Abstract
Purpose
To evaluate longitudinal vision-related quality of life (VRQoL) in patients with noninfectious uveitis.
Design
Cohort study using randomized controlled trial data.
Participants
Patients with active or recently active intermediate uveitis, posterior uveitis, or panuveitis enrolled in the Multicenter Steroid Treatment Trial and Follow-up Study.
Methods
Data from the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) for the first 3 years after randomization were evaluated semiannually. Analyses were stratified by assigned treatment (129 implant therapy vs. 126 systemic therapies) because of substantial differences in trajectories of VRQoL. The impact of baseline measurements of visual function (visual acuity and visual field), demographics, and disease characteristics was assessed using generalized estimating equations.
Main Outcome Measures
Primary outcome was the NEI-VFQ-25 composite score over 3 years after randomization.
Results
Individuals in both treatment groups showed similar improvement in NEI-VFQ-25 scores after 3 years of follow-up (implant: 11.9 points; 95% confidence interval [CI], 8.6–15.2; P < 0.001; systemic: 9.0 points; 95% CI, 5.6–12.3; P < 0.001; P = 0.21 for interaction). Individuals in the implant group showed a substantial improvement during the first 6 months followed by stable scores, whereas individuals in the systemic group showed a steady improvement over the course of follow-up. Worse initial visual acuity and visual fields were associated with lower initial NEI-VFQ-25 scores for both treatment groups. In the systemic group, these differences were maintained throughout follow-up. In the implant group, individuals with initial visual acuity worse than 20/40 showed additional improvement in NEI-VFQ-25 score to come within −7 points (95% CI, −15.0 to 0.9) of those with visual acuity 20/40 or better initially, a clinically meaningful but not statistically significant difference (P = 0.081). Results based on sensitivity analyses showed similar patterns.
Conclusions
Both treatment groups demonstrated significant improvements in NEI-VFQ-25 scores; however, the improvement was immediate for the implant group as opposed to gradual for the systemic group. Poorer visual function was associated significantly with initial differences in NEI-VFQ-25 scores. However, only individuals in the implant group with poor visual acuity were able to overcome their initial deficits by the end of 3 years.
There is an increasing emphasis on patient-reported outcomes such as quality of life in clinical trials. General metrics such as the EQ-5D often are used to allow for comparisons across a wide range of diseases and treatments. However, disease-specific questionnaires also are important because they may be more sensitive to differences in the clinical outcomes used to assess disease severity and response to treatment.1 In ophthalmology, vision-related quality of life (VRQoL) metrics assess both general quality of life and vision-related functioning (e.g., driving, reading), which relies not only on visual acuity, but also on other facets of vision such as visual field, contrast sensitivity, and color vision. One of the most commonly used questionnaires is the 25-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25).2
Chronic eye diseases, such as noninfectious uveitis, are associated with both visual acuity loss and visual field damage, through complications of or treatments for the disease. In uveitic eyes, there is a high prevalence of macular edema, cataracts, and other complications associated with visual acuity loss.3–7 Similarly, the incidence of glaucoma is higher in eyes with uveitis, and some of the therapies for uveitis are know to cause elevated intraocular pressure, both of which are associated with visual field impairment.8
Given the multifaceted impact of uveitis on visual function, a measurement tool such as the NEI-VFQ-25 may play a key role in evaluating the progression of the disease as well as in comparing therapies. Previous research has demonstrated the existence of a strong cross-sectional association between visual acuity and the NEI-VFQ-25 composite score in uveitis.1,9–12 However, the limited research exploring the longitudinal patterns of NEI-VFQ-25 or the relationship between the NEI-VFQ-25 and other components of visual function such as visual field impairment has focused on glaucoma as opposed to uveitis.13–16 The goal of this analysis was to examine the longitudinal trajectories of the NEI-VFQ-25 composite score and to explore the relationship between these trajectories and baseline clinical measurements of visual function and other risk factors using data from patients with noninfectious uveitis enrolled in the Multicenter Uveitis Steroid Treatment (MUST) Trial.
Methods
Details of the MUST Trial (Clinicaltrials.gov identifier, NCT00132691) and Follow-up Study have been described elsewhere.17–19 Individuals were randomized to undergo fluocinolone acetonide implant therapy or systemic corticosteroid therapy with or without immunosuppressive therapy for the treatment of active or recently active (within 6 months) intermediate uveitis, posterior uveitis, or panuveitis. A pars plana vitrectomy could be performed at the time of implant placement, but was not mandated as part of the protocol. Institutional review boards for all centers approved the protocol and all participants provided written informed consent. This analysis is based on data collected at randomization and at semiannual visits for the first 3 years of follow-up, which corresponds to the expected lifespan of the implant.
Vision-Related Quality of Life
Vision-related quality of life was assessed using the NEI-VFQ-25.2,20 The NEI-VFQ-25 includes 12 subscales: general health, general vision, near activities, distance activities, dependency, driving, role difficulties, mental health, social functioning, color vision, ocular pain, and peripheral vision. The overall composite score, our primary outcome, is computed by taking the unweighted average of the 12 subscale scores. Values for the composite score range from 0 to 100, with higher scores representing better VRQoL. A difference of 4 to 6 points is considered clinically meaningful.1,21
Visual Function
Baseline visual function was assessed using best-corrected visual acuity and visual field measurements at randomization. Visual acuity was measured using logarithmic visual acuity charts according to a standardized protocol.17,22 Because the unit of analysis for the NEI-VFQ-25 is the individual, as opposed to the eye, it was necessary to select a person-level summary of visual acuity. For the primary analysis, we focused visual acuity in the better-seeing eye, which is generally accepted as having the greatest influence on an individual’s visual function.4,22 A threshold of 20/40 (70 letters) was used to categorize individuals based on visual acuity in the better-seeing eye at randomization (20/40 or better vs. worse than 20/40). The choice of threshold was based on the Standardization of Uveitis Nomenclature working group recommendations as well as the fact that 20/40 vision is commonly required to obtain a driver’s license.24 Sensitivity analyses based on the visual acuity in the worse-seeing eye using the 20/40 threshold and an alternate threshold of 20/50 for the better-seeing eye also were performed.
