Abstract
Protein Kinase C isoforms have been implicated in regulating multiple processes within the healthy pancreas. Moreover, their dysregulation contributes to all aspects of pancreatic disease. In this review, with a focus on acinar, ductal, and islet cells, we highlight the roles and contributions of the different PKC isoforms to normal pancreas function. We also discuss the contribution of PKC enzymes to pancreatic diseases, including insulin resistance and diabetes mellitus, as well as pancreatitis and the development and progression of pancreatic cancer.
Keywords: Protein Kinase C, PKC, pancreas, pancreatitis, pancreatic cancer
1. INTRODUCTION
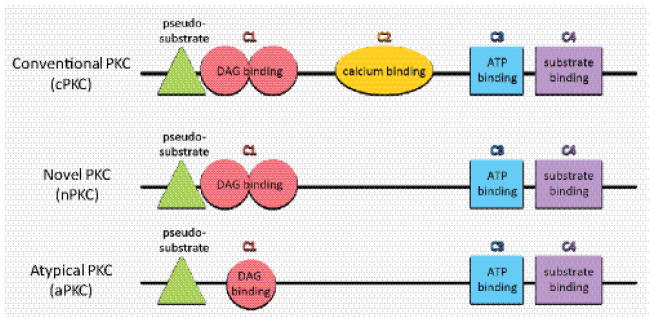
The protein kinase C (PKC) family consists of ten isoforms that are grouped according to their differing structural characteristics and activation requirements (Figure 1). Each isoform has a similar kinase domain, but their regulatory domains differ, reflecting the different requirements for activation (for a detailed review on PKC structure see [1]). The conventional PKCs (cPKCs) PKCα, PKCβI, PKCβII and PKCγ require diacylglycerol (DAG) to bind to the C1 domain, which contains two Cys-rich motifs, and calcium to bind to the C2 domain. Novel PKCs (nPKCs) PKCδ, PKCε, PKCη and PKCθ require DAG to bind to the C1 domain, but as they lack a C2 domain, there is no requirement for calcium to activate this group. The atypical PKCs PKCι and PKCζ have a C1 domain with a single Cys-rich motif, but they lack a C2 domain and are not activated by DAG or calcium. Despite these differences in the regulatory domains of PKCs, there is a pseudosubstrate domain adjacent to the C1 domain in each PKC subgroup. The pseudosubstrate domain prevents phosphorylation of PKC substrates and when this domain is synthetically derived, it can be used as a PKC inhibitor [2].
Figure 1.
PKC structure is similar across family members, although they have different activation requirements. The inhibitory pseudosubstrate domain and the kinase domain, consisting of C3 and C4, are similar amongst PKCs, but the regulatory domains differ. Conventional PKCs (cPKCs) require DAG and calcium binding, while novel PKCs (nPKCs) require DAG binding, and atypical PKCs (aPKCs) require neither DAG nor calcium binding, although they have a single Cys-rich motif (C1).
PKCs are involved in regulating multiple functions within the healthy pancreas, but also have important roles in pancreatic disease. While a healthy pancreas mainly consists of acini, islets and ductal structures, the composition of these cell types changes dramatically during disease. For example, in patients with insulin resistance and diabetes mellitus, islet cell dysfunction and beta cell death occur as a result of inflammation [3, 4]. Moreover, during pancreatitis, acinar cells can undergo metaplasia to duct-like cells or cell death. In addition, fibrotic tissue and presence of inflammatory cells are a hallmark of this disease [5]. In pancreatic cancer, in addition to pancreatitis-related changes (fibrosis, inflammatory cells), differently-progressed pancreatic duct-like lesions can be detected [6]. In the following we will discuss the roles of PKC molecules in the healthy and abnormal pancreas.
2. PKC IN NORMAL PANCREATIC FUNCTIONS
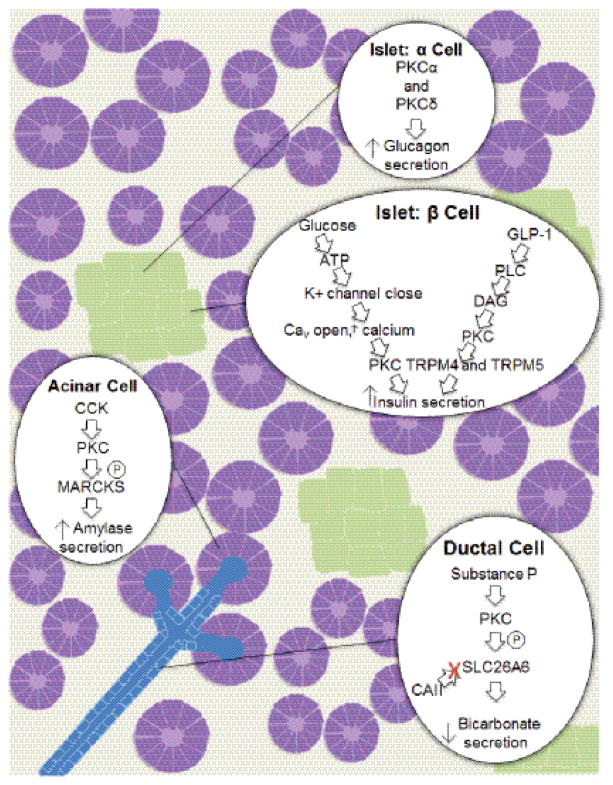
PKC isoenzymes are key regulators of secretion in the normal pancreas. They regulate hormone secretion in the islets, amylase secretion in acinar cells, and bicarbonate secretion in ductal cells (Figure 2). The known roles and functions of individual PKC isoforms are discussed in more detail in the following.
Figure 2.
PKC functions in the normal pancreas. PKCs regulate hormone secretion in the islets, amylase secretion in acinar cells, and bicarbonate secretion in ductal cells of normal pancreatic tissue. In islets, PKCα and PKCδ regulate Glucagon secretion from alpha-cells. In addition PKCs regulate insulin secretion in beta-cells. Amylase secretion is increased in acinar cells after stimulation of the CCK receptor and bicarbonate secretion is downregulated in ductal cells after activation of PKC via Substance P.
2.1 Islets
PKC isoforms with the highest expression in human pancreatic islets include PKCα, ε, θ, and ζ, although all isoforms have shown some expression [7]. Within alpha cells, which produce glucagon, the most abundant PKC isoforms in mouse are α and β1, while η, ε, and ζ are most abundant in human alpha cells [8]. In alpha cells, PKC mediates glucagon secretion, as shown by stimulation with the PKC activator PMA, which drove PKC α and δ (human cells; or δ only in mouse cells) to translocate from the cytosol to the plasma membrane and increase glucagon secretion [8, 9]. Additionally, the PKC inhibitor bisindolylmaleimide (BIM) diminishes glucagon secretion [8].
