Abstract
Background
Quantifying quality of life (QoL) after left ventricular assist device (LVAD) remains challenging. Heart failure-specific health status measures are ideal for assessing symptoms of heart failure; however, if patients’ QoL is limited by other factors, then patients may have an improvement in heart failure-specific QoL with no concurrent improvement in generic QoL. We sought to examine and predict discrepancies between disease-specific and generic QoL measures after LVAD.
Methods and Results
We examined heart failure-specific and generic QoL with the Kansas City Cardiomyopathy Questionnaire (KCCQ) and Euroqol-5D Visual Analog Scale (VAS), respectively among 1888 patients with advanced heart failure who underwent LVAD implant from 2012-2014 as part of the INTERMACS registry. Both measures improved substantially, on average, at 6-months after LVAD, with mean changes of 32.7±25.0 and 27.6±27.4, respectively. Among the 1539 patients (81.5%) with moderate/large improvement in KCCQ, 334 (21.7%) had discordant changes in generic QoL (i.e., VAS did not substantially increase despite improvement in KCCQ). In a multivariable logistic regression model, baseline VAS score was the strongest predictor of KCCQ-VAS discordance while post-LVAD complications were not.
Conclusion
Most patients have large improvements in both heart failure-specific and generic QoL after LVAD implantation, and discordance in these measures after LVAD is uncommon. When it occurred, discordance was primarily observed in patients who reported good generic QoL on the VAS prior to LVAD (despite substantial impairment due to CHF). These results support the continued use of heart-failure specific health status measures to monitor QoL before and after LVAD implantation.
Keywords: heart failure, ventricular assist devices, quality of life
Among patients with advanced heart failure, left ventricular assist devices (LVADs) can prolong survival and markedly improve patients’ symptoms of shortness of breath, edema, and fatigue.1-3 While these benefits are substantial and important, LVADs are also associated with a number of well-described adverse events that can complicate patients’ lives after device implantation.1,4 Moreover, living with an LVAD requires many duties that may impact patients’ overall health-related quality of life (QoL) such as trouble-shooting alarms, managing power sources, and changing driveline exit site dressings, not to mention the potential impact of the device on patients’ social and emotional functioning. Thus, while heart failure symptoms may improve after LVAD implantation, other aspects of QoL may actually worsen.
Most commonly, two distinct measures are used to attempt to fully assess QoL in patients before and after LVAD: a heart failure-specific QoL and a generic QoL measure, each of which has potential limitations. Heart failure-specific QoL measures, such as the Kansas City Cardiomyopathy Questionnaire (KCCQ), are ideal for assessing the primary therapeutic goal of LVAD implantation—namely, the alleviation of the symptoms of heart failure, including dyspnea, fatigue, and edema. As such, the KCCQ is particularly sensitive to change with any heart failure treatment and is able to capture small differences between treatments. However, if QoL is limited by other factors, such as a post-LVAD complications, then patients may have an improvement in heart failure-specific QoL with no change, or even a decline, in generic QoL (mostly commonly assessed in LVAD patients with the EuroQol-5D Visual Analog Scale [VAS]). The discrepancy between heart failure-specific and generic health status measures after LVAD implantation has never been described at the individual patient level, but understanding this relationship could be very informative in defining how best to quantify the patient-centered benefits of treatment. To address this gap in knowledge, we examined concurrent KCCQ and VAS scores in patients before and after LVAD implantation to examine how often patients had an improvement in heart failure symptoms without a similar improvement in generic QoL and what patient factors were most strongly associated with this discordance.
