Abstract
Chronic drug exposure is hypothesized to recruit negative reinforcement processes that increase the magnitude and alter the mechanisms of drug reinforcement. Candidate substrates of negative reinforcement include increased signaling via stress-related neurotransmitters such as corticotropin releasing factor (CRF, acting at CRF receptors) or dynorphin (acting at kappa opioid receptors) and/or decreased signaling via reward-related neurotransmitters such as dopamine. Determinants of drug reinforcement can be examined with choice procedures, in which subjects choose between a drug of interest (e.g. heroin or cocaine) and a non-drug alternative reinforcer (e.g. food). This review summarizes evidence collected from studies of drug choice in rhesus monkeys to address the negative reinforcement hypothesis. In monkeys choosing between heroin and food, chronic heroin exposure and subsequent withdrawal produces a robust increase in heroin choice. This withdrawal-associated increase in heroin choice is blocked by morphine and by other mu opioid agonists used to treat opioid use disorder (methadone, buprenorphine); however, withdrawal-associated increases in heroin choice are not reliably blocked by antagonists of CRF or kappa opioid receptors or by an indirect dopamine agonist. In monkeys choosing between cocaine and food, chronic cocaine exposure and withdrawal fail to increase cocaine choice or alter sensitivity of cocaine choice to treatment with candidate therapeutics including an indirect dopamine agonist and a kappa opioid receptor antagonist. These results support a role for negative reinforcement in self-administration of heroin but not cocaine. The constellation of neurobiological changes that constitutes the negative reinforcing stimulus in opioid-dependent rhesus monkeys remains to be determined.
Introduction: The rationale for studies of drug choice
Drugs of abuse produce reinforcing effects that contribute to their abuse potential. The expression, mechanisms, and modulation of drug reinforcement are commonly studied with drug self-administration procedures (Young and Herling, 1986; Ator and Griffiths, 2003). In these procedures, contingencies are established such that performance of some behavior (e.g. pressing a response lever) results in delivery of a drug dose. In the simplest drug self-administration procedures, the experimental environment contains only one operant manipulandum (e.g. only one response lever), only one behavior is monitored (e.g. pressing the lever), and the primary dependent variable is the rate of responding or drug delivery. A drug is considered to produce reinforcing effects and to function as a reinforcer if delivery of some drug dose maintains higher rates of self-administration than delivery of vehicle. Once self-administration of a given drug is established, then effects of other experimental manipulations on drug self-administration can be evaluated. However, interpretation of effects produced by these manipulations can be challenging, because the primary dependent measure (i.e. rate of drug self-administration) conflates multiple effects of both the self-administered drug and of any manipulation that might modify drug self-administration (Katz, 1989; Negus and Banks, 2011). As one example, drug self-administration dose-effect curves under simple schedules of reinforcement typically display inverted-U shaped dose-effect curves, such that peak rates of drug self-administration are maintained by intermediate drug doses. The decrease in self-administration rates that occurs at higher drug doses could reflect either a decline in reinforcing effects or the recruitment of non-specific behavioral effects (e.g. sedation or stereotypies) that disrupt behavior and impede the subject’s ability to respond. Similarly, an experimental manipulation could decrease drug self-administration either by decreasing the drug’s reinforcing effects, enhancing the drug’s non-specific behavioral effects, or producing its own non-specific behavioral effects. A key challenge in drug self-administration research is dissociation of effects produced by experimental manipulations on drug reinforcement relative to effects on general behavioral competence.
Drug choice procedures provide one approach to mitigating the impact of these interpretive challenges (Negus and Banks, 2011; Banks and Negus, 2012; Banks et al., 2015; Banks and Negus, 2016). In drug choice procedures, the experimental environment typically includes at least two operant manipulanda (e.g. two response levers), and contingencies are established such that responding on one manipulandum produces drug delivery, whereas responding on the second manipulandum produces a non-drug alternative reinforcer, such as food in laboratory animals or money in humans. Although overall rates of responding and reinforcement are measured in choice procedures, the primary dependent variable is not the rate of behavior, but rather the allocation of behavior to the drug and alternative options [e.g. expressed as % Drug Choice = # Drug Choices/Total # of both Drug and Alternative Choices) x 100]. This primary dependent variable provides a measure of a drug’s relative reinforcing efficacy in comparison to the alternative, and this measure is relatively independent of non-specific behavioral effects. For example, drug self-administration dose-effect curves in choice procedures typically show a monotonic increase in % Drug Choice as a function of increasing drug dose. Moreover, manipulations that selectively decrease drug reinforcement reduce % Drug Choice but do not necessarily affect the total number of completed choices (because subjects not only decrease drug choice, but also reallocate their behavior to increase choice of the non-drug alternative). Conversely, manipulations that produce non-selective behavioral disruption decrease the total number of completed choices without necessarily altering % Drug Choice for choices that are completed. In addition to these distinctions with regard to data interpretation, the preclinical use of choice procedures may also promote fidelity of preclinical-to-clinical translation of research results, because human laboratory studies also commonly use choice procedures to assess drug reinforcement, and because drug abuse occurs in settings where both drug and non-drug reinforcers are available (Haney and Spealman, 2008; Huskinson et al., 2015; Czoty et al., 2016).
One application of choice procedures has been to study the impact of chronic drug exposure and subsequent drug withdrawal on drug reinforcement (Negus, 2006; Negus and Rice, 2009; Banks and Negus, 2010). It has been argued that chronic drug exposure and withdrawal produces a “motivational withdrawal syndrome” that augments drug reinforcement and serves as “one of the driving factors for compulsivity in addiction” (Koob and Mason, 2016). Choice procedures provide a useful experimental tool to evaluate this hypothesis, its underlying mechanisms, and its implications for treatment development.