Measurements of the mean deviation were obtained using Humphrey 24-2 visual field testing. Visual field impairment was categorized as mild (>−3 dB), moderate (−3 to −6 dB), or severe (<−6 dB) based on the recommendation of ophthalmologists in the MUST Trial Research Group. A person-level variable was created by selecting the visual field from the eye used to assess visual acuity, that is, the better-seeing eye for the primary analysis. For sensitivity analyses featuring the worse-seeing eye, 2 versions were considered because of the large amount of missing visual field data in the worse-seeing eye: (1) only the visual fields from the worse seeing-eye and (2) visual fields from the worse-seeing eye if available and visual fields from the better-seeing eye otherwise.
Additional Baseline Risk Factors
We also assessed the impact of baseline demographic and clinical characteristics that previously had been shown to be associated with visual function, cross-sectional measurements of the NEI-VFQ-25 composite score, or both.1 Demographic characteristics included age, gender, race (black vs. not black), associated systemic disease (present vs. absent), and obesity (body mass index, ≥30 kg/m2 vs. <30 kg/m2). Clinical characteristics included the type of uveitis (intermediate uveitis vs. posterior uveitis or panuveitis based on the worst diagnosis in either eye) and the presence of associated systemic disease. Time from diagnosis also was considered, but was collinear with age.
Statistical Analysis
The trajectories of quality-of-life measurements including the NEI-VFQ-25 composite score differed substantially for the 2 treatment groups during the MUST Trial and Follow-up Study.18 Therefore, we performed the assessment of risk factors for each treatment group, implant (n = 129) and systemic therapy (n = 126), separately. The interrelationships between measurements of visual function and other baseline risk factors were explored using Wilcoxon rank-sum tests and Fisher exact tests for continuous and categorical variables, respectively. Modeling of the trajectories of NEI-VFQ-25 composite score over time, both unadjusted and adjusted for potential baseline risk factors, was performed using generalized estimating equations to account for repeated measurements within an individual. Given the equal spacing of the visits, a Toeplitz correlation structure was used. We explored quantifying time both as a continuous variable as well as parameterizing individual visits or clusters of visits. Baseline risk factors were included in the model in 2 ways: as a single variable, to quantify the impact on NEI-VFQ-25 composite score at randomization, and as an interaction term, to quantify the impact on the trajectory of NEI-VFQ-25 composite score over time. The primary analyses were based on the principles of intention to treat, that is, patients were analyzed according to their assigned treatment. A per-protocol sensitivity analysis, which included only individuals who underwent the assigned treatment within 6 months of randomization, was used to assess the potential impact of treatment noncompliance and cross-overs. Individuals in the systemic arm were censored at date of the first implantation in either eye. P values of less than 0.05 were considered statistically significant. All analyses were performed using SAS software (SAS/STAT User’s Guide version 9.1; SAS, Inc., Cary, NC), R (The R Project for Statistical Computing, version 3.3.1; http://www.r-project.org/), and STATA version 13 (StataCorp LP, College Station, TX).
Results
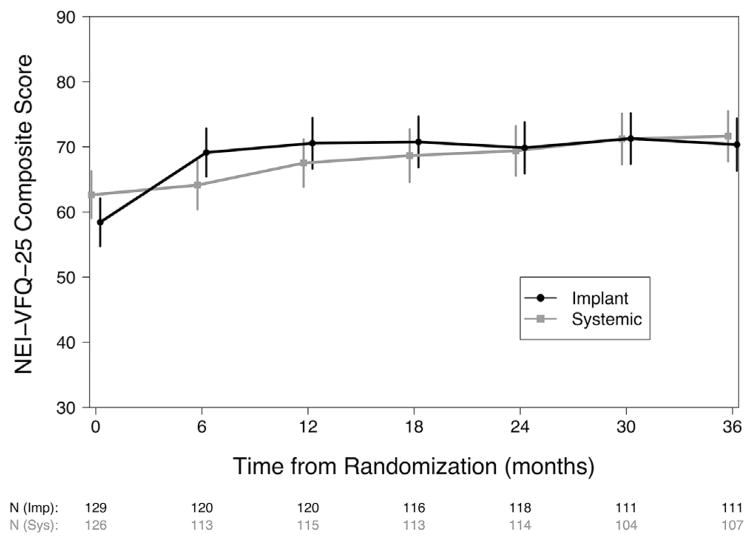
Although the trajectories differed, individuals in both treatment groups showed significant improvement in NEI-VFQ-25 composite score 3 years after randomization (Fig 1). Individuals assigned to undergo implant therapy had, on average, an increase in NEI-VFQ-25 of 11.9 points (95% confidence interval [CI], 8.6–15.2; P < 0.001), with a large gain in the first 6 months followed by relatively stable VFQ scores thereafter. In contrast, individuals assigned to undergo systemic therapy had a cumulative increase in NEI-VFQ-25 of 9.0 points at 3 years (95% CI, 5.6–12.3 points; P < 0.001) with a steady gain in NEI-VFQ-25 score over time. The difference in improvement between the 2 treatment groups at 3 years was neither statistically significant nor clinically meaningful (2.9 points; 95% CI, −1.7 to 7.6 points; P = 0.21).
Figure 1.
