Abstract
Objective
To evaluate the relationship between age- and gender-adjusted dehydroepiandrosterone sulfate (DHEA-S) levels and low-dose adrenocorticotropic hormone (ACTH) stimulation in assessing the integrity of the hypothalamic-pituitary-adrenal (HPA) axis, in patients who were at risk of HPA insufficiency, including those currently being treated with glucocorticoids.
Methods
Forty-six participants with a suspicion of secondary adrenal insufficiency were recruited from the Diabetes and Endocrinology Clinic at Ramathibodi Hospital, Mahidol University, Bangkok. Low-dose (1 μg) ACTH stimulation was performed in every participants, and serum DHEA-S was measured at baseline before ACTH injection.
Results
Individuals with normal age- and gender-specific DHEA-S levels had baseline serum cortisol and peak cortisol levels higher than those with reduced DHEA-S. Normal age- and gender-specific DHEA-S levels predicted intact HPA function with a sensitivity of 87.1%, a specificity of 86.7%, a positive predictive value of 93.1%, and a negative predictive value of 76.5%. To account for the age and gender dependency of DHEA-S, the DHEA-S ratio was calculated by measured DHEA-S divided by the lower limit of the respective reference range for all participants. A DHEA-S ratio of more than 1.78 had 100% sensitivity regarding intact HPA function. Area under the receiver operating characteristic [ROC] curve was 0.920. (95% CI, 0.844–0.997).
Conclusion
Normal age- and gender-specific DHEA-S level or a DHEA-S ratio of more than 1.78 are valuable markers of HPA integrity. Serum DHEA-S may be a candidate for a less costly approach where ACTH stimulation is unavailable.
Keywords: ACTH stimulation, Dehydroepiandrosterone sulfate, DHEA-S, HPA, Insufficiency
Introduction
The hypothalamic-pituitary-adrenal axis (HPA) is a complex process of influences and feedback interactions among three endocrine glands – the hypothalamus, the pituitary gland and the adrenal glands – which is important in maintaining many vital organ functions. The impairment of HPA function as a result of decreased corticotropin-releasing hormone (CRH) and/or adrenocorticotropic hormone (ACTH) secretion from the hypothalamus and pituitary gland, respectively, is called secondary adrenal insufficiency. Nowadays, establishing a diagnosis of secondary adrenal insufficiency remains challenging. Dynamic testing with low-dose (1 μg) ACTH stimulation is the most commonly used method to define HPA integrity [1], [2], [3]. The exact diagnostic serum cortisol cutoffs of low-dose ACTH stimulation are still debated, and depend on assay-specific local reference ranges. In clinical practice, they are set at 18–20 μg/dL, with very low false negative results [4], [5]. However, low-dose ACTH stimulation is usually troublesome for many internists and patients, especially in primary care setting, because of its time-consuming protocol and the unavailability of synthetic ACTH.
Dehydroepiandrosterone sulfate (DHEA-S) is the most abundant steroid hormone in the circulation; almost all is secreted by the zona reticularis of the adrenal cortex. DHEA-S secretion is mediated by the trophic effect of ACTH. Only minimal concentrations of DHEA-S are contributed by the testes [6], [7]. DHEA-S measurement is quite convenient, with a widely available assay. Blood sampling can be performed at any time of the day because of the long half-life of DHEA-S and the lack of circulating diurnal variations [8], [9], [10], [11]. Recent evidence has suggested that DHEA-S level is a good predictive marker of HPA impairment [12], [13]. When DHEA-S level is normal, the diagnosis of HPA impairment is extremely unlikely. However, this finding was based on patients with a large pituitary tumor. A relationship between serum DHEA-S level and low-dose ACTH stimulation in real-world populations – including patients currently being treated with glucocorticoids, with or without a pituitary lesion – is still questionable.
