Abstract
Cholesterol (Ch) is like other unsaturated lipids in being susceptible to peroxidative degradation upon exposure to strong oxidants like hydroxyl radical or peroxynitrite generated under conditions of oxidative stress. In the eukaryotic cell plasma membrane, where most of the cellular Ch resides, peroxidation leads to membrane structural and functional damage from which pathological states may arise. In lipoprotein LDL, Ch and phospholipid peroxidation has long been associated with atherogenesis. Among the many intermediates/products of Ch oxidation, hydroperoxide species (ChOOHs) have a number of different fates and deserve special attention. These fates include (a) damage-enhancement via iron-catalyzed one-electron reduction, (b) damage containment via two-electron reduction, and (c) inter-membrane, inter-lipoprotein, and membrane-lipoprotein translocation, which allows dissemination of one-electron damage or off-site suppression thereof depending on antioxidant location and capacity. In addition, ChOOHs can serve as reliable and conveniently detected mechanistic reporters of free radical- versus non-radical (e.g. singlet oxygen)-mediated reactions. Iron-stimulated peroxidation of Ch and other lipids underlies a newly discovered form of regulated cell death called ferroptosis. These and other deleterious consequences of radical-mediated lipid peroxidation will be discussed in this review.
Keywords: Reactive oxygen species, cholesterol, cholesterol hydroperoxide, hydroperoxide translocation, chain lipid peroxidation, glutathione peroxidase-type 4
Introduction
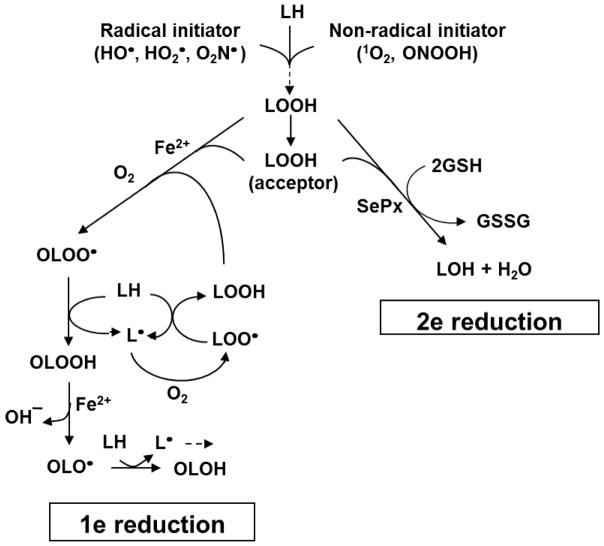
Unsaturated lipids, including phospholipids, glycolipids, and cholesterol in cell membranes, lipoproteins, and other organized systems are susceptible to non-enzymatic peroxidation under oxidative stress conditions [1–3]. This can occur in conjunction with a (i) natural metabolic process such as mitochondrial electron transport or NADPH oxidase activation, or (ii) exposure to an external oxidative insult such as ultraviolet or ionizing radiation. Free radical-propagated or chain lipid peroxidation (LPO) is a degenerative process that negatively affects membrane and lipoprotein structure/function and can give rise to a variety of pathological conditions, including atherosclerosis, neurodegeneration, and carcinogenesis [1–3]. LPO can be initiated by a variety of oxidants, including free radicals such as hydroxyl radical (HO·), hydroperoxyl radical (HO2·), and nitrogen dioxide (·NO2), and non-radicals such as singlet molecular oxygen (1O2), ozone (O3), and peroxynitrous acid (ONOOH) (Fig. 1). Peroxidation is triggered by abstraction of an allylic hydrogen atom from an unsaturated lipid, e.g. a phospholipid sn-2 fatty acyl group or cholesterol at the C7 position. The resulting lipid radical (L·) with a delocalized free electron reacts rapidly with ground state O2 to give a peroxyl radical (LOO·). The latter can abstract a hydrogen atom from a neighboring unsaturated lipid and this initiates a round of chain peroxidation. Concurrently, the (LOO·) is converted to a hydroperoxide intermediate/product (LOOH) (Eq. 1–3). Chain length and LOOH yield depends on factors such as lipid composition, local O2 concentration, and availability/concentration of chain breaking antioxidants such as ascorbate (AH−) (Eq. 4). In contrast to free radical initiation, the non-radical 1O2 can add directly
Fig. 1.
