Abstract
Aims
Despite pharmacokinetic monitoring of calcineurin inhibitors, the long‐term outcome after transplantation (Tx) is still hampered by the side effects of these drugs. The aim of the present study was to characterize nuclear factor of activated T cells (NFAT)‐regulated gene expression as a potential pharmacodynamic biomarker for further individualization of tacrolimus (Tac) therapy.
Methods
In 29 renal allograft recipients, samples were drawn once pre‐Tx, and before and 1.5 h after Tac dosing at approximately 1 week, 6 weeks and 1 year post‐Tx. Tac concentrations were measured by immunoassay, while the expression of genes encoding NFAT‐regulated cytokines [interleukin 2 (IL2), interferon gamma (IFNG), colony stimulating factor 2 (CSF2)] and cytochrome P450 3A5 (CYP3A5) genotyping were determined by real‐time polymerase chain reaction.
Results
The cytokine response after Tac dosing varied up to 46‐fold between patients and changed significantly with time post‐engraftment. Tac concentrations 1.5 h postdose (C1.5) >15 μg l–1 were associated with strong cytokine inhibition and residual gene expression (RGE) ≤10%, while lower Tac C1.5 resulted in more variable responses (RGE 2.5–68.7%). Patients with ongoing subclinical acute rejection (n = 5) demonstrated limited cytokine inhibition (RGE 39.7–72.6%), while patients with polyoma virus viraemia (n = 3) had relatively strong inhibition of cytokines (RGE 2.5–32.5%). By contrast, there was no association between Tac exposure and rejection or viraemia.
Conclusions
The findings of our study support the potential of NFAT‐regulated gene expression measurements as a pharmacodynamic tool for additional monitoring of Tac therapy, especially in the context of overimmunosuppression and viraemia.
Keywords: acute rejection, calcineurin inhibitors, NFAT‐regulated gene expression, pharmacodynamic drug monitoring, renal transplantation, tacrolimus, viraemia
What is Already Known about this Subject
Nuclear factor of activated T cells (NFAT)‐regulated gene expression has been reported to be a promising pharmacodynamic biomarker of the calcineurin inhibitor response.
The majority of studies so far have investigated long‐term stable transplant recipients treated with cyclosporine.
What this Study Adds
We have studied NFAT‐regulated cytokine expression in patients treated with tacrolimus in the early phase after transplantation, and describe that the cytokine response changed significantly with time.
Patients with ongoing subclinical acute rejection demonstrated limited inhibition, while patients with polyoma virus viraemia had relatively strong inhibition of NFAT‐regulated cytokines.
Introduction
The calcineurin inhibitors (CNIs) tacrolimus (Tac) and cyclosporin A (CsA) are cornerstone immunosuppressive drugs for solid organ transplantation (Tx). Owing to the narrow therapeutic range and large pharmacokinetic variability of CNIs, therapeutic drug monitoring is necessary to balance sufficient efficacy with minimal toxicity. However, despite concentration‐based dosing, CNI‐related toxicity is still a considerable challenge limiting the optimal long‐term outcome after Tx 1.
The immunosuppressive effect of CNIs is primarily caused by inhibition of the calcineurin–nuclear factor of activated T cells (NFAT) signalling pathway in T helper cells. Distribution of CNIs into T cells and the drug sensitivity varies between individuals. Therefore, measurement of systemic CNI trough concentrations does not necessarily accurately predict the biological activity of the drugs in immune cells, and measurement of pharmacodynamic biomarkers related to the inhibition of calcineurin may better reflect the biological effect of CNIs in individual patients.
A promising biomarker of the clinical response to CNIs is the expression of NFAT‐regulated cytokines, interleukin 2 (IL2), interferon‐gamma (INFG) and colony‐stimulating factor 2 (CSF2), in ex vivo stimulated immune cells 2. Studies in renal, heart and liver transplant recipients have shown stronger cytokine inhibition among patients with recurrent infections or viraemia 3, 4, 5, 6, 7, 8. Furthermore, strong cytokine suppression after CNI dosing has been associated with an increased risk of malignancies 4, 6, 9, 10. On the other hand, low cytokine inhibition has been associated with an increased risk of rejection 8, 11, 12. However, the association between the CNI cytokine response and clinical outcome is not consistent between studies.
The majority of studies so far have characterized the NFAT‐regulated gene expression among long‐term stable patients receiving CsA‐based immunosuppression 2, 13. The aim of the present study was to characterize NFAT‐regulated gene expression during Tac therapy in renal Tx recipients in the early phase after engraftment, and to study the cytokine expression response in relation to Tac concentrations and short‐term outcome including renal function, rejection episodes and viraemia.
Materials and methods
Patients and samples
The patients were enrolled at Oslo University Hospital, Rikshospitalet in the period from February 2012 to March 2014. The inclusion criteria were renal Tx with a graft from a living donor, planned Tac‐based immunosuppression and recipient age ≥18 years. Patients were excluded if there were any medical limitations of blood sampling. The planned number of subjects for inclusion was 30. However, within the inclusion period, only 29 patients fulfilled the inclusion criteria and were admitted to the hospital at a time when there was staff available for the extra sample handling. The study was approved by the Regional Committee for Medical and Health Research Ethics (2011/1282 D) and performed in accordance with the Declaration of Helsinki and relevant regulations. Prior to inclusion, written informed consent was obtained from all study participants and they were free to withdraw from the study at any time.
Blood samples were drawn in heparin and ethylenediaminetetraacetic acid (EDTA) tubes pre‐Tx (0–4 days before Tx) and before and 1.5 h after Tac dosing approximately 1 week (6–9 days), 5–7 weeks (referred to as 6 weeks) and 1 year post‐Tx. The postdose sampling time point was based on a previous study demonstrating that the strongest inhibition of NFAT‐regulated cytokines occurs approximately 1.5 h after Tac dosing 11. The exact time points for the sampling and dose administration were recorded.