“The Dark Side of Addiction” as a heuristic framework
A prominent contemporary theory of drug addiction posits the existence of two interacting mechanisms of “positive” and “negative” reinforcement that evolve with increasing levels of drug exposure (Koob and Le Moal, 2008). In subjects with relatively limited drug exposure, the reinforcing effects of drugs are thought to be mediated primarily by increased dopamine signaling in mesolimbic dopamine projections from ventral tegmental area to nucleus accumbens. Increased endogenous opioid signaling in this system may also contribute to drug reinforcement, independent of dopamine, for some drugs. Drug reinforcement in subjects with limited drug exposure is often described as “positive reinforcement,” because drug delivery increases both the magnitude of drug stimulus presented to the subject and the level of signaling by the presumed neural substrate within the subject (mesolimbic dopamine/opioid systems). (See inset for commentary on use of the terms “positive” and “negative” reinforcement in relation to drug self-administration.)
Positive and Negative Reinforcement in Drug Self-Administration.
1A “reinforcer” is operationally defined as any stimulus that increases the probability of behavior preceding its delivery. A stimulus is considered to be a “positive reinforcer” or a “negative reinforcer” depending on whether its presentation or its removal, respectively, increases the probability of preceding behavior. For example, operant behavior in laboratory animals is often maintained by food delivery. In this context, food is defined as a positive reinforcer, because behavior by the animal results in food presentation. By contrast, operant behavior is also sometimes maintained by termination of electric shock. Here, shock is defined as a negative reinforcer, because behavior by the animal results in shock removal. In using this terminology, the qualifiers “positive” and “negative” are most precisely applied to external stimuli under the direct control of the investigator (e.g. food or shock in the examples above). The terminology is less precise and is potentially misleading when it is used to refer to the internal state of the organism as the source for the stimulus. For example, regimens of food deprivation can elicit physiological and neurobiological responses inside an organism, produce the subjective state of “hunger” (at least in humans), and increase the reinforcing effectiveness of food presentation. However, even when food reinforcement maintains responding in a food-deprived subject, we typically refer to food delivery as a positive reinforcer rather than to the food-deprivation state and its neurobiological correlates as a negative reinforcer that can be terminated by responding for and delivery of food.
In drug self-administration procedures, behavior by the subject results in delivery of a drug dose, and as a result, drugs that increase responding leading to their presentation are functioning as “positive reinforcers.” When the concept of “negative reinforcement” is invoked, as in the heuristic framework described as “the dark side of addiction,” the negative reinforcer of interest is not the drug, but rather a hypothetical internal state. However, to qualify as a negative reinforcer, this internal state must meet at least five criteria. First, this state should be defined as a physiological stimulus originating inside the organism. For example, the stimulus could be defined as a specific pattern of activity in specific neural substrates (e.g. increases or decreases in specific neurotransmitter levels in specific regions of the central nervous system). Second, this stimulus should be relatively quiescent in drug-naïve subjects and activated by drug exposure. As a corollary to this point, the stimulus is presumed to have a slower onset and longer duration than brain levels of either drug or drug-induced increases in dopamine/opioid release. This permits cumulative and sustained increases in stimulus magnitude with repeated drug exposures. Third, the stimulus should be at least transiently terminated by renewed drug delivery. Importantly, this suggests that the self-administered drug has a biphasic impact on the negative reinforcing stimulus, producing an initial decrease in its intensity followed by a later increase in its intensity. Fourth, the initial, transient termination of this stimulus should be sufficient to reinforce behavior. Lastly, the initial, transient termination of this stimulus should also be necessary for full expression of drug reinforcement in drug-dependent subjects.
To illustrate research implications of these criteria, consider the hypothesis that increased release of hormone X is a candidate negative reinforcer for maintaining heroin self-administration in heroin-dependent subjects. Supportive experiments might generate the following sorts of data.
-
1)
X levels should be relatively low in heroin-naive subjects.
-
2)
X levels should increase with increasing heroin exposure. Heroin-induced increases in X should have slower onset and longer duration than brain levels of either heroin or of heroin-induced increases in dopamine/opioid levels.
-
3)
During withdrawal in heroin-dependent subjects, levels of X should be high, and re-administration of heroin should transiently reduce levels of X.
-
4a)
In heroin-naive subjects, direct administration of X (e.g. by programmed infusion) should function as a negative reinforcer, such that responding to terminate or delay X administration, or to administer an antagonist of X, maintains responding.
-
4b)
During withdrawal in heroin-dependent subjects, antagonists of X should maintain responding.
-
5a)
During withdrawal in heroin-dependent subjects, antagonists of X should reduce heroin self-administration.
-
5b)
During withdrawal in heroin-dependent subjects, heroin self-administration should decline or extinguish if heroin fails to reduce X.
A goal of the heuristic framework is to identify candidate neural systems that meet these criteria and that might serve as targets for substance use disorder treatments. Choice procedures offer an experimental tool to address the last two of these criteria.
More extensive regimens of drug exposure are proposed to alter the state of the subject and thereby alter the mechanisms and increase the magnitude of drug reinforcement. Specifically, extended drug exposure has been shown to (1) decrease basal activity of dopamine and/or opioid reward systems, and (2) recruit activation of other neural systems, sometimes described as “stress” or “anti-reward” systems, that use neurotransmitters including corticotropic releasing factor (CRF) and dynorphin (Koob and Le Moal, 2008; Koob and Mason, 2016). Together, these two processes of decreased reward system function and enhanced stress system function are hypothesized to have three key characteristics. First, their expression occurs with a slower onset and longer duration than the initial stimulation of mesolimbic dopamine/opioid signaling, and as a result of their longer duration of action, their expression can accumulate with repeated drug exposure. Second, they are hypothesized to mediate negative and aversive affective states that occur during drug withdrawal. Lastly, this aversive state is hypothesized to be temporarily reversible by re-exposure to the abused drug, either because the drug transiently increases depressed dopamine/opioid signaling, reduces stress hormone signaling, or both. Overall, then, consumption of any single dose of abused drug is envisioned to produce an initial increase in dopamine/opioid signaling as drug levels rise and peak, followed by a longer period of depressed dopamine/opioid signaling and enhanced stress hormone signaling that together mediate negative affective states of drug withdrawal. Increasingly intensive regimens of drug exposure are hypothesized to produce a cumulative increase in the latter effects and increasingly intense aversive subjective states. Under these circumstances, drug-induced reinforcing effects are often described as “negative,” because the drug is now hypothesized to produce its reinforcing effects not by increasing dopamine/opioid signaling from a normal basal level, but rather by alleviating the aversive state produced by a depressed reward system and activated stress system. Drug self-administration maintained by putative negative reinforcement has been called “the dark side of addiction.”