Primary and secondary reactions of lipid peroxidation. Possible free radical and non-radical initiators are shown. Three different fates for LOOH intermediates are described: (i) iron-mediated one-electron reduction, resulting in chain amplification; (ii) selenoperoxidase (SePx)-catalyzed two-electron reduction, resulting in chain suppression; (iii) translocation to a membrane/lipoprotein acceptor where processes (i) or (ii) can take place.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
to an unsaturated lipid (‘ene’ reaction) to give an allylic LOOH in which all atoms of the –OOH group derive from 1O2 and LH (Eq. 5) [4]. Although this type of peroxidation does not involve free radicals at the outset, such species can appear secondarily due to one-electron turnover of 1O2-derived LOOHs (see below). What follows is a discussion of various LOOH fates in biological systems with an emphasis on cholesterol hydroperoxide (ChOOH) fates and their implications on disorders such as atherogenesis and impaired steroid hormone synthesis.
One-electron reduction of lipid hydroperoxides
Non-esterified cholesterol (Ch) is found in all mammalian cells, most of it in the plasma membrane (~45 mol % of total lipid). Free Ch is also found in lipoproteins such as LDL, where it comprises ~10% of total lipid and ~25% of the cholesteryl ester (CE) content. As a monounsaturated lipid, Ch is susceptible to spontaneous oxidation, but at a lower rate than phospholipids or cholesteryl esters bearing polyunsaturated fatty acyl chains [5,6]. This oxidation can give rise to a variety of potentially mutagenic and cytotoxic products, including peroxides, diols, ketones, and epoxides [5,7]. One of the best known examples is oxidatively modified LDL (oxLDL), which contains numerous Ch, CE, and other lipid products, and is directly linked with atherogenesis due to uncontrolled uptake by cells in the vascular wall [8,9].
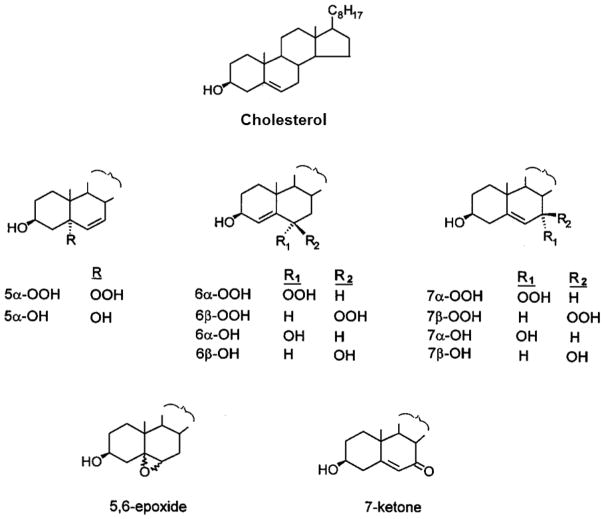
Hydroperoxides (ChOOHs) are the earliest non-radical species to be generated during Ch oxidation [5]. In free radical-mediated reactions, two ChOOHs are generated: 7α-OOH and 7β-OOH (Fig. 2), the latter being more thermodynamically stable [5,10,11]. These hydroperoxides are usually accompanied by downstream species such as the 7α-OH and 7β-OH diols, 5,6-epoxide, and 7-ketone (Fig. 2). Collectively, these oxidized Ch species are referred to as “ChOX”. In 1O2-mediated reactions, different positional hydroperoxides are formed: 5α-OOH, 6α-OOH, and 6β-OOH, the 5α-OOH yield always being far greater than that of the others [3,4,12]. Like all LOOHs, ChOOHs are subject to one-electron reduction if redox-active iron and suitable reductants and available. In the process, ChOOH is converted to an oxyl radical intermediate (ChO·), which could induce chain peroxidation by abstracting an allylic hydrogen from a proximal membrane or lipoprotein LH. One more likely alternative [2,13] is rapid rearrangement with O2 addition to give an initiating epoxyallylic peroxyl radical (OChOO·), as illustrated for general LOOH reduction in Fig. 1. Chain peroxidation induced by one-electron reduction of primary (or “priming”) LOOHs gives rise to new LOOHs which feed into the overall process (Fig. 1), thus amplifying the overall damage and dysfunction.
Fig. 2.
Structures of cholesterol (cholest-5-en-3β-ol), relevant cholesterol hydroperoxide positional isomers, and other cholesterol oxidation products. Identification of 5α-OOH, 6α-OOH, 6β-OOH, and the corresponding alcohols signifies singlet oxygen involvement in a reaction. Identification of 7α-OOH, 7β-OOH and their alcohols, along with 7-ketone and 5,6-epoxide, signifies free radical involvement.
