Abstract
Background
Clinical practice guidelines recommend referral to nephrology once estimated glomerular filtration rate (eGFR) falls below 30 ml/min/1.73 m2; however, evidence for benefits of nephrology care are mixed.
Study Design
Observational cohort using landmark analysis.
Settings & Participants
A national cohort of veterans with advanced CKD, defined by an outpatient eGFR ≤30 ml/min/1.73m2 for January 1, 2010 through December 31, 2010 and a prior eGFR <60 ml/min/1.73m2, using administrative and laboratory data from the Department of Veterans Affairs and the US Renal Data System.
Predictor
Receipt and frequency of outpatient nephrology care over 12 months.
Outcomes
Survival and progression to ESRD (receipt of dialysis or kidney transplantation) were the primary outcomes. In addition, control of associated clinical parameters over 12 months were intermediate outcomes.
Results
Of 39,669 patients included in the cohort, 14,983 (37.8%) received nephrology care. Older age, heart failure, dementia, depression, and rapidly declining kidney function were independently associated with the absence of nephrology care. Over a mean follow up of 2.9 years, 14,719 (37.1%) patients died and 4,310 (10.9%) progressed to ESRD. In models adjusting for demographics, comorbidities, and trajectory of kidney function, nephrology care was associated with a lower risk of death (HR, 0.88; 95% CI, 0.85-0.91), but a higher risk of ESRD (HR, 1.48; 95% CI, 1.38-1.58). Among patients with clinical parameters outside of guideline recommendations at cohort entry, a significantly higher adjusted proportion of patients who received nephrology care had improvement in control of hemoglobin, potassium, albumin, calcium and phosphorus compared to those who did not receive nephrology care.
Limitations
May not be generalizable to non-veterans.
Conclusions
Among patients with advanced CKD, nephrology care was associated with lower mortality, but was not associated with a lower risk for progression to ESRD.
Keywords: Chronic kidney disease (CKD), end-stage renal disease (ESRD), nephrology, delivery of health care, nephrology care, disease trajectory, guideline-concordant care, CKD progression, intensity of care, nephrology referral, landmark analysis, immortal time bias
Chronic kidney disease (CKD) is a common condition among US adults and confers a high risk of mortality and progression to end-stage renal disease (ESRD).1 Clinical practice guidelines recommend referral to nephrology when the estimated glomerular filtration rate (eGFR) falls below 30 ml/min/1.73m2, in order to provide treatments which slow progression of CKD, prevent or ameliorate metabolic complications, and prepare for dialysis and transplantation.2,3 Facilitated by the implementation of automated eGFR reporting by many laboratories, the proportion of older patients who have seen a nephrologist at least one year prior to renal replacement therapy increased from 30.0% to 48.5% from 1996 to 2006.4,5 However, there is conflicting evidence regarding benefits of such care, in part because the risk for progression to ESRD varies widely among patients with a low eGFR.6,7 Whereas some studies suggest that earlier referral to nephrology (patients having seen a nephrologist six to twelve months prior to initiation of dialysis) is associated with lower mortality after initiation of dialysis8-11; other studies have not.5,12
Most previous studies were retrospective analyses of patients who initiated dialysis.5,13,14 As such, the effect of nephrology care on outcomes such as progression to ESRD and mortality prior to ESRD could not be assessed. Among the few studies that have examined the association between nephrology care and outcomes in patients with earlier stages of CKD, several, but not all, suggest nephrology care is associated with better progression-free survival.15-17 However, the studies suggesting favorable effects were restricted to patients with diabetes mellitus or limited to a single center, and did not account for the trajectory of kidney function.
Importantly, prior studies were susceptible to survivor treatment bias or immortal time bias, because patients who receive nephrology care have a minimum survival time imposed, whereas no such requirement is imposed on patients who do not receive nephrology care.18 The landmark method is a well-established method used in time-to-event analysis to address survivor treatment bias by defining a fixed time period for identifying the exposure of interest, in this case, nephrology care.19-21
We sought to determine the relations among frequency of nephrology care, survival and progression to ESRD in a national cohort of veterans with advanced CKD using landmark analysis. We hypothesized that nephrology care would be associated with lower risks for death and progression to ESRD.
Methods
Cohort
We constructed a cohort of US veterans with advanced CKD using laboratory and administrative data from the Veterans Health Administration, Department of Veterans Affairs (VA); Medicare claims; and the US Renal Data System (USRDS), a national registry of patients receiving therapy for ESRD (dialysis or kidney transplantation). We used laboratory data from the VA Decision Support System Laboratory Results file to identify veterans with an outpatient measurement of serum creatinine during 2010. We calculated eGFR from the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.22 Individuals with missing race (1.2%) were assigned black race to estimate GFR. We identified 91,544 veterans with advanced CKD, defined as an outpatient eGFR ≤30 ml/min/1.73m2 for January 1, 2010 through December 31, 2010 and at least one additional outpatient eGFR <60 ml/min/1.73m2 within one year prior to the index eGFR.