Methods
Study Population and Protocol
Our study cohort was derived from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS)—a multicenter, observational registry of patients receiving mechanical circulatory support in the U.S. and Canada that began in 2005.5 Administered through a contract from the National Heart, Lung and Blood Institute to the University of Alabama at Birmingham, INTERMACS is focused on quality improvement and scientific research of patients with LVADs. All adult patients who receive Food and Drug Administration-approved devices are eligible for enrollment. For this analysis, we excluded patients who received right or bi-ventricular assist devices and total artificial hearts. Data are collected through medical chart abstraction by trained research personnel and patients’ self-reported QoL. Patients are assigned an INTERMACS profile by local site clinicians at the time of LVAD implant that indicates the severity of heart failure, ranging from 1 (critical cardiogenic shock) to 7 (advanced New York Heart Association Class III)6. Follow-up is attempted on all surviving patients at 1 week, 1 month, 3 months, 6 months, and every 6 months thereafter for the life of the device or until transplant or LVAD explant.7 All participating sites obtained Institutional Review Board approval. As INTERMACS is considered a quality improvement registry, the Institutional Review Board at the University of Alabama at Birmingham granted a waiver of individual patient informed consent.
Quality of Life Data
Although INTERMACS began in 2005, heart failure-specific QoL measures were not routinely collected until May 2012. Heart failure-specific and generic QoL in INTERMACS is assessed with the KCCQ8 and the VAS,9,10 respectively. The KCCQ is a 23-item questionnaire that assesses specific health domains pertaining to heart failure—physical limitation, symptoms, QoL, social limitation, and self-efficacy—the first 4 of which are combined into an overall summary score.8 A shortened 12-item version of the KCCQ was developed in 2013,11 which INTERMACS began collecting in 2014; for consistency, the 12-item version of the summary score (KCCQ12-os) was used for all analyses (See Supplemental Appendix for methodological details).7 Values for the KCCQ12-os range from 0 to 100, with higher scores indicating less symptom burden, better functional status, and better QoL. A change in KCCQ-os of 5, 10, and 20 points represents a small, moderate, and large change, respectively.12 The VAS13 captures patients’ “self-rating” of their health on a 20-cm vertical scale with anchors of the “best imaginable health state” (corresponding score=100) and “worst imaginable health state” (corresponding score=0).14 The minimum clinically important difference for the VAS ranges from 7 to 12.15
Statistical Analysis
The expected change after LVAD in both disease-specific and generic health status is large.16,17 However, there may be circumstances in which the LVAD improves heart failure symptoms but overall QoL does not improve. As such, our goal was to examine changes in generic health status from baseline to 6 months after LVAD implant among patients with improvement in KCCQ in order to 1) determine how often discordant changes occur (e.g., improvement in KCCQ without improvement in EQ-5D VAS) and 2) examine patient characteristics associated with discordance. Patients were included if they had both KCCQ and EQ-5D VAS measured at baseline and 6 months after LVAD.
To facilitate comparison of the KCCQ12-os and EQ-5D VAS, change scores were standardized by dividing by the standard deviation of the corresponding baseline score (thereby converting the change scores into effect sizes). Standardized changes for both scores were categorized as follows: <0 (worsening), 0 to <0.5 (small improvement), 0.5 to <1 (moderate/large improvement), and ≥1 (large/very large improvement), with discordance defined as a 2-category or greater difference in standardized changes.18 In this way, we attempted to capture large deviations between the measures, as discordance would require at least a 0.5 difference in standardized changes between the measures. Among patients with a moderate or large improvement in the KCCQ12-os, we used multivariable logistic regression to examine factors associated with discordance. Covariates were selected a priori based on clinical judgment and included demographic characteristics, comorbidities, severity of heart failure at the time of LVAD implantation, baseline health status, and post-LVAD bleeding, infection and stroke.