Materials and methods
Study design
We conducted a cross-sectional study in adult participants, 18–59 years of age, with a suspicion of HPA insufficiency or secondary adrenal insufficiency (e.g. known pituitary or hypothalamic tumors, other central nervous system tumors which structurally involve the pituitary gland and/or hypothalamus, and previous history of consecutive glucocorticoid intake or injections more than 3 months). All participants were sent for HPA axis evaluation using low-dose ACTH stimulation test. The testing was performed at least 3 months after the onset of HPA axis insult to ensure that adrenal atrophy had occured. Participants were recruited from the Diabetes and Endocrinology Clinic at Ramathibodi Hospital, Mahidol University, Bangkok. Written informed consent was obtained from all participants. The study was approved by the Mahidol University Institutional Review Board.
Exclusion criteria included known primary adrenal insufficiency, known ACTH-producing tumor, or current use of medications known to have significant effects on cortisol and DHEA-S measurements, including oral contraceptive drugs, central nervous system agents that induce hepatic enzymes (e.g. carbamazepine, clomipramine, imipramine, phenytoin), dopaminergic drugs (e.g. levodopa/dopamine, bromocriptine), neuroleptic agents, danazol, and nicotine [14]. Patients with serum albumin of less than 25 g/L, which can cause falsely low cortisol levels, were also excluded.
Baseline data collected from all participants included gender, age, current height and weight, indication for HPA axis evaluation, and a detailed medical history including current medications. If patients were currently on glucocorticoid treatment, the regimen must be stopped for at least 24 h before testing. Any food or caloric intake was prohibited for at least 8 h before testing.
ACTH preparation
Synacthen is a brand name of tetracosactide (1–24 synthetic ACTH) used at Ramathibodi Hospital. It is available in the form of a vial containing 250 μg of tetracosactide per 1 mL. One mL of Synacthen was drawn from the vial and was reinjected into a 49 mL bag of normal saline. After mixing, Synacthen was refrigerated at 4 °C. The drug was discarded after being used by 10 participants, or more than 2 months after mixing.
Low-dose ACTH stimulation
Low-dose ACTH stimulation was performed in an ambulatory setting, in the early morning (mostly between 8.00 and 9.00 a.m.). Synacthen (0.2 mL, equivalent to 1 μg) was drawn from the already-mixed solution, diluted with normal saline to a volume of 1 mL, and injected intravenously into each participant. Serum cortisol, albumin and DHEA-S levels were measured at baseline before the injection. Serum cortisol was measured at 20, 30 and 40 min after the injection.
Laboratory methods
Serum DHEA-S was analyzed by a solid-phase, competitive chemiluminescent enzyme immunoassay on an IMMULITE® immunoassay system (Siemens Healthcare, Erlangen, Germany). The intra-assay coefficient of variation of serum DHEA-S level was 5.8%. Normal DHEA-S levels were defined as values greater than the 5th percentile of the manufacturer’s reference data.
Serum cortisol was analyzed by IMMULITE 2000 immunoassay using a chemiluminescent technique (Siemens Healthcare). The lower limit of detectability was 0.2 μg/dL.
Statistical analysis
Data were analyzed using SPSS version 18 (SPSS, Chicago, IL). Data were summarized using mean ± SD if the data were normally distributed, or median (interquartile range) if the data were not normally distributed. Two-independent-sample t-test was used to analyze differences in continuous variables between study groups where the variables were normally distributed. Mann–Whitney U test was used to analyze differences in continuous variables between study groups where the variables were not normally distributed. Significance was determined according to P < 0.05. Diagnostic values, including sensitivity, specificity and predictive value, were assessed by confusion matrix calculations. Receiver operating characteristic (ROC) curves were plotted for DHEA-S ratios, which were derived by dividing the measured DHEA-S by the lower limit (5th percentile) of the respective reference range for all participants, using peak cortisol response after low-dose ACTH stimulation of more than 18 μg/dL as the definition of an intact HPA axis.