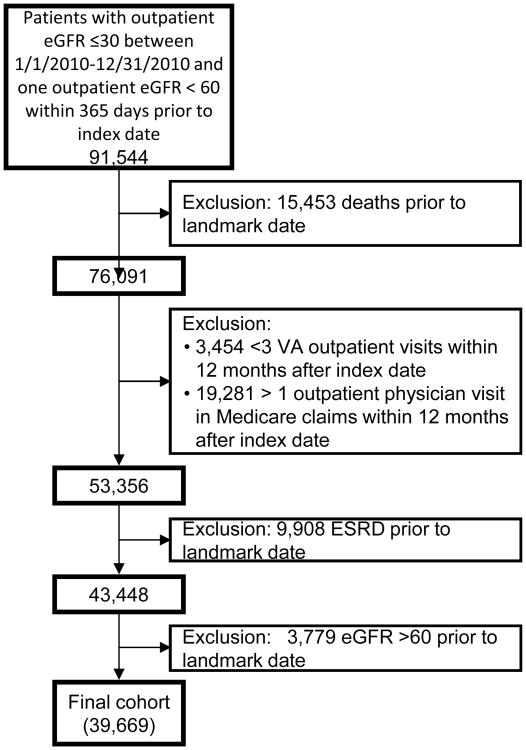
We used landmark analysis19 to determine the relationships among outpatient nephrology care and clinical outcomes. We used a landmark period of 12 months after the index eGFR date to ascertain the presence or absence of nephrology care. Figure S1 (provided as online supplementary material) illustrates the ascertainment of exposure and outcomes. We excluded 1) patients who died prior to the landmark date, 2) patients with fewer than three total outpatient VA visits or more than one Medicare outpatient visit, to ensure that the cohort was comprised of regular VA users, and 3) patients who progressed to ESRD prior to the landmark date. Finally, we excluded patients whose eGFR rose above 60 ml/min/1.73m2 at the end of the landmark period. Baseline characteristics were ascertained at the landmark date. The final analytic cohort had 39,669 patients (Figure 1). This study was approved by the Institutional Review Board at Stanford University (IRB-30539) and was deemed exempt from informed consent.
Figure 1.
Cohort entry diagram.
Nephrology Care
The key exposure of interest was an outpatient visit to a VA nephrology provider within a 12-month period from an index eGFR measurement ≤30 ml/min/1.73m2, which we ascertained based on the provider type and clinic location from the VA Medical SAS datasets, hereafter referred to as ‘nephrology care’. Our primary analysis compared any nephrology visits to none; we conducted secondary analyses comparing zero, 1-2, 3-4, and 5 or more visits, to gauge associations by the frequency of visits.
Outcomes
The primary outcomes were death from any cause, ascertained from the VA Vital Status files; progression to ESRD, defined as the initiation of maintenance dialysis or kidney transplantation, ascertained from USRDS; and the composite outcome of death or progression to ESRD. To assess whether the ESRD outcome was influenced by the use or timing of renal replacement therapy, we used two alternative ESRD definitions in sensitivity analyses. First, we defined ESRD as initiation of maintenance dialysis, kidney transplantation, or progression to a sustained eGFR below 15 ml/min/1.73m2 (two or more measurements separated by at least five days, with at least one outpatient measurement). Second, we assessed the eGFR at dialysis initiation reported to the USRDS on the Medical Evidence Form. We ascertained death and ESRD through September 30, 2014. We also assessed management of CKD as an intermediate outcome in the relationship between nephrology care and death or ESRD. To assess management of CKD, we abstracted the first and last serum potassium, albumin, hemoglobin, calcium, phosphorus, and blood pressure measurements between the index date and the landmark date. For patients who received nephrology care, we required the first measurement be taken before a nephrology visit and the last measurement to occur after at least one nephrology visit (Figure S2).
Covariates
We ascertained age, sex, place of residence, and VA copay status as a proxy for socioeconomic status, from the Medical SAS files and ascertained race with a combination of Medicare and VA files, using a previously described method.23 VA facility complexity was derived from the VA facility survey, categorized as low, mid, or high complexity. We ascertained the following comorbidities from the VA Medical SAS files over the 24 months prior to landmark date: diabetes mellitus, myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease (stroke and transient ischemic attacks), chronic liver disease, cancer, depression and post-traumatic stress disorder (PTSD). Prior hospitalizations and outpatient visits to non-nephrology VA providers were ascertained over the 12-month period prior to landmark date as proxies of overall illness severity. We assessed the trajectory of kidney function by calculating the change in eGFR from index to the last outpatient eGFR prior to the landmark date, divided by the days elapsed. When fewer than 30 days elapsed between the two eGFR measurements, we categorized the trajectory of kidney function as missing. In a sensitivity analysis, we used the absolute change in eGFR from index to the last outpatient eGFR prior to the landmark date, rather than the trajectory of kidney function. We assessed the change in clinical parameters by calculating the change from first measurement after index date to last measurement available before the landmark date, and categorized this change as missing when fewer than 30 days elapsed in between. Patients were considered to have an improvement in the control of clinical parameters if such parameters were not within guideline recommended ranges at the first measurement but were within guideline recommended ranges at the last measurement. We created missing categories for observations with missing values in our model.
Statistical Analysis
We compared baseline characteristics according to nephrology care (ever/never) using t-tests and chi-squared tests. We constructed logistic regression models to determine the relationships among nephrology care and selected patient characteristics. We used modified Poisson models to estimate the adjusted proportion of patients with clinical parameters within normal or guideline recommended ranges at cohort entry,3,24-26 and the adjusted proportion of patients with improvement in control of clinical parameters, by receipt of nephrology care.27,28 We tested for differences in adjusted proportions according to receipt of nephrology care using adjusted risk differences, adjusting for the same variables included in the multivariable-adjusted cause-specific hazard models.
