Abstract
Cerebral tissue oxygenation (oxygen tension, pO2) is a critical parameter that is closely linked to brain metabolism, function, and pathophysiology. In this work, we have used electron paramagnetic resonance (EPR) oximetry with a deep-tissue multi-site oxygen-sensing probe, called implantable resonator (IR), to monitor temporal changes in cerebral pO2 simultaneously at 4 sites in a rabbit model of ischemic stroke induced by embolic clot. The pO2 values in healthy brain were not significantly different among the 4 the sites measured over a period of 4 weeks. During exposure to 15% O2 (hypoxia), a sudden and significant decrease in pO2 was observed in all four sites. On the other hand, brief exposure to breathing carbogen gas (95% O2 + 5% CO2) showed a significant increase in the cerebral pO2 from baseline value. During ischemic stroke, induced by embolic clot in the left brain, a significant decline in the pO2 of the left cortex (ischemic core) was observed without any change in the contralateral sites. While the pO2 in the non-infarct regions returned to baseline at 24-h post-stroke, pO2 in the infarct core was consistently lower compared to the baseline and other regions of the brain. The results demonstrated that EPR oximetry with the IR can repeatedly and simultaneously report temporal changes in cerebral pO2 at multiple sites. This oximetry approach can be used to develop interventions to rescue hypoxic/ischemic tissue by modulating cerebral pO2 during hypoxic and stroke injury.
Keywords: Cerebral ischemia, hypoxia, EPR oximetry, embolic stroke, implantable oxygen sensor, pO2
Introduction
The cerebral tissue is the critical site for oxygen-dependent physiological and pathophysiological processes in the brain. Cerebral tissue oxygen tension (pO2) is closely linked with normal as well as pathophysiological processes, such as ischemic stroke, that are involved in brain injury and recovery. A precise knowledge of pO2 dynamics in the cerebral tissue immediately after an ischemic insult will be immensely useful for understanding the process of cerebral injury and to develop salvage therapies for stroke. Several methods are currently available for assessment of tissue pO2 but with limited temporal capability. The OxyLite and Licox techniques are invasive and require the insertion of probe into the tissue of interest for each measurement [1-9]. Similarly, 19F-MRI, 15O-PET and EPR imaging require the injection of probes for each measurement [10-19]. On the other hand, techniques such as BOLD MRI or MRI measurements of perfusion and diffusion use endogenous probes, but cannot provide direct measurement of tissue pO2; instead, they derive oxygen information from the levels of parameters that are indirectly related to tissue pO2 such as deoxyhemoglobin in the blood [20-25]. We have focused on the development of electron paramagnetic resonance (EPR) oximetry using oxygen-sensing crystalline probe, such as lithium phthalocyanine (LiPc), which require one-time implantation of it using a minimally invasive procedure, but rest of the method for pO2 measurement is entirely non-invasive and can be repeated as desired [26-29]. The basis of EPR oximetry is the paramagnetic nature of molecular oxygen, which affects the EPR linewidth of other paramagnetic species (oximetry probe), in its vicinity by altering their relaxation rates. The magnitude of change in the EPR linewidth is directly related to the amount of oxygen (pO2) that is present in the environment of the probe. The useful properties of LiPc for EPR oximetry are their stability and the strong response of their spectra to the presence of oxygen. The physicochemical properties of the LiPc crystals have been described earlier [30, 31]. EPR oximetry with paramagnetic oxygen-sensing materials has shown several advantages, especially for the repetitive and accurate measurement of localized tissue pO2. However, these studies have also indicated the areas in which progress is needed, particularly the need for technological developments that can enable measurements in tissues at depths of greater than 10 mm. We have developed EPR oximetry using implantable oxygen sensors to repeatedly measure cerebral pO2 in the regions directly affected by ischemic stroke as well as the contralateral regions of the rat brain [32]. We have also systematically developed sensors that can be used to assess pO2 at depths greater than 10 mm from the surface [33]. In this paper, we report the application of an implantable resonator (IR) with four sensory tips to assess the dynamic changes in the regional oxygen levels at different depths (10 mm and 15 mm) in rabbit cerebral tissue for 4 weeks. We also have studied the effect of embolic stroke on cerebral pO2 in ischemic and peripheral areas in rabbits. These results indicate that EPR oximetry with multiple sensors can provide crucial information on the temporal changes in the rabbit cerebral pO2 during hypoxic/hyperoxic challenges and embolic stroke. We anticipate that this oximetry approach can also be used to study the effect of other pathologies, such as traumatic brain injury and intracranial hemorrhage on cerebral pO2 and develop interventions that can improve oxygen levels to minimize tissue damage.
Materials and Methods
Animal and experimental groups
The Dartmouth College Animal Care and Use Committee approved the animal protocol used in the study. Female New Zealand rabbits (Charles River Laboratory, Wilmington, MA), weighing 3-4 kg, were randomly divided into two experimental groups: (i) Hypoxic (15% O2) or hyperoxic (carbogen)-challenge group (N=6); (ii) Embolic-stroke group (30% O2, N=5). The animals had the oxygen sensor placed 3 and 7 days prior to baseline EPR measurements for group (i) and group (2), respectively. The hypoxic/hyperoxic challenge for group (i) was repeated once a week for four weeks and animals with embolic stroke were subjected to a 24 h of ischemia and sacrificed right after last EPR measurements.