The trajectory of the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) over the first 3 years after randomization for individuals assigned to receive implant (black line, circle) or systemic (grey line, square) therapy in the Multicenter Steroid Treatment Trial. The estimated means with 95% confidence intervals are plotted for each treatment group.
Twenty-five–Item National Eye Institute Visual Functioning Questionnaire for Implant Therapy over Time
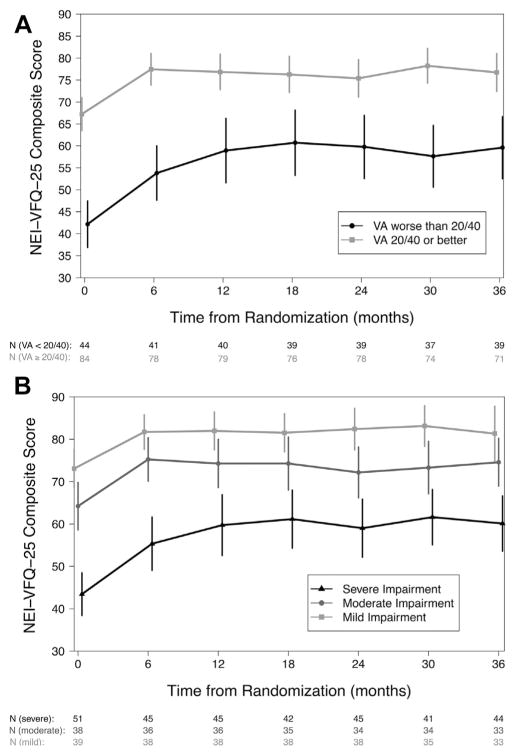
A total of 127 of the 129 individuals assigned to undergo implant therapy were included in the risk factor analysis; 2 were excluded (1 missing baseline visual acuity and 1 missing baseline visual field impairment). Of these, 109 (86%) completed 3 years of follow-up and 120 (94%) received an implant within the first 6 months of follow-up. The baseline visual acuity and visual field impairment both had a significant impact on the NEI-VFQ-25 composite scores for individuals assigned to implant therapy (Fig 2; Table 1). At randomization, individuals with visual acuity worse than 20/40 had NEI-VFQ-25 composite scores that were 15.0 points lower than those with a visual acuity of 20/40 or better. Overall, the average improvement was 10.5 points during the first 6 months regardless of the initial visual acuity. However, although the individuals with a visual acuity of 20/40 or better at baseline had stable NEI-VFQ-25 composite scores after 6 months and beyond (−1.23 points; 95% CI, −3.89 to 1.43 points; P = 0.36), individuals with a visual acuity worse than 20/40 gained an additional 8 points by 1 year (95% CI, 3.1–12.8; P = 0.001), a gain that was maintained through the rest of follow-up. Individuals with moderate and severe visual field impairment had scores that were significantly lower NEI-VFQ-25 composite scores at randomization (−5.2 and −17.4 points, respectively) than individuals with mild impairment. Unlike visual acuity, the degree of visual field impairment did not affect the amount of change in NEI-VFQ-25 composite scores during follow-up. Men had significantly higher NEI-VFQ-25 scores at randomization than women. The NEI-VFQ-25 score at randomization was 2 points lower for each additional 10 years of age beyond 45 years. These differences were maintained throughout follow-up. Although both posterior uveitis and panuveitis (−7.95 points; P = 0.02) and obesity (−7.83 points; P = 0.02) were associated with lower NEI-VFQ-25 composite scores at randomization when considered alone, neither was significant after adjusting for the variables described above. A similar pattern was observed in the sensitivity analyses based on the worse-eye analysis, per protocol analysis set, and when using a visual acuity threshold of 20/50 (Table 2, available at www.aaojournal.org).
Figure 2.
The relationship between the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) composite score and visual function, measured by (A) visual acuity (VA) and (B) visual field mean deviation, over the first 3 years after randomization for eyes assigned to implant therapy. Visual acuity is categorized as 20/40 or better (grey line, square) or worse than 20/40 (black line, circle), and visual field mean deviation is categorized as mild impairment (>−3 dB; light grey line, square), moderate impairment (−3 to −6 dB; grey line, circle), or severe impairment (<−6 dB; black line, triangle). The estimated means with 95% confidence intervals are plotted for each category.
Table 1.
Factors Influencing the Initial and Trajectory of 25-Item National Eye Institute Visual Functioning Questionnaire Composite Scores during the First 3 Years of Follow-up for 127 Individuals Assigned to Undergo Implant Therapy in the Multicenter Steroid Treatment Trial and Follow-up Study
| Characteristic | No. (%) at Randomization | 25-Item National Eye Institute Visual Functioning Questionnaire Score at Randomization: Estimated Mean Difference at Randomization in Points (95% Confidence Interval)* | P Value |
|---|---|---|---|
| Visual acuity worse than 20/40 | 43 (34) | −14.95 (−21.83 to −8.07) | <0.001 |
| Visual field impairment | |||
| Moderate (−3 to −6 dB) | 38 (30) | −5.24 (−10.78 to 0.29) | 0.063 |
| Severe (<−6 dB) | 50 (39) | −17.39 (−24.92 to −9.85) | <0.001 |
| Male | 37 (29) | 7.30 (1.93–12.68) | 0.008 |
| Black | 35 (28) | −1.63 (−8.34 to 5.08) | 0.63 |
| For each 10 yrs older than 45 yrs at randomization | 127 (100) | −2.12 (−3.61 to −0.64) | 0.005 |
| Posterior uveitis or panuveitis | 77 (61) | −3.09 (−8.57 to 2.38) | 0.27 |
|
| |||
| No (%) at Follow-up Visits | Change in 25-Item National Eye Institute Visual Functioning Questionnaire Scores from Randomization to Follow-up: Estimated Mean Difference from Randomization to Follow-up in Points (95% Confidence Interval)* | ||
|
| |||
| Individuals with visual acuity 20/40 or better at randomization† | |||
| At 6 mos | 78 (66) | 10.51 (7.91–13.11) | <0.001 |
| One yr or later | 378 (67) | 9.27 (6.19–12.36) | <0.001 |
| Individuals with visual acuity worse than 20/40 at randomization† | |||
| At 6 mos | 40 (34) | 10.51 (7.91–13.11) | <0.001 |
| One yr or later | 189 (33) | 17.18 (12.48–21.89) | <0.001 |
Visual acuity and visual field impairment was assessed in the better-seeing eye.