We used cause-specific hazard models to determine the relationships between nephrology care and the risks for death and composite outcome of death and ESRD. We confirmed the proportionality assumption by evaluating the Kaplan-Meier curves for categorical covariates and examining Schoenfeld residuals for continuous covariates. For the outcome of ESRD, we confirmed the proportionality assumption by evaluating the cumulative incidence curves for categorical variables and examining Schoenfeld residuals for continuous variables and constructed competing risk models using Fine and Gray methods to account for the occurrence of death prior to progression to ESRD. For each outcome, we constructed an unadjusted model and a multivariable-adjusted model. The multivariable-adjusted model accounted for demographic characteristics and comorbidities: age, sex, race, marital status, place of residence, copay status, median income (within the zip code of residence), VA facility complexity, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, cancer, liver disease, dementia, depression, PTSD, prior hospitalization, visits to non-nephrology VA providers, and the trajectory of kidney function. We performed a sensitivity analysis using propensity score matching. We calculated the probability of nephrology care (versus no nephrology care) using logistic regression, with the same variables in the multivariable-adjusted models. We used a greedy match algorithm to match veterans who received nephrology care with those who did not, in a 1:1 ratio, using a caliper distance <0.03, 29,30 and stratified the analysis by matched pairs. Analyses were conducted using SAS Enterprise Guide Version 7.1 and Stata/MP Version 14.2.
Results
Correlates of Nephrology Care
The majority of patients in the cohort were male (97.1%), white (73.6%), and lived in urban areas (83.7%) (Table 1). Comorbidities were common, including diabetes mellitus (56.2%), heart failure (29.6%), cerebrovascular disease (15.5%) and cancer (20.2%). The mean index eGFR was 25.1±4.5 (standard deviation) ml/min/1.73m2. The majority of patients (62.2%) had no nephrology visits, 9.3% had one visit, 10.6% had two visits, 5.8% had three visits, 5.0% had four visits, and 7.0% had more than 5 visits over a 1-year period.
Table 1. Characteristics of veterans with advanced CKD.
| All (N=39,669) | Nephrology care | P-value | ||
|---|---|---|---|---|
| Yes (n=14,983) | No (n=24,686) | |||
| Age, y | 75.9 ±11.0 | 72.5 ±10.7 | 77.9 ±10.6 | <0.001 |
| Age group | <0.001 | |||
| 21-64 y | 8,172 (20.6%) | 4,308 (28.8) | 3,864 (15.7%) | |
| 65-74 y | 8,910 (22.5%) | 4,096 (27.3%) | 4,814 (19.5%) | |
| ≥75 y | 22,587 (56.9%) | 6,579 (43.9) | 16,008 (64.9%) | |
| Sex | 0.001 | |||
| Female | 1,166 (2.9%) | 387 (2.6%) | 779 (3.2%) | |
| Male | 38,503 (97.1%) | 14,596 (97.4%) | 23,907 (96.8%) | |
| Race | <0.001 | |||
| White | 29,180 (73.6%) | 10,110 (67.5%) | 19,070 (77.3%) | |
| Black | 6,453 (16.3%) | 3,382 (22.6%) | 3,071 (12.4%) | |
| Other | 3,165 (8.0%) | 1,239 (8.3%) | 1,926 (7.8%) | |
| Missing | 871 (2.2%) | 252 (1.7%) | 619 (2.5%) | |
| Marital status | 0.6 | |||
| Married | 21,379 (53.9%) | 8,050 (53.7%) | 13,329 (54.0%) | |
| Other | 18,290 (46.1%) | 6,933 (46.3%) | 11,357 (46.0%) | |
| Residence | 0.01 | |||
| Suburban, rural, small town | 5,552 (14.0%) | 1,897 (12.7%) | 3,655 (14.8%) | |
| Urban | 33,197 (83.7%) | 12,741 (85.0%) | 20,456 (82.9%) | |
| Missing | 920 (2.3%) | 345 (2.3%) | 575 (2.3%) | |
| Copay status+ | 0.3 | |||
| No copay | 29,820 (75.2%) | 10,663 (71.2%) | 19,157 (77.6%) | |
| Copay | 9,789 (24.7%) | 4,295 (28.7%) | 5,494 (22.3%) | |
| Missing | 60 (0.2%) | 25 (0.1%) | 35 (0.1%) | |
| Zip code median annual income | 0.001 | |||
| <$35,000 | 6,794 (17.1%) | 2,706 (18.1%) | 4,088 (16.6%) | |
