Abstract
Intracellular metabolism in skeletal muscle has been studied for more than a century and is the stuff of textbooks. In contrast, the extracellular secretion of metabolites by muscle cells, and their effects on non-muscle cells near or far, has been investigated much less extensively. Here, we describe a number of cases in which striated muscle secretes a metabolite that elicits complex responses in other cells or tissues, with involvements in normal physiology as well as obesity, type II diabetes, and cardiac remodeling. We focus on two recently identified secreted catabolic products of branched chain amino acid breakdown, β-aminoisobutyric acid and 3-hydroxyisobutyrate, and discuss common themes of inter-cellular signaling pathways driven by secreted metabolites.
Introduction
The notion that skeletal muscle secretes products of intermediate metabolism is not new. The most extensively documented such case is that of lactic acid and its role in the Cori Cycle. In a series of seminal papers1–3, Carl and Gerti Cori described a system in which the lactic acid produced by anaerobic glycolysis in skeletal muscle is shuttled out to the liver, where it is converted back to glucose-6-phosphate (G6P). G6P is then converted into liver glycogen or glucose, the latter of which is exported and can be taken up by muscle for consumption. This is an important process that keeps the muscle replenished during times of intense physical activity, in which lactate cannot be combusted, either because the process is too slow or the tissue is too hypoxic. Alanine can similarly be shuttled to the liver, simultaneously allowing for export of nitrogen to the liver.
The Cori cycle essentially represents partitioning of glucose and lactate metabolism, but can lactate also act as a signaling molecule? Two recent studies indicate that it can. Liu et al. have demonstrated both in vitro and in vivo that lactate binds the G-protein coupled receptor GPR81 on adipocytes4, inhibiting lipolysis. Earlier studies showed a correlation between rising plasma lactate, particularly during exercise, and decreased fatty acid oxidation5,6. Thus lactate may serve as a muscle-derived signaling metabolite that blunts fatty acid release from adipose stores when lactate itself cannot be combusted, i.e. indication that fatty acids also likely cannot be combusted because acetyl-CoA combustion is limited. Similarly, Chang et al. have shown that lactate binds to Olfr78, an olfactory GPCR expressed in the glomus cells of carotid bodies7. Glomus cells control lung ventilation, and elevated lactate in the blood under hypoxic conditions leads to Olfr78 activation and increased ventilation. Considering that skeletal muscle likely produces most of the lactate in the blood, especially while exercising, this autocrine metabolite-driven signaling pathway may thus allow skeletal muscle to increase oxygen delivery when limiting local oxygen concentrations prevent the oxidation of lactate.
Adenosine and vasodilation
The most extensively studied bioactive metabolite secreted by striated muscle is undoubtedly adenosine. Berner first proposed in the 1960's the “adenosine hypothesis”, whereby adenosine is secreted from muscle that is experiencing supply/demand mismatch, in turn triggering vasodilation and reactive hyperemia8,9. Details of the hypothesis remain controversial, in part because studies differ widely in methodologies, model organisms, and muscle beds studied10. Overall, however, there is consensus that interstitial adenosine contributes significantly to hyperemia during exercise in many if not most muscle beds, in large part via activation of A2A adenosine receptors on arteriolar smooth muscle cells11,12. NO-dependent vasodilation, via activation of A1 receptors on endothelial cells, may also contribute13. The source of adenosine remains somewhat controversial, because muscle cells, red blood cells, and endothelial cells all can secrete nucleotides. Overall, it appears that the majority of interstitial adenosine stems from secretion from muscle cells of nucleotides, including ATP, ADP, and AMP, which are then catabolized extracellularly to adenosine via ecto-5′-nucleotidase (NT5E), a process that is accentuated under exercise-induced conditions like local acidosis9,14. Indeed, interstitial adenosine and ATP concentrations are tightly coupled to skeletal muscle blood flow15. Adenosine and adenine nucleotides are thus the clearest examples of muscle-secreted metabolites with critical physiological paracrine effects.
