Abstract
Aims
Few studies have investigated the link between individual antibiotics and major congenital malformations (MCMs) including specific malformations owing to small sample size. We aimed to quantify the association between exposure to gestational antibiotic and the risk of MCMs.
Methods
Using the Quebec pregnancy cohort (1998–2008), we included a total of 139 938 liveborn singleton alive whose mothers were covered by the “Régie de l'assurance maladie du Québec” drug plan for at least 12 months before and during pregnancy. Antibiotic exposure was assessed in the first trimester and MCMs were identified within the first year of life.
Results
After adjusting for potential confounders, clindamycin exposure was associated with an increased risk of MCMs (aOR 1.34, 95% CI 1.02–1.77, 60 exposed cases), musculoskeletal system malformations (aOR 1.67, 95% CI 1.12–2.48, 29 exposed cases) and ventricular/atrial septal defect (aOR 1.81, 95% CI 1.04–3.16, 13 exposed cases). Doxycycline exposure increased the risk of circulatory system malformation, cardiac malformations and ventricular/atrial septal defect (aOR 2.38, 95% CI 1.21–4.67, 9 exposed cases; aOR 2.46, 95% CI 1.21–4.99, 8 exposed cases; aOR 3.19, 95% CI 1.57–6.48, 8 exposed cases, respectively). Additional associations were seen with quinolone (1 defect), moxifloxacin (1 defect), ofloxacin (1 defect), macrolide (1 defect), erythromycin (1 defect) and phenoxymethylpenicillin (1 defect). No link was observed with amoxicillin, cephalosporins and nitrofurantoin. Similar results were found when penicillins were used as the comparator group.
Conclusions
Clindamycin, doxycycline, quinolones, macrolides and phenoxymethylpenicillin in utero exposure were linked to organ‐specific malformations. Amoxicillin, cephalosporins and nitrofurantoin were not associated with MCMs.
Keywords: antibiotics, cardiac defect, craniosynostosis, major congenital malformations, pregnancy
What is Already Known about this Subject
Several studies have looked at the risk of major birth defect associated with the use of many antibiotics classes (macrolides, quinolones, etc.) during pregnancy.
Findings from many of them should be interpreted with caution due to methodological flaws including small sample sizes, recall or indication bias.
Few studies have investigated the link between individual antibiotics and major congenital malformations (MCMs) including specific malformations.
What this Study Adds
Clindamycin, doxycycline, quinolones, macrolides and phenoxymethylpenicillin in utero exposure were linked to an increased risk of some organ‐specific malformations.
Amoxicillin, cephalosporins and nitrofurantoin were not associated with major or specific birth defects.
Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available.
Introduction
Antibiotics are widely used during pregnancy worldwide to treat common infections including urinary and respiratory tract infections. In the United States, nitrofurantoin and metronidazole are the most prescribed medication dispensed among pregnant women enrolled in the US Medicaid Program 1. In Quebec province, it has been reported that there is an increasing trend for macrolides, quinolones, tetracycline and nitrofurantoin use during pregnancy 2. In animal studies, current evidence suggests a link between quinolone use during pregnancy and cartilage damage 3. Association between tetracyclines use and fetal tooth discoloration and bone growth inhibition was also observed 4. Furthermore, animal studies have shown that clarithromycin could induce fetal loss in rabbits and monkeys when used in very low dosages and high dosages, respectively 5. Conversely, several reproduction studies have been performed in rabbits and rats at doses up to six times the human dose and have revealed no evidence of harm to the fetus exposed to nitrofurantoin 6. Neither embryotoxic nor teratogenic effects were seen following metronidazole exposure in mice and rats despite the fact that some fetal deaths were noted after intraperitoneal administration in mice 7. Findings from animal studies cannot be extrapolated in human for several reasons including metabolism differences between humans and other species 8, 9, 10.
Although, several human studies have been conducted to evaluate the risk of major congenital malformations (MCMs), evidence regarding fetal safety of these antibiotics remains inconclusive. Nine studies failed to show an association between macrolide exposure during pregnancy and the risk of MCMs or cardiac malformations 5, 11, 12, 13, 14, 15, 16, 17, 18. However, two studies showed an increased risk of cardiac defects associated with erythromycin use 19, 20. Eight studies did not find an association of MCMs with quinolone use 17, 21, 22, 23, 24, 25, 26, 27. Four studies reported an increased risk of MCMs associated with tetracyline use specifically for cardiac malformations and oral clefts 28, 29, 30, 31. However, one study failed to show an association between tetracycline use and MCMs 17. A meta‐analysis based on three cohort studies did not show an increased risk of MCMs associated with nitrofurantoin use but when restricted to three case control studies an increased risk of MCMs has been found 32. Recently another study has reported that nitrofurantoin exposure was associated with an increased risk of oral clefts 33. A systematic review based on 13 studies failed to show an association between metronidazole use and the risk of MCMs 34. Findings from many of these studies should be interpreted with caution due to methodological flaws including small sample sizes, recall or indication bias. To our knowledge, the risk of organ‐specific malformations associated with individual antibiotics such as clindamycin has not yet been investigated. Therefore our study aimed to quantify the association between gestational exposure to antibiotic classes and types and the risk of MCMs including organ‐specific malformations.
Material and methods
Setting
We carried out a population‐based cohort study using data from the Quebec pregnancy cohort (QPC). The QPC has been described previously in Bérard and Sheehy's article 35. Briefly, it is an ongoing population‐based cohort with prospective data collection on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance, from January 1998 to December 2009, in the province of Quebec. Information for each pregnancy is obtained from province‐wide databases and linked using unique personal identifiers. All pregnancies included in the QPC were identified in the “Régie de l'Assurance Maladie du Québec” (RAMQ) and the Quebec hospitalization archives (MedEcho) databases. The first day of the last menstrual period (defined as the first day of gestation, 1DG) was determined using data on gestational age (GA), which was validated by patient charts and ultrasound measures 36. Prospective follow‐up data were available from 1 year before the 1DG, during pregnancy and until December 2009.
The data sources for this study included the medical service database (RAMQ: diagnoses, medical procedures, socio‐economic status of women and prescribers), the Quebec Public Prescription Drug Insurance database (drug name, start date, dosage and duration), the hospitalization archive database (Med Echo: in‐hospital diagnoses and procedures, and GA), and the Quebec Statistics database (ISQ: patient socio‐demographic and birth weight).
Study cohort
Pregnancies from the QPC meeting the following inclusion criteria were included in our cohort.
Pregnancies ending with a live‐born singleton. Pregnancies with multiplicity were excluded as it is a known risk factor for MCMs.
Pregnancies with a continuous prescription drug insurance coverage of ≥12 months before the 1DG, during pregnancy and with at least 12 months of follow‐up after birth. This avoided a misclassification of MCMs that cannot be diagnosed at birth.
Pregnancies with exposure to known teratogens 37, 38 during the first trimester, chromosomal aberrations and minor malformations were also excluded.
Given that we aimed to compare pregnancies exposed to only one class of antibiotics with unexposed pregnancies, during the first trimester, those exposed to multiple antibiotics or exposed to other anti‐infective drugs were excluded as well. The study was approved by the Quebec Data Access Agency and the CHU Sainte‐Justine Institutional Review Board.
Antibiotics exposure
We defined exposure to antibiotics as either having filled at least one prescription for any type of antibiotics within the first trimester of pregnancy or as having filled a prescription for an antibiotic before pregnancy but with a duration that overlapped the 1DG. Only exposure during the first trimester was considered of interest given that organogenesis occurs during that period.
The following classes of antibiotics, defined according to the American Hospital Formulary Service (AHFS) categories, were considered: cephalosporins (AHFS 8:12:06), macrolides (AHFS 8:12:12), penicillins (AHFS 8:12:16), sulfonamides (AHFS 8:12:20), urinary anti‐infectives (AHFS 8:36), other antibacterials (AHFS 8:12:28), tetracyclines (AHFS 8:12.24); quinolones (AHFS 8:12.18) and antiprotozoals (AHFS 8:30.92).
The following individual antibiotics were also taken into account: amoxicillin, amoxicillin/potassium clavulanate, phenoxymethylpenicillin, cephalexin, azithromycin, clarithromycin, ciprofloxacin, norfloxacin, levofloxacin, ofloxacin, clindamycin, doxycycline, minocycline, erythromycin, metronidazole, nitrofurantoin and sulfamethoxazole trimethoprim.
We defined a non‐exposure category as pregnancies with no exposure to antibiotic during the time window of interest.
Filled prescription of antibiotics within the QPC has been validated against maternal reports with high positive and negative predictive value (PPV 86.7% and NPV 92.3%) 39.
Major congenital malformations
MCMs diagnosed in the first year of life were identified in the RAMQ medical file and MedEcho databases and defined according to International Classification of Diseases ICD‐9 and ICD‐10 codes (see Table S1 in the supplementary appendix).
We investigated eight specific organ system MCMs including MCMs for which association with some antibiotic have not yet been studied (see Table S1 in the supplementary appendix). We also assessed four specific defects including cardiac malformations.
MCMs included in the QPC have also been validated against medical charts with high PPV (78.1%) and NPV (94.2%) 40. High PPV (over 80%) have also been reported for specific MCMs including cardiac, cleft, digestive and urinary MCMs 40. All organ systems were considered and defined according to the European Registration of Congenital Anomalies and Twins (EUROCAT) Registry 41.
Chromosomal abnormalities are likely unrelated to exposure. Moreover, diagnosis of a minor malformation among physicians is likely to be subjective. Given that, those malformations were not considered in our study, to avoid MCM misclassification.