Immunosuppressive treatment
All patients received induction therapy consisting of intravenous basiliximab (20 mg) on day 0 and day 4 and intravenous methylprednisolone (250 mg) on day 0. The standard immunosuppressive maintenance regimen consisted of Tac combined with mycophenolate mofetil (MMF, 750 mg twice daily) and prednisolone (20 mg day–1, tapered to 10 mg during the first 4 weeks). Tac was initiated at fixed doses of 0.04 mg kg–1 twice daily, followed by dose adjustments to reach target predose concentrations (C0) of 3–7 μg l–1. Five patients received allografts from HLA‐identical donors and were treated with a standard regimen without MMF. One patient presented donor‐specific antibodies pre‐Tx and received a high‐risk regimen involving higher Tac target levels (8–12 μg l–1 from days 0–30 and 6–10 μg l–1 after day 30), and higher starting doses of methylprednisolone (500 mg on day 0) and prednisolone (80 mg day–1) tapered to 20 mg within 1 week and further tapered to 10 mg by week 8. The high‐risk patient also received additional induction therapy with intravenous rituximab (375 mg m–2) 30 days pre‐Tx and intravenous human immune globulin (400 mg kg–1 day–1) from days 0–4.
Tacrolimus concentration measurements
Tac concentrations were determined in EDTA anticoagulated whole blood using a chemiluminescent microparticle immunoassay on the Architect i2000SR system according to the manufacturer's instructions (Abbott Laboratories, Rungis, France). The validated concentration range of the assay was 1.0–30 μg l–1 , with coefficients of variation ≤10%. Samples with concentrations above 30 μg l–1 were diluted as specified by the manufacturer and reanalysed.
Cytochrome P450 3A5 (CYP3A5) genotyping
CYP3A5 genotyping included analysis of the NM_000777.4:c.219‐237A > G (rs776746, A = *1 and G = *3) variant, and was performed using real‐time polymerase chain reaction (PCR) and melting curve analysis (LightCycler 480 instrument, Roche Applied Science, Penzberg, Germany) as previously described 14.
Gene expression of NFAT‐regulated cytokines
The gene expression of NFAT‐regulated cytokines was determined after ex vivo immune activation using a method modified after Giese et al. 15, 16. After sampling, 150 μl heparinized whole blood was incubated in equal volumes of Roswell Park Memorial Institute (RPMI) 1640 medium containing 100 ng ml–1 phorbol 12‐myristate 13‐acetate (PMA) and 5 μg ml–1 ionomycin (Sigma, St Louis, MO, USA) for 3 h at 37°C. Following ex vivo immune activation, samples were lysed with lysis/binding buffer with 1% (w/v) dithiothreitol (Roche Diagnostics, Mannheim, Germany) and frozen at −70°C until extraction of total RNA using the MagNA Pure LC RNA Isolation Kit – High Performance (Roche Diagnostics) on the MagNA Pure LC instrument (Roche Applied Science, Penzberg, Germany). Total RNA was reverse transcribed into complementary DNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics) and a combination of random hexamer and oligo deoxythymidine primers according to the manufacturer's instructions. MS2 RNA (Roche Diagnostics) was used as carrier RNA in all steps at a final concentration of 10 μg ml–1.
Gene expression measurements were performed by real‐time PCR on the LightCycler 480 instrument using the LightCycler® 480 Probes Master Kit (Roche Diagnostics) and hybridization probes for product detection. The target genes IL2, IFNG and CSF2 and three previously validated reference genes, aminolevulinate delta synthase 1 (ALAS1), β‐2‐microglobulin (B2M) and ribosomal protein L13a (RPL13A), were amplified in triplicate in separate reactions 17. Oligonucleotide sequences, reagent concentrations and amplification conditions are listed in Tables S1 and S2. The PCR results were analysed using the LightCycler 480 Software v.1.5 (Roche Applied Science). Quantification cycles were defined by the second derivative maximum method. Target gene expressions were calculated relative to the geometric mean expression of the reference genes and normalized to a calibrator. The calculations included PCR efficiency correction based on standard curves as previously described 17. Finally, residual gene expression (RGE) values were calculated by normalizing the relative gene expression 1.5 h after dosing to the gene expression predose.
Performance of the gene expression assay
The general reverse transcription–PCR assay (including the same reagent kits, reference genes and instruments) has been thoroughly validated in previous studies, demonstrating within‐run and between‐day coefficients of variation (CV) <15% 17. Furthermore, the between‐day CV of the specific NFAT‐regulated gene expression assay was 14.8% (PCR step repeated 10 times within a 2‐month period).
Clinical data
Clinical data were obtained from the hospital records of each patient. Rejection episodes were biopsy verified and graded according to the Banff classification 18. Polyoma virus viraemia was defined as BK or JC virus levels ≥10 000 DNA copies ml–1 in whole blood. The glomerular filtration rate (eGFR) was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula 19.
Statistical methods
The study was exploratory and the sample size was based on practical constraints (e.g. sample handling). Statistical analyses were performed using IBM® SPSS® Statistics version 21.0 (IBM Corporation, NY, USA). Comparisons between independent groups were performed using the chi‐squared or Fisher exact test for categorical variables and Mann–Whitney U test for continuous variables. The Wilcoxon Rank Sum test was used for comparisons between related samples. Correlations between continuous variables were characterized using the Pearson's product–moment correlation coefficient (r) and the Spearman's rank correlation coefficient (rs). All statistical tests were two sided, and P < 0.05 was considered statistically significant.
Nomenclature of targets and ligands
Key ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 20.
Results
Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Median (range) or number of patients | |
|---|---|
| Age (years) | 58 (20–74) |
| Gender (female/male) | 5/24 |
| Body weight at transplant (kg) | 82 (54–106) |
| Height (cm) | 178 (160–191) |
| Underlying diagnosis | |
| Nephrosclerosis | 9 |
| IgA nephropathy | 4 |
| Polycystic kidney disease | 4 |
| Glomerulonephritis | 4 |
| Diabetes mellitus | 3 |
| Other conditions | 5 |
| Dialysis pretransplant (yes/no) | 10/19 |
| Previous solid organ transplantation (yes/no) | 0/29 |
| Immunological risk (standard/high) | 28/1 |
| HLA‐DR mismatches (mean, standard deviation) | 0.76 (±0.74) |
| Total HLA mismatches (mean, standard deviation) | 1.7 (±1.3) |
| CYP3A5 genotype a | |
| CYP3A5*1/*3 | 5 |
| CYP3A5*3/*3 | 23 |
CYP, cytochrome P450; HLA, human leukocyte antigen; Ig, immunoglobulin
Data from a patient with previous haematopoietic stem cell transplantation are not included
Immunosuppressive treatment
Two patients experienced biopsy‐proven acute rejection (BPAR) within the first week post‐Tx and were receiving rejection therapy with intravenous methylprednisolone (500–125 mg) at the time of the 1‐week gene expression measurement. One of these patients received additional rejection therapy with antithymocyte globulin (ATG). Furthermore, one of the patients initially receiving an MMF‐free regimen experienced subclinical rejection and was started on MMF approximately 6 weeks post‐Tx. Another patient had previously received haematopoietic stem cell Tx and was excluded from the genotyping analyses. At 1 year post‐Tx, 20 of the 29 patients had been switched from the regular twice‐daily formulation of Tac (Prograf®, Astellas Ireland Co. Limited, Killorglin, Kerry, Ireland) to a modified‐release once‐daily formulation (Advagraf®, Astellas Ireland Co. Limited, Killorglin, Kerry, Ireland).