PKC also mediates secretion in beta cells. Expression of PKC isoforms in beta cells includes PKCα, βII, δ, ε, ζ, and λ/ι [10]. Within normal tissue, glucose is the main stimulator of insulin release and this is PKC-dependent as evidenced by usage of the pan-PKC inhibitor Ro 31-8220, which blocks the release of insulin [11]. In contrast, the conventional PKC inhibitor Gö6976 does not block glucose-stimulated insulin secretion, suggesting involvement of novel or atypical PKCs in this process [11]. One mechanism to activate PKC in beta cells involves ATP production from the breakdown of glucose, and this increase of ATP causes the closing of K+ channels. This results in membrane depolarization and subsequent opening of high voltage-gated calcium (CaV) channels, such that the influx of calcium causes PKC activation [12]. Glucose-dependent insulin secretion can also be mediated by PKC in a calcium-independent manner [13]. An additional mechanism of PKC-dependent insulin secretion involves glucagon-like peptide-1 (GLP-1). By activating phospholipase c (PLC), which leads to the production of DAG, GLP-1 activates conventional and novel PKCs [14]. PKC then activates the Na+ permeable channels TRPM4 and TRPM5, causing the membrane to depolarize and allowing for an increased rate of action potential firing followed by insulin secretion [14].
PKC also mediates the regulation of oxidative stress in beta cells. Utilizing the GLP-1 agonist exendin-4 (EX4), of which a synthetic peptide has been developed for use in diabetes mellitus type 2 treatment, PKCδ was activated, and promoted antioxidant gene expression [15]. PKCδ phosphorylates Nrf2 at serine residue 40, which triggers release from Kelch-like ECH-associated protein 1 (KEAP1) and translocation of Nrf2 to the nucleus, where it induces expression of antioxidant genes like GCLC and HMOX1 [15].
2.2 Acinar Cells
PKCγ, λ/ι, and ζ are strongly expressed, while PKCα, δ, and θ show weaker expression in human pancreatic acinar cells [7]. Within rat acini, PKCα, δ, ε, and ζ have been widely studied [16–18]. Of these isoforms, PKCα, δ, and ε undergo translocation to the membrane upon stimulation with physiological levels of cholecystokinin (CCK) [19]. PKC-mediated effects of CCK include amylase secretion, trypsinogen activation, and NF-κB activation [19, 20].
The pancreatic CCKA and CCKB receptors in rodents can exist in different states with high or low binding affinity towards CCK [21, 22]. The low affinity CCKA receptor state leads to translocation and activation of PKCδ downstream of Src family kinases [23]. While PKCδ was initially described as mediating CCK-induced amylase release [19], further studies used a more specific PKCδ inhibitor, δV1-1 [24], as well as PKCδ−/− mice to show that amylase secretion is not dependent on PKCδ expression or activity [20]. The difference in these studies can be explained by the use of rottlerin, which does inhibit amylase secretion [19, 20] and was previously thought to be a specific PKCδ inhibitor, but has since been shown to be a mitochondrial uncoupler that decreases cellular ATP levels [25] and to affect multiple PKCδ-independent pathways [26]. Despite evidence that the PKCδ isoform does not affect amylase secretion, the conventional and novel PKC inhibitor GF109203X does decrease amylase release [19], suggesting an important role for PKC in acinar cell digestive enzyme secretion. Mechanistically, it has been proposed that PKC acts through myristoylated alanine-rich C kinase substrate (MARCKS) to regulate CCK-induced amylase secretion [27]. Upon stimulation with CCK, PKC phosphorylates MARCKS [28], inducing translocation of MARCKS from the plasma membrane to the cytosol [27]. This leads to an increase in amylase secretion, which can be blocked using the PKC inhibitor GF109203X as well as the myristoylated N-terminal sequence of MARCKS (MANS) peptide, which blocks the function of MARCKS [27]. Although the mechanism has not been fully determined, MARCKS translocation is proposed to affect SNAREs [27]. For example, MARCKS associates with GM1a-rich detergent-resistant membrane fractions (DRMs). DRMs contain the t-SNARE Syntaxin2 [27], which likely mediates exocytosis of amylase from acinar cells [29].
Additionally, PKCθ is an important signaling molecule in pancreatic acinar cells that can be activated by CCK [30], as evidenced by phosphorylation at amino-acid residue T538 and translocation from the cytosol to the plasma membrane [31]. This activation occurs through the CCKA receptor such that most activation occurs through the low affinity state, but about 30% of the activation occurs through the high affinity receptor state [30]. While PKCθ does not affect amylase release, active PKCθ affects many downstream targets including Src, PYK2, and FAK [30].
2.3 Ductal Systems
PKCα, β, γ, ε, ζ, ι, and θ are expressed within human pancreatic ductal systems [7]. In ductal epithelial cells PKC regulates tight junctions, which function as a barrier and separate the apical and basolateral surfaces, thereby maintaining polarity [32]. Through 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced activation of PKC, the tight junction proteins Claudin-18, Claudin-4, Claudin-7, Occludin, Zo-1, and Zo-2 are upregulated [33, 34]. This effect is mitigated by the pan-PKC inhibitor GF109203 and partially-inhibited by PKC isoform-specific inhibitors. Using different PKC inhibitors such as Go6976 (PKCα inhibitor), rottlerin (used as a PKCδ inhibitor) and myristoylated PKCθ pseudosubstrate peptide inhibitor, it was shown that the upregulation of Claudin-4 is dependent on PKCα and the upregulation of Claudin-18 depends on PKCα, δ, and θ, while upregulation of Claudin-7, Occludin, Zo-1 and Zo-2 is depend on PKCδ [33, 34].
In addition to regulating structural elements, PKCs have been implicated in modulating secretion in pancreatic ductal cells [35]. For example, substance P, which is produced in periductal nerves, activates PKC in ductal cells [36]. Active PKC then inhibits bicarbonate secretion through phosphorylation of the Cl−/HCO3− exchanger Solute Carrier Family 26 Member 6 (SLC26A6) [37]. SLC26A6 forms a transport metabolon when carbonic anhydrase II (CAII) binds, thereby increasing the rate of bicarbonate transfer out of the cell, as bicarbonate is then produced at the site of the transporter [37]. By phosphorylating SLC26A6 at amino-acid residue S553, PKC decreases bicarbonate secretion by blocking CAII binding [37].