Adjusted for all factors simultaneously.
The change in 25-Item National Eye Institute Visual Functioning Questionnaire score from randomization to 1 year and beyond was significantly different for individuals with visual acuity worse than 20/40 at randomization versus those with visual acuity 20/40 or better at randomization (P = 0.001).
Table 2.
Sensitivity analyses of factors influencing the initial and trajectory of VFQ-25 composite scores during the first 3 years of follow-up for individuals assigned to receive implant therapy in the MUST Trial and Follow-up Study. Worse eye analyses were based upon the visual acuity in the worse seeing eye and the visual field in the same eye only (Version I) or used the visual field for the other eye if not available (Version II). Better eye analyses were based upon the visual acuity in better eye and the corresponding visual field measurements. The Per Protocol analysis only included individuals who received an implant within 6 months of randomization. The visual acuity threshold was 20/40 for all analyses except for the one labeled 20/50.
| Characteristic | Worse Eye Analyses | Better Eye Analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version I (N = 116) |
Version II (N = 127) |
Per Protocol (N = 120) |
20/50 (N = 127) |
|||||||||
| VFQ at Baseline: N (%) Estimate (95% CI) P-value* | ||||||||||||
|
| ||||||||||||
| Worse visual acuity | 73 (63%) | −9.50 (−15.20, −3.79) | 0.001 | 84 (66%) | −10.20 (−16.02, −4.39) | < 0.001 | 41 (34%) | −15.38 (−22.49, −8.26) | < 0.001 | 34 (27%) | −12.30 (−20.13, −4.47) | 0.002 |
| Moderate Visual Field Impairment (−3db to −6db) | 23 (28%) | 0.17 (−5.64, 5.97) | 0.96 | 34 (27%) | −1.06 (−7.05, 4.94) | 0.73 | 36 (30%) | −4.97 (−10.57, 0.62) | 0.081 | 38 (30%) | −6.85 (−12.71, −0.98) | 0.022 |
| Severe Visual Field Impairment (<−6db) | 61 (53%) | −12.40 (−19.01, −5.80) | < 0.001 | 69 (54%) | −14.13 (−20.83, −7.43) | < 0.001 | 46 (39%) | −17.11 (−24.93, −9.28) | < 0.001 | 50 (39%) | −18.60 (−25.94, −11.26) | < 0.001 |
| Male | 37 (32%) | 4.85 (−0.89, 10.59) | 0.10 | 37 (29%) | 6.69 (0.76, 12.61) | 0.027 | 35 (29%) | 7.02 (1.45, 12.59) | 0.013 | 37 (29%) | 7.99 (2.68, 13.30) | 0.003 |
| African American | 31 (27%) | −6.91 (−14.26, 0.45) | 0.066 | 35 (28%) | −3.90 (−11.09, 3.29) | 0.29 | 32 (27%) | −1.72 (−8.54, 5.10) | 0.62 | 35 (28%) | −2.22 (−9.38, 4.95) | 0.54 |
| For each 10 years older than 45 at RZ | 116 (100%) | −2.04 (−3.90, −0.19) | 0.030 | 127 (100%) | −1.98 (−3.77, −0.18) | 0.031 | 120 (100%) | −2.40 (−3.93, −0.87) | 0.002 | 127 (100%) | −2.40 (−3.86, −0.94) | 0.001 |
| Posterior/panuveitis | 68 (59%) | −4.87 (−10.66, 0.92) | 0.10 | 77 (61%) | − −4.59 (−10.64, 1.46) | 0.14 | 73 (61%) | −3.52 (−9.26, 2.22) | 0.23 | 77 (61%) | −3.03 (−8.54, 2.47) | 0.28 |
|
| ||||||||||||
| Change in VFQ from Randomization to Follow-up: N (%) Estimate (95% CI) P-value* | ||||||||||||
|
| ||||||||||||
| Individuals with better visual acuity at randomization | ||||||||||||
| At six months | 40 (37%) | 10.97 (8.42, 13.52) | < 0.001 | 40 (34%) | 10.52 (7.94, 13.10) | < 0.001 | 77 (66%) | 10.59 (7.99, 13.19) | < 0.001 | 87 (74%) | 10.47 (7.87, 13.06) | < 0.001 |
| One year or later | 197 (38%) | 8.53 (4.47, 12.58) | < 0.001 | 197 (35%) | 8.22 (4.13, 12.32) | < 0.001 | 369 (66%) | 8.87 (5.77, 11.98) | < 0.001 | 420 (74%) | 9.97 (6.96, 12.97) | < 0.001 |
| Individuals with better visual acuity at randomization† | ||||||||||||
| At six months wt20/40 | 68 (63%) | 10.97 (8.42, 13.52) | < 0.001 | 78 (66%) | 10.52 (7.94, 13.10) | < 0.001 | 40 (34%) | 10.59 (7.99, 13.19) | < 0.001 | 31 (26%) | 10.47 (7.87, 13.06) | < 0.001 |
| One year or later wt20/40 | 327 (62%) | 14.85 (11.28, 18.42) | < 0.001 | 370 (65%) | 13.84 (10.43, 17.25) | < 0.001 | 189 (34%) | 17.22 (12.50, 21.93) | < 0.001 | 147 (26%) | 17.35 (12.03, 22.67) | < 0.001 |
Adjusted for all factors simultaneously.