Tacrolimus exposure
Daily Tac doses among the standard risk patients (n = 28) were median 0.064 mg kg–1 (ranging 0.024–0.110 mg kg–1), 0.058 mg kg–1 (0.020–0.144 mg kg–1) and 0.045 mg kg–1 (0.015–0.117 mg kg–1) at 1 week, 6 weeks and 1 year, respectively, and were significantly higher at 1 week vs. 1 year post‐Tx (P = 0.014). The corresponding Tac C0 levels were median 5.0 μg l–1 (ranging 2.5–7.8 μg l–1), 6.0 μg l–1 (3.5–9.3 μg l–1) and 5.6 μg l–1 (3.2–8.8 μg l–1), and the concentrations 1.5 h postdose (C1.5) were median 10.3 μg l–1 (3.5–25.3 μg l–1), 8.1 μg l–1 (5.0–18.7 μg l–1) and 9.3 μg l–1 (3.8–19.6 μg l–1). The Tac C0 levels were significantly higher at 6 weeks vs. 1 week (P = 0.005), while the Tac C1.5 levels were lower at 6 weeks (P = 0.029). Furthermore, dose‐normalized Tac C0 was also higher at 6 weeks compared with 1 week [median 202 (μg l–1)/(mg kg–1) vs. 180 (μg l–1)/(mg kg–1), P = 0.002]. By contrast, no significant changes were observed in measured Tac concentrations from 1 week or 6 weeks to 1 year post‐Tx (P ≥ 0.102).
There were no significant differences in Tac concentrations between drug formulations. Patients treated with the twice‐daily formulation 1 year post‐Tx (n = 9, median dose 3.0 mg kg–1) presented a median Tac C0 of 5.9 μg l–1 (3.5–8.8 μg l–1) and C1.5 7.7 μg l–1 (4.7–15.1 μg l–1), while the corresponding levels among standard‐risk patients treated with the extended‐release formulation (n = 19, median dose 4.0 mg kg–1) were 5.4 μg l–1 (3.2–8.3 μg l–1) and 9.6 μg l–1 (3.8–19.6 μg l–1), respectively (P ≥ 0.56).
Dose‐normalized Tac exposure was lower among patients carrying the CYP3A5*1/*3 genotype (n = 5), demonstrating a median Tac C0 of 102 (μg l–1)/(mg kg–1) and C1.5 of 237 (μg l–1)/(mg kg–1) 1 week post‐Tx vs. a median C0 of 185 (μg l–1)/(mg kg–1) and C1.5 of 424 (μg l–1)/(mg kg–1) among patients with the CYP3A5*3/*3 genotype (n = 23, P ≤ 0.033).
Cytokine gene expression response
In total, 18 of the 203 samples for gene expression measurements (10 at 6 weeks and eight at 1 year post‐Tx) were excluded owing to technical problems (failed RNA extraction and immune activation, respectively).
The measured cytokine expression levels varied ≤135‐fold before dosing (E0) and ≤1373‐fold 1.5 h after dosing (E1.5) (Table 2 and Table 3). There was also a large variability in the normalized cytokine response, demonstrating ≤46‐fold differences in RGE between patients (Figure 1). Furthermore, the gene expression response changed significantly with time, showing the strongest inhibition of cytokines 1 week post‐Tx (Figure 1).
Table 2.
Nuclear factor of activated T cells‐regulated cytokine expression and Tac concentrations among patients with and without subclinical rejection
| 6 weeks post‐Tx | 1 year post‐Tx | |||
|---|---|---|---|---|
| BPAR a | Yes (n = 2) | No (n = 22) | Yes (n = 3)b | No (n = 22) |
| RGE, % | 40.6 (39.7–41.6) | 45.3 (5.65–76.2) | 54.3 (44.8–72.6) | 48.1 (17.2–220) |
| E 0 | 1.46 (0.44–2.48) | 1.98 (0.22–4.99) | 1.65 (1.55–2.18) | 1.80 (0.30–5.64) |
| E 1.5 | 0.60 (0.18–1.03) | 0.73 (0.023–2.52) | 0.841 (0.74–1.58) | 0.98 (0.12–3.45) |
| Tac C 0 , μg l –1 | 5.7 (5–6.3) | 5.9 (3.5–9.3) | 4.3 (4.0–7.2) | 5.6 (3.2–8.3) |
| Tac C 1.5 , μg l –1 | 7.0 (6.5–7.5) | 8.7 (5.0–18.7) | 7.1 (4.7–12.7) | 8.9 (3.8–19.6) |
| Tac doses, mg kg –1 day –1 | 0.060 (0.057–0.063) | 0.048 (0.020–0.107) | 0.049 (0.045–0.089) | 0.042 (0.021–0.117) |
BPAR, biopsy‐proven acute rejection; E0, gene expression before dosing; E1.5, gene expression 1.5 h after dosing; RGE, residual gene expression; Tac C0, predose tacrolimus concentrations; Tac C1.5, tacrolimus concentrations 1.5 h postdose; Tx, transplantation
Subclinical biopsy‐proven acute rejection. Gene expression and Tac measurements were performed before the initiation of rejection therapy
One patients experienced concurrent viraemia and subclinical rejection
Table 3.