As this was a comparison of how the KCCQ and VAS change over time in patients from before to after LVAD, only patients who completed both measures at both time points were included—no imputation of missing data was performed. All statistical analyses were performed with SAS, version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.2.0.19
Results
Study Cohort
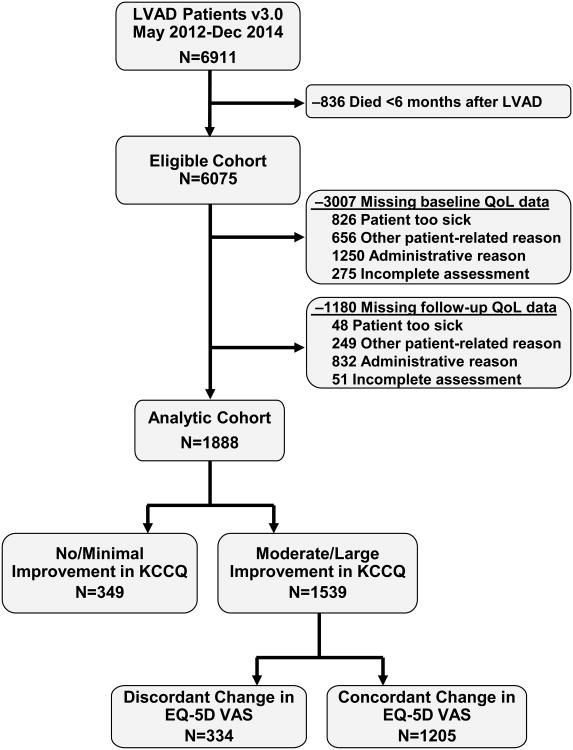
A total of 6911 patients received an LVAD from 5/2012-12/2014 and were included in INTERMACS. We excluded 836 patients who died within the first 6 months after LVAD and 4187 patients who were missing QoL assessments (3007 at baseline and an additional 1180 at follow-up; Figure 1). Most missing QoL assessments were due to administrative issues (e.g., site coordinator too busy to collect the data). However, patients who were eligible for analysis (i.e., survived 6 months) but missing QoL data were generally sicker before implant and more likely to have experienced pump thrombosis in the first 6 months after implant than patients in the analytic cohort (Supplemental Table 2). Among our final analytic cohort of 1888 patients, 80.1% were men, 50.8% were 60 years of age or older, and 43.5% were classified as INTERMACS profile 1-2 (Table 1).
Figure 1. Flow chart of the analytic cohort.
Table 1. Baseline characteristics of analytic cohort.
| n=1888 | |
|---|---|
| Age group (years) | |
| 19-39 | 10.0 |
| 40-49 | 14.0 |
| 50-59 | 25.3 |
| 60-69 | 33.5 |
| 70+ | 17.3 |
| Female | 19.9 |
| Body mass index (kg/m2) | 28.8 ± 6.7 |
| Pulmonary disease | 9.2 |
| Atrial arrhythmia | 21.6 |
| Severe diabetes | 10.7 |
| Major stroke | 3.8 |
| Peripheral vascular disease | 5.3 |
| Cancer | 7.5 |
| Illicit drug use/alcohol abuse | 11.3 |
| Limited social support | 4.1 |
| High school education | 53.9 |
| Severe depression | 2.7 |
| Malnutrition/cachexia | 2.9 |
| # of cardiac hospitalizations in past year | |
| 0-1 | 27.1 |
| 2-3 | 36.6 |
| 4 or more | 18.3 |
| Unknown | 18.0 |
| Previous cardiac surgery | 31.7 |
| Mean arterial pressure (mmHg) | 79.0 ± 10.9 |
| Heart rate (bpm) | 86.5 ± 16.2 |
| Pulmonary systolic pressure (mmHg) | 51.3 ± 14.4 |
| Mod/severe mitral regurgitation | 56.6 |
| Mod/severe tricuspid regurgitation | 39.9 |
| Creatinine (mg/dL) | 1.4 ± 0.8 |
| Albumin (mg/dL) | 3.5 ± 0.6 |
| Hemoglobin (g/dL) | 11.7 ± 2.1 |
| INTERMACS patient profile | |
| 1 Critical Cardiogenic Shock | 5.7 |
| 2 Progressive Decline | 37.8 |
| 3 Stable but Inotrope Dependent | 37.1 |
| 4 Resting Symptoms | 15.9 |