Change in VFQ-25 from randomization to one year and beyond was significantly different for individuals with worse visual acuity at randomization versus those with better visual acuity at randomization in all models (p < 0.02 for all).
RZ = randomization; FU = follow-up; CI = confidence interval.
Twenty-five–Item National Eye Institute Visual Functioning Questionnaire for Systemic Therapy over Time
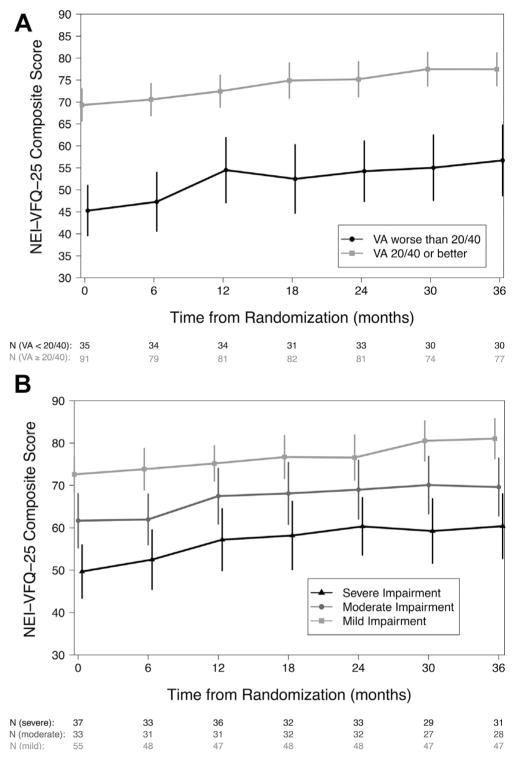
A total of 125 of the 126 individuals assigned to undergo systemic therapy were included in the risk factor analysis; 1 was excluded because of missing baseline visual field impairment. Of these, 106 (85%) completed 3 years of follow-up and 119 (95%) underwent systemic therapy within the first 6 months of follow-up, with 15 censored before 3 years because of implantation. As with the implant group, both visual acuity and visual field impairment had a significant impact on the NEI-VFQ-25 composite score for individuals assigned to systemic therapy (Fig 3; Table 3). However, unlike in the implant group, differences associated with visual acuity that were observed at randomization were sustained throughout follow-up and did not vary over time. At randomization, individuals with visual acuity worse than 20/40 had NEI-VFQ-25 composite scores that were 17.6 points lower than those with a visual acuity of 20/40 or better. Individuals with moderate and severe visual field impairment had NEI-VFQ-25 composite scores that were significantly lower at randomization (−10.3 and −12.7 points, respectively) than individuals with mild impairment. After randomization, individuals had on average a 1.6-point improvement (P < 0.001) for every 6 months of follow-up for a total of 9.5 points (95% CI, 6.3–12.7 points; P < 0.001) at the end of 3 years regardless of the visual function status at randomization. No other risk factors were associated significantly with NEI-VFQ-25 score either at randomization or during follow-up after adjusting for visual acuity and visual field impairment. The results were similar in sensitivity analyses based on the worse eye, the per-protocol analysis set, and when using a visual acuity threshold of 20/50 (Table 4, available at www.aaojournal.org).
Figure 3.
The relationship between the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) composite score and visual function, measured by (A) visual acuity (VA) and (B) visual field mean deviation, over the first 3 years after randomization for eyes assigned to systemic therapy. Visual acuity is categorized as 20/40 or better (grey line, square) or worse than 20/40 (black line, circle), and visual field mean deviation is categorized as mild impairment (>−3 dB; light grey line, square), moderate impairment (−3 to −6 dB; grey line, circle), or severe impairment (<−6 dB; black line, triangle). The estimated means with 95% confidence intervals are plotted for each category.
Table 3.
Factors Influencing the Initial and Trajectory of 25-Item National Eye Institute Visual Functioning Questionnaire Composite Scores during the First 3 Years of Follow-up for 125 Individuals Assigned to Undergo Systemic Therapy in the Multicenter Steroid Treatment Trial and Follow-up Study
| Characteristic | No. (%) at Randomization | 25-Item National Eye Institute Visual Functioning Questionnaire Score at Randomization: Estimated Mean Difference at Randomization in Points (95% Confidence Interval)* | P Value |
|---|---|---|---|
| Visual acuity worse than 20/40 | 34 (27) | −17.61 (−25.05 to −10.16) | <0.001 |
| Visual field impairment | |||
| Moderate (−3 to −6 dB) | 33 (26) | −10.26 (−17.06 to −3.46) | 0.003 |
| Severe (<−6 dB) | 37 (30) | −12.68 (−19.93 to −5.43) | <0.001 |
| Male | 26 (21) | 2.35 (−4.03 to 8.74) | 0.47 |
| Black | 31 (25) | 2.40 (−4.61 to 9.41) | 0.50 |
| For each 10 yrs older than 45 years at randomization | 125 (100) | 0.20 (−1.51 to 1.91) | 0.82 |
| Posterior uveitis or panuveitis | 78 (62) | −1.72 (−7.52 to 4.08) | 0.56 |
|
| |||
| No. (%) at Follow-up Visits | Change in 25-Item National Eye Institute Visual Functioning Questionnaire Scores from Randomization to Follow-up: Estimated Mean Difference from Randomization to Follow-up in Points (95% Confidence Interval)* | ||
|
| |||
| For every additional 6 mos from randomization | 660 (100) | 1.58 (1.05–2.12) | <0.001 |
Visual acuity and visual field impairment were assessed in the better-seeing eye.