Results
A total of 46 patients were included in the study. Among them, 30 were female (65%) with a mean age of 46.5 years; 45.7% of the participants were 50–59 years of age. Most of the participants were sent for HPA axis evaluation due to the presence of pituitary macroadenoma (31 participants; 67.4%). The other indications included past history of prolonged glucocorticoid exposure (10 participants; 21.6%), and other CNS tumors, e.g. meningioma and lymphoma which structurally involve the pituitary gland (5 participants; 11%) (Table 1). For the patients with history of prolonged glucocorticoid exposure, all of them received prednisolone of more than 7.5 mg per day for more than 3 months prior to evaluation.
Table 1.
Baseline characteristics of participants.
| Characteristics | Total, n = 46 (%) |
|---|---|
| Gender | |
| Male | 16 (34.8) |
| Female | 30 (65.2) |
| Age (years) | |
| Median (IQR) = 50 (40–55) | |
| 20–29 | 4 (8.7) |
| 30–39 | 9 (19.6) |
| 40–49 | 12 (26) |
| 50–59 | 21 (45.7) |
| Indication for HPA axis testing | |
| Pituitary macroadenomas | 31 (67.4) |
| Other central nervous system tumors | 5 (11) |
| History of glucocorticoid exposure | 10 (21.6) |
Of all participants, 31 (67.4%) were found to have intact HPA function, defined by peak cortisol response of more than 18 μg/dL after low-dose ACTH stimulation, whereas 15 were found to have less than 18 μg/dL of peak cortisol response, which was defined as abnormal HPA. Participants with abnormal HPA had mean baseline serum cortisol levels that were lower than those with intact HPA function (6.53 ± 3.54 μg/dL and 10.48 ± 3.46 μg/dL, respectively, p = 0.01). Median peak cortisol levels after ACTH stimulation in the abnormal and intact HPA groups were 15.3 μg/dL (IQR = 10.4–17.3) and 21.7 μg/dL (IQR = 19.7–24.1), respectively (p < 0.01) (Table 2).
Table 2.
Biochemical findings in participants with intact HPA axis and abnormal HPA axis, defined by peak cortisol >18 μg/dL after ACTH stimulation.
| Intact HPA | Abnormal HPA | P-value | |
|---|---|---|---|
| Number of participants | 31 | 15 | – |
| Mean baseline cortisol (μg/dL) (±SD) | 10.48 (±3.46) | 6.53 (±3.54) | 0.01 |
| Peak cortisol | |||
| Median (μg/dL) (IQR) | 21.7 (19.7–24.1) | 15.3 (10.4–17.3) | <0.01 |
| Range (μg/dL) (Min–Max) | 18.1–29.9 | 1–17.8 | |
| DHEA-S | |||
| Median (μg/dL) (IQR) | 87.6 (48.7–150) | 30.4 (10.5–55.7) | <0.01 |
| Range (μg/dL) (Min - Max) | 20–292 | 0–80.1 | |
Serum DHEA-S levels
Median serum DHEA-S levels in the abnormal HPA group (30.4 μg/dL, IQR = 10.5–55.7) were apparently lower than those with intact HPA function (87.6 μg/dL, IQR = 48.7–150), p < 0.01 (Table 2). Participants with normal age- and gender-specific DHEA-S levels had baseline serum cortisol levels and peak cortisol levels higher than those with reduced DHEA-S. Median peak cortisol levels after ACTH stimulation in participants with normal and low age- and gender-specific DHEA-S were 22.4 μg/dL (IQR = 19.7–24.1) and 16 μg/dL (IQR = 11.2–17.7), respectively, p < 0.01 (Table 3). 27 of 29 participants (93.1%) with normal DHEA-S had intact HPA function, while 13 of 17 participants (76.5%) with reduced DHEA-S levels had abnormal HPA (Table 4). Normal age- and gender-specific DHEA-S levels predicted intact HPA function with a sensitivity of 87.1%, a specificity of 86.7%, a positive predictive value of 93.1%, and a negative predictive value of 76.5% (Table 4). Separate analysis was performed after exclusion of participants who were currently on glucocorticoid treatment. In this latter cohort, normal age- and gender-specific DHEA-S levels predicted intact HPA function with a sensitivity of 92.6%, a specificity of 77.8%, a positive predictive value of 92.6%, and a negative predictive value of 77.8%.