| $35,000-<$50,000 | 16,005 (40.3%) | 5,925 (39.5%) | 10,080 (40.8%) | |
| $50,000-<$65,000 | 9,484 (23.9%) | 3,601 (24.0%) | 5,883 (23.8%) | |
| ≥$65,000 | 6,692 (16.9%) | 2,483 (16.6%) | 4,209 (17.1%) | |
| Missing | 694 (1.7%) | 268 (1.8%) | 426 (1.7%) | |
| Facility complexity+ | <0.001 | |||
| Low | 3,503 (8.8%) | 983 (6.6%) | 2,520 (10.2%) | |
| Mid | 6,246 (15.7%) | 2,433 (16.2%) | 3,813 (15.4%) | |
| High | 29,541 (74.5%) | 11,454 (76.4%) | 18,087 (73.3%) | |
| Missing | 379 (1.0%) | 113 (0.7%) | 266 (1.1%) | |
| Comorbidities | ||||
| Diabetes | 22,299 (56.2%) | 9,165 (61.2%) | 13,134 (53.2%) | <0.001 |
| Myocardial infarction | 2,676 (6.7%) | 960 (6.4%) | 1,716 (7.0%) | 0.03 |
| Congestive heart failure | 11,741 (29.6%) | 4,360 (29.1%) | 7,381 (29.9%) | 0.09 |
| Peripheral vascular disease | 7,741 (19.5%) | 3,006 (20.1%) | 4,735 (19.2%) | 0.03 |
| Dementia | 2,709 (6.8%) | 675 (4.5%) | 2,034 (8.2%) | <0.001 |
| Cerebrovascular disease | 6,151 (15.5%) | 2,255 (15.0%) | 3,896 (15.8%) | 0.05 |
| Chronic liver disease | 1266 (3.2%) | 607 (4.1%) | 659 (2.7%) | <0.001 |
| Cancer | 8,018 (20.2%) | 2,919 (19.5%) | 5,099 (20.6%) | 0.005 |
| Depression | 8,204 (20.7%) | 3,299 (22.0%) | 4,905 (19.9%) | <0.001 |
| PTSD | 3,104 (7.8%) | 1,344 (9.0%) | 1,760 (7.1%) | <0.001 |
| Prior hospitalization | 10,515 (26.5%) | 4,306 (28.7%) | 6209 (25.2%) | <0.001 |
| Visits to non-nephrology provider | <0.001 | |||
| 0 | 3,940 (9.9%) | 1,144 (7.6%) | 2,796 (11.3%) | |
| 1-2 | 8,763 (22.1%) | 2,731 (18.2%) | 6,032 (24.4%) | |
| 3-4 | 8,774 (22.1%) | 3,014 (20.1%) | 5,760 (23.3%) | |
| ≥5 | 18,192 (45.9%) | 8,094 (54.0%) | 10,098 (40.9%) | |
| Index eGFR (ml/min/1.73 m2) | 25.1 ± 4.5 | 24.8 ± 4.5 | 25.2 ± 4.5 | <0.001 |
| eGFR trajectory | <0.001 | |||
| Improvement | 11,970 (30.2%) | 5,818 (38.8%) | 6,152 (24.9%) | |
| Decline < 10 ml/min/ 1.73m2 per y | 12,136 (30.6%) | 5,129 (34.2%) | 7,007 (28.4%) | |
| Decline ≥10 ml/min/ 1.73m2 per y | 13,609 (34.3%) | 3,816 (25.5%) | 9,793 (39.7%) | |
| Missing or time lapse <30 d | 1,954 (4.9%) | 220 (1.5%) | 1,734 (7.0%) | |
Note: Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation.
Abbreviations: CKD, chronic kidney disease; ESRD – end-stage renal disease, PTSD – post traumatic stress disorder, eGFR – estimated glomerular filtration rate
Copay is based on a veteran's service-connected condition and financial status.33 Department of Veterans Affairs facilities are categorized according to complexity level, which is based on patient characteristics, clinical services offered, educational and research missions and administrative complexity. 34
After adjusting for demographics, comorbidities, and kidney function trajectory, older patients, and patients with a VA copay, heart failure, dementia or depression were less likely to receive nephrology care (Table 2). Conversely, men, black patients (versus white patients), patients who were married, patients who visited facilities with medium or high complexity (versus low complexity), patients who were hospitalized or visited non-nephrology providers, or had diabetes mellitus or peripheral vascular disease were more likely to receive nephrology care. Compared to patients with improving kidney function, patients with varying degrees of declining kidney function or undefined kidney function trajectory were less likely to receive nephrology care.
Table 2. Adjusted association between patient characteristics and nephrology care.
| Adjusted OR (95% CI) | ||
|---|---|---|
| Age group | ||
| 21-64 y | 1.00 (reference) | |
| 65-74 y | 0.80 (0.74-0.85) | |
| ≥75 y | 0.40 | 0.38-0.42 |
| Male Sex | 1.29 | 1.12-1.48 |
| Race | ||
| White | 1.00 (reference) | |
| Black | 1.81 | 1.70-1.93 |
| Other | 1.10 | 1.02-1.20 |
| Married (vs other) | 1.05 | 1.01-1.10 |