NFAT‐regulated cytokine expression and Tac concentrations among patients with and without viremia
| 1 week post‐Tx | 6 weeks post‐Tx | |||
|---|---|---|---|---|
| Viraemia a | Yes (n = 1) | No (n = 28) | Yes (n = 2) | No (n = 22) |
| RGE, % | 2.5 | 18.5 (1.5–68.7) | 24.0 (15.5–32.5) | 45.3 (5.7–76.2) |
| E 0 | 0.596 | 1.28 (0.029–3.90) | 0.50 (0.22–0.78) | 2.11 (0.22–4.99) |
| E 1.5 | 0.015 | 0.14 (0.001–1.37) | 0.14 (0.033–0.25) | 0.97 (0.023–2.52) |
| Tac C 0 | 5.2 | 5.0 (2.5–10.6) | 5.4 (4.3–6.4) | 5.9 (3.5–9.3) |
| Tac C 1.5 | 16.4 | 10.3 (3.5–33.4) | 6.9 (6.7–7.0) | 8.7 (5.0–18.7) |
| Tac doses, mg kg –1 day –1 | 0.11 | 0.064 (0.024–0.16) | 0.059 (0.042–0.075) | 0.054 (0.020–0.107) |
E0, gene expression before dosing; E1.5, gene expression 1.5 h after dosing; RGE, residual gene expression; Tac C0, predose tacrolimus concentrations; Tac C1.5, tacrolimus concentrations 1.5 h postdose; Tx, transplantation
Polyoma virus viraemia defined as BK or JC virus levels ≥10 000 DNA copies ml–1 in whole blood. Measurements were performed at the closest sampling period preceding the viraemia
Figure 1.
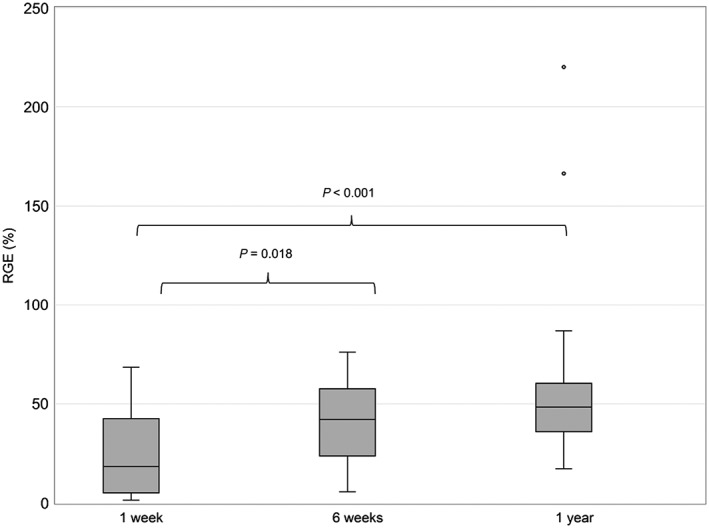
Residual gene expression (RGE) of nuclear factor of activated T cells‐regulated cytokines at 1 week (n = 29), 6 weeks (n = 24) and 1 year (n = 24) after transplantation. Data are shown as median ± interquartile range
The RGE correlated inversely with Tac C1.5 (Figure 2), as well as with Tac C1.5 – C0 (data not shown). At 1 week post‐Tx, Tac C1.5 levels >15 μg l–1 or C1.5 – C0 > 10 μg l–1 were associated with strong cytokine inhibition and RGE <10%, while lower Tac exposure resulted in more variable inhibition, with RGE ranging from 2.5% to 68.7% (Figure 2A). At 6 weeks and 1 year post‐Tx, the majority of patients had a Tac C1.5 <15 μg l–1 and the gene expression response varied considerably, despite similar Tac levels (Figure 2B and C). The two patients who demonstrated postdose increases in cytokine expression 1 year post‐Tx (Figure 2C, RGE of 166% and 220%, respectively) both presented reductions in Tac levels postdose (decline from 4.2 μg l–1 to 3.8 μg l–1 and from 6.9 μg l–1 to 6.4 μg l–1, respectively). Another patient with limited cytokine inhibition 1 year post‐Tx despite a Tac C1.5 of 19.6 μg l–1 (Figure 2C, RGE of 86.9%) demonstrated a Tac C0 of 8.3 μg l–1 and the lowest predose cytokine expression level in the patient population.
Figure 2.
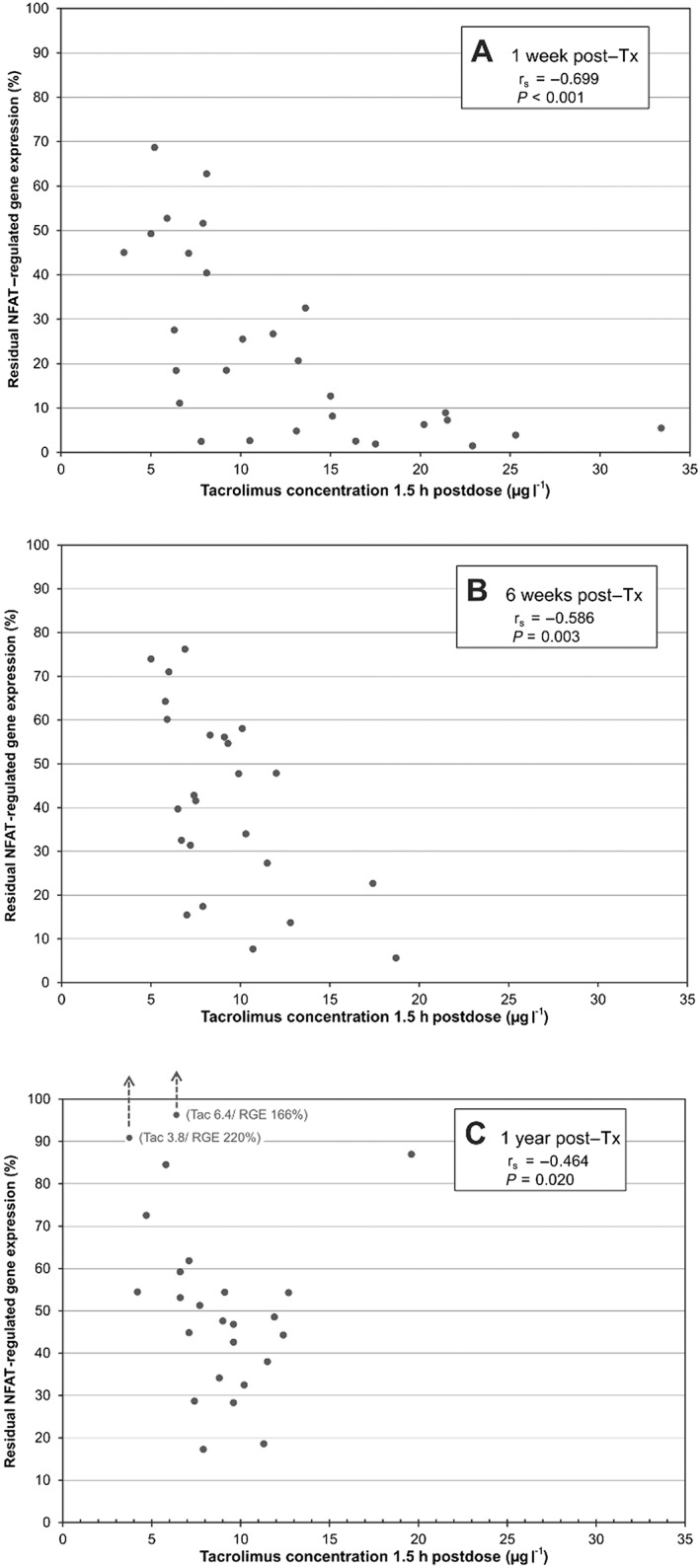
Correlation between tacrolimus (Tac) concentrations 1.5 h post‐dose (C1.5) and the residual gene expression (RGE) of nuclear factor of activated T cells(NFAT)‐regulated cytokines at 1 week (A), 6 weeks (B) and 1 year (C) after transplantation (Tx). rs, Spearman's rank correlation coefficient
The two patients with ongoing rejection therapy 1 week post‐Tx demonstrated lower predose cytokine levels, with an E0 of 0.041 and 0.029 vs. a median of 1.47 (0.14–3.90) among patients without rejection therapy (P = 0.005). Furthermore, both patients showed strong relative inhibition of cytokines after Tac dosing, presenting RGEs of 1.5% and 11.1%, respectively.
There were no significant associations between the gene expression response and Tac C0, regimens with or without MMF, total leucocyte numbers, age or gender.
NFAT‐regulated gene expression and clinical outcome
Five patients demonstrated subclinical BPAR 6 weeks (n = 2) or 1 year (n = 3) post‐Tx. The patients with ongoing subclinical rejection all showed limited cytokine inhibition, with an RGE ≥39.7%. The corresponding cytokine response among patients without ongoing rejection was more variable, demonstrating RGE levels from 5.65% to 220% (Table 2). Tac C0 levels were within, or slightly above, the therapeutic target range in both groups. Furthermore, Tac C1.5 was similar between patients with and without subclinical rejection (Table 2).