Chain lipid peroxidation can be monitored by the relatively simple thiobarbituric acid (TBA) assay, which detects aldehyde by-products, but this assay is insensitive to Ch oxidation and has numerous deficiencies [2,3]. A great improvement came with the authors’ introduction of two highly sensitive and specific new approaches: (i) high performance liquid chromatography with mercury cathode electrochemical detection (HPLC-EC(Hg)) for analyzing individual ChOOHs [14–16] and (ii) high performance thin layer chromatography with phosphorimaging detection (HPTLC-PI) for analyzing [14C]ChOX [17,18]. Using HPLC-EC(Hg), we found that tracking growth of the 7α/7β-OOH signal and decline of the initial 5α-OOH signal provided an excellent means of assessing LOOH-initiated chain peroxidation in cells photodynamically stressed with 1O2 [19]. The HPTLC-PI approach provided additional information. In this case, [14C]Ch is used as a “sensor” for free radical activity in its membrane surroundings. Chain reactions set off by iron-mediated reduction of an unlabeled primary peroxide, e.g. 5α-OOH, result in accumulation of “reporter” [14C]ChOX species, which are HPTLC-separated and detected/quantified by PI [17–20]. In addition to convenience, this approach has the advantage of employing natural Ch as a probe, thus avoiding possible artifacts associated with spin traps, fluorophores and other artificial probes.
Two-electron reduction of lipid hydroperoxides
Newly formed LOOHs, including ChOOHs, may also undergo enzyme-catalyzed two-electron reduction to redox-inactive alcohol (LOH) products (Fig. 1). This is a detoxification process that can compete with cytotoxicity-enhancing one-electron reduction. LOOH detoxification is classified as secondary (reparative) to distinguish it from primary (preventative) detoxification of initiating species, e.g. O2−· by superoxide dismutases or H2O2 by catalase [3,4]. The enzyme most closely associated with cytoprotective LOOH detoxification is selenium-containing glutathione peroxidase-type 4 (GPx4), which uses reduced glutathione (GSH) as a co-substrate. Monomeric GPx4 (~20 kDa) is functionally quite distinct from tetrameric gutathione peroxidase-type 1 (GPx1, ~82 kDa), which is the most abundant SePx in mammalian cells. Whereas GPx4 plus GSH can act directly on LOOHs in membranes and lipoproteins, GPx1 cannot [3,21]. For PLOOHs, hydrolytic release of the peroxidized fatty acyl moiety is required before GPx1 can act [3]. The current consensus is that GPx1 is restricted to relatively polar peroxides such as H2O2 and fatty acid hydroperoxides, whereas GPx4 works best on PLOOHs, ChOOHs and other lower polarity LOOHs [3]. GPx4 is currently the only enzyme known to be capable of catalyzing direct reductive detoxification of ChOOHs [22]. Studies with purified GPx4 revealed a broad range of GPx4 reactivity with different ChOOH isomers, the rate constants increasing in the following order: 5α-OOH≪6α-OOH≈7α/7β-OOH<6β-OOH [23]. The order of toxicity of these ChOOHs for L1210 leukemia cells was found to be the inverse of that for GPx4 detoxification, i.e. 6β-OOH<7α/7β-OOH≪5α-OOH. Thus, 6β-OOH with the shortest lifetime with GPx4 was the least cytotoxic of the ChOOHs examined, whereas 5α-OOH with the longest lifetime was the most cytotoxic. It was subsequently found that 5α-OOH, 6β-OOH, and 7α-OOH in liposomes underwent one-electron decay at the same rate as one another. However, whereas 5α-OOH and 7α-OOH initiated chain peroxidation robustly in the presence of iron and ascorbate, 6β-OOH was unexpectedly very weak in this regard, possibly due to sluggish initiation by 6β-O· [24]. It appears, therefore, that relatively rapid reduction of 6β-OOH by GPx4/GSH (see above) only partially explains this peroxide’s weak cytotoxicity [24]. More advanced studies showed that a breast tumor cell line overexpressing GPx4 in mitochondria were much more resistant to 7α/7β-OOH-induced peroxidative injury and apoptotic death than wild type controls, thus demonstrating GPx4’s key role in cytoprotection against these free radical-generated peroxides [20]. Other studies have shown that mitochondrial GPx4 strongly protected cells against a mitochondrion-targeted photooxidative insult, but considerably less so when the insult was directed elsewhere in the cell [25]. This demonstrated the site-specificity of GPx4 cytoprotection. GPx4’s antiox-peroxidant role has been reported for many other in vitro and in vivo systems, e.g. [26–28], thus highlighting its general importance in this regard. There is also evidence that GPx4 plays a special role in protecting cells against a recently discovered unique form of cell death called ferroptosis, which is distinct from classical apoptosis or necrosis [29,30].