Covariates
We selected a priori variables associated with both antibiotics exposure and MCMs as potential confounders and risk factors for MCMs (Table 1 and Table S2). The following covariates were included: (1) Socio‐demographic variables including maternal age on the 1DG, maternal marital status (living alone or cohabiting), receipt of social assistance during pregnancy, education level in years (≤12 or >12), and area of residence on the 1DG (urban or rural). (2) Maternal chronic co‐morbidities according to physician‐based diagnoses or filled prescriptions of related medications in the year before and during the first trimester of pregnancy (chronic hypertension, depression, diabetes mellitus, asthma, epilepsy, polyarthritis rheumatoid and systemic lupus erythematosus, and thyroid disorders. (3) Endometriosis and maternal infections (urinary tract infection, respiratory tract infection, bacterial vaginosis, and sexually transmitted diseases) determined with physician‐based diagnoses in the year before and during the first trimester of pregnancy. Details on the codes used have been provided in the supplemental files (Table S2). A direct acyclic graph was used to illustrate the structure of confounding with maternal infections 42 (Table S3). (4) Measures of healthcare utilization in the year before pregnancy as markers of general comorbidity. (5) Calendar year of delivery. (6) Infant gender.
Table 1.
Study characteristics
| Study characteristics | Non exposed (n = 124 469) | Penicillins (n = 9106) | Macrolides (n = 2332) | Cephalo‐sporines (n = 1005) | Quinolones (n = 782) | Sulfonamides (n = 164) | Tetracyclines (n = 410) | Other antibacterials a (n = 381) | Anti‐protozoals (n = 412) | Urinary anti‐infectives (n = 877) | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy related: | |||||||||||
| Gestational age (weeks), mean (± SD) | 38.9 ± 1.8 | 38.7 ± 1.9 | 38.7 ± 2.0 | 38.8 ± 1.8 | 38.7 ± 1.9 | 39.0 ± 1.6 | 38.8 ± 2.1 | 38.6 ± 1.9 | 38.6 ± 2.3 | 38.7 ± 1.7 | <.0001 |
| Newborn gender (male), n (%) | 63 918 (51.4) | 4619 (50.7) | 1184 (50.8) | 505 (50.3) | 403 (51.5) | 81 (49.4) | 201 (49.0) | 175 (45.9) | 204 (49.5) | 446 (50.9) | 0.5274 |
| Measure on the first day of gestation | |||||||||||
| Maternal age, years, mean (± SD) | 27.8 ± 5.5 | 27.0 ± 5.5 | 26.4 ± 5.7 | 27.0 ± 5.7 | 27.0 ± 5.8 | 27.7 ± 6.0 | 27.2 ± 6.0 | 26.6 ± 5.3 | 26.0 ± 5.7 | 27.3 ± 5.7 | <.0001 |
| Urban dweller, n (%) | 101 840 (81.8) | 7487 (82.2) | 1959 (84.0) | 819 (81.5) | 661 (84.5) | 139 (84.8) | 342 (83.4) | 318 (83.5) | 346 (84.0) | 741 (84.5) | 0.0237 |
| Welfare recipient, n (%) | 30 411 (24.4) | 2917 (32.0) | 866 (37.1) | 311 (24.4) | 247 (31.0) | 51 (31.1) | 119 (29.0) | 167 (48.8) | 173 (42.0) | 249 (28.4) | <.0001 |
| Living alone, n (%) | 18 086 (14.5) | 1719 (18.9) | 545 (23.4) | 178 (17.7) | 184 (23.5) | 38 (23.2) | 101 (24.6) | 97 (25.5) | 135 (32.8) | 172 (19.6) | <.0001 |
| Education (≤12 years), n (%) | 51 493 (41.4) | 4623 (50.8) | 1244 (53.3) | 523 (52.0) | 379 (48.5) | 82 (50.0) | 210 (51.2) | 246 (64.6) | 247 (60.0) | 428 (48.9) | <.0001 |
| Maternal chronic co‐morbidities in the year before or during the first trimester of pregnancy: | |||||||||||
| Diabetes, n (%) | 1921 (1.5) | 197 (2.2) | 40 (1.7) | 16 (1.6) | 17 (2.2) | 6 (3.7) | 6 (1.5) | 10 (2.7) | 6 (1.5) | 14 (1.6) | 0.0005 |
| Hypertension, n (%) | 3053 (2.5) | 309 (3.4) | 98 (4.2) | 39 (3.9) | 24 (3.1) | 4 (2.4) | 15 (3.7) | 13 (3.4) | 7 (1.7) | 35 (4.0) | <.0001 |
| Thyroid disorders, n (%) | 4437 (3.6) | 371 (4.1) | 92 (4.0) | 44 (4.4) | 26 (3.3) | 4 (2.4) | 25 (6.1) | 19 (5.0) | 9 (2.2) | 38 (4.3) | 0.0070 |
| Asthma, n (%) | 14 296 (11.5) | 1852 (20.3) | 688 (30.0) | 259 (25.8) | 164 (21.0) | 29 (17.7) | 68 (16.6) | 66 (17.3) | 63 (15.3) | 144 (16.4) | <.0001 |
| Depression, n (%) | 16 403 (13.2) | 1716 (18.8) | 501 (21.5) | 193 (19.2) | 180 (23.0) | 43 (26.2) | 81 (19.8) | 66 (17.3) | 79 (19.2) | 156 (17.8) | <.0001 |
| Endometriosis, n (%) | 803 (0.7) | 79 (0.9) | 17 (0.7) | 15 (1.5) | 11 (1.4) | 1 (0.6) | 7 (1.7) | 3 (0.8) | 7 (1.7) | 8 (0.9) | <.0001 |
| Polyarthritis rheumatoid and Systemic lupus erythematosus, n (%) | 81 (0.07) | 6 (0.07) | 2 (0.09) | 1 (0.10) | 0 (0.00) | 2 (1.2) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.11) | <.0001 |
| Epilepsy, n (%) | 681 (0.6) | 72 (0.8) | 23 (1.0) | 5 (0.5) | 12 (1.5) | 2 (1.2) | 5 (1.2) | 7 (1.8) | 5 (1.2) | 11 (1.3) | <.0001 |
| Maternal infections in the year before or during the first trimester of pregnancy: | |||||||||||
| Urinary tract infection, n (%) | 11 707 (9.4) | 1842 (20.2) | 288 (12.4) | 227 (22.6) | 470 (60.1) | 85 (51.8) | 45 (11.0) | 39 (10.2) | 39 (9.5) | 395 (45.0) | <.0001 |
| Respiratory tract infection, n (%) | 35 945 (28.9) | 5010 (55.0) | 1367 (58.6) | 550 (54.7) | 343 (43.9) | 70 (42.7) | 124 (30.2) | 124 (32.6) | 117 (28.4) | 316 (36.0) | <.0001 |
| Sexually transmitted diseases, n (%) | 4462 (3.6) | 412 (4.5) | 120 (5.2) | 47 (4.7) | 43 (5.5) | 6 (3.7) | 24 (5.9) | 17 (4.5) | 24 (5.8) | 39 (4.5) | <.0001 |
| Bacterial vaginosis, n (%) | 9520 (7.7) | 969 (10.6) | 256 (11.0) | 97 (9.7) | 103 (13.2) | 14 (8.5) | 63 (15.4) | 37 (9.7) | 121 (29.4) | 87 (9.9) | <.0001 |
| Health services utilization in the year before pregnancy: | |||||||||||
| Inpatient or emergency visit, n (%) | 23 743 (19.1) | 1986 (21.8) | 471 (20.2) | 257 (25.6) | 168 (21.5) | 26 (15.9) | 90 (22.0) | 87 (22.8) | 69 (16.8) | 169 (19.3) | <.0001 |
| No. of medications used other than anti‐infective | |||||||||||
| 0 | 46 894 (37.7) | 1978 (21.7) | 484 (20.8) | 211 (21.0) | 137 (17.5) | 36 (22.0) | 7 (17.6) | 92 (24.2) | 77 (18.7) | 198 (22.6) | |
| 1–2 | 27 000 (21.7) | 2160 (23.7) | 559 (24.0) | 215 (21.4) | 185 (23.7) | 41 (25.0) | 105 (25.6) | 97 (25.5) | 105 (25.5) | 212 (24.2) | |
| ≥3 | 50 575 (40.6) | 4968 (54.6) | 1289 (55.2) | 579 (57.6) | 460 (58.8) | 87 (53.0) | 233 (56.8) | 192 (50.3) | 230 (55.8) | 467 (53.3) | <.0001 |
| No. of visits to physicians | |||||||||||
| 0 | 15 504 (12.5) | 654 (7.2) | 142 (6.1) | 57 (5.7) | 27 (3.5) | 10 (6.1) | 16 (3.9) | 47 (12.4) | 37 (9.0) | 71 (8.1) | |
| 1–2 | 16 159 (13.0) | 811 (8.9) | 187 (8.0) | 85 (8.5) | 65 (8.3) | 20 (12.2) | 25 (6.1) | 50 (13.1) | 49 (11.9) | 98 (11.2) | |
| ≥3 | 92 806 (74.5) | 7641 (83.9) | 2003 (85.9) | 863 (85.8) | 690 (88.2) | 134 (81.7) | 369 (90.0) | 284 (74.5) | 326 (79.1) | 708 (80.7) | <.0001 |
SD, standard deviation
Other antibacterials included clindamycin and vancomycin
Statistical analysis
Descriptive analyses were performed to summarize the study population characteristics, using one‐way analysis of variance (ANOVA) and chi‐square tests for continuous and categorical variables, respectively. Within our study cohort, we conducted separate analyses for overall major congenital malformations, and for each organ system malformation.
Given that a women could be pregnant several times during the study period and meet the inclusion criteria, we used a conditional generalized estimation equation (GEE) to account for the within‐subject correlation. Thus, pregnancy was the unit of analysis.
We calculated prevalence odds ratios (ORs) and 95% confidence intervals (CIs) after controlling for the potential confounders listed above.
In a sensitivity analysis, penicillin exposure was used as reference group given its indications shared with other antibiotics during pregnancy 43, 44.