Induction of embolic stroke
The procedures for the preparation and injection of blood clots to induce embolic stroke were acquired from the laboratory of Dr. William C. Culp (University of Arkansas for Medical Sciences, Little Rock, AK) [34-36] and modified accordingly. Briefly, blood clots were prepared using fresh rabbit blood, which was placed into a glass tube (1.5-mm inner diameter), incubated at 37°C for 6 h and refrigerated at 4°C for 3 days. The clots were expelled from the tube into a dish containing physiological saline and subsequently cut into several pieces of 4.0-mm in length with an average diameter of 0.6 mm. A single autologous clot was drawn into a syringe containing physiological saline (2 ml) and was injected into the internal carotid artery with a steady flush over 10 - 15 sec to induce ischemia. Extreme care was taken to ensure that no air bubbles were present in the syringe.
Angiography and embolization
Angiography was performed on rabbits in a recumbent position using OEC 9800 cardiac fluoroscope (GE Healthcare, Pittsburg, PA). Using sterile technique, a surgical cut down was performed on a common femoral artery and a 3-Fr vascular sheath was used to introduce an angiographic catheter. The customized 65-cm angled-tip 3-Fr catheter (Slip-Cath, modified JB1; Cook Inc., Bloomington, IN) was advanced using fluoroscopic guidance over a 0.025-in. angle-tip guide wire (Glidewire; Terumo Medical Corp., Elkton, MD) to the aortic arch. The image intensifier was then rotated from the frontal view to a lateral view. Another subtracted fluoroscopy injection identified the ICA for selection. Once engaged, the small catheter usually compromised ICA blood flow until withdrawn. Angiograms using hand injections of 1-2 ml of contrast in both projections showed the detailed anatomy of the entire Circle of Willies (COW). The selected clot was slowly injected with 0.7 - 2.0 ml of saline flush slowly following it down the catheter. A follow-up angiogram confirmed embolus arrival in the COW or a branch and localized the arterial occlusion. The catheter was then pulled back into the CCA and normal ICA flow resumed. The catheter and sheath were eventually removed, the femoral artery ligated and skin sutured.
Evaluation of stroke volume
All rabbits were sacrificed after 24 h of embolic stroke and the brains were carefully removed and sectioned into 4-mm thick coronal sections, starting 4 mm from the frontal pole. The slices were placed in a 2% 2′,3′,5′-triphenyl-2H-tetrazolium chloride (TTC) solution at room temperature to stain viable brain, which demonstrates mitochondrial dehydrogenase activity [37]. The sections were photographed and the volume of the infarct was determined using ImageJ software by an investigator blinded to the groups.
Paramagnetic material and implantable resonators
Lithium phthalocyanine (LiPc) crystals, synthesized in our laboratory [31], were used as oxygen-sensing probe. The implantable resonators were assembled with thin non-magnetic copper wires (0.3-mm wire gauge) consisting of a coupling loop (12-mm diameter) at one end and a transmission line with sensor loops (0.4 - 0.5 mm inner diameter) at the other end (Figure 1). The sensor loops were loaded with 30-50 μg of LiPc crystals. The entire resonator was coated with a gas-permeable and biocompatible Teflon AF2400 [38]. The implantable resonators are well tolerated by the animals (mice, rats, or rabbits) [32, 39-42]. The mean area of the oximetry probe at the surface of each sensor loop was 1.3 - 1.6 mm2. EPR oximetry, therefore, samples a region that includes many capillary segments and provides average pO2 at the site of the sensor loop [5, 26]. We have recently described the procedures for the placement of the implantable resonator and EPR oximetry in rabbits [42]. The diameter and the number of sensors, the length of the transmissions lines and the distance between sensors can be adjusted based on the experimental requirements.
Fig. 1. Anatomy of rabbit brain showing the location of infarct and placement of oxygen sensors.

The infarct (ischemic) region is shown as shaded blue ellipse. The locations of the oxygen sensors are as follows: S4 in the cortex of ischemic core; S3 in the peripheral region; S1 in the contralateral cortex region; and S2 in the contralateral sub-cortex region. S1 and S4 were at a depth of 10 mm, while S2 and S3 were at a depth of 15 mm from the surface of skull. The coupling loop of the resonator was placed underneath the skin. An external surface loop resonator is placed above the coupling loop during pO2 measurements.
Multi-site EPR oximetry with implantable resonator
Surgical placement of the implantable resonator into the brain: The surgical procedure for the placement of implantable resonator in the rabbit brain was used in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth College. The head of the anesthetized rabbit was antiseptically treated with betadine, and 70% alcohol scrubs. A small incision (2-3 cm) was made on the skin and burr holes were gently created by using 23-18G needle on the skull at predefined co-ordinates: AP (anterior-posterior from bregma): -2.0 mm; ML (medial-lateral from midline): 4 mm and 8 mm on each hemisphere; DV (dorsal-ventral from surface of skull): 15 mm and 10 mm in each hemisphere. These co-ordinates were based on the published reports of potential infarct region following ischemia and can be changed depending on the coordinates of the compromised tissue. The sensory points were slowly lowered at the desired depth and the coupling loop was placed on the skull below the skin for inductive coupling with the external surface loop resonator of the EPR spectrometer, as shown in Fig. 1. The incision on the skin was closed with non-absorbable 3-0 nylon suture and the rabbit was monitored for recovery. Repeated measurements of cerebral pO2 was started 72 h after placement of the sensors and the measurements were repeated weekly for 4 weeks.