3. PKC IN PANCREATIC INFLAMMATION
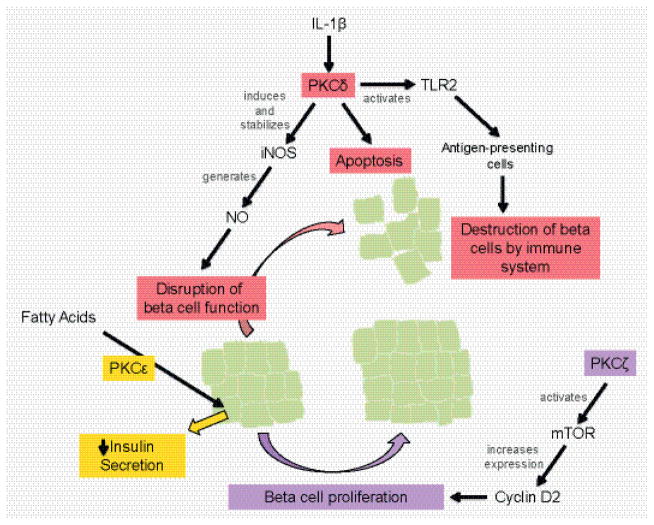
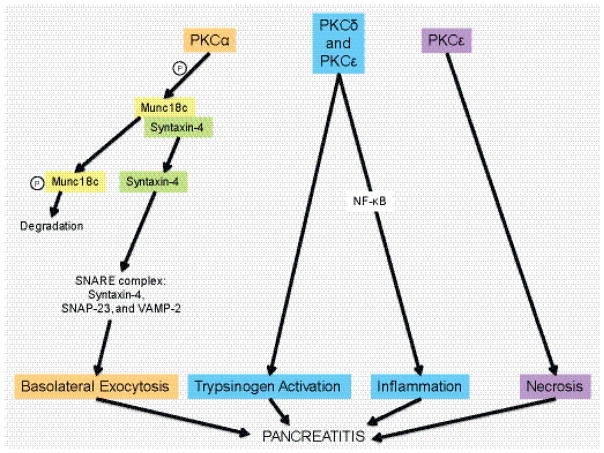
Pancreatic inflammation has consequences on both the endocrine and exocrine tissue, such that it contributes to diabetes mellitus as well as pancreatitis. PKCζ, δ, and ε play key roles in diabetes, such that PKCζ activation could have therapeutic benefit, while PKCδ and ε activity produces negative effects (Figure 3). In pancreatitis, PKCs are involved in regulating cell death pathways, generating an inflammatory response via NF-κB activation, mediating trypsinogen activation, and redirecting exocytosis to the basolateral membrane (Figure 4).
Figure 3.
Roles of PKC isoforms in islet function. In diabetes mellitus development, PKCζ activity promotes beta cell proliferation through mTOR activation, while PKCδ promotes dysfunction and destruction of beta cells via iNOS expression and TLR2 signaling. In a type two diabetes model where islets are pre-treated with fatty acids, PKCε is integral to the resulting decrease in insulin secretion.
Figure 4.
Schematic of how PKD isoforms contribute to pancreatic inflammation (pancreatitis). PKCα can aid basolateral exocytosis via phosphorylation of Munc18c. This leads to Munc18c degradation and release of Syntaxin-4 and formation of the SNARE complex. PKCε has been implicated in necrosis linked to pancreatitis and both, PKCδ, and PKCε also contribute to trypsinogen activation and activation of NF-κB, which drives inflammation.
3.1 Diabetes Mellitus
In type 2 diabetes metabolic stress induces inflammation within the pancreas that negatively affects islet function [38, 39]. Additionally, obesity, which involves chronic inflammation, induces insulin resistance in metabolic tissues [40]. When there is insulin resistance, pancreatic beta cells produce more insulin to try to lower blood glucose levels, but this usually results in exhaustion and eventually type 2 diabetes [41].
In the insulin resistant state, activation of PKCζ seems to act protective, and the expression of constitutively-active PKCζ in beta cells of mice results in insulin secretion as well as beta cell proliferation [42]. In addition, the knockout of PKCζ in beta cells of mice blocks their proliferation in response to insulin resistance [43]. PKCζ drives beta cell proliferation through activation of mammalian target of rapamycin (mTOR) [42, 43]. Activation of mTOR then increases expression of cyclin-D2, which drives proliferation [44]. Thus, in combatting type 2 diabetes, activation of PKCζ may have therapeutic benefit.
In contrast, PKCδ and ε have been shown to contribute to the development of both type 1 and type 2 diabetes. An auto-inflammatory response induces beta cell death in type 1 diabetes. In a model of this condition in which in vitro stimulation of islets with cytokines damaged beta cells, a knockout of PKCδ prevented apoptosis and inhibited toll-like receptor 2 (TLR2) signaling [45]. TLR2 acts as a sensor and activates antigen-presenting cells, thereby aiding type 1 diabetes development, when apoptotic beta cells are not phagocytosed but instead undergo secondary necrosis [46]. Using TLR2 knockout mice it also was shown that this receptor has a role in type 2 diabetes. A high fat diet in these mice exhibited less inflammation and greater insulin sensitivity than wildtype mice [47]. Additionally, PKCδ mediates IL-1β-induced inflammation [48]. Activation of PKCδ via IL-1β is essential in generating and stabilizing inducible nitric oxide synthase (iNOS) expression, which in turn generates nitric oxide (NO) that disrupts beta cell function [48, 49]. Another contributor to beta cell dysfunction, is PKCε, in which knockout of PKCε results in greater insulin secretion in response to glucose when islets are pretreated with fatty acids [50]. This suggests inhibition of PKCε could have therapeutic benefit in type 2 diabetes, which is often modeled by fatty acid treatment that induces a reduction in beta cell mass and insulin secretion [51].
3.2 Pancreatitis
In pancreatitis, PKC isoforms δ and ε have been well characterized in regulating cell death pathways, generating an inflammatory response, mediating trypsinogen activation, and redirecting exocytosis to the basolateral membrane (Figure 4). For example, genetic ablation of PKCε significantly attenuated necrosis and the severity of pancreatic damage in a caerulein-induced pancreatitis mouse model [52]. Furthering pancreatic injury, zymogen activation is increased and apical secretion is decreased in pancreatitis [53, 54]. One particular zymogen, trypsinogen, is activated early in pancreatitis and this induces injury [55]. Upon supraphysiological administration of CCK or caerulein, trypsinogen activation occurs in a PKC-dependent manner [20, 56]. This is mediated via both PKCδ and PKCε, but not PKCα or PKCζ, as shown with PKCδ−/− mice, PKCε−/− mice, and specific PKC isoform inhibitors [20, 52, 56]. Additionally, when secretions occur at the basolateral membrane instead of the apical membrane, injury is induced [57]. PKC mediates a switch from apical secretion to basolateral secretion upon supraphysiological CCK administration, which experimentally induces pancreatitis [58]. By phosphorylating Munc18c, PKCα causes dissociation of Munc18c from Syntaxin-4, which leads to its proteolytic degradation in the cytosol [58–60]. Syntaxin-4 can then form a SNARE complex with SNAP-23 at the membrane and VAMP-2 on zymogen granules, thereby leading to basolateral exocytosis [58–60].
An additional role of PKC is to mediate an inflammatory response in acute pancreatitis [61], which is mediated through the transcription factor nuclear factor-κB (NF-κB) [55]. In CCK-induced pancreatitis models, PKCδ, ε, and ζ are activated, such that the translocation of PKCδ and the activation of PKCε leads to NF-κB activation [62]. This was first shown using the PKC inhibitors GF109203X (pan-PKC inhibitor), Gö 6976 (conventional PKC inhibitor), PKCζ pseudosubstrate (PKCζ inhibitor), δV1-1 (PKCδ translocation inhibitor), and εV1-2 (PKCε translocation inhibitor) [62]. Later, this was confirmed in isolated acinar cells of PKCδ−/− mice stimulated with supraphysiological CCK [20] as well as in vivo in a caerulein-induced pancreatitis model where wildtype mice were treated with the PKCδ translocation inhibitor δV1-1 [63].