| Urban residence (vs other) | 1.07 | 1.00-1.14 |
| Copay (vs no copay) | 0.90 | 0.85-0.95 |
| Zip code median annual income | ||
| <$35,000 | 1.00 (reference) | |
| $35,000-<$50,000 | 1.03 | 0.96-1.10 |
| $50,000-<$65,000 | 1.06 | 0.99-1.14 |
| ≥$65,000 | 1.06 | 0.98-1.15 |
| Facility complexity | ||
| Low | 1.00 (reference) | |
| Mid | 1.56 | 1.41-1.72 |
| High | 1.47 | 1.35-1.61 |
| Prior hospitalization | 1.07 | 1.02-1.13 |
| Visits to non-nephrology provider | ||
| 0 | 1.00 (reference) | |
| 1-2 | 1.10 | 1.00-1.20 |
| 3-4 | 1.16 | 1.06-1.27 |
| ≥5 | 1.68 | 1.55-1.83 |
| Comorbidities | ||
| Diabetes | 1.14 | 1.09-1.20 |
| Myocardial infarction | 0.96 | 0.87-1.05 |
| Congestive heart failure | 0.90 | 0.86-0.95 |
| Peripheral vascular disease | 1.14 | 1.08-1.21 |
| Dementia | 0.71 | 0.65-0.79 |
| Cerebrovascular disease | 1.00 | 0.94-1.06 |
| Chronic liver disease | 1.00 | 0.89-1.14 |
| Cancer | 0.98 | 0.92-1.03 |
| Depression | 0.92 | 0.86-0.99 |
| PTSD | 0.94 | 0.86-1.05 |
| eGFR trajectory | ||
| Improvement | 1.00 (reference) | |
| Decline < 10 ml/min/ 1.73m2 per y | 0.81 | 0.77-0.85 |
| Decline ≥10 ml/min/ 1.73m2 per y | 0.36 | 0.34-0.38 |
| Missing or time lapse <30 d | 0.16 | 0.14-0.19 |
Abbreviations: CI, confidence interval; ESRD – end-stage renal disease, PTSD – post traumatic stress disorder, eGFR – estimated glomerular filtration rate; OR, odds ratio
adjusted for age, sex, race, marital status, place of residence, copay status, zip code median income, facility complexity, prior hospitalization, visits to non-nephrology Department of Veterans Affairs provider, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic liver disease, cancer, depression and PTSD, trajectory of kidney function
Management of CKD
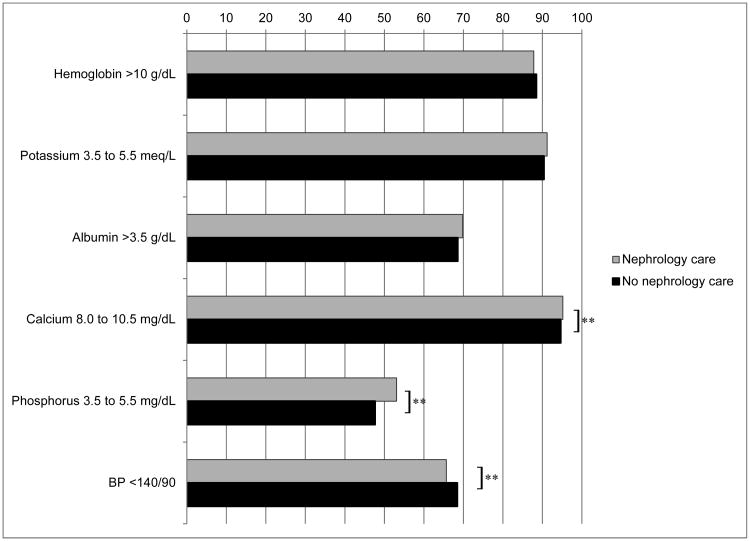
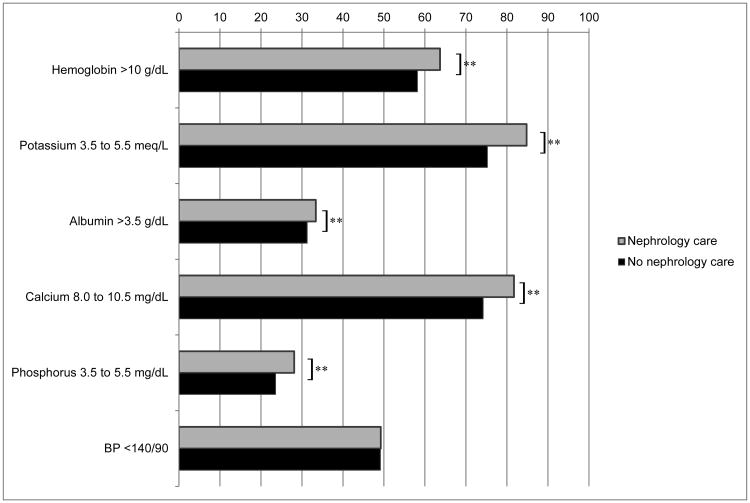
Table S1 shows the mean values of the clinical management parameters. In the adjusted analysis, a higher percentage of patients who received nephrology care had calcium and phosphorus values within guideline recommended ranges compared to those who did not receive nephrology care, and a lower percentage of patients who received nephrology care had blood pressure measurements within guideline recommended ranges compared to those who did not receive nephrology care at cohort entry (Figure 2a). Among patients with clinical parameters outside of guideline-recommended ranges at cohort entry, a significantly higher proportion of patients who received nephrology care had improvement in control of hemoglobin, potassium, albumin, calcium and phosphorus in the nephrology care group compared to the no nephrology care group (p<0.05 for each comparison, Figure 2b), but there was no significant difference in the proportion of patients who had improvement in blood pressure according to nephrology care.
Figure 2.
Clinical indicators according to receipt of nephrology care
Figure 2a: Adjusted proportion of patients with controlled clinical parameters at first measurement, by receipt of nephrology care
Figure 2b: Adjusted proportion of patients with improvement in control of clinical parameters, among patients who were uncontrolled at baseline, by receipt of nephrology care **p<0.05
Percent of laboratory data missing: Hemoglobin 24.9%, potassium 11.6%, albumin 27.5%, calcium 18.1%, phosphorus 53.8%, blood pressure 1.5%
Nephrology Care and Risk of Death
Over a mean follow-up of 2.9±1.1 years, 14,719 (37.1%) patients died. In unadjusted models, receipt of any nephrology care (versus none) was associated with a 20% lower risk of death (Table 3). This association was attenuated but persisted in multivariable adjusted models.
Table 3. Association between nephrology care and risk of death, progression to ESRD and combined outcome of death or ESRD with advanced CKD.