| 5 Exertion Intolerant | 2.5 |
| 6 Exertion Limited | 0.5 |
| 7 Advanced NYHA Class 3 | 0.5 |
Values shown as percentage for categorical variables and mean ± standard deviation for continuous variables
KCCQ-VAS Discordance
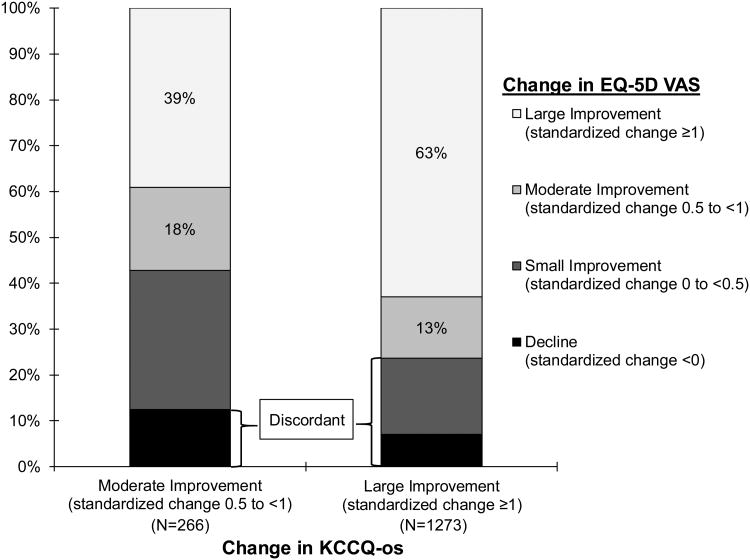
Baseline QoL was substantially impaired in patients prior to LVAD, with mean baseline KCCQ12-os and VAS of 35.5±21.1 and 45.4±24.3, respectively. On average, patients reported substantial improvement in both measures at 6 months after LVAD, with mean changes in the KCCQ of 32.7±25,0 and in the VAS of 27.6±27.4 (Table 2). After converting these measures to standardized differences, the KCCQ was notably more responsive than the VAS (median standardized change: 1.6 vs. 1.0; Table 2). There was a modest correlation between 6-month changes in the two measures (r=0.39, p <0.001; Figure 2). However, once categorized, discordance was relatively uncommon, with 81.5% of patients (n=1539) reporting moderate or greater improvement in KCCQ, of whom 334 (21.7%) had discordant changes on the VAS (Figure 3). Among the 349 patients with limited improvement or worsening on the KCCQ, 133 patients (38.1%) reported discordant changes on the VAS (i.e., heart failure symptoms did not improve or worsened but generic QoL improved; Supplemental Table 2).
Table 2. Baseline and 6-month post-implant KCCQ12-os and EQ-5D VAS.
| n | Baseline Mean ± SD | KCCQ12-os 6-Month Mean ± SD | Median Std Difference | Baseline Mean ± SD | EQ-5D VAS 6-Month Mean ± SD | Median Std Difference | |
|---|---|---|---|---|---|---|---|
| All patients | 1 | 36 ± 21 | 68 ± 20 | 1.6 | 45 ± 24 | 73 ± 20 | 1.0 |
| 8 | |||||||
| 8 | |||||||
| 8 | |||||||
| Patients with strokes | 9 | 35 ± 21 | 62 ± 23 | 1.2 | 44 ± 24 | 69 ± 21 | 0.8 |
| 6 | |||||||
| Patients with bleeding events | 5 | 36 ± 22 | 67 ± 21 | 1.5 | 47 ± 25 | 73 ± 19 | 1.0 |
| 9 | |||||||
| 3 | |||||||
| KCCQ12-os improvers | 1 | 31 ± 18 | 72 ± 17 | 1.9 | 44 ± 24 | 75 ± 19 | 1.2 |
| 5 | |||||||
| 3 | |||||||
| 9 | |||||||
| Concordant patients | 1 | 31 ± 19 | 72 ± 17 | 1.9 | 38 ± 21 | 78 ± 15 | 1.6 |
| 2 | |||||||
| 0 | |||||||
| 5 | |||||||
| Discordant patients | 3 | 32 ± 17 | 72 ± 17 | 1.8 | 65 ± 22 | 63 ± 26 | 0.0 |
| 3 | |||||||
| 4 |
KCCQ12-os, Kansas City Cardiomyopathy Questionnaire-overall summary score; EQ-5D VAS, EuroQoL-5D Visual Analog Scale Values are expressed as mean ± standard deviation
Figure 2. Correlation of changes in KCCQ12-os with EQ-5D VAS from baseline to 6-months after LVAD.