Evidence to support this hypothetical transition from positive to negative reinforcement comes in part from preclinical studies that have used single-operant drug self-administration procedures in which the primary dependent measure is the rate of drug self-administration. Two observations have been especially seminal. First, the recruitment of negative reinforcement processes that occurs with extended drug exposure is hypothesized not only to modify the mechanisms of drug reinforcement, but also to increase its magnitude (i.e. by summing positive and negative reinforcement mechanisms). In support of this hypothesis, regimens of “extended access” (produced by increasing the number of hours per day that subjects can self-administer drug) have been shown to increase rates of self-administration, a phenomenon that has been labeled “escalation” (Koob and Kreek, 2007). Second, the recruitment of these negative reinforcement processes is also hypothesized to render drug self-administration sensitive to manipulations that attenuate stress system signaling. For example, as noted above, CRF acting at CRF1 receptors and dynorphin acting at kappa opioid receptors are two stress-related neurotransmitters implicated in negative reinforcement processes, and both CRF1 receptor antagonists and kappa opioid receptor antagonists were found to decrease escalated self-administration across several drug classes in rats with extended drug access (Koob and Mason, 2016). An implication of these findings has been that CRF1 antagonists, kappa antagonists, or other treatments that reduce stress system signaling might be useful as medications to treat drug addiction.
Despite the appeal of this hypothesis and the accumulation of some data in its favor, several issues remain to be addressed. Three will be mentioned here. First, behavioral data have been collected using single-operant drug self-administration procedures in which the primary dependent variable (i.e. rate of drug self-administration) conflates both reinforcing effects and other behavioral effects produced by the self-administered drug. Consequently, the observation of escalated drug self-administration during extended drug access may reflect an increase in drug reinforcement, but it could also reflect other effects, such as tolerance to non-specific behavioral effects that initially limit responding maintained by the self-administered drug (Zernig et al., 2007). Other explanations for the escalation phenomenon have also been proposed (Goeders et al., 2009; Beckmann et al., 2012). Second, while escalation occurs for self-administration of many abused drugs including both mu opioid agonists (e.g. heroin) and monoamine transporter ligands (e.g. cocaine and methamphetamine), clinical data suggest very different roles for withdrawal as a determinant of abuse for different drugs. For example, physical signs of withdrawal are weaker for cocaine than for mu opioids, expression of withdrawal signs is less likely to be exhibited by patients who meet criteria for cocaine than opioid use disorder, and withdrawal relief is less likely to be endorsed as a reason for continued use of cocaine than opioids (Wu et al., 2009). Third, the effectiveness of some treatments to reduce escalated drug self-administration has not always translated well to clinical studies. For example, CRF1 receptor antagonists have reduced escalated ethanol self-administration in rats (Koob and Kreek, 2007), but the CRF1 receptor antagonist verucerfont failed to produce therapeutically promising effects in ethanol-dependent women despite evidence for CRF1 receptor target engagement (Schwandt et al., 2016). Similarly, both selective kappa opioid receptor antagonists and combinations of buprenorphine+naltrexone (intended to produce kappa receptor antagonism) were effective to block escalated cocaine self-administration in rats (Wee et al., 2012; Koob and Mason, 2016), but a similar buprenorphine+naltrexone combination in humans failed to reduce primary measures of cocaine use in a large clinical trial (Ling et al., 2016).
Choice procedures provide one experimental tool that can be used to address these issues. In particular, the heuristic framework described as “the dark side of addiction” makes two explicit predictions regarding effects of chronic drug exposure and subsequent drug withdrawal on choice between a self-administered drug and an alternative non-drug reinforcer. First, regimens of drug exposure and withdrawal should increase drug reinforcement and thereby increase drug choice. Second, drugs that block stress system signaling hypothesized to mediate aversive withdrawal states should also block withdrawal-associated increases in drug choice. The remainder of this review will evaluate the degree to which these predictions are realized in studies that have examined the effects of extended drug access and drug withdrawal on choice between heroin and food (Negus, 2006; Negus and Rice, 2009) or cocaine and food (Banks and Negus, 2010; Banks et al., 2013a; Hutsell et al., 2016a; Hutsell et al., 2016b) by rhesus monkeys.