Results
After applying inclusion and exclusion criteria, the study population included 139 938 live births (Figure 1). Of this cohort, 15 469 pregnancies were exposed to antibiotics during the first trimester (11%) and 124 469 were unexposed during the same period (89%). Among antibiotics users, 9106 were exposed to penicillins only (58.9%); 2332 to macrolides only (15%); 1005 to cephalosporins only (6.5%); 877 to urinary anti‐infectives only (5.7%); 782 to quinolones only (5%); 412 to antiprotozoal only (2.7%); 410 tetracyclines only (2.7%); 381 other antibacterials only (2.5%) and 164 to sulfonamides only (1%) (Figure 1 and Table 1). We identified 13 852 MCMs diagnosed in the first year of life in the study population (9.9%).
Figure 1.
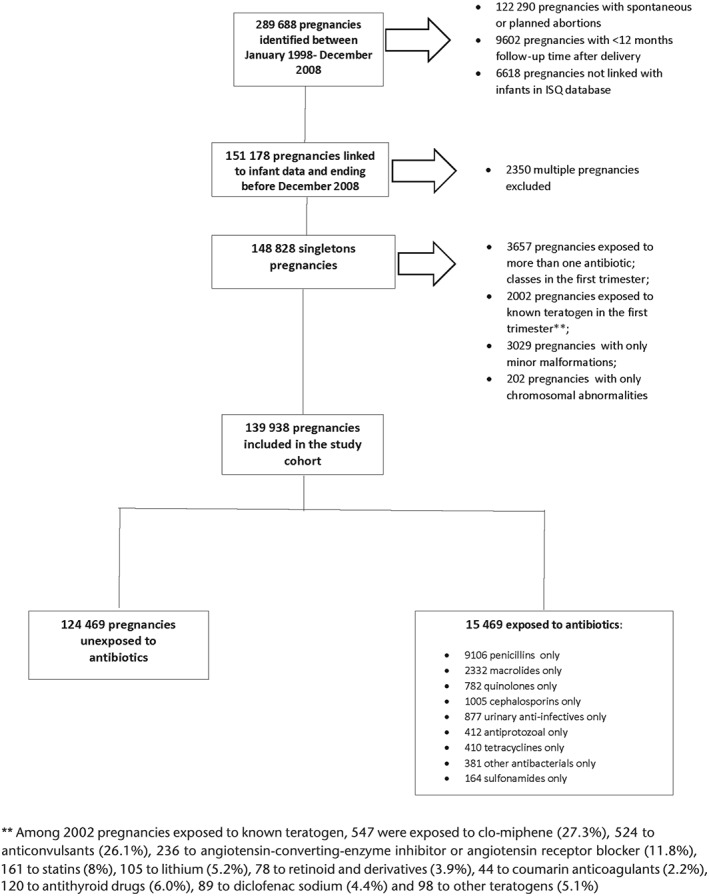
Flow chart of the selection of the study population selection.
Table 1 presents the study population characteristics stratified by antibiotics classes. Overall antibiotics users were more likely to be welfare recipients; less educated; living alone; users of healthcare services as well as more likely to have comorbidities and infections. Table 2 presents antibiotics classes and the risk of MCMs and organ‐specific MCMs.
Table 2.
Antibiotics classes and the risk of Major congenital malformations (MCMs) and organ specific MCMsa
| Types of malformations | Non exposed (n = 124 469) | Penicillins (n = 9106) | Macrolides (n = 2332) | Cephalos‐Porines (n = 1005) | Quinolones (n = 782) | Sulfonamides (n = 164) | Tetracyclines (n = 410) | Other Antibac‐terials a (n = 381) | Anti‐protozoals (n = 412) | Urinary anti‐infectives (n = 877) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Major congenital malformations | n,% | 12 225 (9.82%) | 894 (9.82%) | 265 (11.36%) | 116 (11.54%) | 92 (11.76%) | 14 (8.54%) | 43 (10.49%) | 60 (15.75%) | 47 (11.41%) | 96 (10.95%) |
| ORa | 1.00 | 0.96 | 1.08 | 1.12 | 1.08 | 0.64 | 1.04 | 1.34 | 1.10 | 1.02 | |
| 95 CI | Reference | (0.89–1.03) | (0.95–1.23) | (0.92–1.36) | (0.87–1.35) | (0.37–1.12) | (0.75–1.43) | (1.01–1.76) | (0.81–1.49) | (0.82–1.26) | |
| Nervous system | n,% | 725 (0.58%) | 53 (0.58%) | 16 (0.69%) | 6 (0.60%) | 6 (0.77%) | 2 (1.22%) | 2 (0.49%) | 6(1.57%) | 0 (0.00%) | 9 (1.03%) |
| ORa | 1.00 | 0.92 | 1.00 | 0.95 | 1.16 | 1.62 | 0.79 | 2.17 | NA | 1.59 | |
| 95 CI | Reference | (0.69–1.22) | (0.60–1.66) | (0.43–2.10) | (0.52–2.62) | (0.38–6.90) | (0.20–3.08) | (0.95–4.94) | NA | (0.81–3.14) | |
| Eye, ear, face and neck | n,% | 735 (0.59%) | 51 (0.56%) | 16 (0.69%) | 4 (0.40%) | 4 (0.51%) | 2 (1.22%) | 1 (0.24%) | 2 (0.52%) | 2 (0.49%) | 5 (0.57%) |
| ORa | 1.00 | 0.92 | 1.12 | 0.63 | 0.82 | 1.84 | 0.40 | 0.91 | 0.88 | 0.95 | |
| 95 CI | Reference | (0.69–1.22) | (0.68–1.85) | (0.23–1.72) | (0.30–2.23) | (0.45–7.48) | (0.06–2.83) | (0.23–3.58) | (0.22–3.47) | (0.40–2.30) | |
| Circulatory system | n,% | 2817 (2.26%) | 225 (2.47%) | 58 (2.49%) | 33 (3.28%) | 18 (2.30%) | 3 (1.83%) | 11 (2.68%) | 17 (4.46%) | 14 (3.40%) | 27 (3.08%) |
| ORa | 1.00 | 1.02 | 0.99 | 1.35 | 0.89 | 0.61 | 1.13 | 1.55 | 1.36 | 1.24 | |
| 95 CI | Reference | (0.89–1.18) | (0.76–1.29) | (0.94–1.93) | (0.55–1.43) | (0.19–1.91) | (0.62–2.06) | (0.95–2.52) | (0.80–2.33) | (0.84–1.83) | |
| Respiratory system | n,% | 565 (0.45%) | 45 (0.49%) | 14 (0.60%) | 8 (0.80%) | 5 (0.64%) | 1 (0.61%) | 4 (0.98%) | 5 (1.31%) | 2 (0.49%) | 2 (0.23%) |
| ORa | 1.00 | 1.03 | 1.15 | 1.61 | 1.33 | 1.00 | 2.12 | 2.12 | 0.97 | 0.46 | |
| 95 CI | Reference | (0.75–1.40) | (0.67–1.97) | (0.79–3.26) | (0.55–3.23) | (0.13–7.93) | (0.79–5.17) | (0.88–5.13) | (0.24–3.89) | (0.11–1.89) | |
| Digestive system | n,% | 1099 (0.88%) | 88 (0.97%) | 35 (1.50%) | 10 (1.00%) | 11 (1.41%) | 1 (0.61%) | 3 (0.73%) | 4 (1.05%) | 5 (1.21%) | 9 (1.03%) |
| ORa | 1.00 | 0.99 | 1.46 | 0.99 | 1.33 | 0.52 | 0.75 | 0.88 | 1.13 | 1.01 | |
| 95 CI | Reference | (0.79–1.24) | (1.04–2.06) | (0.53–1.86) | (0.73–2.43) | (0.07–3.68) | (0.24–2.34) | (0.33–2.36) | (0.46–2.75) | (0.52–1.95) | |
| Genital organs system | n,% | 1150 (0.92%) | 75 (0.82%) | 18 (0.77%) | 15 (1.49%) | 10 (1.28%) | 2 (1.22%) | 3 (0.73%) | 5 (1.31%) | 2 (0.49%) | 9 (1.03%) |
| ORa | 1.00 | 0.90 | 0.84 | 1.61 | 1.29 | 1.10 | 0.78 | 1.33 | 0.54 | 1.03 | |
| 95 CI | Reference | (0.71–1.14) | (0.52–1.34) | (0.96–2.70) | (0.64–2.57) | (0.26–4.56 | (0.24–2.53) | (0.55–3.21) | (0.13–2.18) | (0.53–2.01) | |
| Urinary system | n,% | 937 (0.75%) | 79 (0.87%) | 23 (0.99%) | 10 (1.00%) | 14 (1.79%) | 0 (0.00%) | 3 (0.73) | 4 (1.05%) | 5 (1.21%) | 9 (1.25%) |
| ORa | 1.00 | 1.12 | 1.26 | 1.25 | 1.89 | NA | 0.98 | 0.94 | 1.53 | 1.32 | |
| 95 CI | Reference | (0.88–1.42) | (0.83–1.92) | (0.67–2.33) | (1.09–3.28) | NA | (0.31–3.10) | (0.34–2.63) | (0.63–3.71) | (0.72–2.42) | |
| Musculoskeletal system | n,% | 4856 (3.90%) | 335 (3.68%) | 103 (4.42%) | 48 (4.78%) | 28 (3.58%) | 5 (3.05%) | 21 (5.12%) | 29 (7.61%) | 15 (3.64%) | 35 (3.99%) |
| ORa | 1.00 | 0.91 | 1.05 | 1.16 | 0.83 | 0.58 | 1.28 | 1.52 | 0.86 | 0.92 | |
| 95 CI | Reference | (0.81–1.02) | (0.86–1.28) | 0.86–1.55) | (0.57–1.21) | (0.24–1.42) | (0.82–2.00) | (1.03–2.22) | (0.51–1.44) | (0.65–1.29) | |
| Cardiac malformations | n,% | 2416 (1.94%) | 192 (2.11%) | 47 (2.02%) | 30 (2.99%) | 14 (1.79%) | 2 (1.22%) | 9 (2.20%) | 15 (3.94%) | 10 (2.43%) | 24 (2.74%) |
| ORa | 1.00 | 1.02 | 0.93 | 1.43 | 0.81 | 0.48 | 1.07 | 1.61 | 1.13 | 1.29 | |
| 95 CI | Reference | (0.87–1.18) | (0.69–1.25) | (0.98–2.08) | (0.48–1.39) | (0.12–1.95) | (0.55–2.07) | (0.96–2.71) | (0.60–2.12) | (0.86–1.95) | |
| Ventricular/ atrial septal defect | n,% | 1868 (1.50%) | 150 (1.65%) | 35 (1.50%) | 23 (2.29%) | 13 (1.66%) | 1 (0.61%) | 9 (2.20%) | 13 (3.41%) | 8 (1.94%) | 16 (1.82%) |
| ORa | 1.00 | 1.02 | 0.90 | 1.41 | 0.97 | 0.31 | 1.39 | 1.81 | 1.18 | 1.09 | |
| 95 CI | Reference | (0.86–1.21) | (0.64–1.27) | (0.93–2.13) | (0.56–1.68) | (0.04–2.23) | (0.72–2.69) | (1.04–3.14) | (0.58–2.40) | (0.66–1.80) | |
| Craniosynostosis | n,% | 633 (0.51) | 41 (0.45%) | 15 (0.64%) | 9 (0.90%) | 3 (0.38%) | 0 (0.00%) | 1 (0.24%) | 3 (0.79%) | 3 (0.73%) | 2 (0.23%) |
| ORa | 1.00 | 0.94 | 1.26 | 1.82 | 0.77 | NA | 0.49 | 1.19 | 1.37 | 0.43 | |
| 95 CI | Reference | (0.68–1.29) | (0.75–2.12) | (0.94–3.54) | (0.25–2.40) | NA | (0.07–3.47) | (0.38–3.72) | (0.43–4.37) | (0.11–1.72) | |
| Cleft palate | n,% | 194 (0.16%) | 13 (0.14%) | 7 (0.30%) | 3 (0.30%) | 1 (0.13%) | 0 (0.00%) | 1 (0.24%) | 2 (0.52%) | 0 (0.00%) | 1 (0.11%) |
| ORa | 1.00 | 0.84 | 1.70 | 1.70 | 0.80 | NA | 1.56 | 2.98 | NA | 0.71 | |
| 95 CI | Reference | (0.47–1.49) | (0.80–3.60) | (0.54–5.38) | (0.11–5.73) | NA | (0.22–11.23) | (0.72–12.30) | NA | (0.10–5.21) |
Note: CI, confidence interval; OR, odds ratio.