| Death | ESRD | Death or ESRD | ||||
|---|---|---|---|---|---|---|
| Unadj HR (95% CI) | Multivariable-adj HR (95% CI) | Unadj subdistribution HR (95% CI) | Multivariable-adj subdistribution HR (95% CI) | Unadj HR (95% CI) | Multivariable-adj HR (95% CI) | |
| Any nephrology care | 0.80 (0.77-0.83) | 0.88 (0.85-0.91) | 2.77 (2.60-2.94) | 1.48 (1.38-1.58) | 1.07 (1.04-1.10) | 1.00 (0.97-1.04) |
| Frequency of nephrology care | ||||||
| 0 visits | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1-2 visits | 0.80 (0.76-0.83) | 0.87 (0.84-0.91) | 1.95 (1.81-2.11) | 1.22 (1.13-1.32) | 0.95 (0.92-0.99) | 0.93 (0.89-0.97) |
| 3-4 visits | 0.76 (0.72-0.81) | 0.80 (0.80-0.90) | 3.00 (2.77-3.28) | 1.57 (1.45-1.73) | 1.07 (1.02-1.12) | 1.01 (0.96-1.06) |
| ≥5 visits | 0.86 (0.81-0.92) | 0.93 (0.84-0.99) | 4.88 (4.50-5.33) | 2.04 (1.86-2.23) | 1.47 (1.39-1.55) | 1.25 (1.19-1.32) |
Abbreviations: Adj, adjusted; CKD, chronic kidney disease; ESRD, end-stage renal disease; HR – hazard ratio, CI – confidence interval; unadj, unadjusted
Note: N=39,669. The model for ESRD accounted for competing risk of death. Multivariable: adjusted for age, sex, race, marital status, place of residence, copay status, zip code median income, facility complexity, prior hospitalization, visits to non-nephrology Department of Veterans Affairs provider, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic liver disease, cancer, depression post-traumatic stress disorder, and trajectory of kidney function
Nephrology Care and Risk of ESRD
Over the follow-up period, 4,310 patients (10.9%) progressed to ESRD and initiated dialysis (n=4198) or received a kidney transplant (n=112). Compared to patients who did not receive nephrology care, the unadjusted risk for ESRD in competing risk models was 2.77 times higher among patients receiving nephrology care, although this association was attenuated in adjusted models (Table 3). Results were similar in models where the outcome was receipt of renal replacement therapy or progression to a sustained eGFR <15 mL/min/1.73m2: for nephrology care versus none, multivariable adjusted sub-distribution HR was 1.62 (95% confidence interval [CI], 1.55-1.70). Patients who did not receive nephrology care initiated dialysis at a similar eGFR (10.9 mL/min/1.73m2) compared to those who received nephrology care (11.0 mL/min/1.73m2; p= 0.8). Results were not materially different in sensitivity analysis adjusting for the absolute change in eGFR rather than trajectory of kidney function (Table S2).
Nephrology Care and Risk of Death or ESRD
Over the follow up period, 17,805 (44.8%) patients had the composite outcome of death or developing ESRD. There was no significant association between nephrology care (any versus none) and the combined outcome of death or ESRD in multivariable-adjusted models. (Table 3) In analyses categorizing patients by the number of nephrology visits, there was a 25% higher risk of death or ESRD among patients with ≥5 nephrology visits compared to those with none.
Propensity-Matched Analysis
We matched 11,892 veterans who received nephrology care (79.4%) to 11,892 veterans who did not receive nephrology care using propensity scores. The matched cohort was well-balanced on patient characteristics at cohort entry, indicated by standardized differences of <10%. (Table S3)
Among the 23,784 patients included in the propensity-matched analysis, results were similar (Table 4). For example, compared to patients with no nephrology care, the multivariable-adjusted risks for death, ESRD, and death or ESRD were 0.94 (95% CI, 0.89-0.98), 1.38 (95% CI, 1.28-1.48), and 1.02 (95% CI, 0.98-1.06) among patients who received nephrology care.
Table 4.
Association between nephrology care and risk of death, progression to ESRD and combined outcome of death or ESRD among propensity-matched cohort with advanced CKD.
| HR for death (95% CI) | Subdistribution HR for ESRD (95% CI) | HR for death or ESRD (95% CI) | |
|---|---|---|---|
| Any nephrology care | 0.94 (0.89-0.98) | 1.38 (1.28-1.48) | 1.02 (0.98-1.06) |
| Frequency of nephrology care | |||
| 0 visits | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1-2 visits | 0.91 (0.87-0.96) | 1.02 (0.93-1.13) | 0.93 (0.88-0.97) |
| 3-4 visits | 0.90 (0.84-0.96) | 1.50 (1.36-1.67) | 1.02 (0.96-1.08) |
| ≥5 visits | 1.09 (1.01-1.18) | 2.42 (2.12-2.70) | 1.39 (1.30-1.49) |
Note: N = 23,784. The model for ESRD accounted for competing risk of death.
Abbreviations: CKD, chronic kidney disease; HR – hazard ratio, CI – confidence interval; ESRD, end-stage renal disease
Discussion
In this large national cohort of veterans with advanced CKD, we found that nephrology care was independently associated with lower mortality but not with a lower risk of ESRD.
Most previous analyses of patients who initiated dialysis suggest that the timing and duration of nephrology care is associated with mortality after dialysis initiation.5,9,31 For example, Stack et al. found a 68% increased risk for one-year mortality among patients who received later (less than 4 months prior to dialysis initiation) versus earlier nephrology care, and a 25% increased risk among patients who saw a nephrologist only once or not at all in the year before initiation of dialysis versus those who saw a nephrologist at least twice.9 In a contemporary cohort of Canadian patients initiating dialysis, patients who received earlier nephrology care (more than six months prior to initiation of dialysis) and higher cumulative number of nephrology visits had a lower risk of mortality compared to patients who received later care or less frequent care.31 Among a cohort of older veterans who initiated dialysis, Fischer et al. found that those who had a higher number of predialysis visits had lower 2-year mortality after dialysis initiation.11 Conversely, Winkelmayer et al. found no substantial improvement in one-year survival among older adults initiating dialysis during 1996-2006, despite an 18.5% increase in earlier nephrology referrals.5
Fewer studies have examined the relationship between nephrology care and progression of CKD.15-17 In a study of veterans with diabetes mellitus and CKD who received care in 1999, nephrology care was associated with lower mortality and a higher risk of ESRD, similar to our findings.15 Conversely, in a recent analysis of patients in the Chronic Renal Insufficiency Cohort study with a mean eGFR of 45 ml/min/1.73m2, nephrology care was neither associated with slower progression of CKD, nor with more frequent attainment of guideline concordant care.16 Similarly, a study from the National Kidney Foundation–Kidney Early Evaluation Program (NKF-KEEP) found no association between nephrology care and mortality in patients with CKD.17 A limitation of these prior studies is that they did not account for the trajectory of kidney function. Since patients with slower progression of CKD have a longer opportunity to obtain nephrology care, prior analyses may be affected by a form of survival bias. Conversely, patients with rapid progression of CKD or acute kidney injury might appear to be referred to nephrology care “late” due to their accelerated trajectory toward ESRD. In these patients, poor outcomes may be due to the underlying disease process rather than late nephrology care, per se.