4. PKC IN PANCREATIC CANCER
In pancreatic cancer, PKCs have been implicated in both tumor promoting and tumor suppressing roles. PKC isoenzymes contributing to pancreatic cancer development and progression include PKCα, PKCβ, PKCδ, PKCε, PKCζ and PKCι (Figure 5) [7, 64–66]. While PKCβ, δ, ζ, ι, and to a lesser extent PKCε, have been implicated in the malignancy of pancreatic cancer, the role of PKCα remains unclear, as studies have suggested opposing roles [67, 68]. In the following we will discuss the roles of these PKC isoforms in development and progression of pancreatic cancer.
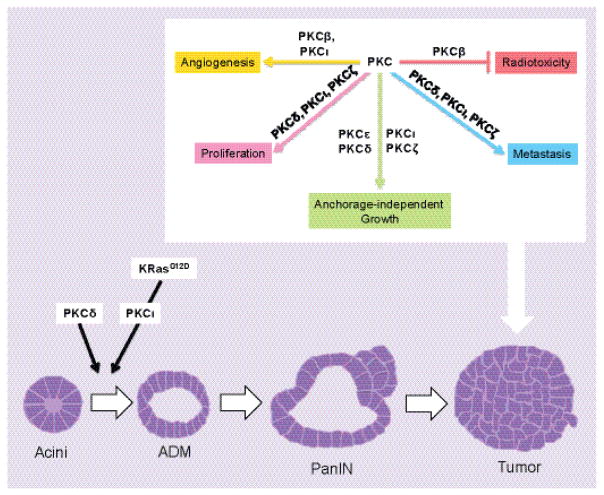
Figure 5.
Schematic of a progression model for pancreatic cancer. Current belief is that acinar cells (acini) can undergo acinar-to-ductal metaplasia (ADM). In presence of an oncogenic KRAS mutation, ADM lesions further develop into pancreatic intraepithelial neoplasia (PanIN), which then can progress to pancreatic cancer. PKCδ and PKCι contribute to the early event of ADM and additional PKCs mediate the growth and progression of pancreatic cancer by aiding angiogenesis, proliferation, anchorage-independent growth, and metastasis, while also preventing radiosensitization.
4.1 PKCs in Pancreatic Cancer Development
Activating KRAS mutations occur in almost all pancreatic ductal adenocarcinoma (PDA) patients [69], and, being present in most acinar-to-ductal metaplasia (ADM) lesions and pancreatic intraepithelial neoplastic lesions (PanIN), are believed to be the initiating mutations leading to development of the cancer [70]. While mutant KRas has thus far not been directly targetable in pancreatic cancer [71], PKCs mediate many of the downstream effects of KRas. For example, in mice, PKCι inhibition can mitigate KrasG12D-driven formation of ADM possibly through upregulation of matrix metalloproteinase-7 (MMP-7) [72]. The likely mechanism of how PKCι and MMP-7 regulate ADM is through Notch, since Notch can be activated by MMP-7-mediated cleavage [73] and is a driver of the ADM process [74]. However, it should be noted that it also was shown that activation of atypical PKCs causes phosphorylation of KrasG12V, thereby decreasing tumorigenesis [75]. Further studies showed that PKCδ contributes to acinar cell dedifferentiation, a first step in the conversion from an acinar to a ductal phenotype [76]. This is interesting because novel PKCs such as PKCδ activate PKD1, which is necessary for oncogenic KRas-mediated ADM and PanIN formation via activation of Notch [74]. The contribution of additional PKC isoforms to pancreatic cancer development remains unclear.
4.2 PKCs in Pancreatic Cancer Progression
The PKC isoforms α, β, δ, ε, ζ and ι have been shown to contribute to an aggressive cancer phenotype. For example, PKCα as well as the novel PKCs PKCε and PKCδ and the atypical PKCs PKCι and PKCζ all have been implicated in regulation of anchorage-independent growth of tumor cells. Anchorage-independent growth and cell proliferation is mediated through both the Raf-MAPK and Akt pathways. For instance, PKCα induces Raf-1 activation via phosphorylation at S499 [77] and indirect activation of Raf-1 occurs via PKCε [78]. Further, conventional and atypical PKCs promote Raf-1 activation via phosphorylation of Raf Kinase Inhibitor Protein (RKIP), which subsequently releases Raf-1 thereby allowing downstream signaling [79], and MAPK phosphorylation/activation is enhanced by PKCδ as well as PKCε [65]. It was shown that in pancreatic cancer cells in response to PMA, neurotensin, and physiological levels of CCK, PKCε translocates to the plasma membrane to activate the MAPK pathway and regulate proliferation [80, 81]. PKCδ has been shown to drive anchorage-independent growth through activation of the PI3K-Akt pathway, and treatment of Panc1 cells overexpressing PKCδ with the PI3K inhibitor LY294002 prevented soft agar colony formation [64]. Besides its roles in cell survival, anchorage independent growth and proliferation, PKCδ is also implicated in cell metastasis [82], and overexpression of PKCδ enhances the metastatic capability of Panc1 cells in xenografts [64].
Additional mediators of anchorage-independent growth and invasion are the atypical isoforms PKCζ and PKCι. In an orthotopic model where Panc1 cells with knockdown of PKCζ were injected, tumor proliferation and metastases were significantly reduced [83]. Additionally, in vitro assays of anchorage-independent growth and invasion indicated that PKCζ mediates these processes through STAT3 activation, but not ERK1/2 signaling [83]. This is not surprising, as STAT3 activity has been implicated in similar processes as PKCζ, such that constant STAT3 activity promotes PanIN and pancreatic cancer progression in a KrasG12D model [84]. Further PKC involvement in PDA progression comes from PKCι, which is associated with reduced survival in patients with high PKCι expression [66, 85]. In vitro, PKCι regulates anchorage-independent growth; and in vivo an orthotopic model using Panc1 cells with knockdown of PKCι results in decreased proliferation, metastases, and angiogenesis. PKCι knockdown also decreased the abundance of Rac1 and vascular endothelial growth factor (VEGF) expression and ERK1/2 phosphorylation [66]. However, in contrast to this, the atypical PKC activator prostratin prevents tumor progression in mice [86].
In addition to regulating tumor growth and progression, PKCs also can mediate resistance to treatment. For example, chemoresistance is caused by TGF-β1-induced PKCα upregulation in BxPC3 cells [87]. In addition, PKCα also mediates TGF-β-induced EMT and knockdown or inhibition of PKCα suppresses Snail and blocks PTEN downregulation [88, 89]. Another conventional PKC, PKCβ, has been implicated as integral in angiogenesis and radioresistance of tumor cells. In a mouse xenograft model the PKCβ inhibitor LY333531 suppressed angiogenesis and induced apoptosis [90]. In an additional study, the PKCβ inhibitor enzastaurin failed to prevent overall tumor growth, but caused a delay in tumor growth when cells were irradiated [91]. In pancreatic cancer cells, resistance to radiotherapy is mediated by glycogen synthase kinase 3β (GSK3β), since expression of a kinase-dead version of this kinase results in radioresistance and expression of a constitutively-active version in radiosensitization of cells [92]. The mechanism of how PKCβ reduces radiation cytotoxicity occurs through mediating an inactivating phosphorylation of GSK3β at amino-acid residue S9 [91, 93, 94].