Effects of Opioid Exposure and Withdrawal on Heroin Choice
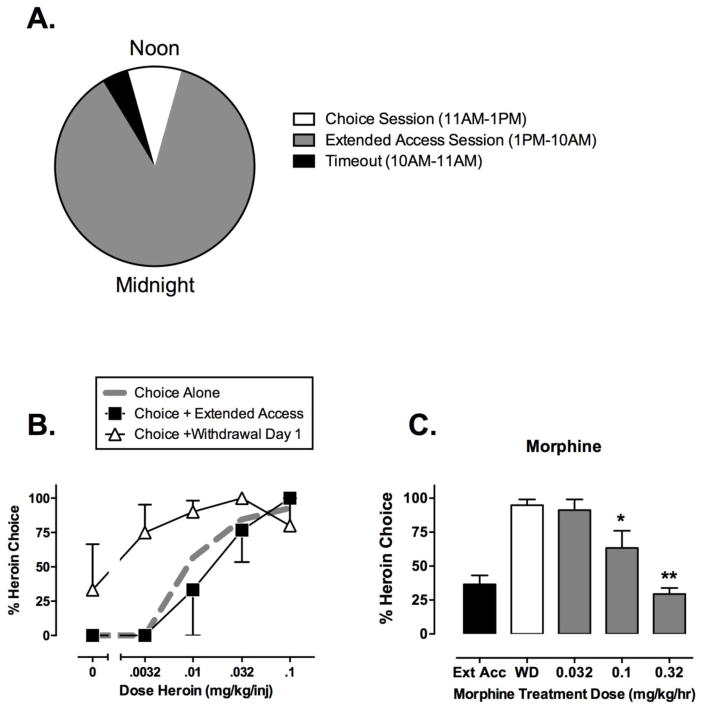
In the first laboratory-animal drug self-administration study ever published, morphine-dependent chimpanzees were afforded the opportunity to choose between subcutaneous morphine injections and bananas, and morphine withdrawal was found to increase morphine choice and decrease banana choice (Spragg, 1940). More recent studies have examined this phenomenon in rhesus monkeys equipped with chronic intravenous catheters and trained under a concurrent schedule of reinforcement for intravenous heroin injections and banana-flavored food pellets (Negus, 2006; Negus and Rice, 2009). Figure 1 shows a diagram of the experimental design along with selected results. Briefly, choice was evaluated during 2-hr behavioral sessions conducted daily from 11am to 1pm. Each daily session was composed of 5 20-min components separated by 5-min time-out periods, and during each component, subjects could press one response key to earn heroin injections or a separate response key to earn food pellets. The available heroin dose increased across components (0, 0.0032, 0.01, 0.032 and 0.1 mg/kg/injection, respectively), and a maximum of 10 total reinforcers was available during each component. This approach permitted determination of heroin choice dose-effect curves each day under various experimental conditions.
Figure 1. Illustrative data from studies of heroin vs. food choice in rhesus monkeys.
Panel A shows the timing of daily choice and extended-access sessions. During choice sessions (11am–1pm), subjects could choose between food and increasing heroin doses. During extended-access sessions (1pm-10am), food was not available, but subjects could respond for 0.1 mg/kg/injection heroin. During time-out periods, neither food nor heroin was available. Panel B shows heroin choice dose-effect curves when heroin was available only during daily choice sessions (“Choice Alone”), during both choice sessions and extended-access sessions (“Choice + Extended Access”), or on the first day after termination of extended-access sessions (“Choice + Withdrawal Day 1). Abscissa: Unit heroin dose in mg/kg/injection, log scale. Ordinate: Percent of total choices allocated to heroin choice during each component of the choice session. Panel C shows effects of morphine treatment on withdrawal-associated increases in heroin choice over the entire choice session. Abscissa: Treatment conditions during extended heroin access (Ext Acc), after 1 day of withdrawal from extended heroin access (WD), or after 1 day of withdrawal during which subjects were also treated with continuous infusions of morphine (dose in mg/kg/hr). Ordinate: Percent of total choices allocated to heroin choice for the entire choice session. Asterisks indicate significantly different from WD (p<0.05). All points show mean ± SEM from three (B) or four (C) monkeys. Data adapted from Negus, 2006; Negus and Rice, 2009.
Initially, heroin was available only during daily choice sessions, and Figure 1B shows the resulting heroin choice dose-effect curve (“Choice Alone,” dotted gray line). Under these conditions, heroin maintained a dose-dependent increase in heroin choice. When low heroin doses were available (0, 0.0032 mg/kg/injection), monkeys allocated all of their responding to the food key, and % Heroin Choice was low. As heroin dose increased across components, monkeys reallocated their behavior to the heroin-associated key, and % Heroin Choice increased to nearly 100% at the highest heroin dose. Daily heroin intakes ranged between 0.5 to 1.0 mg/kg/day, and notably, these subjects did not show signs of opioid withdrawal if heroin access was interrupted, suggesting that these monkeys were not opioid dependent.
During the next phase of the study, daily choice sessions continued, and an additional 21-hr “extended-access” session of heroin self-administration was added each day (1pm to 10am the next morning) for at least seven consecutive days. During extended-access sessions, food was not available, but monkeys could respond on the drug-associated key to earn additional injections of 0.1 mg/kg/injection heroin, and a 15-min time-out period followed each injection. Under these conditions, total daily heroin intake increased to approximately 5.0 mg/kg/day. As shown in Figure 1B (“Choice + Extended Access,” filled squares), this increase in total heroin intake had little effect on the heroin choice dose-effect curve, suggesting little change in the relative reinforcing strength of heroin during the choice session. However, Figure 1B also shows that termination of extended access produced a robust increase in heroin choice (“Choice + Withdrawal Day 1,” open triangles). Termination of extended access also produced general behavioral disruption (expressed as a decrease in the total number of all choices completed) and the emergence of other opioid-withdrawal signs (e.g. unusual postures and vocalizations). All of these withdrawal effects peaked on the first day of withdrawal and gradually subsided over the course of the next seven days. Similar expression of somatic withdrawal signs and withdrawal-associated increases in heroin choice were observed after exposure to and withdrawal from noncontingent opioid agonist delivery (Negus, 2006), suggesting that contingent opioid self-administration during extended-access sessions was not necessary to observe withdrawal-associated increases in heroin choice.