Other antibacterials included clindamycin and vancomycin.
Adjusted for the following variables: maternal age on the 1DG, maternal marital status (living alone or cohabiting), receipt of social assistance during pregnancy, calendar year of delivery, Infant gender, education level in years (≤12 or >12), and area of residence on the 1DG (urban or rural); maternal chronic co‐morbidities assessed using physician‐based diagnoses or filled prescriptions of related medications in the year before and during the first trimester of pregnancy (chronic hypertension, depression, diabetes mellitus, asthma, epilepsy, polyarthritis rheumatoid and systemic lupus erythematosus, thyroid disorders), endometriosis and maternal infections(urinary tract infection, respiratory tract infection, bacterial vaginosis, and sexually transmitted diseases) assessed using physician‐based diagnoses in the year before and during the first trimester of pregnancy; Use of healthcare services in the year before pregnancy; Significant prevalence OR were bolded
Other antibacterials exposure during the first trimester of pregnancy was associated with an increased risk of MCMs (aOR 1.34, 95% CI 1.01–1.76, 60 exposed cases). The most common antibiotics classes (penicillins, macrolides, cephalosporines, quinolones and urinary anti‐infectives) did not increase the risk of MCMs.
Other antibacterials exposure was associated with an increased risk of musculoskeletal system malformation (aOR 1.52, 95% CI 1.03–2.22, 29 exposed cases) and ventricular/atrial septal defect (aOR 1.81, 95% CI 1.04–3.14, 13 exposed cases).
Macrolides exposure was associated with an increased risk of digestive system malformations (aOR 1.46, 95% CI 1.04–2.06, 35 exposed cases) and quinolone exposure increased the risk of urinary system malformations (aOR 1.89, 95% CI 1.09–3.28, 14 exposed cases).
Tables 3 and 4 present antibiotics types and the risk of MCMs and organ‐specific MCMs. Clindamycin and ofloxacin exposures were associated with an increased risk of MCMs (aOR 1.34, 95% CI 1.02–1.77, 60 exposed cases; aOR 8.30, 95% CI 1.60–43.00, 3 exposed cases). Phenoxymethylpenicillin exposure was associated with an increased risk of nervous system malformations (aOR 1.85, 95% CI 1.01–3.39, 11 exposed cases). Erythromycin exposure increased the risk of urinary system malformations (aOR 2.12, 95% CI 1.08–4.17, 9 exposed cases). Moxifloxacin exposure was associated with an increased risk of respiratory system malformations (aOR 5.48, 95% CI 1.32–22.76, 2 exposed cases).
Table 3.
Risk of major congenital malformations and organ‐specific malformations following gestational exposure to amoxicillin, amoxicillin/potassium‐clavulanate, phenoxymethylpenicilline, azithromycin, clarithromycin, ciprofloxacin, norfloxacin, levofloxacin and erythromycina
| Types of malformations | Non‐exposed (n = 124 469) | Amoxicillin (n = 5950) | Amoxicillin + potassium clavulanate (n = 68) | Phenoxy‐Methyl penicilline (n = 854) | Azithro‐mycin (n = 883) | Clarithro‐mycin (n = 658) | Erythro‐mycin (n = 697) | Ciprofloxa‐cin (n = 608) | Norflo‐xacin (n = 37) | Levofloxacin (n = 70) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Major congenital malformations | n,% | 12 225 (9.82%) | 584 (9.82%) | 2 (2.94%) | 103 (12.06%) | 118 (13.36%) | 77 (11.70%) | 64 (9.18%) | 71 (11.68%) | 3 (8.11%) | 6 (8.57%) |
| ORa | 1.00 | 0.96 | 0.37 | 0.95 | 1.20 | 1.11 | 0.98 | 1.08 | 0.79 | 0.85 | |
| 95 CI | Reference | (0.88–1.05) | (0.08–1.36) | (0.77–1.17) | (0.98–1.46) | (0.87–1.41) | (0.75–1.27) | (0.84–1.40) | (0.24–2.57) | (0.37–1.97) | |
| Nervous system | n,% | 725 (0.58%) | 33 (0.55%) | 1 (1.47%) | 11 (1.29%) | 8 (0.91%) | 4 (0.61%) | 2 (0.29%) | 5 (0.82%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | 0.87 | 2.63 | 1.85 | 1.24 | 0.87 | 0.46 | 1.30 | NA | NA | |
| 95 CI | Reference | (0.61–1.25) | (0.36–18.98) | (1.01–3.39) | (0.60–2.55) | (0.32–2.34) | (0.11–1.90) | (0.54–3.15) | NA | NA | |
| Eye, ear, face and neck | n,% | 735 (0.59%) | 36 (0.61%) | 0 (0.00%) | 2 (0.23%) | 8 (0.91%) | 4 (0.61%) | 4 (0.57%) | 3 (0.49%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | 1.00 | NA | 0.36 | 1.46 | 0.96 | 1.00 | 0.79 | NA | NA | |
| 95 CI | Reference | (0.71–1.40) | NA | (0.09–1.51) | (0.72–2.97) | (0.36–2.54) | (0.38–2.67) | (0.25–2.55) | NA | NA | |
| Circulatory system | n,% | 2817 (2.26%) | 143 (2.40%) | 0 (0.00%) | 27 (3.16%) | 24 (2.72%) | 16 (2.43%) | 17 (2.44%) | 14 (2.30%) | 1 (2.70%) | 1 (1.43%) |
| ORa | 1.00 | 1.00 | NA | 1.08 | 1.01 | 0.96 | 1.09 | 0.91 | 1.07 | 0.59 | |
| 95 CI | Reference | (0.84–1.20) | NA | (0.73–1.59) | (0.67–1.52) | (0.59–1.58) | (0.67–1.78) | (0.53–1.55) | (0.14–8.31) | (0.08–4.24) | |
| Respiratory system | n,% | 565 (0.45%) | 30 (0.50%) | 0 (0.00%) | 1 (0.12%) | 6 (0.68%) | 4 (0.61%) | 4 (0.57%) | 3 (0.49%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | 1.05 | NA | 0.18 | 1.18 | 1.12 | 1.26 | 1.03 | NA | NA | |
| 95 CI | Reference | (0.72–1.53) | NA | (0.02–1.27) | (0.52–2.70) | (0.41–3.07) | (0.46–3.44) | (0.33–3.22) | NA | NA | |
| Digestive system | n,% | 1099 (0.88%) | 54 (0.91%) | 0 (0.00%) | 15 (1.76%) | 15 (1.70%) | 10 (1.52%) | 10 (1.43%) | 10 (1.64%) | 0 (0.00%) | 1 (1.43%) |
| ORa | 1.00 | 0.93 | NA | 1.52 | 1.54 | 1.44 | 1.56 | 1.61 | NA | 1.55 | |
| 95 CI | Reference | (0.70–1.23) | NA | (0.91–2.55) | (0.92–2.60) | (0.77–2.70) | (0.83–2.92) | (0.86–3.03) | NA | (0.22–11.13) | |
| Genital organs system | n,% | 1150 (0.92%) | 44 (0.74%) | 1 (1.47%) | 9 (1.05%) | 9 (1.02%) | 7 (1.06%) | 2 (0.29%) | 7 (1.15%) | 1 (2.70%) | 0 (0.00%) |
| ORa | 1.00 | 0.81 | 2.16 | 0.98 | 1.04 | 1.15 | 0.34 | 1.14 | 2.64 | NA | |
| 95 CI | Reference | (0.59–1.09) | (0.29–15.94) | (0.51–1.89) | (0.54–2.01) | (0.55–2.42) | (0.08–1.40) | (0.49–2.67) | (0.34–20.36) | NA | |
| Urinary system | n,% | 937 (0.75%) | 58 (0.97%) | 0 (0.00%) | 8 (0.94%) | 12 (1.36%) | 2 (0.30%) | 9 (1.29%) | 9 (1.48%) | 1 (2.70%) | 2 (2.86%) |
| ORa | 1.00 | 1.27 | NA | 0.79 | 1.49 | 0.40 | 2.12 | 1.49 | 3.26 | 4.24 | |
| 95 CI | Reference | (0.97–1.67) | NA | (0.39–1.59) | (0.84–2.64) | (0.10–1.58) | (1.08–4.17) | (0.75–2.96) | (0.46–22.93) | (1.00–18.01) | |
| Musculoskeletal system | n,% | 4856 (3.90%) | 230 (3.87%) | 0 (0.00%) | 36 (4.22%) | 48 (5.48%) | 30 (4.56%) | 25 (3.59%) | 23 (3.78%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | 0.97 | NA | 0.78 | 1.17 | 1.07 | 1.03 | 0.89 | NA | NA | |
| 95 CI | Reference | (0.84–1.11) | NA | (0.56–1.10) | (0.87–1.57) | (0.74–1.54) | (0.68–1.53) | (0.58–1.36) | NA | NA | |
| Cardiac malformations | n,% | 2416 (1.94%) | 117 (1.97%) | 0 (0.00%) | 25 (2.93%) | 19 (2.15%) | 12 (1.82%) | 15 (2.15%) | 11 (1.81%) | 1 (2.70%) | 1 (1.43%) |
| ORa | 1.00 | 0.96 | NA | 1.19 | 0.95 | 0.84 | 1.11 | 0.84 | 1.24 | 0.70 | |
| 95 CI | Reference | (0.79–1.18) | NA | (0.80–1.77) | (0.60–1.50) | (0.48–1.49) | (0.66–1.87) | (0.46–1.54) | (0.16–9.63) | (0.10–5.02) | |
| Ventricular/atrial septal defect | n,% | 1868 (1.50%) | 93 (1.56%) | 0 (0.00%) | 18 (2.11%) | 14 (1.59%) | 9 (1.37%) | 11 (1.58%) | 10 (1.64%) | 1 (2.70%) | 1 (1.43%) |
| ORa | 1.00 | 0.98 | NA | 1.10 | 0.91 | 0.82 | 1.05 | 0.98 | 1.62 | 0.92 | |
| 95 CI | Reference | (0.79–1.22) | NA | (0.69–1.76) | (0.53–1.56) | (0.43–1.59) | (0.58–1.92) | (0.52–1.84) | (0.21–12.74) | (0.13–6.62) | |