Using landmark analysis, an established method for addressing survivor bias, we demonstrate that nephrology care is associated with lower mortality risk among veterans with advanced CKD. This finding persisted after accounting for multiple potential confounders, including kidney function trajectory. Nephrology care might contribute to lower mortality through better management of heart failure and fluid overload, reduced use of temporary central venous catheters at initiation of dialysis, or greater attention to management of electrolyte and mineral metabolism disorders, although due to the number of patients with missing laboratory data, these improvements should be interpreted cautiously. Nephrology care was not associated with differences in other clinical parameters, including blood pressure control, which is considered a key risk factor both for CKD progression and for mortality. We were unable to determine whether this reflected confounding by indication (i.e. that patients with more difficult to control blood pressure are more often referred to nephrology care) or less aggressive blood pressure management.
Apart from direct or indirect nephrology interventions that may result in improved survival, we could not exclude the possibility that this finding might be explained by differential nephrology referral patterns due to factors not captured in administrative data. Propensity-score matching is often used to reduce this form of bias, but may not eliminate it. For example, a patient with poor functional status may not be referred to nephrology due to ill health or lack of recognition of advanced CKD by the provider, or they may decline nephrology care because it is not consistent with their overall goals.
Similar to the study by Tseng et al,32 we observed a higher risk of ESRD with nephrology care. To account for the possibility that a higher risk of ESRD was due to a higher proportion of patients who received dialysis, we conducted sensitivity analyses which included progression to a sustained eGFR <15 ml/min/1.73m2 in our definition of ESRD, and found similar results. Moreover, patients who received nephrology care initiated dialysis at similar eGFRs compared to patients who received no nephrology care. Hence, the higher risk of ESRD in the group receiving nephrology care cannot be explained by more frequent or earlier provision of dialysis. A higher risk of ESRD was only apparent in patients with three or more nephrology visits in the propensity-matched analysis. Blood pressure control at cohort entry was less optimal in patients who received nephrology care, but the magnitude of this difference was small. In addition, there may be other risk factors for ESRD in patients receiving nephrology care for which we were unable to account.
A novel observation from our study was that kidney function trajectory was a strong correlate of nephrology care – patients whose kidney function declined at a rate of >10 ml/min/1.73m2 per year were 64% less likely to have received nephrology care compared to patients with improving kidney function. Improving access to nephrology care for patients who progress rapidly should be a key priority for quality improvement initiatives and an important area for future research. In this cohort of veterans receiving care in the VA health system, demographic characteristics, such as age, sex, race, and geography, were stronger predictors of nephrology care than socioeconomic status and most comorbidities, though certain psychiatric comorbidities, prior hospitalization and visits to non-nephrology providers were strongly associated with the absence of nephrology care.
The strengths of our study include the large contemporary national sample, the ability to account for multiple potential confounders including kidney function trajectory, and the application of a landmark design to address survival bias and propensity-score matching to address confounding by indication. There were also several important limitations. Our study was limited to veterans who received regular care in the VA, and may not be generalizable to other healthcare systems. We could not determine the length or intensity of each nephrology visit or the management decisions implemented. We were unable to verify the reasons behind referral and non-referral, or whether patients kept their appointments. While we adjusted for multiple comorbidities and sociodemographic factors, unmeasured confounders may affect observed results. While the landmark approach helps to address survival bias, there are some disadvantages to this method, such as the inability to account for events prior to the landmark period.19 Finally, as this was an observational study, we were unable to determine causality.
In summary, nephrology care is associated with lower mortality in patients with advanced CKD but not with a lower risk of ESRD. Future studies are needed to elucidate the optimal pattern of healthcare delivery to this vulnerable population and to develop novel strategies to lower mortality, prevent associated complications, and slow progression of CKD.
Supplementary Material
Table S1: Values of clinical parameter measurements.
Table S2: Association between nephrology care and risk of ESRD in patients with advanced CKD, accounting for competing risk of death, adjusting for absolute eGFR change.
Table S3: Characteristics of propensity score–matched cohort.
Figure S1: Schematic of landmark analysis.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Figure S2: Schematic of assessment of clinical parameters.
Supplementary Table S1 (PDF). Values of clinical parameter measurements.
Supplementary Table S2 (PDF). Association between nephrology care and risk of ESRD in patients with advanced CKD, accounting for competing risk of death, adjusting for absolute eGFR change.
Supplementary Table S3 (PDF). Characteristics of propensity score–matched cohort.
Supplementary Figure S1 (PDF). Schematic of landmark analysis.
Supplementary Figure S2 (PDF). Schematic of assessment of clinical parameters.