cAMP reduces cardiac hypertrophy and fibrosis
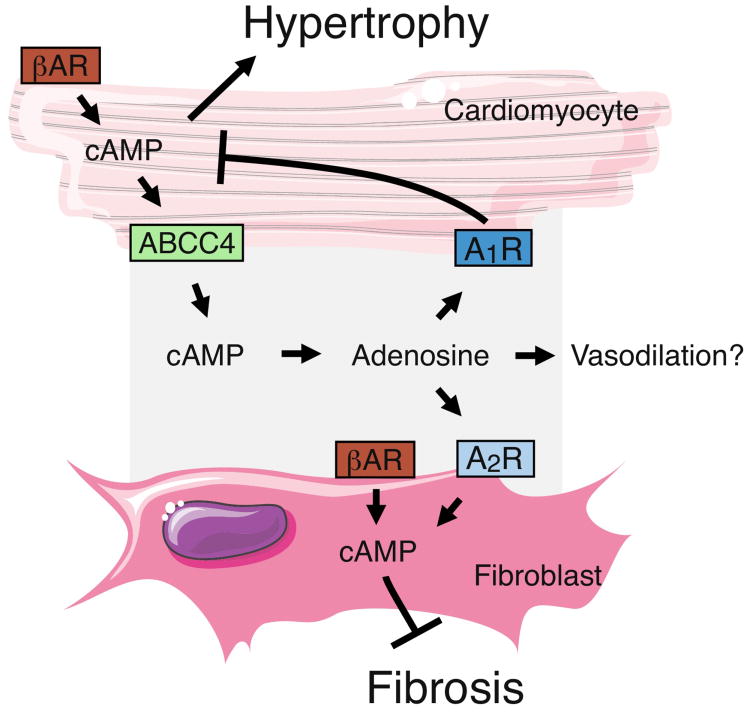
Interestingly, Sassi et al. recently also showed that cardiac muscle secretes cyclic AMP (cAMP), which acts in both an autocrine and paracrine pathway to elicit intracellular signaling16 (figure 1). Intracellular cAMP is essential for increasing cardiac output when demand is high, but prolonged cAMP signaling contributes to pathophysiological conditions such as myocardial hypertrophy and fibrosis. Leveraging the prior observation that cardiomyocytes export cAMP (via MRP4/ABCC4)17, the authors hypothesized that such secretion might direct intracellular signaling that lead to cardioprotective benefits. In support of this idea, the group observed that administration of exogenous cAMP protected mice from the hypertrophy and fibrosis generated by chronic adrenergic stimulation. Cardiomyocytes express the transmembrane proteins Ectonucleotide Pyrophosphatase/Phosphodiesterase 1 (ENPP1) and NT5E, which convert extracellular cAMP to AMP and AMP to adenosine, respectively, suggesting that adenosine may be the bioactive metabolite generated by extracellular cAMP. To explore this notion, the authors employed a panoply of pharmaceutical antagonists of the adenosine receptors (A1R, A2AR, A2BR, A3R) and found that two mechanisms were at work: 1) blocking the cardiomyocyte-specific A1R receptor reduced cardiomyocyote hypertrophy, while 2) blocking the fibroblast-specific A2Rs decreased fibrosis. Thus extracellular adenosine derived from secreted cAMP binds A1R receptors on cardiomyocytes to create an autocrine negative feedback loop, and binds A2R receptors on the neighboring cardiac fibroblasts to reducing fibrosis. This extracellular cAMP/adenosine pathway may thus present a novel target for therapies to prevent hypertrophy and fibrosis.
Figure 1.
Cardiomyocytes secrete cAMP. Chronic adrenergic activation via βadrenergic receptors (βAR) drives hypertrophy and fibrosis. Excess cAMP in cardiomyocytes can be exported via ABCC4. Extracellular cAMP is then converted to adenosine, which acts on adenosine receptors found on cardiomyocytes and fibroblasts. In cardiomyocytes, A1R suppresses intracellular cAMP levels to reduce hypertrophy. In fibroblasts, A2R increases intracellular cAMP to inhibit fibrosis.