EPR measurements
EPR measurements were performed using an L-band (1.2 GHz) spectrometer equipped with a surface-loop resonator and a set of gradient coils for multi-site oximetry [43, 44]. Rabbit, anesthetized using 2.5% isoflurane in 30% O2, was positioned in the EPR magnet and surface-loop resonator was placed over the head region. A magnetic field gradient of 1.7 G/cm was used to separate the EPR spectra from each sensor for simultaneous multisite oximetry [45, 46]. Analysis of the EPR spectra was performed using software written using Matlab (Mathworks, Inc., Natick, MA). The peak-to-peak line-width of the EPR spectra was used to determine pO2 using a standard curve. The rabbit was maintained at 38±1.0°C (monitored via a rectal probe) by keeping the animal warm with warm air during the EPR measurements. The EPR settings were: incident microwave power: 0.4-1.2 mW; modulation frequency 24 kHz; magnetic field center 410 G; scan time 10 sec, scan range 8-12 G, and modulation amplitude not exceeding one-third of the line width.
Three-to-seven days after placement of the implantable resonator, anesthesia was administered via inhalation using 3.5% isoflurane and maintained with 2.5-3.0% of isoflurane with 30% oxygen through a sealed nose cone at a flow rate of 2.0 l/min. Baseline EPR measurements were made in all rabbits for a period of 30 min to measure the pO2. During EPR measurements, the animal temperature was maintained using a thermostatically-controlled heated pad and warm air forced through the core of the magnet. The heart rate and blood oxygen saturation were continuously measured using a pulse oximeter (Nonin 8600V Pulse Oximeter, Phoenix, Arizona) throughout the EPR measurements.
Hypoxic/Hyperoxic challenge
The rabbits were anesthetized with 2-3.0% isoflurane in 30% oxygen through a nose cone and a baseline pO2 was measured for 25 min using EPR oximetry as described above. After the baseline pO2 measurements, animals were subjected sequentially to mild hypoxia (15% O2 for 18 min), normoxia (30% O2 for 20 min), normobaric hyperoxia (carbogen for 20 min) and normoxia (30% O2 for 15 min).
Embolic clot stroke
Seven days after placement of the sensor, the animals were anesthetized (2.5%-3.0% isoflurane with 30% oxygen) and a pre-treatment (baseline) pO2 measurements was made for 30 min at the four sites. Thereafter, embolic clot stroke was induced and pO2 measurements were repeated for 50 min, during which all the animals continued to breathe 30% oxygen. At 24 hours of post-embolic-clot stroke, pO2 measurements were repeated for 60 min during which all the animals continued to breathe 30% oxygen.
Statistical analysis
A paired t-test was used to compare the pO2 values at four sites and during baseline, ypoxia/hyperoxia challenges and embolic clot stroke within the same group. The paired comparison reduces the effects of animal-to-animal heterogeneity and eliminates differences in the baseline pO2. The tests were two-sided and a change with a p-value <0.05 was considered statistically significant. All data are expressed as Mean ± SEM.
Results and Discussion
Cerebral pO2 dynamics during hypoxic and hyperoxic interventions
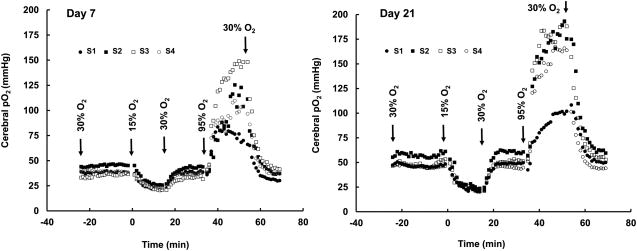
We tested the capability of EPR oximetry for temporal monitoring of cerebral pO2 under normal and altered oxygen-inhalation conditions. Cerebral pO2 was continuously measured while the breathing gas was cycled through 30% O2 (normoxia), 15% O2 (to simulate hypoxia during cerebral ischemia), and 95% O2 (hyperoxia). We used 30% O2 inhalation instead of room air (∼21% O2) breathing to maintain normoxic pO2 level by compensating for the loss of tissue oxygenation under isoflurane anesthesia. Fig. 2 shows the cerebral pO2 values measured from a rabbit on day 7 and repeated on day 21 at multiple locations as shown in Fig. 1. The results showed a quick decline in cerebral pO2 at all four sites when the composition of the breathing gas was switched from 30% to 15% O2. A sharp increase in the cerebral pO2 was observed when the breathing gas was switched from 30% to 95% O2 (carbogen gas). The responses to hypoxia and hyperoxia were similar among the 4 sites; however, the magnitudes of change during hyperoxia were significantly different. An exponential quadratic function of time analyses indicated that it took 10-14 min to reach equilibrium after hypoxic or hyperoxic challenge (data not shown). It should be noted that similar responses were observed on both days suggesting that neither the tissue nor integrity of the sensors was changed during the two-week period.