These results from a heroin-vs.-food choice procedure in rhesus monkeys agree with findings from other choice procedures in nonhuman primates (Spragg, 1940; Griffiths et al., 1975) and rats (Lenoir et al., 2013), and opioid withdrawal also increased breakpoints maintained by opioid agonist self-administration under progressive-ratio schedules in rhesus monkeys and rats (Yanagita, 1978; Carrera et al., 1999). Together these results provide evidence to support the hypothesis that opioid exposure and subsequent opioid withdrawal can increase the reinforcing strength of heroin in a manner consistent with the negative-reinforcement hypothesis. Additionally, Figure 1C shows that morphine administered by continuous infusion during the withdrawal period dose-dependently and completely prevented withdrawal-associated increases in heroin choice (Negus and Rice, 2009). Morphine also blocked behavioral disruption and expression of other heroin-withdrawal signs. Methadone and buprenorphine, which are used clinically as maintenance medications to treat opioid use disorder, produced similar effects, and consistent with methadone’s higher efficacy at mu opioid receptors, methadone produced more complete and reliable effects than buprenorphine (Negus, 2006). Conversely, the mu opioid antagonist naltrexone effectively precipitated withdrawal-associated increases in heroin choice, behavioral disruption, and other withdrawal signs in heroin-dependent monkeys (Negus, 2009). Together, these results confirm the sensitivity of withdrawal-associated increases in heroin choice to mu agonist medications and support the general notion that mu agonist withdrawal may serve as a negative reinforcer that can be effectively terminated by opioid self-administration.
Subsequent studies then examined effects of drugs targeting candidate neural substrates that might mediate the negative reinforcing stimulus of opioid withdrawal (Negus and Rice, 2009). Specifically, four drugs were tested: (1) antalarmin: a CRF1 receptor antagonist to block potentially enhanced CRF signaling, (2) 5’-guanidinonaltrindole (GNTI): a kappa opioid receptor antagonist to block potentially enhanced dynorphin signaling, (3) clonidine: an alpha-2 noradrenergic receptor agonist that binds noradrenergic autoreceptors to reduce noradrenergic signaling, and (4) amphetamine: a substrate at dopamine and norepinephrine transporters to increase potentially depressed mesolimbic dopamine levels. None of these drugs was effective to recapitulate the effects of mu agonists and reduce withdrawal-associated increases in heroin choice. In one of three monkeys tested, antalarmin dose-dependently decreased both physical withdrawal signs and withdrawal-associated decreases in heroin choice; however, in the other two monkeys, antalarmin produced only general behavioral disruption without altering the allocation of residual behavior between heroin and food choices. GNTI, clonidine, and amphetamine produced similar effects of behavioral disruption without producing systematic or reliable changes in heroin choice. Taken together, these results fail to support a major role either for enhanced CRF, dynorphin, or norepinephrine signaling, or for depressed dopamine signaling, in mediating the negative reinforcing effects of heroin withdrawal. Additionally, these results fail to support utility of these particular drugs as non-mu opioid medications for treatment of opioid use disorder. Several caveats to these conclusions warrant mention. First, these results were collected in a relatively small number of subjects, and antalarmin was effective in one of three monkeys tested, so CRF signaling was implicated as a potential mediator of negative reinforcement in at least this monkey. The basis of individual differences in treatment effectiveness warrants further study. Second, amphetamine increases both dopamine and norepinephrine levels, and enhanced norepinephrine levels may have obscured effects that might have been produced by enhanced dopamine. It would be of interest to study more selective dopaminergic drugs, especially insofar as amphetamine and cocaine alleviate the discriminative stimulus effects of opioid withdrawal in rhesus monkeys (Sell and France, 2002), and some signs of opioid withdrawal are attenuated by cocaine in humans (Kosten and Kosten, 1989; Rosen et al., 1992). Lastly, it is possible that negative reinforcing effects of mu opioid withdrawal are mediated by multiple neural substrates that would require intervention with multiple drugs.
Overall, these results with mu opioid receptor agonists suggest the following major conclusions related the negative-reinforcement hypothesis. (1) Mu opioid agonist withdrawal in dependent subjects can increase the relative reinforcing effectiveness of opioid agonists. (2) Neither enhanced CRF, dynorphin, or norepinephrine signaling, nor depressed dopamine signaling, is sufficient to serve as the internal negative reinforcing stimulus that mediates effects of opioid withdrawal. (3) These results illustrate one preclinical strategy for use of choice procedures to investigate mechanisms that contribute to the positive reinforcing effects of drugs and the putative negative reinforcing effects of aversive and drug-reversible internal states during drug withdrawal. Of particular importance, choice procedures permit a dissociation of the effects of drug exposure, drug withdrawal, and treatment with candidate therapeutics on measures of relative drug reinforcement (quantified by measures of behavioral choice between drug and food choices) and general behavioral competence (quantified by measures of overall behavioral rate). Only mu opioid agonists blocked withdrawal-induced increases in heroin choice, behavioral disruption, and expression of other withdrawal signs. Other medications failed to reliably block withdrawal-induced increases in heroin choice or withdrawal signs, and they often exacerbated withdrawal-induced behavioral disruption.