Another PKC that is activated by irradiation is PKCδ. Ionizing radiation causes cleavage and activation of caspases 3 and 7, of which caspase 3 cleaves PKCδ, resulting in an active, unregulated kinase fragment [95, 96]. This active PKCδ fragment then activates Akt to drive tumor cell proliferation [82]. While PKCε can be activated by caspase 3 by a similar mechanism, it so far has not been implicated in this pathway [96].
Taken together, PKCs have been implicated in almost all aspects of pancreatic cancer progression, including angiogenesis, anchorage-independent growth, radioresistance and metastasis, suggesting inhibition could have a positive therapeutic role.
4.3. Targeting PKC in pancreatic cancer
Most work in pancreatic cell lines and mouse models have shown activation of PKCs to increase malignancy. Therefore, therapeutic approaches have focused on inhibiting PKCs. However many PKC mutations in cancer are loss-of-function mutations [97]. For example, the PKCγ mutation P524R prevents activation in response to stimuli and this residue in an analogous position in PKCδ, ε, and θ is also mutated such that it loses function in pancreatic cancer [97]. Additionally, many PKC inhibitors have not been successful in the clinic (for a review of PKC therapeutic strategies, see [98]). For example, bryostatin-1, which initially activates PKC but then downregulates it upon further exposure [99], was an initial promising target as shown by its efficacy in increasing apoptosis following gemcitabine treatment of human pancreatic cancer cell lines [100]. However, in a phase II clinical trial where bryostatin-1 was used in conjunction with paclitaxel in advanced stage pancreatic adenocarcinoma patients, bryostatin-1 was not effective in improving patient survival or decreasing tumor burden [101].
In contrast, PKCα, βI, and δ show increased expression in pancreatic cancer tissue versus normal tissue, suggesting inhibition of these isoforms has therapeutic potential [100]. For example, the orthotopic injection of HPAC cells overexpressing PKCα increased the mortality rate in mice, while down-regulating PKCα expression increased survival [102]. This suggests that inhibition of PKCα may be beneficial to patients. However, it should be noted that PKCα also has been shown to inhibit pancreatic cancer cell proliferation in cell lines [67] and induce increased expression of Bad as well as the TRAIL receptors DL4 and DL5, thereby making cells sensitive to apoptosis [68]. Taken together, the often contradictory results obtained by using animal models, cell lines or inhibitors clearly indicate that more studies are needed to understand the roles of PKC isoforms in the development and progression of this cancer, before meaningful therapeutic targeting strategies can be developed.
5. CONCLUSION
PKCs regulate many pancreatic functions in normal acinar cells, ductal cells, and islets, as well as in disease states, including insulin resistance, diabetes mellitus, pancreatitis and pancreatic ductal adenocarcinoma (PDA). In the normal pancreas PKCs regulate secretory mechanisms, as seen by amylase secretion in acinar cells, bicarbonate secretion in ductal cells, and glucagon as well as insulin secretion in islets. PKCs are also essential in insulin resistance and diabetes mellitus development where they regulate β-cell proliferation and function as well as insulin secretion and β-cell destruction. During pancreatitis, PKCs are key players contributing to pancreatic damage and inflammation, as well as trypsinogen activation and basolateral exocytosis. Eventually PKCs also contribute to PDA development by contributing to acinar cell dedifferentiation (PKCδ) [76] and acinar-to-ductal metaplasia (PKCι) [72]. During the progression of PDA, PKCα, PKCδ, PKCε and the atypical PKCs PKCζ and PKCι regulate proliferation and enhance anchorage-independent growth [65, 66, 77–79, 83]. In addition, atypical PKCs also have been implicated in regulating metastasis [66, 83]. In summary, PKC isoforms have diverse roles in maintaining normal pancreatic function, but they can also contribute to the induction and progression of pancreatic disease.
Highlights.
PKCs play key roles in acinar, ductal, and islet cells of the pancreas.
PKCs are involved in pancreatic inflammation, contributing to insulin resistance, diabetes mellitus, and pancreatitis.
PKCs mediate pancreatic cancer development and progression.
Acknowledgments
This work was supported by the NIH/NCI grant CA200572 to PS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funders had no role in decision to publish, or preparation of the manuscript. All authors have no conflict of interest.
ABBREVIATIONS
- ADM
acinar-to-ductal metaplasia
- BIM
bisindolylmaleimide
- CCK
cholecystokinin
- DAG
diacylglycerol
- DRM
detergent-resistant membrane fraction
- EX4
exendin-4
- GLP-1
glucagon-like peptide-1
- GSK3β
glycogen synthase kinase 3β
- iNOS
inducible nitric oxide synthase
- KEAP1
Kelch-like ECH-associated protein 1
- MANS
myristoylated N-terminal sequence of MARCKS
- MARCKS
myristoylated alanine-rich C kinase substrate
- MMP-7
matrix metalloproteinase-7
- NF-κB
transcription factor nuclear factor-κB
- NO
nitric oxide
- PKC
protein kinase C
- PLC
phospholipase c
- RKIP
Raf Kinase Inhibitor Protein
- SLC26A6
Solute Carrier Family 26 Member 6
- TLR2
toll-like receptor 2
- TPA
12-O-Tetradecanoylphorbol-13-acetate
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newton AC. Protein kinase C: structure, function, and regulation. The Journal of biological chemistry. 1995;270(48):28495–8. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 2.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238(4834):1726–8. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307(5708):380–4. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 4.Quan W, Jo EK, Lee MS. Role of pancreatic beta-cell death and inflammation in diabetes. Diabetes Obes Metab. 2013;15(Suppl 3):141–51. doi: 10.1111/dom.12153. [DOI] [PubMed] [Google Scholar]
- 5.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol. 2015;31(5):395–9. doi: 10.1097/MOG.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storz P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2017;14(5):296–304. doi: 10.1038/nrgastro.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JD, Cornford PA, Dodson A, Neoptolemos JP, Foster CS. Expression patterns of protein kinase C isoenzymes are characteristically modulated in chronic pancreatitis and pancreatic cancer. Am J Clin Pathol. 2003;119(3):392–402. doi: 10.1309/bkpc9dx98r781b87. [DOI] [PubMed] [Google Scholar]
- 8.De Marinis YZ, Zhang E, Amisten S, Taneera J, Renstrom E, Rorsman P, Eliasson L. Enhancement of glucagon secretion in mouse and human pancreatic alpha cells by protein kinase C (PKC) involves intracellular trafficking of PKCalpha and PKCdelta. Diabetologia. 2010;53(4):717–29. doi: 10.1007/s00125-009-1635-x. [DOI] [PubMed] [Google Scholar]