| Craniosyno‐stosis | n,% | 633 (0.51%) | 27 (0.45%) | 0 (0.00%) | 3 (0.35%) | 8 (0.91%) | 4 (0.61%) | 3 (0.43%) | 1 (0.16%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | 0.95 | NA | 0.49 | 1.52 | 1.24 | 1.01 | 0.33 | NA | NA | |
| 95 CI | Reference | (0.64–1.41) | NA | (0.15–1.54) | (0.74–3.11) | (0.46–3.31) | (0.32–3.17) | (0.05–2.26) | NA | NA | |
| Cleft palate | n,% | 194 (0.16%) | 5 (0.08%) | 0 (0.00%) | 3 (0.35%) | 2 (0.23%) | 2 (0.30%) | 3 (0.43%) | 1 (0.16%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | 0.48 | NA | 1.91 | 1.27 | 1.57 | 2.49 | 1.01 | NA | NA | |
| 95 CI | Reference | (0.20–1.19) | NA | (0.60–6.07) | (0.32–5.08) | (0.39–6.41) | (0.80–7.74) | (0.14–7.22) | NA | NA |
Note: CI, confidence interval; OR, odds ratio.
Adjusted for the following variables: maternal age on the 1DG, maternal marital status (living alone or cohabiting), receipt of social assistance during pregnancy, calendar year of delivery, Infant gender, education level in years (≤12 or >12), and area of residence on the 1DG (urban or rural); maternal chronic co‐morbidities assessed using physician‐based diagnoses or filled prescriptions of related medications in the year before and during the first trimester of pregnancy (chronic hypertension, depression, diabetes mellitus, asthma, epilepsy, polyarthritis rheumatoid and systemic lupus erythematosus, thyroid disorders), endometriosis and maternal infections (urinary tract infection, respiratory tract infection, bacterial vaginosis, and sexually transmitted diseases) assessed using physician‐based diagnoses in the year before and during the first trimester of pregnancy, Use of healthcare services in the year before pregnancy; Significant prevalence OR were bolded
Table 4.
Risk of major congenital malformations and organ‐specific malformations following gestational exposure to cephalexin, moxifloxacin, ofloxacin, clindamycin, doxycycline, minocycline, TMP‐SMX, metronidazole and nitrofurantoina
| Types of malformations | Non exposed (n = 124 469) | Moxifloxacin (n = 55) | Ofloxacin (n = 6) | Doxycy‐cline (n = 164) | Minocycline (n = 166) | Clindamycin (n = 380) | Cephalexin (n = 124) | Nitrofu‐rantoin (n = 874) | TMP‐SMX (n = 158) | Metronida‐zole (n = 412) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Major congenital malformations | n,% | 12 225 (9.82%) | 7 (12.73%) | 3 (50.00%) | 23 (14.02%) | 15 (9.04%) | 60 (15.79%) | 13 (10.48%) | 96 (10.98%) | 12 (7.59%) | 47 (8.57%) |
| ORa | 1.00 | 0.94 | 8.30 | 1.46 | 0.86 | 1.34 | 0.83 | 1.02 | 0.57 | 1.10 | |
| 95 CI | Reference | (0.42–2.11) | (1.60–43.00) | (0.93–2.28) | (0.50–1.46) | (1.02–1.77) | (0.47–1.46) | (0.82–1.26) | (0.31–1.03) | (0.81–1.49) | |
| Nervous system | n,% | 725 (0.58%) | 1 (1.82%) | 0 (0.00%) | 1 (0.61%) | 1 (0.60%) | 6 (1.58%) | 1 (0.81%) | 59 (1.03%) | 2 (1.27%) | 0 (0.00%) |
| ORa | 1.00 | 2.21 | NA | 1.00 | 0.94 | 2.19 | 1.17 | 1.64 | 1.75 | NA | |
| 95 CI | Reference | (0.25–19.45) | NA | (0.14–6.94) | (0.14–6.52) | (0.96–4.99) | (0.17–8.13) | (0.83–3.23) | (0.41–7.50) | NA | |
| Eye, ear, face and neck | n,% | 735 (0.59%) | 1 (1.82%) | 0 (0.00%) | 1 (0.61%) | 0 (0.00%) | 2 (0.53%) | 0 (0.00%) | 5 (0.57%) | 0 (0.00%) | 2 (0.49%) |
| ORa | 1.00 | 2.85 | NA | 1.02 | NA | 0.90 | NA | 0.96 | NA | 0.88 | |
| 95 CI | Reference | (0.40–20.45) | NA | (0.14–7.54) | NA | (0.23–3.57) | NA | (0.40–2.31) | NA | (0.22–3.47) | |
| Circulatory system | n,% | 2817 (2.26%) | 2 (3.64%) | 0 (0.00%) | 9 (5.49%) | 2 (1.20%) | 17 (4.47%) | 4 (3.23%) | 27 (3.09%) | 3 (1.90%) | 14 (3.40%) |
| ORa | 1.00 | 1.20 | NA | 2.38 | 0.49 | 1.56 | 1.15 | 1.24 | 0.64 | 1.36 | |
| 95 CI | Reference | (0.28–5.09) | NA | (1.21–4.67) | (0.12–2.02) | (0.96–2.54) | (0.43–3.04) | (0.84–1.83) | (0.20–2.01) | (0.80–2.33) | |
| Respiratory system | n,% | 565 (0.45%) | 2 (3.64%) | 0 (0.00%) | 1 (0.61%) | 2 (1.20%) | 5 (1.32%) | 1 (0.81%) | 2 (0.23%) | 1 (0.63%) | 2 (0.49%) |
| ORa | 1.00 | 5.48 | NA | 1.31 | 2.62 | 2.13 | 1.32 | 0.47 | 1.02 | 0.97 | |
| 95 CI | Reference | (1.32–22.76) | NA | (0.18–9.34) | (0.65–10.56) | (0.88–5.16) | (0.19–9.14) | (0.12–1.90) | (0.13–8.09) | (0.24–3.89) | |
| Digestive system | n,% | 1099 (0.88%) | 0 (0.00%) | 0 (0.00%) | 1 (0.61%) | 1 (0.60%) | 4 (1.05%) | 2 (1.61%) | 9 (1.03%) | 1 (0.63%) | 5 (1.21%) |
| ORa | 1.00 | NA | NA | 0.62 | 0.62 | 0.88 | 1.42 | 1.04 | 0.55 | 1.13 | |
| 95 CI | Reference | NA | NA | (0.09–4.50) | (0.09–4.44) | (0.33–2.37) | (0.35–5.78) | (0.54–2.00) | (0.08–3.90) | (0.46–2.75) | |
| Genital organs system | n,% | 1150 (0.92%) | 1 (1.82%) | 1 (16.67%) | 2 (1.22%) | 0 (0.00%) | 5 (1.32%) | 2 (1.61%) | 9 (1.03%) | 2 (1.27%) | 2 (0.49%) |
| ORa | 1.00 | 1.75 | 17.40 | 1.33 | NA | 1.35 | 1.46 | 1.03 | 1.15 | 0.54 | |
| 95 CI | Reference | (0.24–12.51) | (1.52–198.82) | (0.31–5.64) | NA | (0.56–3.25) | (0.36–5.85) | (0.53–2.01) | (0.28–4.80) | (0.13–2.18) | |
| Urinary system | n,% | 937 (0.75%) | 0 (0.00%) | 1 (16.67%) | 1 (0.61%) | 1 (0.60%) | 4 (1.05%) | 0 (0.00%) | 11 (1.26%) | 0 (0.00%) | 5 (1.21%) |
| ORa | 1.00 | NA | 22.69 | 0.80 | 0.80 | 0.94 | NA | 1.30 | NA | 1.53 | |
| 95 CI | Reference | NA | (2.88–335.32) | (0.11–5.84) | (0.11–5.94) | (0.34–2.63) | NA | (0.71–2.39) | NA | (0.63–3.71) | |
| Musculoskeletal system | n,% | 4856 (3.90%) | 3 (5.45%) | 1 (16.67%) | 11 (6.71%) | 8 (4.82%) | 29 (7.63%) | 5 (4.03%) | 35 (4.00%) | 4 (2.53%) | 15 (3.64%) |
| ORa | 1.00 | 0.95 | 4.42 | 1.72 | 1.16 | 1.67 | 0.76 | 0.92 | 0.48 | 0.86 | |
| 95 CI | Reference | (0.29–3.12) | (0.49–39.58) | (0.93–3.21) | (0.57–2.37) | (1.12–2.48) | (0.31–1.88) | (0.65–1.29) | (0.18–1.30) | (0.51–1.44) | |
| Cardiac malformations | n,% | 2416 (1.94%) | 1 (1.82%) | 0 (0.00%) | 8 (4.88%) | 1 (0.60%) | 15 (3.95%) | 3 (2.42%) | 24 (2.75%) | 2 (1.27%) | 10 (2.43%) |
| ORa | 1.00 | 0.72 | NA | 2.46 | 0.29 | 1.63 | 1.02 | 1.30 | 0.51 | 1.13 | |
| 95 CI | Reference | (0.10–5.25) | NA | (1.21–4.99) | (0.04–2.10) | (0.97–2.74) | (0.33–3.14) | (0.86–1.96) | (0.13–2.05) | (0.60–2.12) | |
| Ventricular/atrial septal defect | n,% | 1868 (1.50%) | 1 (1.82%) | 0 (0.00%) | 8 (4.88%) | 1 (0.60%) | 13 (3.42%) | 1 (0.81%) | 16 (1.83%) | 1 (0.63%) | 8 (1.94%) |
| ORa | 1.00 | 0.92 | NA | 3.19 | 0.38 | 1.81 | 0.44 | 1.10 | 0.33 | 1.18 | |
| 95 CI | Reference | (0.13–6.71) | NA | (1.57–6.48) | (0.05–2.67) | (1.04–3.16) | (0.06–2.98) | (0.67–1.82) | (0.05–2.35) | (0.58–2.40) | |
| Craniosynostosis | n,% | 633 (0.51%) | 1 (1.82%) | 1 (16.67%) | 0 (0.00%) | 1 (0.60%) | 3 (0.79%) | 1 (0.81%) | 2 (0.23%) | 0 (0.00%) | 3 (0.73%) |
| ORa | 1.00 | 2.77 | 41.36 | NA | 1.17 | 1.19 | 1.18 | 0.43 | NA | 1.37 | |
| 95 CI | Reference | (0.39–19.51) | (4.57–374.20) | NA | (0.16–8.34) | (0.38–3.73) | (0.17–8.35) | (0.11–1.70) | NA | (0.43–4.37) | |
| Cleft palate | n,% | 194 (0.16%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.60%) | 2 (0.53%) | 0 (0.00%) | 1 (0.11%) | 0 (0.00%) | 0 (0.00%) |
| ORa | 1.00 | NA | NA | NA | 4.01 | 2.95 | NA | 0.71 | NA | NA | |
| 95 CI | Reference | NA | NA | NA | (0.56–28.99) | (0.72–12.16) | NA | (0.10–5.21) | NA | NA |
Note: CI, confidence interval; OR, odds ratio TMP‐SMX, Trimethoprim/sulfamethoxazole;