This new signaling may also be present in other tissue where chronic cAMP activation drives significant changes. Notably, skeletal muscle effluxes cAMP via the same transporter, ABCC4, with consequences on intracellular cAMP levels, though the mechanism by which this occurs remains unclear, and may be mediated by adenosine18,19. It will be of interest to understand if, and how, these autocrine and paracrine pathways compartmentalize from the vasodilatory effects of adenosine described above. It will also be interesting to see if a similar mechanism is at work in the beiging of adipose tissue, and other tissues similarly highly responsive to cAMP signaling.
β-aminoisobutyric acid increases adipocyte thermogenesis and hepatic β-oxidation
Beyond adenosine and adenine nucleotides, there have been surprisingly few discoveries of other secreted muscle-borne metabolites that act in an endocrine or paracrine fashion. Recent simultaneous metabolomic profiling of human skeletal muscle and plasma revealed strong correlations of a few metabolites between the two20,21, suggesting that muscle may dictate plasma levels of these metabolites. Most of the stronger candidates were found among the amino acids, including the branched-chain amino acid (BCAA) valine. Intriguingly, two metabolite products of valine catabolism, β-aminoisobutyric acid and 3-hydroxyisobutyrate, were recently identified as muscle-borne metabolites with signaling functions outside of the muscle. These stories are described below. The discovery of β-aminoisobutyric acid (BAIBA)22 as a secreted product came from screening cultured myotubes that over-expressed the protein PGC1α, a transcriptional co-activator that regulates a wide array of metabolic process23. In muscle, PGC1α plays a major role in the adaptive response to exercise, contributing to mitochondrial biogenesis, increased angiogenesis, and increased glucose transport and β-oxidation24–29. Interestingly, transgenic mice with skeletal muscle-specific enhanced expression of PGC1α (MCKα mice) also have higher expression of brown adipocyte tissue (BAT) genes (such as UCP1 and CIDEA) in white adipose tissue (WAT) depots. To investigate whether this potential endocrine effect is mediated by a metabolite, Roberts et. al. performed LC-MS metabolic profiling of cultured medium from C2C12 myotubes that over-express PGC1α, and found elevated levels of BAIBA compared to controls. MCKα mice contain 11-fold higher plasma levels of BAIBA, and wild-type mice exercised for three weeks revealed increased concentrations of skeletal muscle BAIBA. The source of BAIBA was presumed to be valine, especially in light of the noted induction of BCAA catabolic genes, but it is important to note that BAIBA can also be produced by the degradation of pyrimidines30 (figure 2).
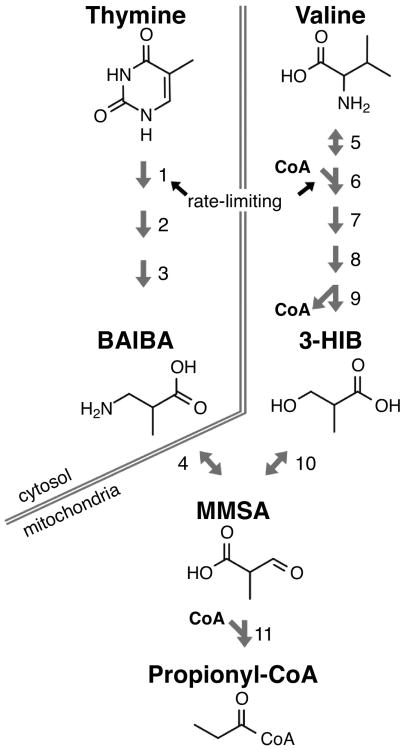
Figure 2.
Production of 3-HIB and BAIBA from valine and thymine. While 3-HIB is solely a product of valine catabolism, BAIBA can be derived from breakdown of thymine or from further processing of 3-HIB through the short-lived methylmalonate semialdehyde (MMSA) intermediate. MMSA is further reduced to propionyl-CoA and eventually succinyl-CoA and entry into the TCA cycle (not pictured). The numbered enzymes are as follows: 1) DPYD, 2) DPYS 3) UPB1, 4) AGXT2/ABAT, 5) BCAT1/2, 6) BCDKH (complex), 7)ACAD, 8) HADHA, 9) HIBCH, 10) HIBADH, 11) ALDH6A1. *Note: while this reaction is reversible, it is heavily favored in the direction of producing MMSA.