Fig. 2. Changes in cerebral pO2 measured in a rabbit subjected to hypoxic and hyperoxic breathing.
An implantable resonator with 4 sensory tips (S1-S4) was implanted at two sites in each hemisphere, as shown in Fig. 1. pO2 measurements were made on the same rabbit on day 7 and day 21 after sensor placement. Oxygen content in the inhalation gas was varied as indicated. The results show a consistent variation of pO2 among all 4 sites up on exposure to different oxygen levels.
To determine the reliability of the multi-site sensor for long-term monitoring of tissue pO2, measurements were repeated on a group of rabbits for four consecutive weeks following the sensor placement. Fig. 3 shows the average pO2 values at each of the 4 sites obtained from a total of 5 rabbits during a 4-week period. The data showed, in general, a 40% decline during hypoxic (15% O2) intervention and a 60% surge during hyperoxic (95% O2) intervention. The mean baseline pO2 data obtained from each of the 4 sites over the 4-week period were in the range 34-57 mmHg (Fig. 4). The pO2 averaged over all sites and animals showed a consistent variation up on hypoxia or hyperoxia intervention suggesting that, in general, there were no substantial variations in the cerebral pO2 among the sites in the normal brain. We also observed a similar increase in cerebral tissue pO2 in mice and rats breathing carbogen [39, 47-49]. Overall, the results established the capability of EPR oximetry to obtain reliable measurements repeatedly and simultaneously from multiple sites in the brain.
Fig. 3. Cerebral pO2 measured over a 4-week period.
The pO2 measurements were performed simultaneously at 4 sites (S1-S4) using EPR oximetry under conditions of breathing normal (□ 30% O2), low (■ 15% O2) and high (■ 95% O2) oxygen. Data represent Mean ± SEM obtained from 5 rabbits.
Fig. 4. Cerebral pO2 and its response to oxygen breathing.
(A) Mean pO2 values obtained from the four sites (S1-S4) while the rabbits (N=5) were breathing 30% O2. (B) Mean pO2 from the four sites during baseline (30% O2), inhalation of 15% O2, recovery from 15% O2 breathing, inhalation of 95% O2 (carbogen) and recovery. Data represent Mean±SEM, N=5. *p<0.01 compared to baseline pO2 on each day (paired t-test).
Cerebral pO2 during embolic-clot stroke
We used a minimally invasive angiographic approach to induce embolic-clot stroke in rabbits [34, 50-53]. This approach involves trans-arterial injection of pre-formed blood clot to model human cerebral thromboembolism. The angiographic access to the internal carotid artery (ICA) is obtained via cannulation of the femoral artery in the hind limb of the rabbits. This approach is less invasive than other approaches that involve major surgery in the neck and/or brain region to induce stroke [54, 55]. The angiographic approach also avoids surgical distortions in the regional arteries supplying the brain, does not occlude the common carotid artery (CCA) thereby preserving inflow, and provides a pure embolic defect. The injection of large pre-aged clots (4-mm length, 3 days) not only mimics major stroke, but can also be lysed by tPA (tissue plasminogen activator). This model simulates the human embolic stroke in which occlusive material, usually clot or atheroma, is dislodged and passed into the large cerebral arteries. This model also allows one to immediately confirm the presence and location of the occlusion (Fig. 5).
Fig. 5. Angiographic images showing arterial occlusion following ischemic stroke.
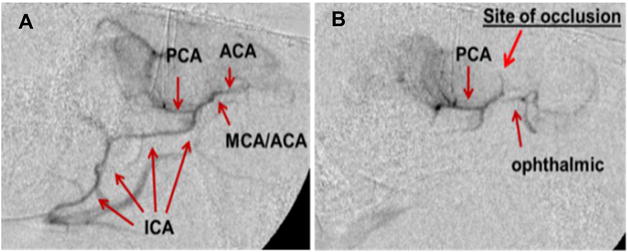
Arterial network in (A) pre-stroke and (B) post-stroke induced by embolic blood clot as described in the methods section. ICA: Internal carotid artery; MCA: Middle cerebral artery; ACA: Anterior cerebral artery; PCA: Posterior cerebral artery.
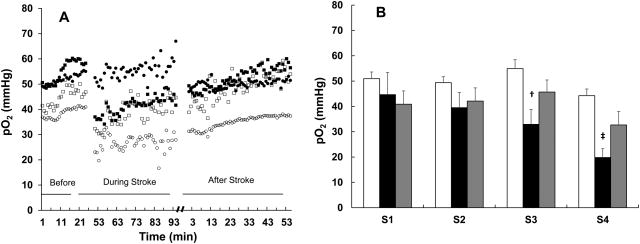
To perform EPR oximetry in the emboli-stroke model a, we first determined the typical locations of infarct core and penumbra in 5 test rabbits, which were not part the study groups. Using this information, we could target the resonator implantation so that the sensors could be in each of the following locations: infarct core (S4), ischemic penumbra (S3) and matching regions in the other cerebral hemisphere (S2, S1). After acquiring pO2 measurements in rabbits breathing 30% O2 (baseline), clot was injected to induce ischemic stroke. Cerebral pO2 was monitored immediately after the occlusion and 24 h later (Fig. 6). A significant and rapid decline in the pO2 of the infarct core and ischemic penumbra was evident (Fig. 6). No change in the pO2 of the contralateral region was observed. Tissue pO2 in the penumbra (S3) returned to baseline at 24-h post-stroke; however, low levels of pO2 in the infarct core persisted during this period. The results demonstrated the capability of EPR oximetry for real-time monitoring of ischemia induction and oxygen dynamics during the development of embolic clot-induced stroke. Such direct and repeated measurements of oxygen levels in the brain will provide valuable insight on the temporal changes during ischemic stroke and will facilitate evaluation of the hyperoxic strategies to improve oxygen levels and treatment outcome.