Patients with polyoma virus viraemia (n = 3) showed relatively strong inhibition of cytokines and RGEs from 2.5% to 32.5% at the closest measurement preceding the viraemia (Table 3). In addition to the strong inhibition of cytokines, patients who later experienced viraemia also demonstrated relatively low cytokine expression levels before dosing (E0, Table 3). Tac C0 levels were within the therapeutic target. Furthermore, two of the patients with viraemia demonstrated a Tac C1.5 in the lower range of concentrations, while the third patient presented a Tac C1.5 above the median concentration (Table 3).
One of the patients experienced persistent BK virus viraemia from 23 weeks post‐Tx and throughout the study period. Furthermore, 1 year post‐Tx this patient had also developed subclinical cellular rejection. The RGE of this patient increased from 32.5% 6 weeks post‐Tx (preceding viraemia) to 54.2% 1 year post‐Tx (during concurrent viraemia and rejection). Despite less cytokine inhibition, Tac levels tended to be higher 1 year post‐Tx, with a Tac C0 of 7.2 μg l–1 and a C1.5 of 12.7 μg l–1 vs. 6.4 μg l–1 and 6.7 μg l–1 6 weeks post‐Tx, respectively.
There were no significant associations between eGFR and cytokine response or Tac levels.
Discussion
The results of the present study indicate that there is a possibility of utilizing NFAT‐regulated gene expression measurements as a tool for improvement of current Tac monitoring practice. Several previous studies have reported RGE as a promising pharmacodynamic biomarker of the CNI response 2. However, the majority of studies so far have focused on long‐term stable transplant patients treated with CsA 13. The present study investigated NFAT‐regulated gene expression in 29 renal allograft recipients treated with Tac in the early phase after Tx.
There was a large variability in cytokine gene expression levels between patients before (E0) and 1.5 h after (E1.5) Tac dosing. The observed variability in baseline expression (E0) may be related to differences in the underlying disease, comorbidities, drug therapy, genetics, immune status and the response to surgery. Importantly, normalization of E1.5 to E0, expressed as RGE, largely corrects for the variability in baseline cytokine levels and allows more accurate measurement of the drug response after dosing 21.
The observed correlation between RGE and Tac C1.5 (Figure 2), as well as with Tac C1.5 – C0, indicates that the variability in the cytokine response after dosing is partly explained by differences in Tac levels. However, the considerable variability in RGE in the lower range of Tac concentrations (C1.5 <15 μg l–1 or C1.5 – C0 <10 μg l–1) suggests that there may be individual differences in the sensitivity to Tac. Furthermore, the variability in cytokine inhibition may be related to different sensitivities or exposure to other immunosuppressive drugs (e.g. glucocorticoids), or differences in the relationship between whole blood and intracellular Tac concentrations. Patients with Tac C1.5 >15 μg l–1, or C1.5 – C0 >10 μg l–1, demonstrated strong cytokine inhibition and RGE <10% (Figure 2). Similar to our findings, Sommerer et al. (2010) reported that patients with Tac C1.5 – C0 ≥11.4 μg l–1 had strong cytokine inhibition (RGE <30%) 11. The lack of additional inhibitory effect by increasing Tac levels suggests that in individual patients the total Tac exposure may be reduced, while maintaining sufficient immunosuppressive effect.