Table 3.
Biochemical findings in participants with normal and less than normal age- and gender-specific DHEA-S levels.
| Normal DHEA-S | Reduced DHEA-S | P-value | |
|---|---|---|---|
| Number of participants | 29 | 17 | – |
| Mean baseline cortisol (μg/dL) (±SD) | 10.33 (±3.34) | 7.25 (±4.16) | 0.015 |
| Peak cortisol | |||
| Median (μg/dL) (IQR) | 22.4 (19.7–24.1) | 16 (11.2–17.7) | <0.01 |
| Range (μg/dL) (Min–Max) | 16.8 – 29.9 | 1–21.5 | |
Table 4.
Diagnostic value of normal DHEA-S in predicting intact HPA status.
| Intact HPA | Abnormal HPA | Total, n = 46 | |
|---|---|---|---|
| Normal DHEA-S | 27 | 2 | 29 |
| Reduced DHEA-S | 4 | 13 | 17 |
Sensitivity = 87.1%.
Specificity = 86.7%.
Positive predictive value = 93.1%.
Negative predictive value = 76.5%.
ROC curve for DHEA-S ratio
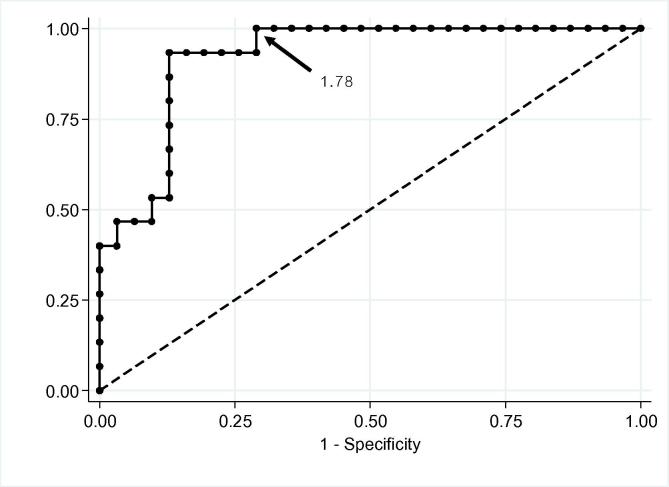
After adjusting for age- and gender-specific DHEA-S levels by calculating the DHEA-S ratio – derived from dividing measured DHEA-S by the lower limit (5th percentile) of the respective reference range for all participants – the baseline DHEA-S ratio was plotted in an ROC curve. The curve was drawn based on a peak cortisol response of more than 18 μg/dL as the gold standard for defining intact HPA function. The area under the curve was 0.920 (95% CI, 0.844–0.997), with 100% sensitivity when the DHEA-S ratio was above 1.78 (Fig. 1).
Fig. 1.
Receiver operating characteristic curve for baseline DHEA-S ratio (dividing measured DHEA-S by the lower limit [5th percentile] of the respective reference range). The curve was drawn based on a peak cortisol response of more than 18 μg/dL as the definition of an intact HPA axis. The area under the curve was 0.920 (95% CI, 0.844–0.997). DHEA-S = dehydroepiandrosterone sulfate; HPA = hypothalamic–pituitary–adrenal.