Fig. 6. Cerebral pO2 at 4 different sites in the rabbit brain with stroke.
(A) Changes in pO2 in the ischemic core (S4; ○), contralateral cortex (S1; ●), peripheral region (S3, □) and contralateral sub-cortex (S2, ■) before, during and after induction of stroke. (B) Mean pO2 levels at each site during baseline, at 30-min post-stroke and at 24-h post-stroke. Data represent Mean±SEM (N = 3-5). †p<0.05; ‡p<0.01, compared with baseline pO2 (paired t-test).
Measurement of physiology and infarct volume
The arterial oxyhemoglobin saturation, heart rate, and core temperature of animals breathing 30% O2 were stable. SpO2 values significantly decreased during hypoxia (15% O2) as compared to the baseline. On the other hand, a significant increase in arterial oxyhemoglobin saturation after carbogen inhalation was observed when compared to the baseline (data not shown). The arterial oxyhemoglobin saturation, heart rate, and core temperature of animals at 30 min and 24 hours after embolic clot stroke were stable (data not shown). Fig. 7 shows the axial view of whole rabbit brain with the track of the implantable sensor on the surface and infarction in rostral and caudal coronal sections of the brain after 24 h of embolization. A close examination of the tissue sections revealed that the sensors S4 and S3 were in the infarct core and ischemic penumbra regions, respectively, in all 5 rabbits. Quantitation of the infarct volume indicated a significant infarct in the ischemic hemisphere of all surviving rabbits in embolic clot stroke group.
Fig 7. Coronal sections and infarct size.
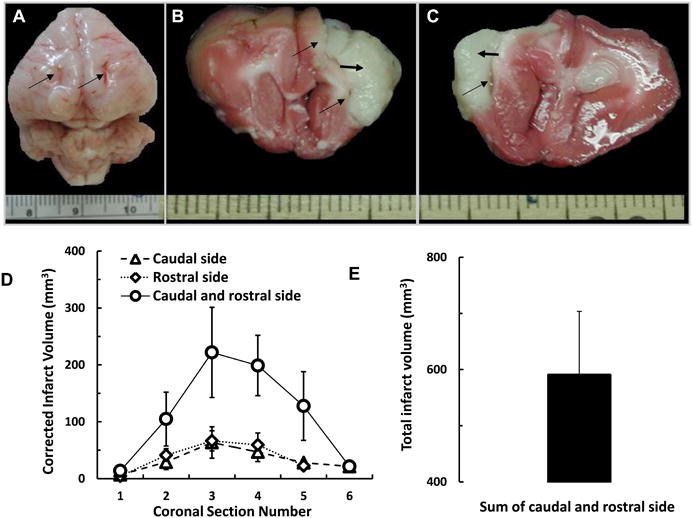
(A) Axial view of whole rabbit brain with the track of the implantable sensor on the surface indicated by long arrows. (B) Rostral and (C) caudal coronal sections of the brain after 24 h of arterial embolization showing the infarct region indicated by short arrow. (D) Infarct volume in each of the 6 coronal sections for rostral and caudal sides. (E) Total infarct volume summed over the 6 sections for rostral and caudal sides. Corrected infarct area = measured infarct area × area of contralateral corresponding structure/area of ipsilateral corresponding structure. Infarction volumes for continuous macrosections = Σ(thickness × ½ (corrected infarct area of a section's rostral surface + corrected infarct area of a section's caudal surface). The thickness of each section is 5 mm and was stained with the vital dye TTC. Viable areas stain red and non-viable areas remain white indicated with black short arrow. The scale bars represent the millimeters.
The results presented in this report is part of our systematic study to characterize the changes in brain pO2 during inhalation of hypoxic and hyperoxic gases using in vivo EPR oximetry. We have previously reported the effect of breathing hypoxic (10-15% O2) and hyperoxic (100% O2, carbogen and hyperbaric oxygen therapy) challenges on the brain pO2 in rats and rabbits with the goal to understand the sensitivity of IRs to local changes of brain pO2 and how improved oxygen levels protect brain from ischemic stroke in the brain [28,29,32,33].
Conclusions
The results of the present study demonstrated the capability of EPR oximetry to perform repeated measurements of cerebral pO2 simultaneously at multiple sites. The method enables reliable measurements of temporal changes in cerebral pO2 under pathophysiological conditions over a period of weeks. The dynamic information of cerebral pO2 can be used to test and to optimize strategies for improving treatment outcome of ischemic stroke.