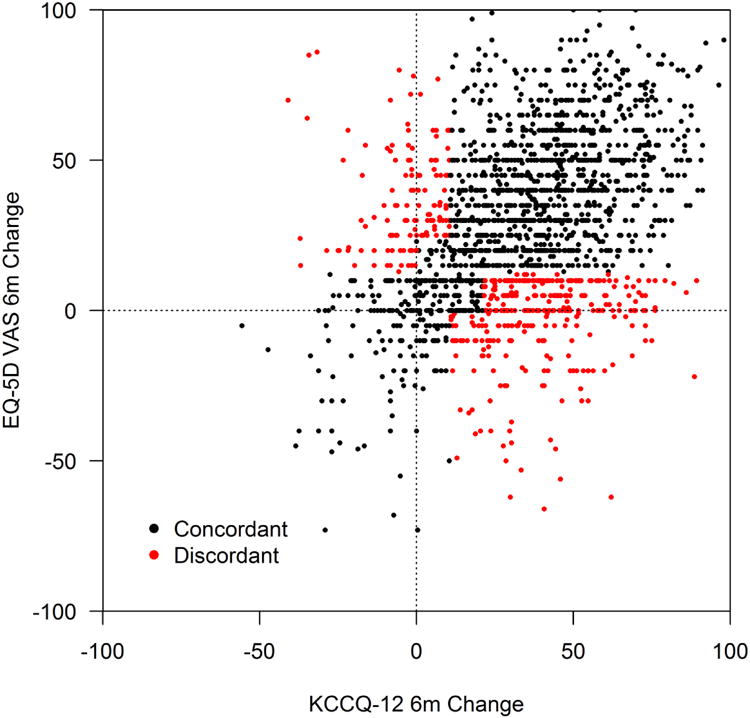
Black dots=concordant changes; red dots=discordant changes
Figure 3. Distribution of changes in EQ-5D VAS among patients with improvement in KCCQ12-os.
Association of Baseline Patient Characteristics with KCCQ-VAS Discordance
Among KCCQ improvers, patients with discordant (vs. concordant) changes in VAS were more likely to be female and had lower body mass indices (Supplemental Table 3). Baseline KCCQ was similarly impaired between groups (KCCQ12-os: 32.3 vs. 31.1, p=0.280), but patients with discordant changes in EQ-5D VAS had markedly higher baseline EQ-5D VAS scores (65.4 vs. 37.6, p<0.001) (Table 2). In the multivariable model, among the KCCQ improvers, the only clinical factor associated with a greater odds of KCCQ-VAS discordance was higher mean arterial pressure (OR 1.08 per 5 mmHg, 95% CI 1.02-1.36; Table 3). The most important characteristic associated with KCCQ-VAS discordance, however, was baseline EQ-5D VAS scores. Compared to those with a baseline VAS score of <25, patients with a baseline of 50-74 and >75 were nearly 8 and 87 fold more likely to have KCCQ-VAS discordance at 6 months (OR 7.81, 95% CI 4.66-13.10 and OR 87.39, 95% CI 46.56-164.01 0.43, respectively).