Effects of Cocaine Exposure and Withdrawal on Cocaine Choice
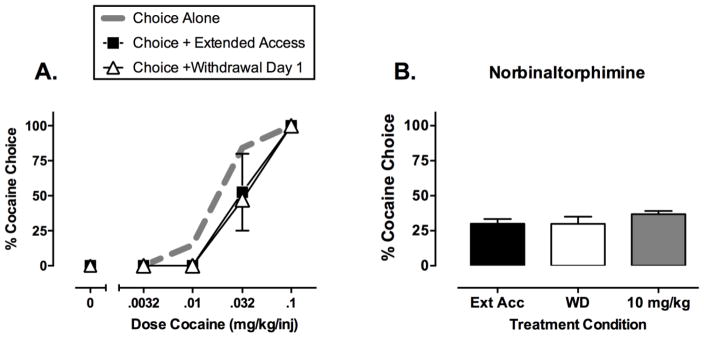
Similar studies have been conducted to evaluate effects of drug exposure, drug withdrawal, and treatment with candidate medications on cocaine vs. food choice (Banks and Negus, 2010; Banks et al., 2013a; Hutsell et al., 2016a; Hutsell et al., 2016b). As with the heroin choice studies, daily behavioral sessions lasted 2 hr and consisted of five components, during which rhesus monkeys could choose between increasing doses of intravenous cocaine (0, 0.0032, 0.01, 0.032, 0.1 mg/kg/injection) and food. When cocaine was available only during daily 2-hr choice sessions, cocaine maintained a dose-dependent increase in % Cocaine Choice (Figure 2A, “Choice Alone,” dotted gray line). Under these conditions, total daily cocaine intake was approximately 1.2 mg/kg/day. When extended-access sessions were introduced (21-hr sessions during which 0.1 mg/kg/injection cocaine was available with a 15-min time-out period after each injection), total daily cocaine intake increased to approximately 6 mg/kg/day, but there was no significant change in the cocaine choice dose-effect curve (Figure 2A, “Choice + Extended Access,” filled squares). Lastly, and most importantly, termination of extended cocaine access failed to increase % Cocaine Choice (Figure 2A, “Choice + Withdrawal Day 1,” open triangles). Withdrawal from this regimen of extended cocaine access also failed to increase % Cocaine Choice at later times of withdrawal up to 1 week, and withdrawal from other regimens of extended cocaine access also failed to increase % Cocaine Choice, even when daily cocaine intakes increased to more than 11 mg/kg/day. Cocaine withdrawal has also failed to increase cocaine choice in rats (Lenoir et al., 2007) and breakpoints maintained by cocaine self-administration in rhesus monkeys and rats responding under progressive-ratio schedules (Yanagita, 1978; Li et al., 1994; Liu et al., 2005; Czoty et al., 2006). These results highlight a key difference between the effects of drug withdrawal on the reinforcing effects of mu opioid agonists and cocaine. Specifically, mu opioid withdrawal clearly increases the relative reinforcing effects of mu opioid agonists in a manner consistent with the hypothesis that withdrawal functions as a negative reinforcer. Conversely, the failure of cocaine withdrawal to increase cocaine reinforcement suggests that cocaine withdrawal does not function as a negative reinforcer.
Figure 2. Illustrative data from studies of cocaine vs. food choice in rhesus monkeys.
Panel A shows cocaine choice dose-effect curves when cocaine was available only during daily choice sessions (“Choice Alone”), during both choice sessions and extended-access sessions (“Choice + Extended Access”), or the first day after termination of extended-access sessions (“Choice + Withdrawal Day 1). Abscissa: Unit cocaine dose in mg/kg/injection, log scale. Ordinate: Percent of total choices allocated to cocaine choice during each component of the choice session. Panel B shows effects of treatment with the kappa opioid receptor antagonist norbinaltorphimine on cocaine choice during withdrawal from extended cocaine access. Abscissa: treatment conditions during extended cocaine access (Ext Acc), after 1 day of withdrawal from extended cocaine access (WD), or after 1 day of withdrawal from extended cocaine access during which subjects were also treated with 10 mg/kg intramuscular norbinaltorphimine. Ordinate: Percent of total choices allocated to heroin choice for the entire choice session. All points show mean ± SEM from four monkeys. Data adapted from Banks and Negus, 2010; Hutsell et al., 2016a.
The failure of cocaine withdrawal to alter cocaine vs. food choice in our studies does not result from a general insensitivity of cocaine choice to any experimental manipulation. We and others have described numerous environmental and pharmacological manipulations that can either increase or decrease cocaine choice (for reviews, see Negus and Banks, 2011; Banks and Negus, 2012). For example, cocaine choice can be increased by reducing the price for cocaine delivery (i.e. the ratio requirement), combining cocaine with an additional reinforcer, or chronically treating subjects with a kappa opioid receptor agonist or dopamine receptor antagonist (Negus, 2003; Negus, 2004; Negus, 2005). Thus, withdrawal from extended cocaine access is less effective than many other manipulations to alter cocaine vs. food choice.
Although cocaine withdrawal does not increase the reinforcing effects of cocaine, it remains possible that chronic cocaine exposure and withdrawal may alter the underlying neurobiological mechanisms of cocaine reinforcement. However, existing data from drug choice procedures do not support this possibility. For example, maintenance on the monoamine releaser prodrug phendimetrazine decreases cocaine choice in both the absence and presence of extended cocaine access (Banks et al., 2013a; Banks et al., 2013b), whereas the kappa opioid receptor antagonist norbinaltorphimine fails to alter cocaine choice in both the absence and presence of extended cocaine access (Negus, 2004; Hutsell et al., 2016a). Figure 2B illustrates the results of one study with norbinaltorphimine. In this study, monkeys initially had access to cocaine during both choice and extended-access sessions. Withdrawal from extended access did not increase % Cocaine Choice, and administration of norbinaltorphimine during withdrawal failed to decrease % Cocaine Choice. Effects of CRF antagonists or other medications targeting candidate negative-reinforcement substrates on cocaine choice have not been examined; however, neither acute nor chronic administration of the CRF1 antagonist antalarmin decreased cocaine self-administration in rhesus monkeys responding under a multiple schedule of sequential access to cocaine and food reinforcement (Mello et al., 2006).
In summary, data from cocaine vs. food choice studies fail to reveal withdrawal-associated increases in cocaine reinforcement or changes in the sensitivity of cocaine choice to treatment with medications targeting candidate negative-reinforcement substrates. As such, these results do not support the negative reinforcement hypothesis. Importantly, these results leave open the possibility that regimens of cocaine exposure could activate stress systems, desensitize reward systems, or produce physical dependence as indicated by the emergence of physiological or behavioral abstinence signs during cocaine withdrawal. However, any such effects of chronic cocaine treatment do not appear to increase the magnitude or alter the mechanisms of cocaine reinforcement, suggesting that treatment of cocaine withdrawal signs may not reduce cocaine reinforcement in laboratory studies or cocaine use by patients with cocaine use disorder.