Further testing showed that BAIBA alone (at low micromolar concentrations) was sufficient to increase the expression of UCP1 and CIDEA in cultured white adipocytes, without changing the expression of white-specific genes such as adiponectin. Mice given BAIBA in their drinking water for two weeks revealed higher levels of UCP1 and CIDEA in inguinal WAT depots, demonstrating in vivo activity of BAIBA. Furthermore, consistent with a putative metabolic protective role of “browning” of WAT31,32, mice given BAIBA had lower percent body fat and improved glucose tolerance. Using a combination of genetic knockouts and pharmacological inhibitors, Roberts et al, showed that BAIBA in part activates a PPARα-mediated mechanism in adipocytes, as well as in hepatocytes, where BAIBA increases β-oxidation (figure 3). Recent work suggests a role for AMPK downstream of BAIBA as well33. Altogether, these data indicate that a physiologically relevant endocrine signaling pathway exists that originates from the release of skeletal muscle BAIBA and affects WAT and liver metabolic function. Plasma concentrations of BAIBA are increased in exercised individuals, as well as those with relatively lower cardiometabolic risk factors, suggesting that BAIBA may contribute to metabolic health in humans.
Figure 3.
Skeletal Muscle secretes BAIBA and 3-HIB. PGC-1α, a key transcriptional coactivator in the skeletal muscle adaptation to exercise, increases production and secretion of BAIBA and 3-HIB. BAIBA induces β-oxidation in the liver and thermogenesis in adipocytes via PPARα-dependent mechanisms. 3-HIB induces fatty acid transport across the endothelial barrier and into skeletal muscle, and this increased lipid uptake promotes insulin resistance.
3-hydroxyisobutyrate induces transendothelial fatty acid uptake and transport
In light of the role of BAIBA described above, and that PGC-1α is largely thought of as beneficial to skeletal muscle metabolism, it is somewhat paradoxical that MCKA mice are in fact prone to insulin resistance34. Part of the answer to this paradox can be found in another metabolite heightened in the plasma and skeletal muscle of MCKα mice: 3-hydroxyisobutyrate (3-HIB)35. Jang et al. used a combination of column chromatography and tandem MS-MS to isolate and identify 3-HIB from PGC1α-overexpressing myotubes, similar to the case of BAIBA. 3-HIB is a catabolic product of valine, and unlike BAIBA, cannot also be derived from thymine (figure 2). Indeed, culturing cells in 13C-labeled valine led to complete labeling of 3HIB in media, demonstrating that 100% of 3-HIB is derived from valine.
3-HIB plays a completely different physiological role from that of BAIBA: it induces, in a paracrine fashion, the adjacent endothelium to transport fatty acids from blood to skeletal muscle. 3-HIB-treated endothelial cells demonstrated higher uptake of the fluorescent fatty acid analog BODIPY-FA in a dose-dependent manner, as well as increased trans-endothelial transport of long chain fatty acids. This activity could be competed out with unlabeled oleic acid and the fatty acid uptake inhibitor sulfo-succinimidyl oleate (SSO), and could be elicited only in endothelial cells, demonstrating significant specificity. To show the relevance of 3-HIB in vivo, luciferase-expressing mice were treated with 3-HIB and then assayed for uptake of a luciferin-tagged fatty acid molecule36, demonstrating large induction of fatty acid uptake in heart and skeletal muscle in response to 3-HIB. Together, these data demonstrate another physiologically relevant metabolite-mediated signaling pathway that originates from skeletal muscle, this one paracrine and involving the modulation of endothelial fatty acid transport (figure 3).