Discussion
The ACTH stimulation test, also known as the short synacthen test or cosyntropin test, is commonly used for the diagnosis of adrenal insufficiency. This test assesses the serum cortisol response to acute ACTH stimulation with either a 250-μg dose (high or standard dose) or 1-μg dose (low dose). Clinically, low-dose ACTH stimulation is preferable to high-dose ACTH stimulation in establishing the diagnosis of secondary adrenal insufficiency. Based on the assumption that diminished endogenous ACTH in secondary adrenal insufficiency leads to adrenal cortex atrophy, cortisol secretion would not respond appropriately to certain dose of exogenously administered ACTH. Therefore, 250-μg dose of ACTH stimulation, which considered as a supraphysiological stimulus, might overcome the suboptimal cortisol response, and would result in a false negative result. Recent meta-analysis in 2016 showed that high-dose ACTH stimulation had lower sensitivity (64%) than low-dose ACTH stimulation (83%) in detecting secondary adrenal insufficiency [15]. Although low-dose ACTH stimulation is not gold standard testing, it remains the most comprehensive and practical method to validate HPA axis integrity. The cut point of peak cortisol levels of more than 18 μg/dL is widely accepted in clinical practice. Nowadays, in patients with suspected HPA insufficiency, baseline morning serum cortisol levels are of limited value in guiding the diagnosis, and ACTH stimulation is frequently needed in many patients. This may lead to unnecessary time-consuming procedures, especially in patients with low pre-test probability of adrenal insufficiency.
DHEA-S is a promising adjunctive tool in determining HPA status. Impairment of adrenal androgen secretion precedes loss of glucocorticoid secretion in patients with impaired HPA function [16], [17]. The explanations of this phenomenon have been previously proposed by Topor et al. (2011). In vitro study revealed that cortisol at physiological intra-adrenal concentration stimulated DHEA secretion in a dose-dependent fashion, which may be due to its ability to inhibit 3β-hydroxysteroid dehydrogenase type II activity [18]. Therefore, only a minimal reduction of intra-adrenal cortisol concentration in early adrenal insufficiency will result in lower DHEA secretion due to increased 3β-hydroxysteroid dehydrogenase type II activity. In other words, normal secretion of adrenal androgen should imply normal cortisol secretion from adrenocortical cells. Our data demonstrate that participants with normal age- and gender-specific DHEA-S levels are likely to have peak cortisol levels after 1 μg ACTH stimulation of more than 18 μg/dL, which normally defines intact HPA function, with a sensitivity of 87.1%, a specificity of 86.7%, a positive predictive value of 93.1% and a negative predictive value of 76.5%. However, this result should be interpreted cautiously because we did not perform an insulin tolerance test, which is considered a gold standard method in defining HPA integrity. The implication from this result is that normal DHEA-S levels, can predict the likelihood of HPA integrity, defined by adequate response to 1 μg ACTH stimulation. In clinical situations where pre-test probability of adrenal insufficiency is low, the presence of normal DHEA-S levels would provide additional support for HPA integrity and adequate adrenocortical function, especially in primary care medical centers with limited capacity to perform ACTH stimulation.
Normally, DHEA and DHEA-S are released simultaneously with cortisol from the adrenal cortex [19]. Only minimal concentrations of DHEA-S are contributed by the testes, hence rendering DHEA-S levels almost unaltered by gonadal status [6]. However, several concerns are worth mentioning. First, DHEA-S levels decline with age [20], requiring age-specific references. Normal values in the elderly are quite low, especially in those more than 60 years of age. This implies that assessment of DHEA-S levels in extreme old age may not be accurate for determining the integrity of adrenocortical function. Based on the ROC curve created from our data, a DHEA-S ratio of more than 1.78, which gives 100% sensitivity in identifying intact HPA function, should be considered as the minimal requirement to define normal value in any group or gender. Second, similar to endogenous cortisol secretion, long-acting glucocorticoid supplementation may result in suppression of DHEA-S secretion, which persists even after cortisol secretion returns to normal [21]. However, this was not observed in our findings. Although a certain number of our participants (21.6%) had been exposed to consecutive doses of glucocorticoid, DHEA-S measurement still exhibited fairly good prediction of HPA status.
