed] [Google Scholar]
- 50.Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008;31(6):1357–1367. doi: 10.1183/09031936.00171307. [DOI] [PubMed] [Google Scholar]
- 51.Engstrom G, Melander O, Hedblad B. Population-based study of lung function and incidence of heart failure hospitalisations. Thorax. 2010;65(7):633–638. doi: 10.1136/thx.2010.135392. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs DR, Jr, Yatsuya H, Hearst MO, et al. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59(2):219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuttica MJ, Colangelo LA, Shah SJ, et al. Loss of Lung Health from Young Adulthood and Cardiac Phenotypes in Middle Age. Am J Respir Crit Care Med. 2015;192(1):76–85. doi: 10.1164/rccm.201501-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Brancati FL. Vital capacity as a predictor of incident type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(6):1472–1479. doi: 10.2337/diacare.28.6.1472. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization: Recent news from WHO. Bull World Health Organ. 2010;88:885–886. doi: 10.2471/BLT.10.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol. 2014;795:17–29. doi: 10.1007/978-1-4614-8603-9_2. [DOI] [PubMed] [Google Scholar]
- 57.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 58.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Thresholds of race- and sex-specific quartiles of percent-predicted FVC and FEV1/FVC.
Table S2: Baseline characteristics according to race- and sex-specific quartiles of FEV1/FVC.
Table S3: Adjusted cause-specific HRs for incident CKD by quartiles of percent-predicted FVC and FEV1/FVC.
Table S4: Adjusted subhazard ratios of incident ESRD according to quartiles of percent-predicted FVC and FEV1/FVC in subdistribution hazards models.
Table S5: Adjusted cause-specific HRs for incident ESRD according to quartiles of percent-predicted FVC and FEV1/FVC.
Table S6: Combined associations of percent-predicted FVC and FEV1/FVC with incident ESRD.
Table S7: Adjusted cause-specific HRs for incident CKD by lung function patterns.
Table S8: Adjusted subhazard ratios of incident ESRD according to lung function patterns in subdistribution hazards models.
Table S9: Adjusted cause-specific HRs for incident ESRD according to lung function patterns.
Figure S1: Flow diagram.
Figure S2: Cumulative incidence curves for incident CKD by percent-predicted FVC and FEV1/FVC.
Figure S3: Cumulative incidence curves for incident CKD by lung function patterns.
Figure S4: Adjusted cause-specific HRs of ESRD associated with lung function patterns in predefined subgroups.