Adjusted for the following variables: maternal age on the 1DG, maternal marital status (living alone or cohabiting), receipt of social assistance during pregnancy, calendar year of delivery, Infant gender, education level in years (≤12 or >12), and area of residence on the 1DG (urban or rural); maternal chronic co‐morbidities assessed using physician‐based diagnoses or filled prescriptions of related medications in the year before and during the first trimester of pregnancy (chronic hypertension, depression, diabetes mellitus, asthma, epilepsy, uterine malformations, polyarthritis rheumatoid and systemic lupus erythematosus, thyroid disorders), endometriosis and maternal infections (urinary tract infection, respiratory tract infection, bacterial vaginosis, and sexually transmitted diseases) assessed using physician‐based diagnoses in the year before and during the first trimester of pregnancy; Use of healthcare services in the year before pregnancy; Significant prevalence OR were bolded
Doxycycline exposure was associated with an increased risk of circulatory system malformation, cardiac malformations and ventricular/atrial septal defect, respectively (aOR 2.38, 95% CI 1.21–4.67, 9 exposed cases; aOR 2.46, 95% CI 1.21–4.99, 8 exposed cases; aOR 3.19, 95% CI 1.57–6.48, 8 exposed cases). Clindamycin exposure increased the risk of musculoskeletal system malformation (aOR 1.67, 95% CI 1.12–2.48, 29 exposed cases) and ventricular/atrial septal defect (aOR 1.81, 95% CI 1.04–3.16, 13 exposed cases). Amoxicillin, nitrofurantoin, cephalexin and metronidazole did not increase the risk of MCMs or organ‐specific MCMs.
In sensitivity analyses, when penicillin exposure was used as comparator group, similar estimates were found but with less precision (Tables S4–S6).
Discussion
To our knowledge, this is the largest cohort study investigating the risk of MCMs including organ‐specific malformations associated with the use of antibiotics classes and types during pregnancy. Penicillin and most specifically amoxicillin were not associated with an increased risk of MCMs and organ‐specific MCMs. These findings are consistent with the current literature 45, 46.
Similar to the study results of Denker et al. 47, phenoxymethylpenicillin exposure was not associated with MCMs nor cardiac malformations. However, we found an 85% increased risk of nervous system malformations following in utero exposure to phenoxymethylpenicillin during the first trimester of pregnancy, though residual confounding or chance finding could not be ruled out. This finding requires further investigation.
Macrolides exposure was not associated with an increased risk of MCMs nor cardiac malformations as previously reported in other studies 5, 11, 12, 13, 14, 15, 16, 17, 18. However we found a 46% increased risk of digestive system malformations. There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis 48, 49. Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy despite the fact that organogenesis occurs during that period. Therefore, our results should be considered as exploratory and require further research on specific digestive malformations such as orofacial cleft. Erythromycin use was associated with an increased risk of major urinary system malformations. However, chance finding or residual confounding could also be an explanation for this finding. Compared to penicillin, no association was found due to a lack of power.
Similarly to previous studies 17, 21, 22, 23, 24, 25, 26, 27, quinolones use was not associated with an increased risk of MCMs. However, we found a 89% increased risk of urinary system malformations associated with quinolone exposure. Compared to penicillins use, quinolone exposure still tended to increase the risk of urinary system malformations though it did not reach statistical significance.
Moxifloxacin exposure was associated with a 5‐fold increased risk of respiratory system malformations and ofloxacin use with an 8‐fold increased risk of MCMs. However, these results should be interpreted with caution given the small number of exposed cases.
Teratogenicity of quinolone has been reported in the literature in animal and experimental studies 50, 51. Indeed, quinolones can act as DNA gyrase inhibitors and also as mitotic inhibitors 52. This may partially damage DNA and induce fetal malformation, which supports our findings 52.
Doxycycline use was associated with a 2‐fold increased risk of circulatory system malformation and cardiac malformations and 3‐fold increased risk of ventricular/atrial septal defect. These results are consistent with a previous study that showed a 2‐fold non‐significant increased risk of heart defects associated with the use of tetracycline as a class 28. It is known that tissue remodelling is involved in placenta development 53. Given that, doxycycline may inhibit the production of pro‐inflammatory cytokines and matrix metalloproteinases known to play a role in tissue remodelling 54. This leads to placental anomalies that have been linked to birth defects as well 55.
Clindamycin exposure was associated with a 34% increased risk of MCMs including a 67% increased risk of musculoskeletal system malformation and an 81% increased risk of ventricular/atrial septal defect. To our knowledge, no previous studies have investigated the risk of MCMs or organ defects associated specifically with this antibiotic. Clindamycin has been classified as a nitrosatable drug (tertiary amine) given its chemical structure 56, 57. Studies reported that nitrosatable drugs were associated with an increased risk of many congenital malformations including heart and musculoskeletal system malformations 56, 57. Nitrosatable drugs form N‐nitroso compounds in the presence of nitrite under highly acidic environments such as human stomach 58. These N‐nitroso compounds may therefore induce congenital abnormalities through DNA alkylation of embryonic cells 59, 60.
Nitrofurantoin exposure was not associated with MCMs or organ‐specific MCMs. This result was consistent with a meta‐analysis that found similar results with cohort studies 32. Conversely, our findings were different from the meta‐analysis based on case control studies 32. These discrepancies may be explained by the potential recall bias found in case control studies.
Metronidazole use did not increase the risk of MCMs or organ‐specific MCMs as in previous studies 34. However, this finding should be interpreted with caution given that our team has recently shown a link between spontaneous abortion (SA) and metronidazole exposure 61. Given that SA are proxies of severe birth defects 62, ours results may have underestimated the true risk.
The strengths of our study included the use of QPC, which is a population‐based cohort including all pregnancies in Quebec. This allowed us to avoid a potential selection bias and to evaluate the association between individual antibiotics and specific malformations. Our cohort included also information on filled prescription and physician records collected prospectively which limits the potential for recall and detection bias. Another strength encompassed the use of validated data on antibiotics prescriptions and MCMs, which were validated against maternal reports and medical charts, respectively 39, 40. We also used a validated gestational age which allowed us to determine an accurate time window of interest 35. We included several potential confounders including maternal infections, and we conducted a sensitivity analysis using penicillin as a comparator group to further take into account confounding by indication. We investigated the relation between individual antibiotics and organ‐specific MCMs.