As noted above, 3-HIB is a catabolic product of valine, a branched-chain amino acid (BCAA). BCAAs have been implicated in the development of insulin resistance, most notably through strong epidemiological correlations between elevated BCAA levels and insulin resistance37–40 and rodent studies with supplementation of BCAAs in daily chow or genetic perturbation of BCAA metabolism41–44. However, the mechanism by which BCAAs contribute to insulin resistance in not understood. The discovery of 3-HIB's role in trans-endothelial fatty acid transport may now provide a mechanistic explanation: excess BCAAs lead to excess valine catabolism in skeletal muscle, in turn raising 3-HIB levels and thus fatty acid import into skeletal muscle, ultimately leading to intramuscular lipid accumulation. The accumulation of non-esterifed non-oxidized lipid intermediates in skeletal muscle is now well-established as a proximate cause of insulin resistance (although some controversy exists over which specific lipid species are the main cause)45–48. Consistent with this notion, mice given 3-HIB for two weeks had higher tri- and di-acylglycerols in their skeletal muscle, and had decreased glucose tolerance and impaired intracellular insulin signaling. In addition, diabetic mice (db/db genotype) and type II diabetic patients both have elevated levels of 3-HIB in their blood and skeletal muscle35. These findings also likely explain the seeming paradox that MCKA mice are insulin resistant: PGC-1α overexpression activates valine catabolism, increasing 3HIB and driving fatty acid influx, which in the absence of exercise accrues and causes lipotoxicity47,49. In contrast, in the presence of exercise, the muscle combusts the fat, and in fact MCKA mice have improved insulin sensitivity compared to non-transgenic controls after exercise50. The discovery of 3-HIB and its role in transendothelial fatty acid transport thus reveals novel connections between BCAA flux, intramuscular lipid deposition and insulin resistance. Targeting 3-HIB signaling may have therapeutic potential to treat intramuscular lipid accumulation and insulin resistance.
Despite the similarities of BAIBA and 3-HIB with regards to upstream regulation, pathway of production (Figure 2), physiological source, and chemical structure, their activities strikingly differ. BAIBA has no effect on endothelial transport of fatty acids, and 3-HIB has no effect on UCP1 expression in fat cells. One mechanistic explanation may lie in different receptors, but neither metabolite's method of signaling on target cells is yet known. Physiologically, whereas BAIBA promotes insulin sensitivity, 3-HIB seems to cause insulin resistance. However, in an important sense, these two molecules ultimately work toward the same goal of driving the usage of fatty acids as an energy source in different organs; perhaps even through linked pathways, as 3-HIB may be directing the transendothelial transport of fatty acids that were liberated from adipose depots by BAIBA. An imbalance in the production of the two molecules may underlie inappropriate physiology in insulin resistance, either by shunting 3HIB and BAIBA to different extents from the valine catabolism pathway, or by changes in thymine metabolism that will only affect BAIBA. These conjectures will require testing.
Conclusions
These examples of bioactive metabolites secreted by striated muscle are likely just the tip of the iceberg. Why have more such metabolites not been described? A number of hurdles exist to working with metabolites. First, assays are limited largely to bulk identification via mass spectrometry; immune-based assays such as Western blotting and assays with localization information like immunohistochemistry are not readily available. Second, whereas extracellular peptide-based factors typically interact with cognate receptors with dissociation constants (Kd) in the nanomolar range, metabolites commonly circulate in the high micromolar range and thus interact with receptors in the same range. These low-affinity interactions render capture-based assays, such as receptor purification, nearly impossible. Third, metabolites are usually more labile than peptides, often interconverting within seconds with other metabolites both intra- and extra-cellularly. Finding novel secreted metabolites is thus a challenging feat. However, the throughput and the precision of mass spectrometry technology is advancing at an unprecedented pace, rapidly increasing our ability to hunt down these elusive metabolites. Such discoveries may reveal new pathways that give us both insight into present diseases and new opportunities for pharmaceutical intervention.
Highlights.
Muscle secretes several metabolites with paracrine and endocrine signaling functions.
Skeletal muscle secretes adenosine to increase vasodilation.