Adjusted for all factors simultaneously.
Table 4.
Sensitivity analyses of factors influencing the initial and trajectory of VFQ-25 composite scores during the first 3 years of follow-up for individuals assigned to receive systemic therapy in the MUST Trial and Follow-up Study. Worse eye analyses were based upon the visual acuity in the worse seeing eye and the visual field in the same eye only (Version I) or used the visual field for the other eye if not available (Version II). Better eye analyses were based upon the visual acuity in better eye and the corresponding visual field measurements. The Per Protocol analysis only included individuals who received systemic therapy within 6 months of randomization and censored follow-up at the time of first implantation. The visual acuity threshold was 20/40 for all analyses except for the one labeled 20/50.
| Characteristic | Worse Eye Analyses | Better Eye Analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version I (N = 117) |
Version II (N = 125) |
Per Protocol (N = 119) |
20/50 (N = 125) |
|||||||||
| VFQ at Baseline: N (%) Estimate (95% CI) P-value* | ||||||||||||
|
| ||||||||||||
| Worse visual acuity | 71 (61%) | −5.90 (−12.39, 0.60) | 0.075 | 79 (63%) | −6.71 (−13.21, −0.21) | 0.043 | 33 (28%) | −16.39 (−24.17, −8.61) | < 0.001 | 24 (19%) | −16.00 (−24.27, −7.72) | < 0.001 |
| Moderate Visual Field Impairment (−3db to −6db) | 41 (35%) | −7.89 (−15.22, −0.56) | 0.035 | 42 (34%) | −9.31 (−16.35, −2.27) | 0.009 | 33 (28%) | −8.97 (−15.85, −2.10) | 0.010 | 33 (26%) | −9.87 (−16.63, −3.10) | 0.004 |
| Severe Visual Field Impairment (<−6db) | 52 (44%) | −10.92 (−18.13, −3.72) | 0.003 | 58 (46%) | −12.69 (−19.74, −5.63) | < 0.001 | 34 (29%) | −12.67 (−20.48, −4.85) | 0.001 | 37 (30%) | −13.14 (−20.54, −5.73) | < 0.001 |
| Male | 24 (21%) | 4.40 (−2.28, 11.07) | 0.20 | 26 (21%) | 2.88 (−4.35, 10.12) | 0.43 | 23 (19%) | 0.27 (−6.39, 6.93) | 0.94 | 26 (21%) | 1.54 (−4.97, 8.04) | 0.64 |
| African American | 27 (23%) | 0.43 (−7.77, 8.64) | 0.92 | 31 (25%) | 1.59 (−6.07, 9.24) | 0.68 | 31 (26%) | 2.67 (−4.57, 9.91) | 0.47 | 31 (25%) | 2.57 (−4.55, 9.70) | 0.48 |
| For each 10 years older than 45 at RZ | 117 (100%) | −0.85 (−2.90, 1.20) | 0.41 | 125 (100%) | −0.77 (−2.75, 1.21) | 0.44 | 119 (100%) | −0.02 (−1.82, 1.79) | 0.98 | 125 (100%) | −0.26 (−2.02, 1.50) | 0.77 |
| Posterior/panuveitis | 74 (63%) | 2.96 (−3.36, 9.28) | 0.36 | 78 (62%) | 0.10 (−5.93, 6.12) | 0.97 | 73 (61%) | −1.13 (−7.19, 4.93) | 0.72 | 78 (62%) | −1.58 (−7.46, 4.30) | 0.60 |
|
| ||||||||||||
| Change in VFQ from Randomization to Follow-up: N (%) Estimate (95% CI) P-value* | ||||||||||||
|
| ||||||||||||
| For every additional 6 months from randomization | 616 (100%) | 1.62 (1.07, 2.17) | < 0.001 | 660 (100%) | 1.57 (1.04, 2.11) | < 0.001 | 583 (100%) | 1.49 (0.89, 2.08) | < 0.001 | 660 (100%) | 1.57 (1.04, 2.11) | < 0.001 |
Adjusted for all factors simultaneously.
RZ = randomization; FU = follow-up; CI = confidence interval.
Discussion
During the first 3 years after randomization in the MUST Trial and Follow-up Study, individuals in both treatment groups demonstrated clinically meaningful and statistically significant improvements in NEI-VFQ-25 composite scores. Although both therapies achieved a similar increase in VRQoL, the patterns of improvement differed substantially. A number of factors are likely related to the different trajectories observed. First, the overall pattern of initial improvement in NEI-VFQ-25 was similar to that seen in visual acuity in the better eye, an important factor in the NEI-VFQ-25, as well as immediate control of inflammation and macular edema as compared with more steady improvement in the systemic arm.19 Second, the patient expectations and enthusiasm likely initially would be higher for implant therapy because it was the more novel therapy and had the potential to replace systemic corticosteroids and immunotherapy. However, the patients in the systemic therapy arm were able to achieve stable therapeutic doses with low side-effect profiles and maintained good visual acuity,18 which likely contributed to the steady improvement in NEI-VFQ-25 score. Sheppard et al25 observed a NEI-VFQ-25 pattern similar to that observed in the implant group for patients treated with adalimumab, a treatment with a similar visual acuity and expectation profile. These differences underscore the importance of quantifying quality of life both within a specific disease, but also for classes of treatments for that disease.