The genotyping analysis showed that the dose‐normalized Tac concentrations pre‐ and postdose were 1.8 times higher among homozygous carriers of the nonfunctional CYP3A5*3 allele than in heterozygous carriers of the CYP3A5*1 allele. This is in agreement with several previous reports in transplant recipients treated with Tac 22. Furthermore, the variability in Tac pharmacokinetics (Table 2) may have been related to factors such as differences in body weight, age, gender, haematocrit and drug interactions 14, 23.
The patients demonstrated significantly less inhibition of cytokines (higher RGE) with increasing time since Tx (Figure 1). Similar changes in cytokine response and calcineurin activity have also been observed in previous studies of CNI‐treated renal and liver allograft recipients 10, 11, 12, 24, 25. However, other studies have shown relatively unchanged cytokine responses with time after Tx 4, 16, 26. The difference in findings may be related to the timing of measurements, and the observed increase in RGE with time (Figure 1) may partly have been related to alterations in Tac pharmacokinetics in the early phase post‐Tx. The actual and dose‐normalized Tac C0 levels were higher at 6 weeks vs. 1 week post‐Tx. Previous studies have reported an increase in bioavailability and a reduction in steady‐state clearance of CNIs after Tx 27, 28, which in turn may be related to changes in immune status and glucocorticoid exposure 28, 29. Other studies have shown that the time‐dependent increase in Tac C0 early post‐Tx largely can be explained by a simultaneous increase in haematocrit 14. Importantly, the observed time‐dependent changes in gene expression response indicate that single gene expression measurements may not be sufficient to predict the pharmacodynamic response of Tac.
The different Tac formulations may also affect Tac exposure and RGE. One year post‐Tx, 19 of the standard‐risk patients had been switched to an extended‐release formulation. However, Tac concentrations did not tend to differ between formulations, and the higher RGE observed at 1 year vs. 1 week post‐Tx (Figure 1) is probably not explained by the switch in formulations. Nevertheless, the increasing use of the extended‐release formulation emphasizes the need for further investigations of NFAT‐regulated gene expression during treatment with the once‐daily formulation.
The limited correlation between RGE and Tac levels in the lower range of concentrations (Figure 2) suggests that the change in cytokine response is not explained by Tac exposure alone. Other factors with a potential impact on the cytokine response may be comedication, the immune status of the patient and homeostatic processes counteracting the cytokine inhibition. Giese et al. (2004) reported the NFAT‐regulated gene expression assay to be relatively CNI specific 16. However, other studies have shown reduced expression of NFAT‐regulated cytokines during treatment with drugs such as glucocorticoids and mycophenolic acid (MPA) 30, 31. It is likely that the gene expression measurements 1 week post‐Tx may have been more influenced by the induction therapy, comprising basiliximab and methylprednisolone, than later measurements. Moreover, the initial prednisolone doses were 20 mg day–1 vs. 10 mg day–1 6 weeks post‐Tx. Glucocorticoids inhibit the binding of transcription factors [e.g. activator protein‐1 (AP‐1) and nuclear factor kappa B (NF‐kB)] that are required for the transcription of proinflammatory cytokines, including IL2, INFG and CSF2 30, 32. Furthermore, glucocorticoids are reported to inhibit cytokine secretion by reducing mRNA stability 33, and by suppressing the cytokine‐producing cell populations 34. We observed that the two patients with ongoing rejection therapy with methylprednisolone (500–125 mg) presented significantly reduced E0, as well as strong inhibition of cytokines after dosing (RGE 1.5% and 11.1%). Basiliximab, and specifically ATG, have long‐lasting immunosuppressive effects, with a potential impact on both E0 and E1.5 levels. The effect of these drugs on cytokine expression is probably largely corrected by the calculation of remaining gene expression relative to predose expression. However, basiliximab and ATG have dramatic effects on the composition of leukocyte subpopulations 35, 36, which in turn may affect the relative inhibition of cytokine expression after Tac dosing. MPA interferes with proinflammatory signalling, including the p38, Jun N‐terminal kinase (JNK) and NF‐κB pathways, which in turn interact with the NFAT signalling pathway 37. In addition to the effects on signalling, MPA inhibits the clonal expansion of activated T lymphocytes, and may thereby also reduce the number of cytokine‐producing cells 31. Millan et al. (2003) reported that immunosuppressive regimens with Tac and MMF caused stronger inhibition of IL2 than Tac‐based regimens without MMF 31. Altogether, this emphasizes the need for further studies to characterize the impact of glucocorticoids and other drugs on NFAT‐regulated gene expression.
We observed that the patients with ongoing subclinical rejection (n = 5) had weak cytokine inhibition, with RGE ranging from 39.7% to 72.6% (Table 2). The patients without ongoing rejection demonstrated highly variable cytokine inhibition, with an RGE between 5.7% and 220% (Table 2). These results are in line with previous studies in CsA‐treated 5, 12, 21, 26, as well as Tac‐treated 8, 11, 12, kidney and liver recipients.
Two of the patients in the present study demonstrated postdose increases in cytokine expression and RGEs of 166% and 220%, respectively, 1 year post‐Tx (Figure 2C). However, despite the apparent lack of Tac response after dosing, the patients did not experience rejection. Similar findings have also been observed in other studies of long‐term stable Tx patients 7, 10. The increase in cytokine expression after dosing may be partly explained by differences in Tac pharmacokinetics (e.g. delayed absorption or elimination), implying that measurements predose and 1.5 h post‐dose might not be sufficient to reflect the Tac response in these patients. Furthermore, Tac has been associated with higher RGE than CsA in long‐term stable Tx patients 7, 10, 11, 38. However, Tac has not been shown to be inferior to CsA 39, and the difference in cytokine responses may be related to differences in molar concentrations, binding to immunophilins, alterations of cytokine profiles, cytokine receptor blockade or additional mechanisms of action.
In the present study, we observed that the three patients with polyoma virus viraemia (≥10 000 DNA copies ml–1) experienced relatively strong cytokine inhibition and RGEs ranging from 2.55% to 32.5% before the development of viraemia (Table 3). Furthermore, the patients with viraemia demonstrated relatively low E0, while there was no difference in Tac levels (Table 3). These findings are in agreement with previous reports describing associations between low RGE and infectious complications 3, 4, 5, 6, 7, 8, 11. Additionally, strong cytokine suppression has been reported to be a risk factor for the development of malignancies 4, 6, 9, 10. Altogether, this indicates that the gene expression of NFAT‐regulated cytokines may be used to identify overimmunosuppression, even when the Tac levels are within therapeutic ranges.