- 9.Hii CS, Stutchfield J, Howell SL. Enhancement of glucagon secretion from isolated rat islets of Langerhans by phorbol 12-myristate 13-acetate. Biochem J. 1986;233(1):287–9. doi: 10.1042/bj2330287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian YM, Urquidi V, Ashcroft SJ. Protein kinase C in beta-cells: expression of multiple isoforms and involvement in cholinergic stimulation of insulin secretion. Mol Cell Endocrinol. 1996;119(2):185–93. doi: 10.1016/0303-7207(96)03811-7. [DOI] [PubMed] [Google Scholar]
- 11.Harris TE, Persaud SJ, Jones PM. Atypical isoforms of pKc and insulin secretion from pancreatic beta-cells: evidence using Go 6976 and Ro 31-8220 as Pkc inhibitors. Biochem Biophys Res Commun. 1996;227(3):672–6. doi: 10.1006/bbrc.1996.1567. [DOI] [PubMed] [Google Scholar]
- 12.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18(6):451–63. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 13.Lee IS, Hur EM, Suh BC, Kim MH, Koh DS, Rhee IJ, Ha H, Kim KT. Protein kinase A- and C-induced insulin release from Ca2+-insensitive pools. Cell Signal. 2003;15(5):529–37. doi: 10.1016/s0898-6568(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 14.Shigeto M, Ramracheya R, Tarasov AI, Cha CY, Chibalina MV, Hastoy B, Philippaert K, Reinbothe T, Rorsman N, Salehi A, Sones WR, Vergari E, Weston C, Gorelik J, Katsura M, Nikolaev VO, Vennekens R, Zaccolo M, Galione A, Johnson PR, Kaku K, Ladds G, Rorsman P. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest. 2015;125(12):4714–28. doi: 10.1172/JCI81975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MH, Kim EH, Jung HS, Yang D, Park EY, Jun HS. EX4 stabilizes and activates Nrf2 via PKCdelta, contributing to the prevention of oxidative stress-induced pancreatic beta cell damage. Toxicol Appl Pharmacol. 2017;315:60–69. doi: 10.1016/j.taap.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick F, Pandol S, Thrower E. Protein kinase C in the pancreatic acinar cell. J Gastroenterol Hepatol. 2008;23(Suppl 1):S37–41. doi: 10.1111/j.1440-1746.2007.05282.x. [DOI] [PubMed] [Google Scholar]
- 17.Pollo DA, Baldassare JJ, Honda T, Henderson PA, Talkad VD, Gardner JD. Effects of cholecystokinin (CCK) and other secretagogues on isoforms of protein kinase C (PKC) in pancreatic acini. Biochimica et biophysica acta. 1994;1224(1):127–38. doi: 10.1016/0167-4889(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 18.Bastani B, Yang L, Baldassare JJ, Pollo DA, Gardner JD. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochimica et biophysica acta. 1995;1269(3):307–15. doi: 10.1016/0167-4889(95)00120-0. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Chen X, Williams JA. Regulation of CCK-induced amylase release by PKC-delta in rat pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G764–71. doi: 10.1152/ajpgi.00111.2004. [DOI] [PubMed] [Google Scholar]
- 20.Thrower EC, Wang J, Cheriyan S, Lugea A, Kolodecik TR, Yuan J, Reeve JR, Jr, Gorelick FS, Pandol SJ. Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas. 2009;38(8):930–5. doi: 10.1097/MPA.0b013e3181b8476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SC, Fortune KP, Wank SA, Kopin AS, Gardner JD. Multiple affinity states of different cholecystokinin receptors. The Journal of biological chemistry. 1994;269(42):26121–6. [PubMed] [Google Scholar]
- 22.Talkad VD, Patto RJ, Metz DC, Turner RJ, Fortune KP, Bhat ST, Gardner JD. Characterization of the three different states of the cholecystokinin (CCK) receptor in pancreatic acini. Biochimica et biophysica acta. 1994;1224(1):103–16. doi: 10.1016/0167-4889(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Tapia JA, Bragado MJ, Garcia-Marin LJ, Jensen RT. Cholecystokinin-stimulated tyrosine phosphorylation of PKC-delta in pancreatic acinar cells is regulated bidirectionally by PKC activation. Biochimica et biophysica acta. 2002;1593(1):99–113. doi: 10.1016/s0167-4889(02)00346-4. [DOI] [PubMed] [Google Scholar]
- 24.Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochimica et biophysica acta. 2007;1773(4):483–501. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. The Journal of biological chemistry. 2001;276(41):37986–92. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- 26.Tapia JA, Jensen RT, Garcia-Marin LJ. Rottlerin inhibits stimulated enzymatic secretion and several intracellular signaling transduction pathways in pancreatic acinar cells by a non-PKC-delta-dependent mechanism. Biochimica et biophysica acta. 2006;1763(1):25–38. doi: 10.1016/j.bbamcr.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Satoh K, Narita T, Katsumata-Kato O, Sugiya H, Seo Y. Involvement of myristoylated alanine-rich C kinase substrate phosphorylation and translocation in cholecystokinin-induced amylase release in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2016;310(6):G399–409. doi: 10.1152/ajpgi.00198.2015. [DOI] [PubMed] [Google Scholar]
- 28.Stumpo DJ, Graff JM, Albert KA, Greengard P, Blackshear PJ. Molecular cloning, characterization, and expression of a cDNA encoding the “80- to 87-kDa” myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989;86(11):4012–6. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickett JA, Campos-Toimil M, Thomas P, Edwardson JM. Identification of SNAREs that mediate zymogen granule exocytosis. Biochem Biophys Res Commun. 2007;359(3):599–603. doi: 10.1016/j.bbrc.2007.05.128. [DOI] [PubMed] [Google Scholar]
- 30.Sancho V, Berna MJ, Thill M, Jensen RT. PKCtheta activation in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors is needed for stimulation of numerous important cellular signaling cascades. Biochimica et biophysica acta. 2011;1813(12):2145–56. doi: 10.1016/j.bbamcr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparatore B, Passalacqua M, Pedrazzi M, Ledda S, Patrone M, Gaggero D, Pontremoli S, Melloni E. Role of the kinase activation loop on protein kinase C theta activity and intracellular localisation. FEBS Lett. 2003;554(1–2):35–40. doi: 10.1016/s0014-5793(03)01073-1. [DOI] [PubMed] [Google Scholar]
- 32.Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262(6 Pt 1):L647–61. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, Koizumi J, Murata M, Nagayama M, Nobuoka T, Tanaka S, Hirata K, Sawada N. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol. 2010;177(2):698–712. doi: 10.2353/ajpath.2010.091226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K, Sawada N. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem. 2011;112(7):1761–72. doi: 10.1002/jcb.23095. [DOI] [PubMed] [Google Scholar]