The NEI-VFQ-25 was designed to capture multiple facets of visual function simultaneously. Its success is demonstrated by the fact that worse baseline measurements of both of the primary clinical measures of visual function, visual acuity and visual fields, were associated independently with lower initial NEI-VFQ-25 composite scores regardless of treatment type. These additive effects may be more prominent in moderate to severe uveitis because the disease is rarely localized, and we saw significant visual field defects at baseline (28% moderate and 35% severe at randomization). In a study at the Aravind Eye Hospital in Tamil Nadu, India, the presence of glaucoma was associated independently with NEI-VFQ-25 composite scores after adjustment for visual acuity, which also supports the importance of visual field loss independent of visual acuity.26 One of the strengths of VRQoL measures is that they have the potential to integrate the multifaceted effects of disease on visual function. This ability makes the NEI-VFQ-25 and its corresponding preference-based index27 potentially valuables tools for cost-effectiveness analyses by providing a method for capturing disease-specific trade-offs to complement the more commonly described general health trade-offs assessed using metrics such as the EQ-5D. However, the NEI-VFQ was not originally designed for patients with uveitis, so it may not be able to capture the impact on quality of life from disease-specific visual symptoms.
Although it is important to know that individuals with poor initial visual function have lower VRQoL, an important question is whether the loss is permanent or can be reclaimed with appropriate treatment. The initial deficit in NEI-VFQ-25 composite scores was erased only for those with poor visual acuity in the implant group, who demonstrated a larger improvement in NEI-VFQ-25 composite scores than those with good visual acuity. In fact by 3 years, the difference in NEI-VFQ-25 composite scores between those with poor initial visual acuity and good initial visual acuity was no longer statistically significant (−7 points; P = 0.081). However, it should be noted that the magnitude of the difference would be considered clinically meaningful. The fact that the large majority of participants had good vision probably made it harder to detect such a difference in our study.
None of the demographic or disease characteristics were associated significantly with the longitudinal pattern of NEI-VFQ-25 composite scores for individuals assigned to systemic therapy. For implant therapy, men had significantly higher NEI-VFQ-25 composite scores throughout the study similar to what was observed among glaucoma patients by Ekici et al.28 The relationship had the same direction, but was not statistically significant in the systemic group or in the sensitivity analyses. Older age was associated significantly with lower NEI-VFQ-25 composite scores in the implant group, although not the systemic group. However, the magnitude of the effect was small and achieved clinical significance only for differences of 3 or more decades, similar to what was shown in the Aravind study.26 Both of these results are difficult to interpret because we would expect the influence at baseline to be similar for the 2 treatment arms. However, they are supported by larger cohorts, although not specific to uveitis.
There are a number of limitations for our study. First, individuals enrolled in the MUST Trial had relatively good visual acuity and quality of life at baseline. Sixty-seven percent had visual acuity better than 20/40, which limited the potential for observing meaningful improvement. Second, the sample size for examining each treatment group was relatively small. Although clearly it was important to evaluate each treatment separately, this did limit our ability to detect potential complex associations or associations for small subsets. Third, quality of life is measured for the individual, whereas visual function measurements are performed for each eye. We based our primary analysis on the better-seeing eye, which is a commonly used surrogate for binocular vision, but does not capture all of the visual outcomes for the individual and may be dependent on other factors.29 We also performed sensitivity analyses based on the worse-seeing eye. These analyses showed a similar pattern of results for the relationship between visual function and the NEI-VFQ-25 composite score; however, magnitudes of the effect sizes were slightly attenuated. This supports both the robustness of our findings and the concept that most of the information is captured by the better eye in patient populations with high rates of bilateral disease like ours. Fourth, this article focuses on the relationship between visual function and VRQoL. As demonstrated by Frick et al,1 there is only a moderate association between general health quality of life metrics and the NEI-VFQ-25 composite score. So, the results should not be generalized to general health metrics. Finally, missing data are always a concern when monitoring long-term follow-up. Our retention at 3 years was high (n = 218; 85%), and sensitivity analyses including complete case analysis and mixed effects models showed similar results.
In conclusion, both treatments for noninfectious uveitis resulted in significant improvements in NEI-VFQ-25 composite scores over the course of 3 years of follow-up; however, the longitudinal patterns differed. Multiple facets of visual function were associated with VRQoL. Individuals who started with poor visual acuity or visual fields in general were unable to achieve levels of NEI-VFQ-25 composite score similar to those who started with good values by the end of 3 years. In fact, only those in the implant group who started with poor visual acuity were able to make up some ground. It will be important to investigate the association between short- and long-term changes in NEI-VFQ-25 composite scores and measurements of visual function to determine what the barriers are to regaining similar levels of quality of life.
Supplementary Material
Acknowledgments
Supported by collaborative agreements from the National Eye Institute, National Institutes of Health, Bethesda, Maryland (D.A.J., J.T.H.). Bausch & Lomb donated fluocinolone implants for participants randomized to receive implant therapy who were uninsured or otherwise unable to pay for implants or were located at a site where implants could not be purchased. A representative of the National Eye Institute participated in the conduct of the study, including the study design and the collection, management, analysis and interpretation of the data, and in the review and approval of this manuscript.
Abbreviations and Acronyms
- CI
confidence interval
- MUST
Multicenter Steroid Treatment
- NEI-VFQ-25
25-item National Eye Institute Visual Functioning Questionnaire
- VRQoL
vision-related quality of life
Footnotes
Presented at: Association of Research in Vision and Ophthalmology Annual Meeting, May 2016, Seattle, Washington.
Supplemental material available at www.aaojournal.org.