Cardiomyocytes secrete cAMP which ultimately reduces cardiac hypertrophy and fibrosis.
Skeletal muscle secretes BAIBA to activate beta oxidation in liver and browning in adipose tissue.
Skeletal muscle secretes 3-HIB to induce the adjacent endothelium to transport fatty acids from blood into skeletal muscle.
Acknowledgments
Work described here was supported by grants from the National Institutes of Health (HL094499 DK107667 to Z.A.), and an Established Investigator Award the American Heart Association (Z.A.).
Footnotes
Present Address at: Department of Medicine and Cardiovascular Institute, Perelman School of Medicine, University of Pennsylvania.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cori CF, 1, Cori GT. Fate of Glucose and Other Sugars in the Eviscerated Animal. Exp Biol Med. 1929;26:432–432. [Google Scholar]
- 2.Cori CF, 1, Cori GT. Glycogen formation in the liver from d-and l-lactic acid. J Biol Chem. 1929;81:389–403. [Google Scholar]
- 3.Cori CF, 1, Cori GT. Carbohydrate Metabolism. Annu Rev Biochem. 1933;2:129–146. doi: 10.1146/annurev.bi.15.070146.001205. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, 1, et al. Lactate Inhibits Lipolysis in Fat Cells through Activation of an Orphan G-protein-coupled Receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 5.Boyd AE, 1, Giamber SR, Mager M, Lebovitz HE. Lactate inhibition of lipolysis in exercising man. Metabolism. 1974;23:531–542. doi: 10.1016/0026-0495(74)90081-x. [DOI] [PubMed] [Google Scholar]
- 6.Achten J, 1, Jeukendrup AE. Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities. Int J Sports Med. 2004;25:32–37. doi: 10.1055/s-2003-45231. [DOI] [PubMed] [Google Scholar]
- 7.Chang AJ, 1, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature. 2015;527:240–244. doi: 10.1038/nature15721. This provocative paper demonstrates that circulating lactate can signal through an olfactory receptor located in the carotid body to stimulate breathing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobson JG, 1, Rubio R, Berne RM. Role of adenine nucleotides, adenosine, and inorganic phosphate in the regulation of skeletal muscle blood flow. Circ Res. 1971;29:375–384. doi: 10.1161/01.res.29.4.375. [DOI] [PubMed] [Google Scholar]
- 9.Berne RM., 1 Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. This classic paper describes for the first time the idea that a metabolite, in this case adenosine, can be secreted from myocytes and impact signaling in nearby cells, in this case smooth muscle cells, to affect physiology, in this case vasodilation. [DOI] [PubMed] [Google Scholar]
- 10.Joyner MJ, 1, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95:549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard HJ, 1, Cotterrell D, Karim F. Appearance of adenosine in venous blood from thecontracting gracilis muscle and its role in vasodilatation in the dog. J Physiol. 1987;387:401–413. doi: 10.1113/jphysiol.1987.sp016580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall JM., 1 The roles of adenosine and related substances in exercise hyperaemia. J Physiol. 2007;583:835–845. doi: 10.1113/jphysiol.2007.136416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall JM, 1, Ray CJ. Contribution of non-endothelium-dependent substances to exercise hyperaemia: are they O(2) dependent? J Physiol. 2012;590:6307–6320. doi: 10.1113/jphysiol.2012.240721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen SP, 1, Saltin B. Regulation of the skeletal muscle blood flow in humans. Exp Physiol. 2014;99:1552–1558. doi: 10.1113/expphysiol.2014.081620. [DOI] [PubMed] [Google Scholar]
- 15.Hellsten Y, 1, Maclean D, Rådegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Sassi Y, 1, et al. Cardiac myocyte–secreted cAMP exerts paracrine action via adenosine receptor activation. J Clin Invest. 2014;124:5385–5397. doi: 10.1172/JCI74349. This paper describes a novel role for extracellular cAMP, secreted from cardiomyocytes, and affecting both adjacent fibroblasts in a paracrine fashion, and cardiac cells themselves in an autocrine fashion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sassi Y, 1, et al. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. 2012;26:1009–1017. doi: 10.1096/fj.11-194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiavegatti T, 1, Costa VL, Araújo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP–adenosine pathway. Br J Pharmacol. 2008;153:1331–1340. doi: 10.1038/sj.bjp.0707648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godinho RO, 1, Costa-Jr VL. Regulation of intracellular cyclic AMP in skeletal muscle cells involves the efflux of cyclic nucleotide to the extracellular compartment. Br J Pharmacol. 2003;138:995–1003. doi: 10.1038/sj.bjp.0705130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazelzadeh P, 1, et al. The Muscle Metabolome Differs between Healthy and Frail Older Adults. J Proteome Res. 2016;15:499–509. doi: 10.1021/acs.jproteome.5b00840. [DOI] [PubMed] [Google Scholar]