Although many mechanistic details are still lacking, ferroptosis depends on iron-catalyzed lipid peroxidation and is stimulated by inhibitors of GSH synthesis or GPx4 activity. There is accumulating evidence that ferroptosis plays a unique role in a variety of normo- and patho-physiological processes [29,30]. Although most current thinking about GPx4 centers on its cytoprotective antioxidant effects, there is also evidence that it plays a key regulatory role in the activities of lipoxygenase (LOX) and cyclooxygenase (COX) enzymes. This is based on knowledge that these enzymes require a low level of pre-existing PLOOH or ChOOH (a peroxide tone) for optimal activity and that GPx4 can negatively affect this. In support of this, it was found, for example, that (i) 5-LOX expression/activity was strongly upregulated in GPx4-deficient (Se-starved) cells, and (ii) 15-LOX-induced lipid peroxidation in membranes and LDL was significantly suppressed by GSH/GPx4 [31,32].
Cholesterol hydroperoxide translocation and trafficking
Studies in our laboratories have revealed that induction of chain peroxidation is not necessarily restricted to a nascent LOOH’s immediate membrane or lipoprotein surroundings, but can be disseminated via LOOH translocation [33–36]. Since all LOOHs, including ChOOHs, are more polar than parent lipids, they should desorb more readily into the aqueous compartment, allowing intracellular as well as extracellular transfer to acceptor sites. Using a model system consisting of photoperoxidized erythrocyte (RBC) ghosts as ChOOH donors, unilamellar liposomes as acceptors, and HPLC-EC(Hg) for analysis, we found that the first-order rate constants for spontaneous transfer decreased in the following order: 7α/7β-OOH≫5α-OOH>6β-OOH, which corresponded to the decreasing hydrophilicities of these ChOOHs [34]. Transfer was completely independent of any physical contact between donors and acceptors. The same general trend was observed with a variety of transfer models, including liposome-to-cell and RBC ghost-to-LDL systems [34–36]. Interestingly, experiments with wild-type COH-BR1 cells, which are GPX4-deficient, revealed that the cytolethality of three different ChOOHs in liposome donors decreased in parallel with their rates of transfer uptake, i.e. 7α/7β-OOH>5α-OOH>6β-OOH [35]. This demonstrated for the first time that transfer rate-limited cytotoxicity is possible for exogenous LOOHs. Although the focus here is on ChOOHs, PLOOHs have also been shown to translocate spontaneously. Like ChOOHs, phosphatidylcholine-, phosphatidylethanolamine-, and sphingomyelin-derived PLOOHs all migrated much more rapidly than their parent lipids [35,36]. Collectively, however, PLOOHs were found to be much less mobile than ChOOHs. Since circulating RBCs are under relative high oxidative pressure and are limited in antioxidant capacity [1], transfer to an acceptor like LDL might result in cellular protection against peroxidative damage. If antioxidant capacity of LDL is exceeded, this could promote formation of atherogenic oxLDL.
Transfer proteins play crucial roles in lipid metabolism and membrane biogenesis and metabolism. A well-known intracellular example is sterol carrier protein-2 (SCP-2), which can transport sterols as well as phospholipids between membranes [37]. Our studies were the first to demonstrate that a lipid transfer protein, SCP-2 in this case, could accelerate ChOOH transfer between membranes [38]. Using unilamellar liposomes as [14C]7α-OOH donors and isolated liver mitochondria as acceptors, we found that SCP-2 accelerated peroxide uptake and that this stimulated both chain peroxidation and loss of mitochondrial membrane potential [38]. Subsequent work showed that a transfect clone of hepatoma cells expressing ~10-fold more SCP-2 than a vector control was much more sensitive to 7α-OOH-induced mitochondrial damage and lethality than the control [39,40]. 7α-OH (an SCP-2 ligand, but redox-inactive) and tert-butyl hydroperoxide (redox-active, but not a ligand) showed no such effects. This was the first reported evidence that a cellular lipid trafficking protein could exacerbate LOOH redox damage and cytotoxicity.