A potential limitation included missing information on potentially important confounders such as smoking, folic acid and alcohol intake. However, we indirectly adjusted for lifestyle by design given that we used penicillin exposure as comparator groups.
Though we adjusted for many potential confounders including maternal infections and comorbidities, residual confounding could not be completely ruled out.
Given that many comparisons were made in our study, we cannot rule out chance finding. However, by reducing the type 1 error, multiple test adjustments may increase the type II error (i.e. the probability of accepting the null hypothesis when the alternative is true) for those associations that are not null 63, 64. Therefore we did not adjust for multiple testing in our study to avoid missing important findings 64. In addition, our results were consistent with previous published studies on major congenital malformations, and biological plausibility explained our finding as well.
Like in previous studies, only pregnancies with live singleton birth were considered in our research. Exclusion of SA, a determinant of severe defects, may have underestimated the true risk of MCM following gestational antibiotic exposure, as these drugs could have caused SA. Lately, our team has recently reported that metronidazole, azithromycin, clarithromycin, sulfonamides, quinolones and tetracyclines increased the risk of SA 61. Nonetheless, even if our estimates may have been underestimated, they should remain conservative.
The prevalence of MCMs in our cohort was higher than in other studies (3–5%). However, our rate is consistent with what is expected in the province of Quebec, due to increased genetic risk stemming from the ‘founding’ French ancestor 65. Therefore, as the prevalence of MCMs is high irrespective of our exposure status, there is no reason to believe that it will affect our internal validity given that it will be cancelled out when comparing exposed and unexposed groups.
Some of our analyses investigating the association between individual antibiotics and specific malformations were underpowered given the small number of exposed cases.
Absolute risks for musculoskeletal system malformations and cardiac malformation were 1.3% and 1% respectively in Quebec 66. Therefore a 67% increase of musculoskeletal system malformations in pregnant women exposed to clindamycin would result in an increase in the absolute risks from 1.3% to 2.1%. Similarly a twofold increase of the risk of cardiac malformations following an exposure to doxycycline during pregnancy would increase the absolute risks to 2%. Despite the fact that the absolute risks remain small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible.
Finally, our cohort included women of low socio‐economic status insured by the RAMQ for their medications. Bérard and Lacasse 67 have shown that socioeconomic status was an effect modifier in the QPC. Therefore, internal validity of our results should not be affected.
Conclusion
In this large population‐based cohort, we found that in utero exposure to clindamycin, doxycycline, macrolide, quinolone and phenoxymethylpenicillin increased the risk of organ‐specific MCMS in infants. Reassuringly amoxicillin, cephalosporins and nitrofurantoin did not increase the risk of MCMs or any organ‐specific malformation. Although the absolute risk for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available.
Competing Interests
A.B. is a consultant for plaintiffs in litigation involving antidepressants and birth defects. F.T.M. and O.S. report no conflicts of interest.
This work was supported by the Réseau Québécois de recherche sur l'usage des médicaments. F.T.M. is the recipient of the Sainte‐Justine Hospital Foundation and the Foundation of Stars scholarship from the Faculty of Pharmacy of the University of Montreal and is scholarship holder of the Canadian Network for Advanced Interdisciplinary Methods for comparative effectiveness research (CAN‐AIM). A.B. is recipient of a career award from the FRSQ and is on the endowment Research Chair of the Famille Louis‐Boivin on Medications, Pregnancy and Lactation at the Faculty of Pharmacy of the University of Montreal. The funding body had no involvement in the data collection or analysis, the preparation of the manuscript, or the decision to submit the paper for publication.
Contributors
All authors conceived and designed this study. Data were acquired by A.B. Statistical analyses were carried out by F.T.M. under the supervision of O.S. and A.B. and all authors interpreted the data. The manuscript was drafted by F.T.M. and all authors were involved in the critical revision and approval of the final manuscript.
Supporting information
Table S1 ICD‐9 and ICD‐10 Diagnostic codes used to identify major congenital malformation by organ system
Table S2 Diagnostic codes and medication (American Hospital Formulary Service (AHFS) or The Anatomical Therapeutic Chemical (ATC) for covariates)
Table S3 Illustration of the structure of confounding with maternal infections using a direct acyclic graph
Table S4 Antibiotics classes and the risk of major congenital malformations (MCMs) and organ specific MCMs
Table S5 Antibiotics types and the risk of major congenital malformations (MCMs) and organ specific MCMs*+
Table S6 Antibiotics types and the risk of major congenital malformations (MCMs) and organ specific MCMs**+
Muanda, F. T. , Sheehy, O. , and Bérard, A. (2017) Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. Br J Clin Pharmacol, 83: 2557–2571. doi: 10.1111/bcp.13364.
References
- 1. Palmsten K, Hernandez‐Diaz S, Chambers CD, Mogun H, Lai S, Gilmer TP, et al. The most commonly dispensed prescription medications among pregnant women enrolled in the U.S. Medicaid program. Obstet Gynecol 2015; 126: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santos F, Sheehy O, Perreault S, Ferreira E, Berard A. Trends in anti‐infective drugs use in pregnancy. J Popul Ther Clin Pharmacol 2012; 19: e460–e465. [PubMed] [Google Scholar]
- 3. Maslanka T, Jaroszewski JJ, Chrostowska M. Pathogenesis of quinolone‐induced arthropathy: a review of hypotheses. Pol J Vet Sci 2004; 7: 323–331. [PubMed] [Google Scholar]
- 4. Demers P, Fraser D, Goldbloom RB, Haworth JC, LaRochelle J, MacLean R, et al. Effects of tetracyclines on skeletal growth and dentition. A report by the Nutrition Committee of the Canadian Paediatric Society. Can Med Assoc J 1968; 99: 849–854. [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen JT, Petersen M, Jimenez‐Solem E, Broedbaek K, Andersen NL, Torp‐Pedersen C, et al. Clarithromycin in early pregnancy and the risk of miscarriage and malformation: a register based nationwide cohort study. PLoS One 2013; 8: e53327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prytherch JP, Sutton ML, Denine EP. General reproduction, perinatal‐postnatal, and teratology studies of nitrofurantoin macrocrystals in rats and rabbits. J Toxicol Environ Health 1984; 13: 811–823. [DOI] [PubMed] [Google Scholar]
- 7. Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol 2011; 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP‐mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2006; 2: 875–894. [DOI] [PubMed] [Google Scholar]
- 9. Bracken MB. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med 2009; 102: 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruelius HW. Extrapolation from animals to man: predictions, pitfalls and perspectives. Xenobiotica 1987; 17: 255–265. [DOI] [PubMed] [Google Scholar]
- 11. Berard A, Sheehy O, Zhao JP, Nordeng H. Use of macrolides during pregnancy and the risk of birth defects: a population‐based study. Pharmacoepidemiol Drug Saf 2015; 24: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 12. Lin KJ, Mitchell AA, Yau WP, Louik C, Hernandez‐Diaz S. Safety of macrolides during pregnancy. Am J Obstet Gynecol 2013; 208: 221.e1–221.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bar‐Oz B, Weber‐Schoendorfer C, Berlin M, Clementi M, Di Gianantonio E, de Vries L, et al. The outcomes of pregnancy in women exposed to the new macrolides in the first trimester: a prospective, multicentre, observational study. Drug Saf 2012; 35: 589–598. [DOI] [PubMed] [Google Scholar]
- 14. Bahat Dinur A, Koren G, Matok I, Wiznitzer A, Uziel E, Gorodischer R, et al. Fetal safety of macrolides. Antimicrob Agents Chemother 2013; 57: 3307–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bar‐Oz B, Diav‐Citrin O, Shechtman S, Tellem R, Arnon J, Francetic I, et al. Pregnancy outcome after gestational exposure to the new macrolides: a prospective multi‐center observational study. Eur J Obstet Gynecol Reprod Biol 2008; 141: 31–34. [DOI] [PubMed] [Google Scholar]
- 16. Romoren M, Lindbaek M, Nordeng H. Pregnancy outcome after gestational exposure to erythromycin – a population‐based register study from Norway. Br J Clin Pharmacol 2012; 74: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper WO, Hernandez‐Diaz S, Arbogast PG, Dudley JA, Dyer SM, Gideon PS, et al. Antibiotics potentially used in response to bioterrorism and the risk of major congenital malformations. Paediatr Perinat Epidemiol 2009; 23: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. A population‐based case‐control teratologic study of oral erythromycin treatment during pregnancy. Reprod Toxicol 1999; 13: 531–536. [DOI] [PubMed] [Google Scholar]
- 19. Kallen BA, Otterblad Olausson P, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol 2005; 20: 209–214. [DOI] [PubMed] [Google Scholar]
- 20. Kallen B, Danielsson BR. Fetal safety of erythromycin: an update of Swedish data. Eur J Clin Pharmacol 2014; 70: 355–360. [DOI] [PubMed] [Google Scholar]