- 35.Evans RL, Ashton N, Elliott AC, Green R, Argent BE. Interactions between secretin and acetylcholine in the regulation of fluid secretion by isolated rat pancreatic ducts. J Physiol. 1996;496(Pt 1):265–73. doi: 10.1113/jphysiol.1996.sp021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegyi P, Rakonczay Z, Jr, Tiszlavicz L, Varro A, Toth A, Racz G, Varga G, Gray MA, Argent BE. Protein kinase C mediates the inhibitory effect of substance P on HCO3- secretion from guinea pig pancreatic ducts. Am J Physiol Cell Physiol. 2005;288(5):C1030–41. doi: 10.1152/ajpcell.00430.2003. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J. 2005;24(14):2499–511. doi: 10.1038/sj.emboj.7600736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boni-Schnetzler M, Ehses JA, Faulenbach M, Donath MY. Insulitis in type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):201–4. doi: 10.1111/j.1463-1326.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 39.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–31. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Chen R, Wang H, Liang F. Mechanisms Linking Inflammation to Insulin Resistance. Int J Endocrinol. 2015;2015:508409. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–49. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velazquez-Garcia S, Valle S, Rosa TC, Takane KK, Demirci C, Alvarez-Perez JC, Mellado-Gil JM, Ernst S, Scott DK, Vasavada RC, Alonso LC, Garcia-Ocana A. Activation of protein kinase C-zeta in pancreatic beta-cells in vivo improves glucose tolerance and induces beta-cell expansion via mTOR activation. Diabetes. 2011;60(10):2546–59. doi: 10.2337/db10-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakshmipathi J, Alvarez-Perez JC, Rosselot C, Casinelli GP, Stamateris RE, Rausell-Palamos F, O’Donnell CP, Vasavada RC, Scott DK, Alonso LC, Garcia-Ocana A. PKCzeta Is Essential for Pancreatic beta-Cell Replication During Insulin Resistance by Regulating mTOR and Cyclin-D2. Diabetes. 2016;65(5):1283–96. doi: 10.2337/db15-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. The Journal of biological chemistry. 2009;284(12):7832–42. doi: 10.1074/jbc.M807458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantley J, Boslem E, Laybutt DR, Cordery DV, Pearson G, Carpenter L, Leitges M, Biden TJ. Deletion of protein kinase Cdelta in mice modulates stability of inflammatory genes and protects against cytokine-stimulated beta cell death in vitro and in vivo. Diabetologia. 2011;54(2):380–9. doi: 10.1007/s00125-010-1962-y. [DOI] [PubMed] [Google Scholar]
- 46.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27(2):321–33. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rutti S, Schuit FC, Lutz TA, Boni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53(8):1795–806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 48.Carpenter L, Cordery D, Biden TJ. Protein kinase Cdelta activation by interleukin-1beta stabilizes inducible nitric-oxide synthase mRNA in pancreatic beta-cells. The Journal of biological chemistry. 2001;276(7):5368–74. doi: 10.1074/jbc.M010036200. [DOI] [PubMed] [Google Scholar]
- 49.Eizirik DL, Flodstrom M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia. 1996;39(8):875–90. doi: 10.1007/BF00403906. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, Pedersen DJ, Braun U, Cooney GJ, Leitges M, Biden TJ. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab. 2007;6(4):320–8. doi: 10.1016/j.cmet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93(2):870–6. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Yuan J, Tan T, Jia W, Lugea A, Mareninova O, Waldron RT, Pandol SJ. Genetic inhibition of protein kinase Cepsilon attenuates necrosis in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2014;307(5):G550–63. doi: 10.1152/ajpgi.00432.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grady T, Mah’Moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol. 1998;275(5 Pt 1):G1010–7. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhuri A, Kolodecik TR, Gorelick FS. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol. 2005;288(2):G235–43. doi: 10.1152/ajpgi.00334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141(6):2210–2217e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR, Jr, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(6):G1344–53. doi: 10.1152/ajpgi.00020.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheele G, Adler G, Kern H. Exocytosis occurs at the lateral plasma membrane of the pancreatic acinar cell during supramaximal secretagogue stimulation. Gastroenterology. 1987;92(2):345–53. doi: 10.1016/0016-5085(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 58.Gaisano HY, Lutz MP, Leser J, Sheu L, Lynch G, Tang L, Tamori Y, Trimble WS, Salapatek AM. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108(11):1597–611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosen-Binker LI, Lam PP, Binker MG, Gaisano HY. Alcohol-induced protein kinase Calpha phosphorylation of Munc18c in carbachol-stimulated acini causes basolateral exocytosis. Gastroenterology. 2007;132(4):1527–45. doi: 10.1053/j.gastro.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 60.Cosen-Binker LI, Lam PP, Binker MG, Reeve J, Pandol S, Gaisano HY. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. The Journal of biological chemistry. 2007;282(17):13047–58. doi: 10.1074/jbc.M611132200. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122(2):448–57. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 62.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, Shimosegawa T, Pandol SJ. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G582–91. doi: 10.1152/ajpgi.00087.2004. [DOI] [PubMed] [Google Scholar]
- 63.Ramnath RD, Sun J, Bhatia M. PKC delta mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. J Mol Med (Berl) 2010;88(10):1055–63. doi: 10.1007/s00109-010-0647-9. [DOI] [PubMed] [Google Scholar]
- 64.Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A, Pallotta MG, Kazanietz MG, Bal de Kier Joffe ED, Puricelli LL. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas. 2010;39(1):e31–41. doi: 10.1097/MPA.0b013e3181bce796. [DOI] [PubMed] [Google Scholar]
- 65.Ishino K, Fukazawa H, Shikano M, Ohba M, Kuroki T, Uehara Y. Enhancement of anchorage-independent growth of human pancreatic carcinoma MIA PaCa-2 cells by signaling from protein kinase C to mitogen-activated protein kinase. Mol Carcinog. 2002;34(4):180–6. doi: 10.1002/mc.10063. [DOI] [PubMed] [Google Scholar]
- 66.Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010;70(5):2064–74. doi: 10.1158/0008-5472.CAN-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Detjen KM, Brembeck FH, Welzel M, Kaiser A, Haller H, Wiedenmann B, Rosewicz S. Activation of protein kinase Calpha inhibits growth of pancreatic cancer cells via p21(cip)-mediated G(1) arrest. J Cell Sci. 2000;113(Pt 17):3025–35. doi: 10.1242/jcs.113.17.3025. [DOI] [PubMed] [Google Scholar]
- 68.Farrow B, Thomas RP, Wang XF, Evers BM. Activation of conventional PKC isoforms increases expression of the pro-apoptotic protein Bad and TRAIL receptors. Int J Gastrointest Cancer. 2002;32(2–3):63–72. doi: 10.1385/IJGC:32:2-3:63. [DOI] [PubMed] [Google Scholar]
- 69.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, Kern SE. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57(9):1731–4. [PubMed] [Google Scholar]