Unlike SCP-2, which recognizes many different lipid ligands, steroidogenic acute regulatory (StAR) transfer proteins are highly specific for Ch and other sterols [41], and also ChOOHs [42]. StAR family proteins play a crucial role in steroid hormone synthesis by trafficking Ch to/into mitochondria of steroidogenic cells, where it is converted to pregnenolone by the CYP11A1 system [43]. Cytosolic StarD4 and mitochondrial StarD1 have been strongly implicated in this Ch trafficking. Our studies with testicular MA-10 cells revealed that StarD1 and StarD4 were strongly upregulated after cAMP stimulation [44]. Compared with non-stimulated controls, this resulted in (i) greater delivery of Ch as well as 7α-OOH to mitochondria; (ii) grater loss of membrane potential and progesterone output during 7α-OOH exposure; and (iii) more extensive apoptotic cell death. This was the first known evidence for ChOOH impairment of hormone synthesis through engagement in a natural trafficking pathway. StAR proteins are also known to play a key role in early stage reverse cholesterol transport (RCT) in vascular macrophages, which limits accumulation of potentially atherogenic Ch [45,46]. We hypothesized that under pathological conditions associated with oxidative stress, oxLDL-supplied 7α/7β-OOH would be caught up in StAR-mediated Ch trafficking to/into macrophage mitochondria, thereby inducing peroxidative damage that impairs RCT and ultimately proves cytotoxic. Testing this first on stimulated mouse RAW264.7 macrophages, we found that 7α-OOH uptake in mitochondria was StarD1-dependent and induced lipid peroxidation, membrane depolarization, and intrinsic apoptosis [47]. In recent work of greater relevance to cardiovascular disease, we used human THP-1 monocyte-derived macrophages, showing that cAMP-stimulation resulted in upregulation of mitochondrial StarD1 and plasma membrane ABCA1/G1, which mediate Ch efflux [48]. Major functional consequences of exposing stimulated cells to 7α-OOH were: (i) greater mitochondrial uptake of 7α-OOH compared with unstimulated cells; (ii) greater mitochondrial chain lipid peroxidation; (iii) activity loss of mitochondrial 27-hydroxylase (CYP27A1), which generates 27-hydroxycholesterol (27-OH), a key agonist for ABCA1/G1 expression and RCT function; (iv) reduced 27-OH output, and (v) diminished ABCA1/G1 expression. Correspondingly, 7α-OOH-challenged THP-1 macrophages exported less Ch to acceptors (e.g. apoA-I, HDL) than 7α-OH- of 7-ketone-treated controls and succumbed to apoptosis more readily [48]. These findings further supported our novel hypothesis that a redox active ChOOH like 7α-OOH can integrate into a natural Ch trafficking pathway, and in so doing induce mitochondrial damage that disrupts Ch homeostasis. New insights into mechanisms of vascular macrophage oxidative damage/dysfunction and its pathologic implications are apparent for this work [47,48].
Lipid hydroperoxides and signal transduction
The most widely studied redox signaling mediator, H2O2, can traverse cell membranes via aquaporin channels, but has no known other protein transporters. Thus, it is not clear how H2O2, if it diffuses freely on its own, might distinguish between various sensor target proteins in different cellular compartments. Our discovery that ChOOHs and PLOOHs do not necessarily migrate randomly from sites of origin, but can be delivered to acceptors by transfer proteins [38–40,44,47,48], suggests a new paradigm for peroxide signaling. One can postulate that sensor proteins on or near membranes would be the preferred targets of mobilized amphiphilic LOOHs, whereas highly polar H2O2 would preferably target cytosolic sensors. Thioredoxins, peroxiredoxins, and protein tyrosine phosphatases are all good examples of the latter. Although transfer protein-mediated ChOOH or PLOOH signaling would be slow relative to signaling by free H2O2 [49], it would have the following clear advantages: (i) longer peroxide lifetime in transit due to sequestration and protection against one- or two-electron turnover; (ii) precise delivery due to specific transfer protein-sensor protein interactions. Except for two examples [50,51], little is known about LOOH-mediated signaling, and a greater understanding of this from a mechanistic perspective is eagerly awaited.
Conclusions and perspectives
Like all LOOHs generated by non-enzymatic lipid peroxidation, ChOOHs have a variety of fates, including (i) iron-stimulated one-electron reduction, which exacerbates peroxidative damage/dysfunction; (ii) SePx-catalyzed two-electron reduction, which attenuates this damage/dysfunction; and (iii) spontaneous or protein-mediated translocation to membrane/lipoprotein acceptors, where processes (i) or (ii) may take place. In addition, ChOOHs, like PLOOHs, may function as redox signaling mediators, particularly when delivered to specific sensor targets by transfer proteins. Compared to H2O2 signaling, little is currently known about ChOOH/PLOOH signaling (underlying mechanisms, sub-cellular locations, specific biological effects, etc.) and a better understanding of this should be an important goal of future investigations.