Table 3. Patient factors associated with EQ-5D VAS discordance among patients with improved KCCQ12-os.
| OR (95% CI) | P-value | |
|---|---|---|
| Age | 0.58 | |
| 19-39 | Reference | |
| 40-49 | 0.94 (0.50, 1.75) | |
| 50-59 | 1.25 (0.70, 2.23) | |
| 60-69 | 0.96 (0.54, 1.73) | |
| 70+ | 1.21 (0.64, 2.31) | |
| Female | 1.18 (0.81, 1.72) | 0.39 |
| Body mass index (per +5 kg/m2) | 0.89 (0.79, 1.01) | 0.07 |
| Pulmonary disease | 1.00 (0.59, 1.70) | 0.99 |
| Atrial arrhythmia | 1.11 (0.76, 1.62) | 0.59 |
| Severe diabetes | 1.25 (0.76, 2.05) | 0.37 |
| Major stroke | 0.41 (0.17, 1.01) | 0.05 |
| Peripheral vascular disease | 0.99 (0.49, 2.03) | 0.99 |
| Cancer | 1.15 (0.65, 2.04) | 0.63 |
| Illicit drug use/alcohol abuse | 1.18 (0.73, 1.90) | 0.50 |
| Limited social support | 0.65 (0.30, 1.41) | 0.28 |
| Greater than high school education | 1.13 (0.78, 1.65) | 0.51 |
| Severe depression | 1.21 (0.51, 2.87) | 0.66 |
| Malnutrition/cachexia | 1.02 (0.45, 2.33) | 0.96 |
| # of cardiac hospitalizations in past year | 0.44 | |
| 0-1 | Reference | |
| 2-3 | 1.18 (0.80, 1.74) | |
| 4 or more | 1.33 (0.85, 2.09) | |
| Unknown | 1.41 (0.90, 2.21) | |
| Previous cardiac surgery | 0.72 (0.51, 1.03) | 0.07 |
| Mean arterial pressure (per +10 mmHg) | 1.16 (1.01, 1.34) | 0.04 |
| Pulmonary systolic pressure (per +10 mmHg) | 0.91 (0.80, 1.04) | 0.17 |
| Heart rate (per +10 bpm) | 0.95 (0.85, 1.05) | 0.28 |
| Mod/severe mitral regurgitation | 0.84 (0.59, 1.20) | 0.34 |
| Mod/severe tricuspid regurgitation | 1.13 (0.80, 1.58) | 0.49 |
| Creatinine (per +1 mg/dL) | 1.14 (0.96, 1.36) | 0.14 |
| Albumin (per +1 mg/dL) | 1.21 (0.91, 1.62) | 0.19 |
| Hemoglobin (per +1 mg/dL) | 1.00 (0.92, 1.08) | 0.95 |
| INTERMACS Patient Profile | 0.71 | |
| 1 Critical Cardiogenic Shock | Reference | |
| 2 Progressive Decline | 0.94 (0.48, 1.82) | |
| 3 Stable but Inotrope Dependent | 0.74 (0.37, 1.47) | |
| 4 Resting Symptoms | 1.02 (0.48, 2.15) | |
| 5 Exertion Intolerant | 0.82 (0.25, 2.66) | |
| 6 Exertion Limited | 1.08 (0.13, 9.16) | |
| 7 Advanced NYHA Class 3 | 0.22 (0.01, 4.14) | |
| Baseline KCCQ12-os (per + 10 pts) | <0.001 | |
| 0-<25 (reference) | 1.00 | |
| 25-<50 | 0.66 (0.47, 0.93) | |
| 50-<75 | 0.31 (0.19, 0.51) | |
| 75-100 | 0.03 (0.01, 0.15) | |
| Baseline EQ-5D VAS (per + 10 pts) | <0.001 | |
| 0-<25 (reference) | 1.00 | |
| 25-<50 | 1.62 (0.93, 2.85) | |
| 50-<75 | 7.81 (4.66, 13.10) | |
| 75-100 | 87.39 (46.56, 164.01) | |
| Post-LVAD infection | 1.09 (0.77, 1.56) | 0.62 |
| Post-LVAD respiratory failure | 0.90 (0.51, 1.58) | 0.71 |
| Post-LVAD psychiatric episode | 1.77 (0.94, 3.34) | 0.08 |
| Post-LVAD stroke | 1.53 (0.76, 3.06) | 0.236 |
| Post-LVAD bleeding | 0.90 (0.64, 1.26) | 0.539 |
KCCQ12-os, Kansas City Cardiomyopathy Questionnaire-overall summary score; EQ-5D VAS, EuroQoL-5D Visual Analog Scale
Association of Post-LVAD Complications with KCCQ-VAS Discordance
In the 6 months following LVAD implantation, 5.1% of patients had a stroke and 31.4% had a bleeding event. Patients with bleeding events had, on average, large improvements in both KCCQ and VAS scores, with mean 6-month changes of 31.0 and 26.2 points, respectively, and standardized differences in scores that were quite similar to those in the overall population (Table 2). Patients who had a stroke after LVAD also reported, on average, large improvements on both the KCCQ and VAS (6-month changes 27.3 and 25.1 points, respectively), although these standardized differences in scores were slightly blunted from the overall population. In the multivariable model in patients with improvement in KCCQ scores, neither strokes, infections nor bleeds were associated with an increased odds of KCCQ-VAS discordance (p=0.54, 0.62 and 0.43, respectively; Table 3).