Acknowledgments
Support: Drs Fung, Chang, and Chertow are supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (F32 DK107111, K23 DK095914, and K24 DK085446, respectively). Drs Asch and Kurella Tamura are supported by HX001262 from the Department of Veterans Affairs. Views expressed are those of the authors and not necessarily those of the VA. The funders of this study did not have direct role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: EF, MKT; data acquisition: I-CT, MKT; data analysis/interpretation: EF, MKT; statistical analysis: EF, I-CT; supervision or mentorship: TIC, GMC, SMA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by two external peer reviewers, a Statistics/Methods Editor, an Associate Editor, and Editor-in-Chief Feldman.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Renal Data System. Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2014. [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 3.KDIGO CKD Work Group. ‘Chapter 5: Referral to specialists and models of care’ in KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl (2011) 2013;3(1):112–119. doi: 10.1038/kisup.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303(12):1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Liu J, Chertow GM, Tamura MK. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med. 2011;171(15):1371–1378. doi: 10.1001/archinternmed.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 7.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 8.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137(6):479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis. 2003;41(2):310–318. doi: 10.1053/ajkd.2003.50038. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmayer WC, Owen WF, Jr, Levin R, Avorn J. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol. 2003;14(2):486–492. doi: 10.1097/01.asn.0000046047.66958.c3. [DOI] [PubMed] [Google Scholar]
- 11.Fischer MJ, Stroupe KT, Kaufman JS, et al. Predialysis nephrology care and dialysis-related health outcomes among older adults initiating dialysis. BMC Nephrol. 2016;17(1):103. doi: 10.1186/s12882-016-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roubicek C, Brunet P, Huiart L, et al. Timing of nephrology referral: influence on mortality and morbidity. Am J Kidney Dis. 2000;36(1):35–41. doi: 10.1053/ajkd.2000.8241. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MJ, Stroupe KT, Kaufman JS, et al. Predialysis nephrology care among older veterans using Department of Veterans Affairs or Medicare-covered services. Am J Manag Care. 2010;16(2):e57–66. [PubMed] [Google Scholar]
- 14.Gillespie BW, Morgenstern H, Hedgeman E, et al. Nephrology care prior to end-stage renal disease and outcomes among new ESRD patients in the USA. Clin Kidney J. 2015;8(6):772–780. doi: 10.1093/ckj/sfv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Ramirez HR, Jalomo-Martinez B, Cortes-Sanabria L, et al. Renal function preservation in type 2 diabetes mellitus patients with early nephropathy: a comparative prospective cohort study between primary health care doctors and a nephrologist. Am J Kidney Dis. 2006;47(1):78–87. doi: 10.1053/j.ajkd.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Ricardo AC, Roy JA, Tao K, et al. Influence of Nephrologist Care on Management and Outcomes in Adults with Chronic Kidney Disease. J Gen Intern Med. 2016;31(1):22–29. doi: 10.1007/s11606-015-3452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saab G, Chen SC, Li S, et al. Association of physician care with mortality in Kidney Early Evaluation Program (KEEP) participants. Am J Kidney Dis. 2012;59(3 Suppl 2):S34–39. doi: 10.1053/j.ajkd.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19(5):841–843. doi: 10.1681/ASN.2007121354. [DOI] [PubMed] [Google Scholar]
- 19.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 20.Glesby MJ, Hoover DR. Survivor treatment selection bias in observational studies: examples from the AIDS literature. Ann Intern Med. 1996;124(11):999–1005. doi: 10.7326/0003-4819-124-11-199606010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Alawadi Z, Phatak UR, Hu CY, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer. 2017;123(7):1124–1133. doi: 10.1002/cncr.30230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroupe KT, Tarlov E, Zhang Q, Haywood T, Owens A, Hynes DM. Use of Medicare and DOD data for improving VA race data quality. J Rehabil Res Dev. 2010;47(8):781–795. doi: 10.1682/jrrd.2009.08.0122. [DOI] [PubMed] [Google Scholar]
- 24.KDIGO Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl (2011) 2012;2(4):279–335. [Google Scholar]
- 25.KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl (2011) 2012;2(5):377–414. [Google Scholar]
- 26.KDIGO CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD) Kidney Int. 2009;76113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 27.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 28.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 29.Kosanke J, Bergstralh E. Locally written SAS Macros Match 1 or more controls to cases using the GREEDY algorithm. [Accessed April 12, 2017];2004 http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros.
- 30.Chang TI, Shilane D, Brunelli SM, Cheung AK, Chertow GM, Winkelmayer WC. Angiotensin-converting enzyme inhibitors and cardiovascular outcomes in patients on maintenance hemodialysis. Am Heart J. 2011;162(2):324–330. doi: 10.1016/j.ahj.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singhal R, Hux JE, Alibhai SM, Oliver MJ. Inadequate predialysis care and mortality after initiation of renal replacement therapy. Kidney Int. 2014;86(2):399–406. doi: 10.1038/ki.2014.16. [DOI] [PubMed] [Google Scholar]
- 32.Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med. 2008;168(1):55–62. doi: 10.1001/archinternmed.2007.9. [DOI] [PubMed] [Google Scholar]
- 33.US Department of Veterans Affairs. Health Benefits. [Accessed 4/18/2017]; https://www.va.gov/healthbenefits/cost/copays.asp.
- 34.Office of Quality Safety and Value. VHA Facility Quality and Safety Report. [Accessed 4/28/2017];2012 https://www.va.gov/HEALTH/docs/2012_VHA_Facility_Quality_and_Safety_Report_FINAL508.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Values of clinical parameter measurements.
Table S2: Association between nephrology care and risk of ESRD in patients with advanced CKD, accounting for competing risk of death, adjusting for absolute eGFR change.
Table S3: Characteristics of propensity score–matched cohort.
Figure S1: Schematic of landmark analysis.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Figure S2: Schematic of assessment of clinical parameters.
Supplementary Table S1 (PDF). Values of clinical parameter measurements.
Supplementary Table S2 (PDF). Association between nephrology care and risk of ESRD in patients with advanced CKD, accounting for competing risk of death, adjusting for absolute eGFR change.
Supplementary Table S3 (PDF). Characteristics of propensity score–matched cohort.
Supplementary Figure S1 (PDF). Schematic of landmark analysis.
Supplementary Figure S2 (PDF). Schematic of assessment of clinical parameters.