Acknowledgments
Studies in the authors’ laboratories were supported by USPHS Grants CA72630, TW001386, and CA70823 (to A.W.G.) and Polish National Center for Science Grant NCN-2014/13/B/NZ3/00833 (to W.K.). We thank Peter Geiger, Andrew Vila, Tamas Kriska, Vlad Levchenko, Jared Schmitt, Magda Niziolek, Kasia Wawak, and Anna Pilat for their many valuable contributions to the research supported by these grants.
Abbreviations
- Ch
cholesterol
- ChOOH
cholesterol hydroperoxide
- ChOH
cholesterol hydroxide
- ChOX
oxidized cholesterol species
- cAMP
cyclic-AMP
- LOOH
lipid hydroperoxide
- PLOOH
phospholipid hydroperoxide
- GPx4
glutathione peroxidase type-4
- GSH
reduced glutathione
- LDL
low density lipoprotein
- oxLDL
oxidatively modified LDL
- RBC
red blood cell
- SePx
selenoperoxidase
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Clarendon Press; Oxford, U.K: 1999. [Google Scholar]
- 2.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 3.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of Lipid Research. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 4.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. Journal of Photochemistry and Photobiology B: Biology. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 5.Smith LL. Cholesterol Autoxidation. Plenum Press; New York: 1981. [Google Scholar]
- 6.Doleiden FH, Farenholtz SR, Lamola AA, Trozzolo AM. Reactivity of cholesterol and some fatty acids toward singlet oxygen. Photochemistry and Photobiology. 1974;20:519–21. doi: 10.1111/j.1751-1097.1974.tb06613.x. [DOI] [PubMed] [Google Scholar]
- 7.Fielding CJ, Fielding PE. In: Biochemistry of Lipids and Membranes. Vance DE, Vance JE, editors. Benjamin/Cummings; Menlo Park, California: 1985. p. 404. [Google Scholar]
- 8.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. New England Journal of Medicine. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 9.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiological Reviews. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 10.Teng JI, Kulig MJ, Smith LL, Kan G, Van Lier JE. Sterol metabolism. XX. Cholesterol 7-hydroperoxide. Journal of Organic Chemistry. 1973;38:119–23. doi: 10.1021/jo00941a024. [DOI] [PubMed] [Google Scholar]
- 11.Smith LL, Teng JL, Julig MJ, Hill FL. Sterol metabolism. 23. Cholesterol oxidation by radiation-induced processes. Journal of Organic Chemistry. 1973;38:1763–5. doi: 10.1021/jo00949a041. [DOI] [PubMed] [Google Scholar]
- 12.Kulig MJ, Smith LL. Sterol metabolism. XXV. Cholesterol oxidation by singlet molecular oxygen. Journal of Organic Chemistry. 1973;38:3639–42. doi: 10.1021/jo00960a050. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox AL, Marnett LJ. Polyunsaturated fatty acid alkoxyl radicals exist as carbon-centered epoxyallylic radicals: a key step in hydroperoxide-amplified lipid peroxidation. Chemical Research in Toxicology. 1993;6:413–416. doi: 10.1021/tx00034a003. [DOI] [PubMed] [Google Scholar]
- 14.Korytowski W, Bachowski GJ, Girotti AW. Analysis of cholesterol and phospholipid hydroperoxides by high-performance liquid chromatography with mercury drop electrochemical detection. Analytical Biochemistry. 1993;213:111–9. doi: 10.1006/abio.1993.1393. [DOI] [PubMed] [Google Scholar]
- 15.Korytowski W, Geiger PG, Girotti AW. High-performance liquid chromatography with mercury cathode electrochemical detection: application to lipid hydroperoxide analysis. Journal of Chromatography B: Biomedical Applications. 1995;670:189–97. doi: 10.1016/0378-4347(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 16.Korytowski W, Geiger PG, Girotti AW. Lipid hydroperoxide analysis by high-performance liquid chromatography with mercury cathode electrochemical detection. Methods in Enzymology. 1999;300:23–33. doi: 10.1016/s0076-6879(99)00109-3. [DOI] [PubMed] [Google Scholar]
- 17.Korytowski W, Wrona M, Girotti AW. Radiolabeled cholesterol as a reporter for assessing one-electron turnover of lipid hydroperoxides. Analytical Biochemistry. 1999;270:123–32. doi: 10.1006/abio.1999.4070. [DOI] [PubMed] [Google Scholar]