Conclusions and Future Directions
Data reviewed here support a role for negative reinforcement in self-administration of heroin but not cocaine. With regard to heroin, extended-access to heroin self-administration followed by heroin withdrawal produced a robust increase in heroin-vs.-food choice that could be blocked by mu opioid agonists but not by medications targeting candidate negative reinforcement substrates related to enhanced stress system function or decreased dopamine reward system function. These findings suggest that none of these candidate substrates is sufficient to mediate negative reinforcing effects of opioid withdrawal. With regard to cocaine, extended-access to cocaine self-administration followed by cocaine withdrawal failed to increase cocaine-vs.-food choice or alter sensitivity of cocaine choice to treatment drugs that have been tested to date.
Results from heroin-vs.-food choice studies do not rule out a role for altered stress or reward system activity in the negative reinforcing effects of opioid withdrawal. In particular, the CRF1 receptor antagonist antalarmin completely blocked withdrawal-associated increases in heroin choice in one of three monkeys, and this finding supports the hypothesis that antalarmin or other CRF1 receptor antagonists may be useful as a non-opioid treatment for opioid use disorder in some subjects. The expression and mechanisms of such individual variability warrant further study, and genetic differences such as polymorphisms in the CRF receptor gene may be one contributing factor (Treutlein et al., 2006). More generally, it is possible that negative reinforcing effects of opioid withdrawal observed here are mediated by changes in other neural systems or require simultaneous changes in multiple systems. The constellation of neurobiological changes that constitutes the negative reinforcing stimulus of opioid withdrawal in opioid-dependent rhesus monkeys remains to be determined; however, it is clear that mu agonist treatment was sufficient to completely block withdrawal-associated increases in heroin choice.
In studies of cocaine-vs.-food choice, the failure of cocaine withdrawal to increase cocaine choice does not imply an absence of physiological or behavioral abstinence signs during cocaine withdrawal. Although abstinence signs are milder for withdrawal from cocaine than from opioids or some other classes of abused drugs, cocaine withdrawal is associated with subtle but significant abstinence signs in animals (Fung and Richard, 1994; Markou et al., 1992) and humans (Foltin and Fischman, 1997; Gawin and Kleber, 1986; Walsh et al., 2009). However, results of the present study suggest that these signs do not function as a negative reinforcing stimulus in rhesus monkeys. This conclusion from preclinical studies is consistent with conclusions from clinical studies that have examined the contribution of different diagnostic criteria to cocaine use disorder (Bryant et al., 1991; Wu et al, 2009). For example, one study of patients with cocaine use disorder concluded that “Thoughts about obtaining and using cocaine seem to be linked more closely with achieving and sustaining positive reinforcement than with the perceived need to relieve withdrawal symptoms” (Bryant et al., 1991). An implication of these findings is that medications development for cocaine use disorder many not benefit from research on putative negative reinforcing effects of cocaine withdrawal.
Several aspects of these results using a choice procedure in rhesus monkeys differ from results that have been obtained on measures of escalated drug self-administration in rats (Koob and Mason, 2016). First, extended access to and withdrawal from cocaine fails to increase cocaine choice in monkeys but does produce escalation of cocaine self-administration in rats. Second, both CRF and kappa opioid receptor antagonists fail to reliably alter heroin or cocaine choice in monkeys but do reduce escalated heroin or cocaine self-administration in rats. The reasons for these discrepancies remain to be determined and may involve procedural differences in experimental design or engagement of negative reinforcement substrates by chronic drug exposure. Regardless of the reason, the distinction has implications for preclinical testing of candidate medications. In our view, escalated drug self-administration in single-operant drug self-administration procedures can be influenced by a host of factors independent of drug reinforcement (see above), and as a result, effects of test treatments on escalated drug self-administration are difficult to interpret. Drug-vs.-food choice procedures provide an alternative measure of drug reinforcement (% Drug Choice) expressed as behavioral allocation rather than behavioral rate. Measures of drug choice can be influenced by factors other than changes in drug reinforcement; for example, drug-vs.-food choice can be increased not only by increases in reinforcing effects of the drug, but also by decreases in the reinforcing effectiveness of food (Negus and Banks, 2011; Banks and Negus, 2012; Banks et al., 2015; Banks and Negus, 2016). Nonetheless, the expression and modulation of withdrawal-associated increases in drug choice appear to translate more reliably to human studies and clinical trials than expression and modulation of escalated drug self-administration (Czoty et al., 2016). Recent disappointing clinical results with the CRF1 antagonist verucerfont in ethanol-dependent patients and with buprenorphine/naloxone+naltrexone in cocaine-dependent patients may provide further support for this proposition (Ling et al., 2016; Schwandt et al., 2016); however, more extensive translational research with other treatments will be useful to clarify optimal preclinical strategies for testing candidate medications. The studies reviewed here illustrate one strategy for use of choice procedures in research on negative reinforcement in drug self-administration.
As a final point, choice studies in rhesus monkeys have been used to examine the role of negative reinforcement for only heroin and cocaine, and it would likely be of interest to apply this approach to studies with other classes of abused drugs. Preliminary studies indicate that extended access to and withdrawal from methamphetamine fails to produce an increase in methamphetamine vs. food choice (M. Banks, unpublished observations), suggesting that, as with cocaine, negative reinforcement may also play little role in maintenance of methamphetamine choice. However, it remains to be determined if withdrawal-associated increases in drug choice could be obtained or pharmacologically modulated in studies with ethanol, nicotine, benzodiazepines, cannabinoids, or other classes of abused drugs.
Highlights.
Choice procedures provide an alternative to conventional drug self-administration procedures for research on determinants of drug reinforcement.
Extended drug access followed by drug withdrawal increases heroin vs. food choice but does not alter cocaine vs. food choice by rhesus monkeys. These results support a role for negative reinforcement in choice of heroin but not cocaine.
Withdrawal-associated increases in heroin choice can be blocked by mu opioid receptor agonists, but not by medications that target candidate negative reinforcement substrates related to enhanced stress system function or decreased dopamine reward system function.