- 21.Mullen E, 1, Ohlendieck K. Proteomic profiling of non-obese type 2 diabetic skeletal muscle. Int J Mol Med. 2010;25:445–458. doi: 10.3892/ijmm_00000364. [DOI] [PubMed] [Google Scholar]
- 22.Roberts LD, 1, et al. β-Aminoisobutyric Acid Induces Browning of White Fat and Hepatic β-Oxidation and Is Inversely Correlated with Cardiometabolic Risk Factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. The authors describe the novel identification and characterization of BAIBA, a metabolite of valine or thymine, that is secreted from skeletal muscle and, in an endocrine fashion, affects lipolysis in adipose tissue and fatty acid oxidation in liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puigserver P, 1, et al. Activation of PPARγ Coactivator-1 Through Transcription Factor Docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 24.Baar K, 1, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 25.Arany Z, 1, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Finck BN, 1, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arany Z, 1, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 28.Chan MC, 1, Arany Z. The many roles of PGC-1α in muscle — recent developments. Metabolism. 2014;63:441–451. doi: 10.1016/j.metabol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michael LF, 1, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink K, 1, Cline RE, Henderson RB, Fink RM. Metabolism of thymine (methyl-C14 or - 2-C14) by rat liver in vitro. J Biol Chem. 1956;221:425–433. [PubMed] [Google Scholar]
- 31.Cao L, 1, et al. White to Brown Fat Phenotypic Switch Induced by Genetic and Environmental Activation of a Hypothalamic-Adipocyte Axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartelt A, 1, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 33.Shi CX, 1, et al. β-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep. 2016;6 doi: 10.1038/srep21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi CS, 1, et al. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang C, 1, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–426. doi: 10.1038/nm.4057. This paper outlines the discovery of a novel role for 3-HIB, an intermediate breakdown of valine, that is secreted from myocytes and in a paracrine fashion modulates fatty acid transport across adjacent endothelial cells, thereby regulating delivery of fatty acids to the myocytes, with consequences on fatty acid consumption and insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henkin AH, 1, et al. Real-Time Noninvasive Imaging of Fatty Acid Uptake in Vivo. ACS Chem Biol. 2012;7:1884–1891. doi: 10.1021/cb300194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felig P, 1, Marliss E, Cahill GF. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 38.Wang TJ, 1, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swerdlow DI., 1 Mendelian Randomization and Type 2 Diabetes. Cardiovasc Drugs Ther. 2016;30:51–57. doi: 10.1007/s10557-016-6638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lotta LA, 1, et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota D, 1, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 42.She P, 1, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon MS., 1 The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients. 2016;8 doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newgard CB., 1 Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodpaster BH, He J, Watkins S, Kelley DE., 1 Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 46.Chavez JA, Summers SA., 1 A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Szendroedi J, 1, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111:9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shulman GI., 1 Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N Engl J Med. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 49.Krssak M, 1, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 50.Summermatter S, 1, et al. PGC-1α Improves Glucose Homeostasis in Skeletal Muscle in an Activity-Dependent Manner. Diabetes. 2013;62:85–95. doi: 10.2337/db12-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]