Discussion
In a real-world registry of nearly 2,000 patients undergoing LVAD implantation, we found that the vast majority of patients had improvements in both heart failure-specific and generic QoL after LVAD implantation. Furthermore, discordance was relatively infrequent, with 83% of patients who had improved heart failure-specific QoL also having similarly large improvements in generic QoL. We hypothesized that KCCQ-VAS discordance would be uncommon but, when present, it would occur in patients with limiting conditions unrelated to heart failure (e.g., peripheral artery disease, depression, malnutrition) and among those with a serious post-LVAD complication. However, KCCQ-VAS discordance appeared to be driven by patients who reported a good generic QoL prior to LVAD, despite their severe heart failure symptoms. In fact, patients with serious post-LVAD complications reported, on average, large improvements in both heart failure and generic QoL. These results indicate that both generic and heart failure-specific measures similarly quantify QoL among LVAD-supported patients and highlight a potential limitation of the VAS for capturing the non-heart failure aspects to QoL.
LVADs are a markedly effective treatment to improve cardiac output and, as a consequence, ameliorate the symptoms of heart failure, including reducing dyspnea, edema, and fatigue.20 However, the trade-offs with receiving an LVAD are a high risk of serious complications as well as the hassle of maintenance of the complicated device,20 both of which would be expected to negatively impact patients’ overall QoL. As such, there has been much discussion on how best to assess QoL before and after LVAD implantation. A heart failure-specific measure is ideal prior to LVAD implantation, when the patient is most debilitated by his or her heart failure (by documenting the impairment in QoL), and is also ideal for demonstrating the effect of the LVAD on heart failure symptoms, physical functioning, and QoL. However, the KCCQ is unlikely to capture many of the LVAD-specific aspects of living with this technology. Thus, by using only the KCCQ to assess QoL, we might be inappropriately labeling some patients as having a good QoL after LVAD when their QoL is impaired due to issues unrelated to heart failure. For these reasons in many studies, the KCCQ has been supplemented by the VAS in an effort to capture the non-heart failure aspects of QoL.
Despite the logic of this reasoning, we found only minimal discordance between the KCCQ and VAS that was not driven by these proposed non-heart failure issues. For example, in patients with serious post-LVAD complications, we expected an improvement in heart failure-specific QoL but not in generic QoL (or at least a blunted response), but we found both measures improved similarly in these patients. This suggests that either the VAS may not be sensitive in detecting the adverse consequences of living with an LVAD or the improvement in heart failure symptoms post LVAD dominates any impact on QoL from non-HF issues. These results highlight a need for an LVAD-specific QoL measure that could more sensitively capture and quantify the burdens on patients living with an LVAD.20
Our findings must be considered in the context of the following potential limitations. First, although INTERMACS is a unique and powerful database given its real-world representation of current U.S. and Canadian LVAD practice (and therefore ensures generalizability), as a registry with minimal support for local data collection, it suffers from significant missing data, particularly with QoL data.21 Some of this is a result of difficulties collecting QoL data from patients who are critically ill prior to surgery; however, particularly after implant, much of this is due to administrative issues which can only be improved with local efforts. We did not impute QoL data in this study, as the QoL data were central to our analysis. Imputation likely would have decreased the frequency of KCCQ-VAS discordance even more, as imputed baseline scores for both measures in patients “too sick” would have substantially improved at follow-up. Accordingly, our results are more generalizable to patients well enough to complete baseline and follow-up health status assessments but may not apply to more critically ill patients. Second, the rates of stroke and pump thrombosis of 5.1% and 0.2%, respectively, at 6 months in our analytic cohort are lower than previously reported,2,4,22 which may have limited our ability to identify associations of these complications with discordance.