The main limitation of the present study was the small number of patients, presenting with different immunological statuses and immunosuppressive regimens. Furthermore, Tac concentration measurements were performed by an immunoassay, which generally has lower analytical sensitivity and specificity than liquid chromatography–tandem mass spectrometry assays. A strength of the study was the single‐centre prospective approach, following participants from before Tx and with repeated measurements at predefined time‐points post‐engraftment.
In conclusion, the findings of our study support the potential of NFAT‐regulated gene expression measurements as a tool for the pharmacodynamic monitoring of Tac therapy in the early phase post‐Tx, especially in the context of overimmunosuppression and viraemia. However, further knowledge is needed, considering different patient populations, interfering parameters, monitoring strategies and target ranges. The assays for measurement of NFAT‐regulated gene expression need further validation and standardization to allow comparisons between studies. Finally, the clinical value of pharmacodynamic monitoring based on NFAT‐regulated gene expression should be evaluated in prospective clinical studies.
Competing Interests
There are no competing interests to declare.
The authors are grateful to Lene Pham, Kristine Hole and Rolf Klaasen for their excellent assistance with sample handling, genetic analyses and extraction of clinical data. The authors would also like to acknowledge Laila Gjerdalen, Torunn Skogseth, Marit Hansen Hallberg, May Ellen Lauritsen and their colleagues for organizing laboratory facilities and assistance with pharmacokinetic measurements.
Contributors
S.B., N.T.V. and St.B. participated in research design. M.S. and K.M. recruited patients. S.B., N.T.V., M.K., E.D.J. and St.B. participated in performance of the research. S.B., N.T.V. and St.B. participated in data analysis and wrote the manuscript. All authors were involved in the discussion of results, critical revision of the manuscript and approval of the final version.
Supporting information
Table S1 Oligonucleotides for the nuclear factor of activated T cells‐regulated gene expression assay
Table S2 Amplification conditions for the nuclear factor of activated T cells‐regulated gene expression assay
Bremer, S. , Vethe, N. T. , Skauby, M. , Kasbo, M. , Johansson, E. D. , Midtvedt, K. , and Bergan, S. (2017) NFAT‐regulated cytokine gene expression during tacrolimus therapy early after renal transplantation. Br J Clin Pharmacol, 83: 2494–2502. doi: 10.1111/bcp.13367.
References
- 1. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009; 4: 481–508. [DOI] [PubMed] [Google Scholar]
- 2. Sommerer C, Meuer S, Zeier M, Giese T. Calcineurin inhibitors and NFAT‐regulated gene expression. Clin Chim Acta 2012; 413: 1379–1386. [DOI] [PubMed] [Google Scholar]
- 3. Konstandin MH, Sommerer C, Doesch A, Zeier M, Meuer SC, Katus HA, et al. Pharmacodynamic cyclosporine A‐monitoring: relation of gene expression in lymphocytes to cyclosporine blood levels in cardiac allograft recipients. Transpl Int 2007; 20: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 4. Sommerer C, Konstandin M, Dengler T, Schmidt J, Meuer S, Zeier M, et al. Pharmacodynamic monitoring of cyclosporine a in renal allograft recipients shows a quantitative relationship between immunosuppression and the occurrence of recurrent infections and malignancies. Transplantation 2006; 82: 1280–1285. [DOI] [PubMed] [Google Scholar]
- 5. Billing H, Giese T, Sommerer C, Zeier M, Feneberg R, Meuer S, et al. Pharmacodynamic monitoring of cyclosporine a by NFAT‐regulated gene expression and the relationship with infectious complications in pediatric renal transplant recipients. Pediatr Transplant 2010; 14: 844–851. [DOI] [PubMed] [Google Scholar]
- 6. Sommerer C, Schnitzler P, Meuer S, Zeier M, Giese T. Pharmacodynamic monitoring of cyclosporin a reveals risk of opportunistic infections and malignancies in renal transplant recipients 65 years and older. Ther Drug Monit 2011; 33: 694–698. [DOI] [PubMed] [Google Scholar]
- 7. Billing H, Breil T, Schmidt J, Tonshoff B, Schmitt CP, Giese T, et al. Pharmacodynamic monitoring by residual NFAT‐regulated gene expression in stable pediatric liver transplant recipients. Pediatr Transplant 2012; 16: 187–194. [DOI] [PubMed] [Google Scholar]
- 8. Sommerer C, Zeier M, Czock D, Schnitzler P, Meuer S, Giese T. Pharmacodynamic disparities in tacrolimus‐treated patients developing cytomegalus virus viremia. Ther Drug Monit 2011; 33: 373–379. [DOI] [PubMed] [Google Scholar]
- 9. Sommerer C, Hartschuh W, Enk A, Meuer S, Zeier M, Giese T. Pharmacodynamic immune monitoring of NFAT‐regulated genes predicts skin cancer in elderly long‐term renal transplant recipients. Clin Transplant 2008; 22: 549–554. [DOI] [PubMed] [Google Scholar]
- 10. Zahn A, Schott N, Hinz U, Stremmel W, Schmidt J, Ganten T, et al. Immunomonitoring of nuclear factor of activated T cells‐regulated gene expression: the first clinical trial in liver allograft recipients. Liver Transpl 2011; 17: 466–473. [DOI] [PubMed] [Google Scholar]
- 11. Sommerer C, Zeier M, Meuer S, Giese T. Individualized monitoring of nuclear factor of activated T cells‐regulated gene expression in FK506‐treated kidney transplant recipients. Transplantation 2010; 89: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 12. Steinebrunner N, Sandig C, Sommerer C, Hinz U, Giese T, Stremmel W, et al. Pharmacodynamic monitoring of nuclear factor of activated T cell‐regulated gene expression in liver allograft recipients on immunosuppressive therapy with calcineurin inhibitors in the course of time and correlation with acute rejection episodes – a prospective study. Ann Transplant 2014; 19: 32–40. [DOI] [PubMed] [Google Scholar]