- 70.Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142(4):730–733e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111(5):817–22. doi: 10.1038/bjc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scotti ML, Smith KE, Butler AM, Calcagno SR, Crawford HC, Leitges M, Fields AP, Murray NR. Protein kinase C iota regulates pancreatic acinar-to-ductal metaplasia. PLoS One. 2012;7(2):e30509. doi: 10.1371/journal.pone.0030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci U S A. 2007;104(49):19327–32. doi: 10.1073/pnas.0705953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liou GY, Doppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, Crawford HC, Fields AP, Murray NR, Wang QJ, Leitges M, Storz P. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun. 2015;6:6200. doi: 10.1038/ncomms7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang MT, Holderfield M, Galeas J, Delrosario R, To MD, Balmain A, McCormick F. K-Ras Promotes Tumorigenicity through Suppression of Non-canonical Wnt Signaling. Cell. 2015;163(5):1237–51. doi: 10.1016/j.cell.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 76.Johnson CL, Peat JM, Volante SN, Wang R, McLean CA, Pin CL. Activation of protein kinase Cdelta leads to increased pancreatic acinar cell dedifferentiation in the absence of MIST1. J Pathol. 2012;228(3):351–65. doi: 10.1002/path.4015. [DOI] [PubMed] [Google Scholar]
- 77.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364(6434):249–52. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 78.Ueffing M, Lovric J, Philipp A, Mischak H, Kolch W. Protein kinase C-epsilon associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity. Oncogene. 1997;15(24):2921–7. doi: 10.1038/sj.onc.1201477. [DOI] [PubMed] [Google Scholar]
- 79.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. The Journal of biological chemistry. 2003;278(15):13061–8. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- 80.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin stimulates protein kinase C-dependent mitogenic signaling in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2003;63(10):2379–87. [PubMed] [Google Scholar]
- 81.Racz GZ, Szucs A, Szlavik V, Vag J, Burghardt B, Elliott AC, Varga G. Possible role of duration of PKC-induced ERK activation in the effects of agonists and phorbol esters on DNA synthesis in Panc-1 cells. J Cell Biochem. 2006;98(6):1667–80. doi: 10.1002/jcb.20913. [DOI] [PubMed] [Google Scholar]
- 82.Cheng J, Tian L, Ma J, Gong Y, Zhang Z, Chen Z, Xu B, Xiong H, Li C, Huang Q. Dying tumor cells stimulate proliferation of living tumor cells via caspase-dependent protein kinase Cdelta activation in pancreatic ductal adenocarcinoma. Mol Oncol. 2015;9(1):105–14. doi: 10.1016/j.molonc.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butler AM, Scotti Buzhardt ML, Li S, Smith KE, Fields AP, Murray NR. Protein kinase C zeta regulates human pancreatic cancer cell transformed growth and invasion through a STAT3-dependent mechanism. PLoS One. 2013;8(8):e72061. doi: 10.1371/journal.pone.0072061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algul H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Kato S, Akimoto K, Nagashima Y, Ishiguro H, Kubota K, Kobayashi N, Hosono K, Watanabe S, Sekino Y, Sato T, Sasaki K, Nakaigawa N, Kubota Y, Inayama Y, Endo I, Ohno S, Maeda S, Nakajima A. aPKClambda/iota is a beneficial prognostic marker for pancreatic neoplasms. Pancreatology. 2013;13(4):360–8. doi: 10.1016/j.pan.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Szallasi Z, Krsmanovic L, Blumberg PM. Nonpromoting 12-deoxyphorbol 13-esters inhibit phorbol 12-myristate 13-acetate induced tumor promotion in CD-1 mouse skin. Cancer Res. 1993;53(11):2507–12. [PubMed] [Google Scholar]
- 87.Chen Y, Yu G, Yu D, Zhu M. PKCalpha-induced drug resistance in pancreatic cancer cells is associated with transforming growth factor-beta1. J Exp Clin Cancer Res. 2010;29:104. doi: 10.1186/1756-9966-29-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kyuno D, Kojima T, Yamaguchi H, Ito T, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K, Sawada N. Protein kinase Calpha inhibitor protects against downregulation of claudin-1 during epithelial-mesenchymal transition of pancreatic cancer. Carcinogenesis. 2013;34(6):1232–43. doi: 10.1093/carcin/bgt057. [DOI] [PubMed] [Google Scholar]
- 89.Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G899–905. doi: 10.1152/ajpgi.00411.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshiji H, Kuriyama S, Ways DK, Yoshii J, Miyamoto Y, Kawata M, Ikenaka Y, Tsujinoue H, Nakatani T, Shibuya M, Fukui H. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999;59(17):4413–8. [PubMed] [Google Scholar]
- 91.Spalding AC, Watson R, Davis ME, Kim AC, Lawrence TS, Ben-Josef E. Inhibition of protein kinase Cbeta by enzastaurin enhances radiation cytotoxicity in pancreatic cancer. Clin Cancer Res. 2007;13(22 Pt 1):6827–33. doi: 10.1158/1078-0432.CCR-07-0454. [DOI] [PubMed] [Google Scholar]
- 92.Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, Bommer GT, Eldar-Finkelman H, Giordano T, Fearon ER, Hammer GD, Lawrence TS, Ben-Josef E. GSK3beta and beta-catenin modulate radiation cytotoxicity in pancreatic cancer. Neoplasia. 2010;12(5):357–65. doi: 10.1593/neo.92112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. The Journal of biological chemistry. 1992;267(24):16878–82. [PubMed] [Google Scholar]
- 94.Neri A, Marmiroli S, Tassone P, Lombardi L, Nobili L, Verdelli D, Civallero M, Cosenza M, Bertacchini J, Federico M, De Pol A, Deliliers GL, Sacchi S. The oral protein-kinase C beta inhibitor enzastaurin (LY317615) suppresses signalling through the AKT pathway, inhibits proliferation and induces apoptosis in multiple myeloma cell lines. Leuk Lymphoma. 2008;49(7):1374–83. doi: 10.1080/10428190802078289. [DOI] [PubMed] [Google Scholar]
- 95.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamen R, Weichselbaum R, et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14(24):6148–56. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koriyama H, Kouchi Z, Umeda T, Saido TC, Momoi T, Ishiura S, Suzuki K. Proteolytic activation of protein kinase C delta and epsilon by caspase-3 in U937 cells during chemotherapeutic agent-induced apoptosis. Cell Signal. 1999;11(11):831–8. doi: 10.1016/s0898-6568(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 97.Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari FB, Hunter T, Brognard J, Newton AC. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160(3):489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Storz P. Targeting protein kinase C subtypes in pancreatic cancer. Expert Rev Anticancer Ther. 2015;15(4):433–8. doi: 10.1586/14737140.2015.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Philip PA, Zonder JA. Pharmacology and clinical experience with bryostatin 1: a novel anticancer drug. Expert Opin Investig Drugs. 1999;8(12):2189–2199. doi: 10.1517/13543784.8.12.2189. [DOI] [PubMed] [Google Scholar]
- 100.El-Rayes BF, Ali S, Philip PA, Sarkar FH. Protein kinase C: a target for therapy in pancreatic cancer. Pancreas. 2008;36(4):346–52. doi: 10.1097/MPA.0b013e31815ceaf7. [DOI] [PubMed] [Google Scholar]
- 101.Lam AP, Sparano JA, Vinciguerra V, Ocean AJ, Christos P, Hochster H, Camacho F, Goel S, Mani S, Kaubisch A. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am J Clin Oncol. 2010;33(2):121–4. doi: 10.1097/COC.0b013e3181a31920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denham DW, Franz MG, Denham W, Zervos EE, Gower WR, Jr, Rosemurgy AS, Norman J. Directed antisense therapy confirms the role of protein kinase C-alpha in the tumorigenicity of pancreatic cancer. Surgery. 1998;124(2):218–23. discussion 223–4. [PubMed] [Google Scholar]