Acknowledgments
Supported by NIH grant R01 DA026946 from the National Institute on Drug Abuse. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013b;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS. Use of Preclinical Drug vs. Food Choice Procedures to Evaluate Candidate Medications for Cocaine Addiction. Curr Treat Options Psychiatry. 2015;2:136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacology. 2010;35:493–504. doi: 10.1038/npp.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Insights from Preclinical Choice Models on Treating Drug Addiction. Trends Pharmacol Sci. 2016 doi: 10.1016/j.tips.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich JA, Bardo MT. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl) 2012;222:257–267. doi: 10.1007/s00213-012-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KJ, Rounsaville BJ, Babor TF. Coherence of the dependence syndrome in cocaine users. Br J Addiction. 1991;88:1299–1310. doi: 10.1111/j.1360-0443.1991.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Schulteis G, Koob GF. Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology (Berl) 1999;144:111–120. doi: 10.1007/s002130050983. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Influence of abstinence and conditions of cocaine access on the reinforcing strength of cocaine in nonhuman primates. Drug Alcohol Depend. 2006;85:213–220. doi: 10.1016/j.drugalcdep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. A laboratory model of cocaine withdrawal in humans: intravenous cocaine. Exp Clin Psychopharmacol. 1997;5:404–411. doi: 10.1037//1064-1297.5.4.404. [DOI] [PubMed] [Google Scholar]
- Fung YK, Richard LA. Behavioural consequences of cocaine withdrawal in rats. J Pharm Pharmacol. 1994;46:150–152. doi: 10.1111/j.2042-7158.1994.tb03761.x. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomology and psychiatric diagnosis in cocaine abusers. Archiv Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goeders JE, Murnane KS, Banks ML, Fantegrossi WE. Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2009;93:67–74. doi: 10.1016/j.pbb.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Woolverton WL, Green L, Myerson J, Freeman KB. Delay discounting of food by rhesus monkeys: Cocaine and food choice in isomorphic and allomorphic situations. Exp Clin Psychopharmacol. 2015;23:184–193. doi: 10.1037/pha0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict Biol. 2016a;21:360–373. doi: 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Negus SS, Banks ML. Effects of 21-day d-amphetamine and risperidone treatment on cocaine vs food choice and extended-access cocaine intake in male rhesus monkeys. Drug Alcohol Depend. 2016b;168:36–44. doi: 10.1016/j.drugalcdep.2016.08.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL. Drugs as reinforcers: pharmacological and behavioral factors. In: Liebman J, Cooper SJ, editors. The Neuropharmacological Basis of Reward. Clarendon Press; Oxford: 1989. pp. 164–213. [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kosten TR. Cocaine abuse and opioid withdrawal. Lancet. 1989;2:165–166. doi: 10.1016/s0140-6736(89)90229-8. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacol. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DH, Depoortere RY, Emmett-Oglesby MW. Tolerance to the reinforcing effects of cocaine in a progressive ratio paradigm. Psychopharmacology (Berl) 1994;116:326–332. doi: 10.1007/BF02245336. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse MP, Saxon AJ, Mooney LJ, Thomas CM, Ang A, Matthews AG, Hasson A, Annon J, Sparenborg S, Liu DS, McCormack J, Church S, Swafford W, Drexler K, Schuman C, Ross S, Wiest K, Korthuis PT, Lawson W, Brigham GS, Knox PC, Dawes M, Rotrosen J. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction. 2016;111:1416–1427. doi: 10.1111/add.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology. 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Rice KC, Mendelson JH. Effects of the CRF1 antagonist antalarmin on cocaine self-administration and discrimination in rhesus monkeys. Pharmacol Biochem Behav. 2006;85:744–751. doi: 10.1016/j.pbb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacol. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Negus SS. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs.-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacolgy. 2005;180:115–124. doi: 10.1007/s00213-004-2133-y. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS. Opioid antagonist effects in animal models related to opioid abuse: drug discrimination and drug self-administration. In: Dean RL, Bilsky EJ, Negus SS, editors. Opiate receptors and antagonists: From bench to clinic. Humana Press; New York: 2009. pp. 201–226. [Google Scholar]
- Negus SS, Banks ML. Making the right choice: lessons from drug discrimination for research on drug reinforcement and drug self-administration. In: Glennon RA, Young R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. John Wiley & Sons; Hoboken, NJ: 2011. pp. 361–388. [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Wallace EA, Sullivan MC, Stine S, Kosten TR. Use of cocaine to prevent opiate withdrawal. Am J Psychiatry. 1992;149:1609. doi: 10.1176/ajp.149.11.1609b. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, Grigoriadis DE, Pich EM, Leggio L, Heilig M. The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, France CP. Cocaine and amphetamine attenuate the discriminative stimulus effects of naltrexone in opioid-dependent rhesus monkeys. J Pharmacol Exp Ther. 2002;301:1103–1110. doi: 10.1124/jpet.301.3.1103. [DOI] [PubMed] [Google Scholar]
- Spragg S. Morphine addiction in chimpanzees. Comp Psychol Mono. 1940;15:5–132. [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Stoops WW, Moody DE, Lin SN, Bigelow GE. Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Exp Clin Psychopharmacol. 2009;17:205–216. doi: 10.1037/a0016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med. 2012;4:146ra110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-T, Pan J-J, Blazer DG, Tai B, Brooner RK, Stitzer ML, Patkar AA, Blaine JD. The construct and measurement equivalence of cocaine and opioid dependences: a national drug abuse treatment clinical trials network (CTN) study. Drug Alcohol Depend. 2009;103:114–123. doi: 10.1016/j.drugalcdep.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita T. Drug dependence studies in laboratory animals. NIDA Res Monogr. 1978;19:179–190. [PubMed] [Google Scholar]
- Young AM, Herling S. Drugs as reinforcers: studies in laboratory animals. In: Goldberg SR, Stolerman IP, editors. Behavioral Analysis of Drug Dependence. Academic Press; Orlando, FL: 1986. pp. 9–67. [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]