- 13. Bremer S, Vethe NT, Bergan S. Monitoring calcineurin inhibitors response based on NFAT‐regulated gene expression A2 In: Personalized Immunosuppression in Transplantation, eds Oellerich M, Dasgupta A. San Diego, CA: Elsevier, 2016; 259–290. [Google Scholar]
- 14. Storset E, Holford N, Midtvedt K, Bremer S, Bergan S, Asberg A. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol 2014; 70: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giese T, Zeier M, Meuer S. Analysis of NFAT‐regulated gene expression in vivo: a novel perspective for optimal individualized doses of calcineurin inhibitors. Nephrol Dial Transplant 2004; 19 ((Suppl 4)): iv55–iv60. [DOI] [PubMed] [Google Scholar]
- 16. Giese T, Zeier M, Schemmer P, Uhl W, Schoels M, Dengler T, et al. Monitoring of NFAT‐regulated gene expression in the peripheral blood of allograft recipients: a novel perspective toward individually optimized drug doses of cyclosporine A. Transplantation 2004; 77: 339–344. [DOI] [PubMed] [Google Scholar]
- 17. Bremer S, Rootwelt H, Bergan S. Real‐time PCR determination of IMPDH1 and IMPDH2 expression in blood cells. Clin Chem 2007; 53: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 18. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8: 753–760. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1D68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herden U, Kromminga A, Hagel C, Hartleb J, Nashan B, Sterneck M, et al. Monitoring of nuclear factor of activated T‐cell‐regulated gene expression in de novo and long‐term liver transplant recipients treated with cyclosporine A. Ther Drug Monit 2011; 33: 185–191. [DOI] [PubMed] [Google Scholar]
- 22. Barry A, Levine M. A systematic review of the effect of CYP3A5 genotype on the apparent oral clearance of tacrolimus in renal transplant recipients. Ther Drug Monit 2010; 32: 708–714. [DOI] [PubMed] [Google Scholar]
- 23. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004; 43: 623–653. [DOI] [PubMed] [Google Scholar]
- 24. Tumlin JA, Roberts BR, Kokko KE, El Minshawy O, Gooch JL. T‐cell receptor‐stimulated calcineurin activity is inhibited in isolated T cells from transplant patients. J Pharmacol Exp Ther 2009; 330: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mortensen DM, Koefoed‐Nielsen PB, Jorgensen KA. Calcineurin activity in tacrolimus‐treated renal transplant patients early after and 5 years after transplantation. Transplant Proc 2006; 38: 2651–2653. [DOI] [PubMed] [Google Scholar]
- 26. Sommerer C, Giese T, Schmidt J, Meuer S, Zeier M. Ciclosporin A tapering monitored by NFAT‐regulated gene expression: a new concept of individual immunosuppression. Transplantation 2008; 85: 15–21. [DOI] [PubMed] [Google Scholar]
- 27. Buchler M, Chadban S, Cole E, Midtvedt K, Thervet E, Prestele H, et al. Evolution of the absorption profile of cyclosporine A in renal transplant recipients: a longitudinal study of the de novo and maintenance phases. Nephrol Dial Transplant 2006; 21: 197–202. [DOI] [PubMed] [Google Scholar]
- 28. Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, et al. Time‐related clinical determinants of long‐term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet 2004; 43: 741–762. [DOI] [PubMed] [Google Scholar]
- 29. Zidek Z, Anzenbacher P, Kmonickova E. Current status and challenges of cytokine pharmacology. Br J Pharmacol 2009; 157: 342–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paliogianni F, Raptis A, Ahuja SS, Najjar SM, Boumpas DT. Negative transcriptional regulation of human interleukin 2 (IL‐2) gene by glucocorticoids through interference with nuclear transcription factors AP‐1 and NF‐AT. J Clin Invest 1993; 91: 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Millan O, Brunet M, Campistol JM, Faura A, Rojo I, Vidal E, et al. Pharmacodynamic approach to immunosuppressive therapies using calcineurin inhibitors and mycophenolate mofetil. Clin Chem 2003; 49: 1891–1899. [DOI] [PubMed] [Google Scholar]
- 32. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med 2005; 353: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 33. Tobler A, Meier R, Seitz M, Dewald B, Baggiolini M, Fey MF. Glucocorticoids downregulate gene expression of GM‐CSF, NAP‐1/IL‐8, and IL‐6, but not of M‐CSF in human fibroblasts. Blood 1992; 79: 45–51. [PubMed] [Google Scholar]
- 34. Oppong E, Cato AC. Effects of glucocorticoids in the immune system. Adv Exp Med Biol 2015; 872: 217–233. [DOI] [PubMed] [Google Scholar]
- 35. Cherkassky L, Lanning M, Lalli PN, Czerr J, Siegel H, Danziger‐Isakov L, et al. Evaluation of alloreactivity in kidney transplant recipients treated with antithymocyte globulin versus IL‐2 receptor blocker. Am J Transplant 2011; 11: 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Praditpornsilpa K, Avihingsanon Y, Kupatawintu P, Songpanich S, Pisitkul T, Kansanabuch T, et al. Monitoring of T‐cell subsets in patients treated with anti‐CD 25 antibody. Transplant Proc 2004; 36: 487s–491s. [DOI] [PubMed] [Google Scholar]
- 37. Andreucci M, Faga T, Lucisano G, Uccello F, Pisani A, Memoli B, et al. Mycophenolic acid inhibits the phosphorylation of NF‐kappaB and JNKs and causes a decrease in IL‐8 release in H2O2‐treated human renal proximal tubular cells. Chem Biol Interact 2010; 185: 253–262. [DOI] [PubMed] [Google Scholar]
- 38. Zucker C, Zucker K, Asthana D, Carreno M, Viciana AL, Ruiz P, et al. Longitudinal induced IL‐2 mRNA monitoring in renal transplant patients immunosuppressed with cyclosporine and in unmodified canine renal transplant rejection. Hum Immunol 1996; 45: 1–12. [DOI] [PubMed] [Google Scholar]
- 39. Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562–2575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Oligonucleotides for the nuclear factor of activated T cells‐regulated gene expression assay
Table S2 Amplification conditions for the nuclear factor of activated T cells‐regulated gene expression assay