Earlier studies showed the diagnostic value of serum DHEA-S in patients with secondary adrenal insufficiency due to a large pituitary tumor [12], [13]. But evidence suggesting its application in other populations, escpecially those with chronic glucocorticoids exposure, is scarce. This may be due to the fact that prior use of glucocorticoids will affect serum level of DHEA-S [22]. However, our data demonstrated the usefulness of DHEA-S in predicting HPA status in both steroid users and non-users. The result of this study should extend the generalizability of DHEA-S utility toward the general population, with or without pituitary tumor, including patients who had previously received glucocorticoid treatment, which enabled us to assess the value of DHEA-S in a population reflecting daily clinical practice. To our knowledge, the report by Fischli et al. (2008) is the only published data that demonstrated similar results of DHEA-S diagnostic value on HPA function in a mixed population [23]. The difference between our study and that of Fischli et al. is that they used z-score as a representative of how far (in units of the population SD) a measured DHEA-S value was from the mean of a population with corresponding age and gender, which was calculated using reference curves provided by the assay manufacturer; whereas our study categorized DHEA-S into normal or reduced DHEA-S levels based on the 5th percentile of the age- and gender-specific assay reference data.
We acknowledge several limitations of our study. First, normal age- and gender-specific DHEA-S levels in our study were based on European Caucasian data. In view of ethnic differences of DHEA-S levels, this raises a concern on application in our cohorts which were Asians. Second, we did not collect certain data such as prolactin level and gonadal status which may interfere with DHEA-S level. Nevertheless, since prolactin or gonadal testing are rarely performed routinely, we consider our result as a “real-world” data which demonstrates the value of DHEA-S in general practice. Finally, the relatively small sample size in our study precluded a strong recommendation of DHEA-S utility, and did not allow further stratification of the subjects. Future research on DHEA-S measurement, including a cost-effectiveness evaluation, are needed.
Conclusion
Normal age- and gender-specific DHEA-S level or a DHEA-S ratio of more than 1.78 are valuable markers of HPA integrity, as defined by peak cortisol response after 1 μg ACTH stimulation of more than 18 μg/dL. Serum DHEA-S may be a candidate for a less costly approach where ACTH stimulation is unavailable, especially in patients with the presence of risk factors including chronic glucocorticoid exposure but clinically low suspicion of adrenal insufficiency.
Funding
This work was supported by Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Dorin R.I., Qualls C.R., Crapo L.M. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 2.Al-Aridi R., Abdelmannan D., Arafah B.M. Biochemical diagnosis of adrenal insufficiency: the added value of dehydroepiandrosterone sulfate measurements. Endocr Pract. 2011;17(2):261–270. doi: 10.4158/EP10262.RA. [DOI] [PubMed] [Google Scholar]
- 3.Thaler L.M., Blevins L.S., Jr. The low dose (1-microg) adrenocorticotropin stimulation test in the evaluation of patients with suspected central adrenal insufficiency. J Clin Endocrinol Metab. 1998;83(8):2726–2729. doi: 10.1210/jcem.83.8.5039. [DOI] [PubMed] [Google Scholar]
- 4.Agha A., Tomlinson J.W., Clark P.M., Holder G., Stewart P.M. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2006;91(1):43–47. doi: 10.1210/jc.2005-1131. [DOI] [PubMed] [Google Scholar]
- 5.Bancos I., Hahner S., Tomlinson J., Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216–226. doi: 10.1016/S2213-8587(14)70142-1. [DOI] [PubMed] [Google Scholar]