- 18.Girotti AW, Korytowski W. Cholesterol as a natural probe for free radical-mediated lipid peroxidation in biological membranes and lipoproteins. Journal of Chromatography B: Analytical Technology and Biomedical Life Sciences. 2016;1019:202–209. doi: 10.1016/j.jchromb.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korytowski W, Zareba M, Girotti AW. Inhibition of free radical-mediated cholesterol peroxidation by diazeniumdiolate-derived nitric oxide: effect of release rate on mechanism of action in a membrane system. Chemical Research in Toxicology. 2000;13:1265–74. doi: 10.1021/tx000160o. [DOI] [PubMed] [Google Scholar]
- 20.Hurst R, Korytowski W, Kriska T, Esworthy RS, Chu FF, Girotti AW. Hyperresistance to cholesterol hydroperoxide-induced peroxidative injury and apoptotic death in a tumor cell line that overexpresses glutathione peroxidase isotype-4. Free Radical Biology and Medicine. 2001;31:1051–65. doi: 10.1016/s0891-5849(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 21.Ursini F, Maiorino M, Brigelius-Flohé R, Aumann KD, Roveri A, Schomburg D, Flohé L. Diversity of glutathione peroxidases. Methods in Enzymology. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- 22.Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation: in situ reduction of phospholipid and cholesterol hydroperoxides. Journal of Biological Chemistry. 1990;265:454–461. [PubMed] [Google Scholar]
- 23.Korytowski W, Geiger PG, Girotti AW. Enzymatic reducibility in relation to cytotoxicity for various cholesterol hydroperoxides. Biochemistry. 1996;35:8670–8679. doi: 10.1021/bi960522k. [DOI] [PubMed] [Google Scholar]
- 24.Korytowski W, Schmitt JC, Girotti AW. Surprising inability of singlet oxygen-generated 6-hydroperoxycholesterol to induce damaging free radical lipid peroxidation in cell membranes. Photochemistry and Photobiology. 2010;86:747–751. doi: 10.1111/j.1751-1097.2010.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriska T, Korytowski W, Girotti AW. Hyperresistance to photosensitized lipid peroxidation and apoptotic killing in 5-aminolevulinate-treated tumor cells overexpressing mitochondrial GPx4. Free Radical Biology and Medicine. 2002;33:1389–1402. doi: 10.1016/s0891-5849(02)01078-x. [DOI] [PubMed] [Google Scholar]
- 26.Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radical Biology and Medicine. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 27.Ran Q, Van Remmen H, Gu M, Qi W, Roberts LJ, Prolla T, et al. Embryonic fibroblasts from Gpx4+/− mice: a novel model for studying the role of membrane peroxidation in biological processes. Free Radical Biology and Medicine. 2003;35:1101–1109. doi: 10.1016/s0891-5849(03)00466-0. [DOI] [PubMed] [Google Scholar]
- 28.Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ, et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. Journal of Biological Chemistry. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 29.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends in Cell Biology. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weitzel F, Wendel A. Selenoenzymes regulate the activity of leukocyte 5-lipoxygenase via the peroxide tone. Journal of Biological Chemistry. 1993;268:6288–6292. [PubMed] [Google Scholar]
- 32.Schnurr K, Belkner J, Ursini F, Schewe T, Kühn HJ. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. Journal of Biological Chemistry. 1996;271:4653–4658. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- 33.Vila A, Korytowski W, Girotti AW. Dissemination of peroxidative stress via intermembrane transfer of lipid hydroperoxides: model studies with cholesterol hydroperoxides. Archives of Biochemistry and Biophysics. 2000;380:208–218. doi: 10.1006/abbi.2000.1928. [DOI] [PubMed] [Google Scholar]
- 34.Vila A, Korytowski W, Girotti AW. Spontaneous intermembrane transfer of various cholesterol-derived hydroperoxide species: kinetic studies with model membranes and cells. Biochemistry. 2001;40:14715–14726. doi: 10.1021/bi011408r. [DOI] [PubMed] [Google Scholar]
- 35.Vila A, Korytowski W, Girotti AW. Spontaneous transfer of phospholipid and cholesterol hydroperoxides between cell membranes and low-density lipoprotein: assessment of reaction kinetics and prooxidant effects. Biochemistry. 2002;41:13705–13716. doi: 10.1021/bi026467z. [DOI] [PubMed] [Google Scholar]