Acknowledgments
This work was supported by National Institutes of Health grants NS082585 and EB004031
Footnotes
Conflict of interest: None
References
- 1.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. British Journal of Radiology. 1999;72(859):627–630. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 2.Braun RD, Lanzen JL, Snyder SA, Dewhirst MW. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280(6):H2533–H2544. doi: 10.1152/ajpheart.2001.280.6.H2533. [DOI] [PubMed] [Google Scholar]
- 3.Seddon BM, Honess DJ, Vojnovic B, Tozer GM, Workman P. Measurement of tumor oxygenation: in vivo comparison of a luminescence fiber-optic sensor and a polarographic electrode in the p22 tumor. Radiat Res. 2001;155(6):837–46. doi: 10.1667/0033-7587(2001)155[0837:motoiv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Urano M, Chen Y, Humm J, Koutcher JA, Zanzonico P, Ling C. Measurements of tumor tissue oxygen tension using a time-resolved luminescence-based optical OxyLite probe: comparison with a paired survival assay. Radiat Res. 2002;158(2):167–73. doi: 10.1667/0033-7587(2002)158[0167:mottot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.O'Hara JA, Hou H, Demidenko E, Springett RJ, Khan N, Swartz HM. Simultaneous measurement of rat brain cortex PtO2 using EPR oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiol Meas. 2005;26(3):203–13. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 6.Vikram DS, Bratasz A, Ahmad R, Kuppusamy P. A comparative evaluation of EPR and OxyLite oximetry using a random sampling of pO(2) in a murine tumor. Radiat Res. 2007;168(3):308–15. doi: 10.1667/RR0854.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengl M, Jaeger M, Renner C, Meixensberger J. Comparing brain tissue oxygen measurements and derived autoregulation parameters from different probes (Licox vs Raumedic) Acta Neurochir Suppl. 2012;114:165–8. doi: 10.1007/978-3-7091-0956-4_31. [DOI] [PubMed] [Google Scholar]
- 8.Keddie S, Rohman L. Reviewing the reliability, effectiveness and applications of Licox in traumatic brain injury. Nurs Crit Care. 2012;17(4):204–12. doi: 10.1111/j.1478-5153.2012.00499.x. [DOI] [PubMed] [Google Scholar]
- 9.Morgalla MH, Haas R, Grozinger G, Thiel C, Thiel K, Schuhmann MU, Schenk M. Experimental comparison of the measurement accuracy of the Licox((R)) and Raumedic ((R)) Neurovent-PTO brain tissue oxygen monitors. Acta Neurochir Suppl. 2012;114:169–72. doi: 10.1007/978-3-7091-0956-4_32. [DOI] [PubMed] [Google Scholar]
- 10.Tran LB, Bol A, Labar D, Jordan B, Magat J, Mignion L, Gregoire V, Gallez B. Hypoxia imaging with the nitroimidazole 18F-FAZA PET tracer: a comparison with OxyLite, EPR oximetry and 19F-MRI relaxometry. Radiother Oncol. 2012;105(1):29–35. doi: 10.1016/j.radonc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Orringer DA, Vago DR, Golby AJ. Clinical applications and future directions of functional MRI. Semin Neurol. 2012;32(4):466–75. doi: 10.1055/s-0032-1331816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna MC, Matsumoto S, Yasui H, Saito K, Devasahayam N, Subramanian S, Mitchell JB. Electron paramagnetic resonance imaging of tumor pO(2) Radiat Res. 2012;177(4):376–86. doi: 10.1667/rr2622.1. [DOI] [PubMed] [Google Scholar]
- 13.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674–87. doi: 10.1038/nrclinonc.2012.171. [DOI] [PubMed] [Google Scholar]
- 14.Giraudeau C, Djemai B, Ghaly MA, Boumezbeur F, Meriaux S, Robert P, Port M, Robic C, Le Bihan D, Lethimonnier F, Valette J. High sensitivity 19F MRI of a perfluorooctyl bromide emulsion: application to a dynamic biodistribution study and oxygen tension mapping in the mouse liver and spleen. NMR Biomed. 2012;25(4):654–60. doi: 10.1002/nbm.1781. [DOI] [PubMed] [Google Scholar]
- 15.Gaertner FC, Souvatzoglou M, Brix G, Beer AJ. Imaging of hypoxia using PET and MRI. Curr Pharm Biotechnol. 2012;13(4):552–70. doi: 10.2174/138920112799436267. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Shah SJ, Wilmes LJ, Feiner J, Kodibagkar VD, Wendland MF, Mason RP, Hylton N, Hopf HW, Rollins MD. Quantitative tissue oxygen measurement in multiple organs using 19F MRI in a rat model. Magn Reson Med. 2011;66(6):1722–30. doi: 10.1002/mrm.22968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baete SH, Vandecasteele J, De Deene Y. 19F MRI oximetry: simulation of perfluorocarbon distribution impact. Phys Med Biol. 2011;56(8):2535–57. doi: 10.1088/0031-9155/56/8/013. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad R, Caia G, Potter LC, Petryakov S, Kuppusamy P, Zweier JL. In vivo multisite oximetry using EPR-NMR coimaging. J Magn Reson. 2010 doi: 10.1016/j.jmr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Runge VM. Current Technological Advances in Magnetic Resonance with Critical Impact for Clinical Diagnosis and Therapy. Invest Radiol. 2013 doi: 10.1097/01.rli.0000434380.71793.d3. [DOI] [PubMed] [Google Scholar]