Financial Disclosure(s):
The author(s) have made the following disclosure(s): J.E.T.: Consultant – Abbvie, Gilead, NightstaRx, Santen, Inc., XOMA; Financial support -Allergan
G.N.H.: Consultant – Genentech, Inc., Novartis International, Santen, Inc., XOMA (US) LLC, Abbvie
R.C.W.: Consultant – Abbvie
Author Contributions:
Conception and design: Sugar, Venugopal, Thorne, Frick, Jabs
Analysis and interpretation: Sugar, Venugopal, Thorne, Frick, Holland, Wang, Almanzor, Jabs
Data collection: Sugar, Holland, Wang, Almanzor
Obtained funding: none
Overall responsibility: Sugar, Venugopal, Thorne, Frick, Holland, Wang, Almanzor, Jabs
References
- 1.Frick KD, Drye LT, Kempen JH, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the Multicenter Uveitis Steroid Treatment Trial. Invest Ophthalmol Vis Sci. 2012;53:1169–1176. doi: 10.1167/iovs.11-8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 3.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A, Suttorpvan Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomkins-Netzer O, Talat L, Bar A, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121:2387–2392. doi: 10.1016/j.ophtha.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–1162. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DS, Holbrook JT, Katz J, et al. Risk of elevated intraocular pressure and glaucoma in patients with uveitis; results of the Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2013;120:1571–1579. doi: 10.1016/j.ophtha.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardiner AM, Armstrong RA, Dunne MC, Murray PI. Correlation between visual function and visual ability in patients with uveitis. Br J Ophthalmol. 2002;86:993–996. doi: 10.1136/bjo.86.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan P, Koh YT, Wong PY, Teoh SC. Evaluation of the impact of uveitis on visual-related quality of life. Ocul Immunol Inflamm. 2012;20:453–459. doi: 10.3109/09273948.2012.723781. [DOI] [PubMed] [Google Scholar]
- 11.Schiffman RM, Jacobsen G, Whitcup SM. Visual functioning and general health status in patients with uveitis. Arch Ophthalmol. 2001;119:841–849. doi: 10.1001/archopht.119.6.841. [DOI] [PubMed] [Google Scholar]
- 12.Venkataraman A, Rathinam SR. A pre- and post-treatment evaluation of vision-related quality of life in uveitis. Indian J Ophthalmol. 2008;56:307–312. doi: 10.4103/0301-4738.39662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Yan HG, Chi Y, et al. Vision-and health-related quality of life in patients with uveitis. Zhonghua Yan Ke Za Zhi. 2016;52:429–436. doi: 10.3760/cma.j.issn.0412-4081.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Alqudah A, Mansberger SL, Gardiner SK, Demirel S. Vision-related quality of life in glaucoma suspect or early glaucoma patients. J Glaucoma. 2016;25:629–633. doi: 10.1097/IJG.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe RY, Diniz-Filho A, Costa VP, et al. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology. 2016;123:552–557. doi: 10.1016/j.ophtha.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azoulay L, Chaumet-Riffaud P, Jaron S, et al. Threshold levels of visual field and acuity loss related to significant decreases in the quality of life and emotional states of patients with retinitis pigmentosa. Ophthalmic Res. 2015;54:78–84. doi: 10.1159/000435886. [DOI] [PubMed] [Google Scholar]
- 17.Multicenter Uveitis Steroid Treatment Trial Research Group. The Multicenter Uveitis Steroid Treatment (MUST) Trial: Rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149:550–61. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Multicenter Uveitis Steroid Treatment Trial Follow-up Study Research Group. Quality of life and risks associated with systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, or panuveitis: fifty-four-month results of the Multi-center Uveitis Steroid Treatment Trial and Follow-up Study. Ophthalmology. 2015;122:1976–1986. doi: 10.1016/j.ophtha.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Multicenter Uveitis Steroid Treatment Trial Follow-up Study Research Group. Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. Ophthalmology. 2015;122:1967–75. doi: 10.1016/j.ophtha.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole SR, Beck RW, Moke PS, et al. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Optic Neuritis Treatment Trial. Invest Ophthalmol Vis Sci. 2000;41:1017–1021. [PubMed] [Google Scholar]
- 21.Suner IJ, Kokame GT, Yu E, et al. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009;50:3629–3635. doi: 10.1167/iovs.08-3225. [DOI] [PubMed] [Google Scholar]
- 22.Ferris FL, III, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103:181–182. doi: 10.1016/s0161-6420(96)30742-2. [DOI] [PubMed] [Google Scholar]
- 23.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80:844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard JD, Joshi A, Mittal M, et al. Effect of adalimumab on visual function (VFQ-25) in VISUAL-1 trial patients with non-anterior non-infectious uveitis. Invest Ophthalmol Vis Sci. 2015;56:1723. [Google Scholar]
- 26.Nirmalan PK, Tielsch JM, Katz J, et al. Relationship between vision impairment and eye disease to vision-specific quality of life and function in rural India: the Aravind Comprehensive Eye Survey. Invest Ophthalmol Vis Sci. 2005;46:2308–2312. doi: 10.1167/iovs.04-0830. [DOI] [PubMed] [Google Scholar]
- 27.Rentz AM, Kowalski JW, Walt JG, et al. Development of a preference-based index from the National Eye Institute Visual Function Questionnaire-25. JAMA Ophthalmol. 2014;132:310–318. doi: 10.1001/jamaophthalmol.2013.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekici F, Loh R, Waisbourd M, et al. Relationships between measures of the ability to perform vision-related activities, vision-related quality of life, and clinical findings in patients with glaucoma. JAMA Ophthalmol. 2015;133:1377–1385. doi: 10.1001/jamaophthalmol.2015.3426. [DOI] [PubMed] [Google Scholar]
- 29.Hirneiss C. The impact of a better-seeing eye and a worse-seeing eye on vision-related quality of life. Clin Ophthalmol. 2014;8:1703–1709. doi: 10.2147/OPTH.S64200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.