- 36.Girotti AW. Translocation as a means of disseminating lipid hydroperoxide-induced oxidative damage and effector action. Free Radical Biology and Medicine. 2008;44:956–968. doi: 10.1016/j.freeradbiomed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scallen TJ, Pastuszyn A, Noland BJ, Chanderbhan R, Kharroubi A, Vahouny GV. Sterol carrier and lipid transfer proteins. Chemistry and Physics of Lipids. 1985;38:239–261. doi: 10.1016/0009-3084(85)90019-2. [DOI] [PubMed] [Google Scholar]
- 38.Vila A, Levchenko VV, Korytowski W, Girotti AW. Sterol carrier protein-2-facilitated intermembrane transfer of cholesterol- and phospholipid-derived hydroperoxides. Biochemistry. 2004;43:12592–12605. doi: 10.1021/bi0491200. [DOI] [PubMed] [Google Scholar]
- 39.Kriska T, Levchenko VV, Korytowski W, Atshaves BP, Schroeder F, Girotti AW. Intracellular dissemination of peroxidative stress. Internalization, transport, and lethal targeting of a cholesterol hydroperoxide species by sterol carrier protein-2-overexpressing hepatoma cells. Journal of Biological Chemistry. 2006;281:23643–23651. doi: 10.1074/jbc.M600744200. [DOI] [PubMed] [Google Scholar]
- 40.Kriska T, Pilat A, Schmitt JC, Girotti AW. Sterol carrier protein-2 (SCP-2) involvement in cholesterol hydroperoxide cytotoxicity as revealed by SCP-2 inhibitor effects. Journal of Lipid Research. 2010;51:3174–3184. doi: 10.1194/jlr.M008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. Journal of Biological Chemistry. 2003;278:22183–22186. doi: 10.1074/jbc.R300003200. [DOI] [PubMed] [Google Scholar]
- 42.Korytowski W, Rodriguez-Agudo D, Pilat A, Girotti AW. StarD4-mediated translocation of 7-hydroperoxycholesterol to isolated mitochondria: deleterious effects and implications for steroidogenesis under oxidative stress conditions. Biochemical and Biophysical Research Communications. 2010;392:58–62. doi: 10.1016/j.bbrc.2009.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrinology Reviews. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 44.Korytowski W, Pilat A, Schmitt JC, Girotti AW. Deleterious cholesterol hydroperoxide trafficking in steroidogenic acute regulatory (StAR) protein-expressing MA-10 Leydig cells: implications for oxidative stress-impaired steroidogenesis. Journal of Biological Chemistry. 2013;288:11509–11519. doi: 10.1074/jbc.M113.452151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 46.Borthwick F, Taylor JM, Bartholomew C, Graham A. Differential regulation of the STARD1 subfamily of START lipid trafficking proteins in human macrophages. FEBS Letters. 2009;583:1147–1153. doi: 10.1016/j.febslet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 47.Korytowski W, Wawak K, Pabisz P, Schmitt JC, Girotti AW. Macrophage mitochondrial damage from StAR transport of 7-hydroperoxycholesterol: implications for oxidative stress-impaired reverse cholesterol transport. FEBS Letters. 2014;588:65–70. doi: 10.1016/j.febslet.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 48.Korytowski W, Wawak K, Pabisz P, Schmitt JC, Chadwick AC, Sahoo D, Girotti AW. Impairment of macrophage cholesterol efflux by cholesterol hydroperoxide trafficking: implications for atherogenesis under oxidative stress. Arteriosclerosis Thrombosis and Vascular Biology. 2015;35:2104–2113. doi: 10.1161/ATVBAHA.115.306210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girotti AW, Korytowski W. Generation and reactivity of lipid hydroperoxides in biological systems. In: Liebman JT, Greer A, editors. The Chemistry and Physics of Peroxides, Part 2. Ch. 18. John Wiley & Sons, Ltd; West Sussex, England: 2014. [Google Scholar]
- 50.Tyurina YY, Tyurin VA, Zhao Q, Djukic M, Quinn PJ, Pitt BR, et al. Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis? Biochemical and Biophysical Research Communications. 2004;324:1059–1064. doi: 10.1016/j.bbrc.2004.09.102. [DOI] [PubMed] [Google Scholar]
- 51.Korytowski W, Basova LV, Pilat A, Kernstock RM, Girotti AW. Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: mechanistic implications for the intrinsic pathway of oxidative apoptosis. Journal of Biological Chemistry. 2011;286:26334–26343. doi: 10.1074/jbc.M110.188516. [DOI] [PMC free article] [PubMed] [Google Scholar]