- 20.Christen T, Bolar DS, Zaharchuk G. Imaging brain oxygenation with MRI using blood oxygenation approaches: methods, validation, and clinical applications. AJNR Am J Neuroradiol. 2013;34(6):1113–23. doi: 10.3174/ajnr.A3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blockley NP, Griffeth VE, Simon AB, Buxton RB. A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism. NMR Biomed. 2013;26(8):987–1003. doi: 10.1002/nbm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vikram DS, Zweier JL, Kuppusamy P. Methods for noninvasive imaging of tissue hypoxia. Antioxid Redox Signal. 2007;9(10):1745–56. doi: 10.1089/ars.2007.1717. [DOI] [PubMed] [Google Scholar]
- 23.Tuunanen PI, Kauppinen RA. Effects of oxygen saturation on BOLD and arterial spin labelling perfusion fMRI signals studied in a motor activation task. Neuroimage. 2006;30(1):102–9. doi: 10.1016/j.neuroimage.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Dunn JF, O'Hara J, Zaim-Wadghiri Y, Zhu H, Lei H, Meyerand ME, Grinberg O, Hou HG, Hoopes PJ, Demidenko E, Swartz HM. Changes in oxygenation of intracranial tumors with carbogen, an MR “BOLD” imaging and EPR oximetry study. J Magn Reson Imaging. 2002;16:511–521. doi: 10.1002/jmri.10192. [DOI] [PubMed] [Google Scholar]
- 25.Demeure RJ, Jordan BF, Yang QX, Beghein N, Smith MB, Gregoire V, Gallez B. Removal of local field gradient artefacts in BOLD contrast imaging of head and neck tumours. Physics in Medicine and Biology. 2002;10:1819–1825. doi: 10.1088/0031-9155/47/10/315. [DOI] [PubMed] [Google Scholar]
- 26.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9(8):1169–82. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou H, Khan N, O'Hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Demidenko E, Lu S, Steffen RP, Swartz HM. Increased oxygenation of intracranial tumors by efaproxyn (efaproxiral), an allosteric hemoglobin modifier: In vivo EPR oximetry study. Int J Radiat Oncol Biol Phys. 2005;61(5):1503–9. doi: 10.1016/j.ijrobp.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 28.Hou H, Grinberg OY, Grinberg SA, Demidenko E, Swartz HM. Cerebral tissue oxygenation in reversible focal ischemia in rats: multi-site EPR oximetry measurements. Physiol Meas. 2005;26(1):131–41. doi: 10.1088/0967-3334/26/1/012. [DOI] [PubMed] [Google Scholar]
- 29.Hou H, Grinberg O, Williams B, Grinberg S, Yu H, Alvarenga DL, Wallach H, Buckey J, Swartz HM. The effect of oxygen therapy on brain damage and cerebral pO(2) in transient focal cerebral ischemia in the rat. Physiol Meas. 2007;28(8):963–76. doi: 10.1088/0967-3334/28/8/017. [DOI] [PubMed] [Google Scholar]
- 30.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43(7):1957–75. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 31.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90(12):5438–42. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou H, Li H, Dong R, Khan N, Swartz H. Real-time monitoring of ischemic and contralateral brain pO2 during stroke by variable length multisite resonators. Magn Reson Imaging. 2014;32(5):563–9. doi: 10.1016/j.mri.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan N, Hou H, Eskey CJ, Moodie K, Gohain S, Du G, Hodge S, Culp WC, Kuppusamy P, Swartz HM. Deep-tissue oxygen monitoring in the brain of rabbits for stroke research. Stroke. 2015;46(3):e62–6. doi: 10.1161/STROKEAHA.114.007324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culp WC, Woods SD, Brown AT, Lowery JD, Hennings LJ, Skinner RD, Borrelli MJ, Roberson PK. Three variations in rabbit angiographic stroke models. J Neurosci Methods. 2013;212(2):322–8. doi: 10.1016/j.jneumeth.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods SD, Flores R, Roberson PK, Lowery JD, Skinner RD, Culp WC. Decreased Serum Levels of S-100B Protein Reflect Successful Treatment Effects in a Rabbit Model of Acute Ischemic Stroke. Open Neurol J. 2011;5:55–7. doi: 10.2174/1874205X01105010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hennings LJ, Flores R, Roberson PK, Brown A, Lowery J, Borrelli M, Culp WC. Persistent penumbra in a rabbit stroke model: incidence and histologic characteristics. Stroke Res Treat. 2011;2011:764830. doi: 10.4061/2011/764830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bederson J, Pitts L, Germano S, Nishimura M, Davis R, Bartkowski H. Evaluation of of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 38.Dinguizli M, Jeumont S, Beghein N, He J, Walczak T, Lesniewski PN, Hou H, Grinberg OY, Sucheta A, Swartz HM, Gallez B. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron. 2006;21(7):1015–22. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Hou H, Dong R, Li H, Williams B, Lariviere JP, Hekmatyar SK, Kauppinen RA, Khan N, Swartz H. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: a multisite EPR oximetry with implantable resonators. J Magn Reson. 2012;214(1):22–8. doi: 10.1016/j.jmr.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou H, Li H, Dong R, Mupparaju S, Khan N, Swartz H. Cerebral oxygenation of the cortex and striatum following normobaric hyperoxia and mild hypoxia in rats by EPR oximetry using multi-probe implantable resonators. Adv Exp Med Biol. 2011;701:61–7. doi: 10.1007/978-1-4419-7756-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Hou H, Sucheta A, Williams BB, Lariviere JP, Khan MN, Lesniewski PN, Gallez B, Swartz HM. Implantable resonators--a technique for repeated measurement of oxygen at multiple deep sites with in vivo EPR. Adv Exp Med Biol. 2010;662:265–72. doi: 10.1007/978-1-4419-1241-1_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan N, Hou H, Eskey CJ, Moodie K, Gohain S, Du G, Hodge S, Culp WC, Kuppusamy P, Swartz HM. Deep-Tissue Oxygen Monitoring in the Brain of Rabbits for Stroke Research. Stroke. 2015;46(3):e62–66. doi: 10.1161/STROKEAHA.114.007324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirata H, Walczak T, Swartz HM. Electronically tunable surface-coil-type resonator for L-band EPR spectroscopy. J Magn Reson. 2000;142(1):159–67. doi: 10.1006/jmre.1999.1927. [DOI] [PubMed] [Google Scholar]
- 44.Salikhov I, Hirata H, Walczak T, Swartz HM. An improved external loop resonator for in vivo L-band EPR spectroscopy. J Magn Reson. 2003;164(1):54–9. doi: 10.1016/s1090-7807(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 45.Smirnov AI, Norby SW, Clarkson RB, Walczak T, Swartz HM. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med. 1993;30(2):213–20. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 46.Hou H, Mupparaju SP, Lariviere JP, Hodge S, Gui J, Swartz HM, Khan N. Assessment of the changes in 9L and C6 glioma pO2 by EPR oximetry as a prognostic indicator of differential response to radiotherapy. Radiat Res. 2013;179(3):343–51. doi: 10.1667/RR2811.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou H, Dong R, Lariviere JP, Mupparaju SP, Swartz HM, Khan N. Synergistic combination of hyperoxygenation and radiotherapy by repeated assessments of tumor pO2 with EPR oximetry. J Radiat Res. 2011;52(5):568–74. doi: 10.1269/jrr.11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan N, Mupparaju S, Hekmatyar SK, Hou H, Lariviere JP, Demidenko E, Gladstone DJ, Kauppinen RA, Swartz HM. Effect of hyperoxygenation on tissue pO2 and its effect on radiotherapeutic efficacy of orthotopic F98 gliomas. Int J Radiat Oncol Biol Phys. 2010;78(4):1193–200. doi: 10.1016/j.ijrobp.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan N, Li H, Hou H, Lariviere JP, Gladstone DJ, Demidenko E, Swartz HM. Tissue pO2 of orthotopic 9L and C6 gliomas and tumor-specific response to radiotherapy and hyperoxygenation. Int J Radiat Oncol Biol Phys. 2009;73(3):878–85. doi: 10.1016/j.ijrobp.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown A, Woods S, Skinner R, Hatton J, Lowery J, Roberson P, Hennings L, Culp WC. Neurological assessment scores in rabbit embolic stroke models. Open Neurol J. 2013;7:38–43. doi: 10.2174/1874205X01307010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown AT, Skinner RD, Flores R, Hennings L, Borrelli MJ, Lowery J, Culp WC. Stroke location and brain function in an embolic rabbit stroke model. J Vasc Interv Radiol. 2010;21(6):903–9. doi: 10.1016/j.jvir.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Culp BC, Culp WC. Rabbit subselective angiography stroke model. Stroke. 2008;39(11):e165. doi: 10.1161/STROKEAHA.108.526335. author reply e166. [DOI] [PubMed] [Google Scholar]
- 53.Culp BC, Brown AT, Erdem E, Lowery J, Culp WC. Selective intracranial magnification angiography of the rabbit: basic techniques and anatomy. J Vasc Interv Radiol. 2007;18(2):187–92. doi: 10.1016/j.jvir.2006.12.720. [DOI] [PubMed] [Google Scholar]
- 54.Chen ZZ, Jiang XD, Zhang LL, Shang JH, Du MX, Xu G, Xu RX. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci Lett. 2008;445(1):36–41. doi: 10.1016/j.neulet.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 55.Lapchak PA. Translational Stroke Research Using a Rabbit Embolic Stroke Model: A Correlative Analysis Hypothesis for Novel Therapy Development. Transl Stroke Res. 2010;1(2):96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Hou H, Sucheta A, Williams BB, Lariviere JP, Khan MN, Lesniewski PN, Gallez B, Swartz HM. Implantable Resonators - A Technique for Repeated Measurement of Oxygen at Multiple Deep Sites with In Vivo EPR. Adv Exp Med Biol. 2010;662:265–72. doi: 10.1007/978-1-4419-1241-1_38. [DOI] [PMC free article] [PubMed] [Google Scholar]