- 6.Kroboth P.D., Salek F.S., Pittenger A.L., Fabian T.J., Frye R.F. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39(4):327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- 7.Nafziger A.N., Bowlin S.J., Jenkins P.L., Pearson T.A. Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med. 1998;131(4):316–323. doi: 10.1016/s0022-2143(98)90181-0. [DOI] [PubMed] [Google Scholar]
- 8.Haning R.V. Using DHEAS to monitor androgen disorders. Contemp Ob/Gyn. 1981;18:117–131. [Google Scholar]
- 9.Sciarra F. Diagnosis of virilizing syndromes: endocrinological parameters. In: Molinatti G., editor. Androgenization in women. Raven Press; New York: 1983. pp. 85–113. [Google Scholar]
- 10.Tourniaire J., Pugeat M. Strategic approach of hyperandrogenism in women. Horm Res. 1983;18(1–3):125–134. doi: 10.1159/000179785. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen A. Androgen secretion by adrenals and gonads. In: Mahesh V., Greenblatt R.B., editors. Hirsutism and virilism. John Wright–PSG; Boston: 1983. pp. 17–34. [Google Scholar]
- 12.Nasrallah M.P., Arafah B.M. The value of dehydroepiandrosterone sulfate measurements in the assessment of adrenal function. J Clin Endocrinol Metab. 2003;88(11):5293–5298. doi: 10.1210/jc.2003-030449. [DOI] [PubMed] [Google Scholar]
- 13.Sayyed Kassem L., El Sibai K., Chaiban J., Abdelmannan D., Arafah B.M. Measurements of serum DHEA and DHEA sulphate levels improve the accuracy of the low-dose cosyntropin test in the diagnosis of central adrenal insufficiency. J Clin Endocrinol Metab. 2012;97(10):3655–3662. doi: 10.1210/jc.2012-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salek F.S., Bigos K.L., Kroboth P.D. The influence of hormones and pharmaceutical agents on DHEA and DHEA-S concentrations: a review of clinical studies. J Clin Pharmacol. 2002;42(3):247–266. doi: 10.1177/00912700222011274. [DOI] [PubMed] [Google Scholar]
- 15.Ospina N.S., Al Nofal A., Bancos I., Javed A., Benkhadra K., Kapoor E. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427–434. doi: 10.1210/jc.2015-1700. [DOI] [PubMed] [Google Scholar]
- 16.Laureti S., Candeloro P., Aglietti M.C., Giordano R., Arvat E., Ghigo E. Dehydroepiandrosterone, 17alpha-hydroxyprogesterone and aldosterone responses to the low-dose (1 micro g) ACTH test in subjects with preclinical adrenal autoimmunity. Clin Endocrinol (Oxf) 2002;57(5):677–683. doi: 10.1046/j.1365-2265.2002.01651.x. [DOI] [PubMed] [Google Scholar]
- 17.Laureti S., Arvat E., Candeloro P., Di Vito L., Ghigo E., Santeusanio F. Low dose (1 microg) ACTH test in the evaluation of adrenal dysfunction in pre-clinical Addison's disease. Clin Endocrinol (Oxf) 2000;53(1):107–115. doi: 10.1046/j.1365-2265.2000.01050.x. [DOI] [PubMed] [Google Scholar]
- 18.Topor L.S., Asai M., Dunn J., Majzoub J.A. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab. 2011;96(1):E31–E39. doi: 10.1210/jc.2010-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld R.S., Hellman L., Gallagher T.F. Metabolism and interconversion of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin Endocrinol Metab. 1972;35(2):187–193. doi: 10.1210/jcem-35-2-187. [DOI] [PubMed] [Google Scholar]
- 20.Orentreich N., Brind J.L., Rizer R.L., Vogelman J.H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 21.Friel P.N., Alexander T., Wright J.V. Suppression of adrenal function by low-dose prednisone: assessment with 24-hour urinary steroid hormone profiles–a review of five cases. Altern Med Rev. 2006;11(1):40–46. [PubMed] [Google Scholar]
- 22.Parker L.N. Control of adrenal androgen secretion. Endocrinol Metab Clin North Am. 1991;20(2):401–421. [PubMed] [Google Scholar]
- 23.Fischli S., Jenni S., Allemann S., Zwahlen M., Diem P., Christ E.R. Dehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2008;93(2):539–542. doi: 10.1210/jc.2007-1780. [DOI] [PubMed] [Google Scholar]