In conclusion, in a large cohort of patients undergoing LVAD for advanced heart failure, we found that most patients had large improvements in both heart failure-specific and generic QoL. Patients who reported improvement in heart failure QoL but no improvement in generic QoL were not those with non-cardiac comorbidities or complications from their LVADs. Rather, KCCQ-VAS discordance was observed primarily in patients who reported discordant QoL prior to LVAD (i.e., severe heart failure impairment but reasonably good generic QoL). These results support the continued use of the KCCQ to monitor QoL before and after LVAD implantation but also highlight the limitations of the VAS for reliably capturing the non-heart failure aspects of QoL. An LVAD-specific QoL measure may be able to better quantify the aspects of living with an LVAD that impact patients’ QoL and are distinct from the symptoms, physical limitations and quality of life consequences of heart failure. The development of such a measure would be ideal to quantify the patient-centered outcomes of LVADs so as to improve our understanding of the comprehensive impact of LVADs on these patients.
Supplementary Material
Acknowledgments
Funding Source: This project has been funded in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C. MEN and TJF: supported by a T32 grant from the NHLBI (T32HL110837). SVA: supported by a Career Development Grant Award from the NHLBI (K23 HL116799).
Footnotes
Conflict of Interest Disclosures: JAS: owns the copyright to the Kansas City Cardiomyopathy Questionnaire and has served as a consultant to Novartis and Janssen Pharmaceuticals. LAA has served as a consultant for Novartis, Janssen, ZS Pharma, and St. Jude, and receives grant support from the NIH, PCORI, and AHA.
The other authors report no relevant conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Griffith BP, Kormos RL, Borovetz HS, et al. Heart Mate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001;71(3 Suppl):S116–120. doi: 10.1016/s0003-4975(00)02639-4. discussion S114-116. [DOI] [PubMed] [Google Scholar]
- 4.Nassif ME, LaRue SJ, Raymer DS, et al. Relationship Between Anticoagulation Intensity and Thrombotic or Bleeding Outcomes Among Outpatients With Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail. 2016;9(5) doi: 10.1161/CIRCHEARTFAILURE.115.002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27(10):1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28(6):535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS®) Protocol 4.0. [Accessed May 18, 2015];2014 http://www.uab.edu/medicine/intermacs/
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 9.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 10.EuroQol--a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands) 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 11.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015 doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spertus J, Safley D, Garg M, Jones P, Peterson ED. The influence of race on health status outcomes one year after an acute coronary syndrome. J Am Coll Cardiol. 2005;46(10):1838–1844. doi: 10.1016/j.jacc.2005.05.092. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 14.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 15.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health and quality of life outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical power analysis for the behavioral sciences (2nd edition) Lawrence Erlbaum Associates. 1988 [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2015 http://www.R-project.org/
- 20.Grady KL, Magasi S, Hahn EA, Buono S, McGee EC, Jr, Yancy C. Health-related quality of life in mechanical circulatory support: Development of a new conceptual model and items for self-administration. J Heart Lung Transplant. 2015;34(10):1292–1304. doi: 10.1016/j.healun.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Gupta BP, Grady KL, Fendler T, Jones PG, Spertus JA. Variation of Quality of Life Data Collection Across INTERMACS Sites. J Card Fail. 2016;22(5):323–337. doi: 10.1016/j.cardfail.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.