- 21. Padberg S, Wacker E, Meister R, Panse M, Weber‐Schoendorfer C, Oppermann M, et al. Observational cohort study of pregnancy outcome after first‐trimester exposure to fluoroquinolones. Antimicrob Agents Chemother 2014; 58: 4392–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaefer C, Amoura‐Elefant E, Vial T, Ornoy A, Garbis H, Robert E, et al. Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European Network of Teratology Information Services (ENTIS). Eur J Obstet Gynecol Reprod Biol 1996; 69: 83–89. [DOI] [PubMed] [Google Scholar]
- 23. Czeizel AE, Sorensen HT, Rockenbauer M, Olsen J. A population‐based case‐control teratologic study of nalidixic acid. Int J Gynaecol Obstet 2001; 73: 221–228. [DOI] [PubMed] [Google Scholar]
- 24. Berkovitch M, Pastuszak A, Gazarian M, Lewis M, Koren G. Safety of the new quinolones in pregnancy. Obstet Gynecol 1994; 84: 535–538. [PubMed] [Google Scholar]
- 25. Wogelius P, Norgaard M, Gislum M, Pedersen L, Schonheyder HC, Sorensen HT. Further analysis of the risk of adverse birth outcome after maternal use of fluoroquinolones. Int J Antimicrob Agents 2005; 26: 323–326. [DOI] [PubMed] [Google Scholar]
- 26. Larsen H, Nielsen GL, Schonheyder HC, Olesen C, Sorensen HT. Birth outcome following maternal use of fluoroquinolones. Int J Antimicrob Agents 2001; 18: 259–262. [DOI] [PubMed] [Google Scholar]
- 27. Loebstein R, Addis A, Ho E, Andreou R, Sage S, Donnenfeld AE, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrob Agents Chemother 1998; 42: 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med 2009; 163: 978–985. [DOI] [PubMed] [Google Scholar]
- 29. Molgaard‐Nielsen D, Hviid A. Maternal use of antibiotics and the risk of orofacial clefts: a nationwide cohort study. Pharmacoepidemiol Drug Saf 2012; 21: 246–253. [DOI] [PubMed] [Google Scholar]
- 30. Czeizel AE, Rockenbauer M. A population‐based case‐control teratologic study of oral oxytetracycline treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol 2000; 88: 27–33. [DOI] [PubMed] [Google Scholar]
- 31. Czeizel AE, Rockenbauer M. Teratogenic study of doxycycline. Obstet Gynecol 1997; 89: 524–528. [DOI] [PubMed] [Google Scholar]
- 32. Goldberg O, Moretti M, Levy A, Koren G. Exposure to nitrofurantoin during early pregnancy and congenital malformations: a systematic review and meta‐analysis. J Obstet Gynaecol Can 2015; 37: 150–156. [DOI] [PubMed] [Google Scholar]
- 33. Ailes EC, Gilboa SM, Gill SK, Broussard CS, Crider KS, Berry RJ, et al. Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Res A Clin Mol Teratol 2016; 106: 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheehy O, Santos F, Ferreira E, Berard A. The use of metronidazole during pregnancy: a review of evidence. Curr Drug Saf 2015; 10: 170–179. [DOI] [PubMed] [Google Scholar]
- 35. Bérard A, Sheehy O. The Quebec Pregnancy Cohort – prevalence of medication use during gestation and pregnancy outcomes. PLoS One 2014; 9: e93870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vilain A, Otis S, Forget A, Blais L. Agreement between administrative databases and medical charts for pregnancy‐related variables among asthmatic women. Pharmacoepidemiol Drug Saf 2008; 17: 345–353. [DOI] [PubMed] [Google Scholar]
- 37. Kulaga S, Zargarzadeh AH, Berard A. Prescriptions filled during pregnancy for drugs with the potential of fetal harm. BJOG 2009; 116: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 38. Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med 1998; 338: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 39. Jobin‐Gervais K, Sheehy O, Berard A. Can we rely on pharmacy claims databases to ascertain maternal use of medications during pregnancy? Pharmacoepidemiol Drug Saf 2013; 22: 155. [DOI] [PubMed] [Google Scholar]
- 40. Blais L, Berard A, Kettani FZ, Forget A. Validity of congenital malformation diagnostic codes recorded in Quebec's administrative databases. Pharmacoepidemiol Drug Saf 2013; 22: 881–889. [DOI] [PubMed] [Google Scholar]
- 41. Lechat MF, Dolk H. Registries of congenital anomalies: EUROCAT. Environ Health Perspect 1993; 101 (Suppl 2): 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10: 37–48. [PubMed] [Google Scholar]
- 43. Lynch CM, Sinnott JT, Holt DA, Herold AH. Use of antibiotics during pregnancy. Am Fam Physician 1991; 43: 1365–1368. [PubMed] [Google Scholar]
- 44. Rosa FW, Baum C, Shaw M. Pregnancy outcomes after first‐trimester vaginitis drug therapy. Obstet Gynecol 1987; 69: 751–755. [PubMed] [Google Scholar]
- 45. Jepsen P, Skriver MV, Floyd A, Lipworth L, Schonheyder HC, Sorensen HT. A population‐based study of maternal use of amoxicillin and pregnancy outcome in Denmark. Br J Clin Pharmacol 2003; 55: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. Use of cephalosporins during pregnancy and in the presence of congenital abnormalities: a population‐based, case‐control study. Am J Obstet Gynecol 2001; 184: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 47. Dencker BB, Larsen H, Jensen ES, Schonheyder HC, Nielsen GL, Sorensen HT. Birth outcome of 1886 pregnancies after exposure to phenoxymethylpenicillin in utero. Clin Microbiol Infect 2002; 8: 196–201. [DOI] [PubMed] [Google Scholar]
- 48. Lund M, Pasternak B, Davidsen RB, Feenstra B, Krogh C, Diaz LJ, et al. Use of macrolides in mother and child and risk of infantile hypertrophic pyloric stenosis: nationwide cohort study. BMJ 2014; 348: g1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cooper WO, Ray WA, Griffin MR. Prenatal prescription of macrolide antibiotics and infantile hypertrophic pyloric stenosis. Obstet Gynecol 2002; 100: 101–106. [DOI] [PubMed] [Google Scholar]
- 50. Linseman DA, Hampton LA, Branstetter DG. Quinolone‐induced arthropathy in the neonatal mouse. Morphological analysis of articular lesions produced by pipemidic acid and ciprofloxacin. Fundam Appl Toxicol 1995; 28: 59–64. [DOI] [PubMed] [Google Scholar]
- 51. Aboubakr M, Elbadawy M, Soliman A, El‐Hewaity M. Embryotoxic and teratogenic effects of norfloxacin in pregnant female albino rats. Adv Pharmacol Sci 2014; 2014: 924706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Corbett JW, Ko SS, Rodgers JD, Gearhart LA, Magnus NA, Bacheler LT, et al. Inhibition of clinically relevant mutant variants of HIV‐1 by quinazolinone non‐nucleoside reverse transcriptase inhibitors. J Med Chem 2000; 43: 2019–2030. [DOI] [PubMed] [Google Scholar]
- 53. Moutier R, Tchang F, Caucheteux SM, Kanellopoulos‐Langevin C. Placental anomalies and fetal loss in mice, after administration of doxycycline in food for tet‐system activation. Transgenic Res 2003; 12: 369–373. [DOI] [PubMed] [Google Scholar]
- 54. Shlopov BV, Stuart JM, Gumanovskaya ML, Hasty KA. Regulation of cartilage collagenase by doxycycline. J Rheumatol 2001; 28: 835–842. [PubMed] [Google Scholar]
- 55. Sadler TW, Rasmussen SA. Examining the evidence for vascular pathogenesis of selected birth defects. Am J Med Genet A 2010; 152A: 2426–2436. [DOI] [PubMed] [Google Scholar]
- 56. Brender JD, Werler MM, Shinde MU, Vuong AM, Kelley KE, Huber JC Jr, et al. Nitrosatable drug exposure during the first trimester of pregnancy and selected congenital malformations. Birth Defects Res A Clin Mol Teratol 2012; 94: 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Olshan AF, Faustman EM. Nitrosatable drug exposure during pregnancy and adverse pregnancy outcome. Int J Epidemiol 1989; 18: 891–899. [DOI] [PubMed] [Google Scholar]
- 58. Gillatt PN, Palmer RC, Smith PL, Walters CL, Reed PI. Susceptibilities of drugs to nitrosation under simulated gastric conditions. Food Chem Toxicol 1985; 23: 849–855. [DOI] [PubMed] [Google Scholar]
- 59. Inouye M, Murakami U. Teratogenic effect of N‐methyl‐N'‐nitro‐N‐nitrosoguanidine in mice. Teratology 1978; 18: 263–267. [DOI] [PubMed] [Google Scholar]
- 60. Givelber HM, DiPaolo JA. Teratogenic effects of N‐ethyl‐N‐nitrosourea in the Syrian hamster. Cancer Res 1969; 29: 1151–1155. [PubMed] [Google Scholar]
- 61. Muanda FT, Sheehy O, Berard A. Use of antibiotics during pregnancy and risk of spontaneous abortion. CMAJ 2017; 189: E625–EE33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Svensson E, Ehrenstein V, Norgaard M, Bakketeig LS, Rothman KJ, Sorensen HT, et al. Estimating the proportion of all observed birth defects occurring in pregnancies terminated by a second‐trimester abortion. Epidemiology 2014; 25: 866–871. [DOI] [PubMed] [Google Scholar]
- 63. Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998; 316: 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–46. [PubMed] [Google Scholar]
- 65. Laberge AM, Michaud J, Richter A, Lemyre E, Lambert M, Brais B, et al. Population history and its impact on medical genetics in Quebec. Clin Genet 2005; 68: 287–301. [DOI] [PubMed] [Google Scholar]
- 66. Zhao JP, Sheehy O, Berard A. Regional variations in the prevalence of major congenital malformations in Quebec: the importance of fetal growth environment. J Popul Ther Clin Pharmacol 2015; 22: e198–e210. [PubMed] [Google Scholar]
- 67. Bérard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol 2009; 16: e360–e369. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ICD‐9 and ICD‐10 Diagnostic codes used to identify major congenital malformation by organ system
Table S2 Diagnostic codes and medication (American Hospital Formulary Service (AHFS) or The Anatomical Therapeutic Chemical (ATC) for covariates)
Table S3 Illustration of the structure of confounding with maternal infections using a direct acyclic graph
Table S4 Antibiotics classes and the risk of major congenital malformations (MCMs) and organ specific MCMs
Table S5 Antibiotics types and the risk of major congenital malformations (MCMs) and organ specific MCMs*+
Table S6 Antibiotics types and the risk of major congenital malformations (MCMs) and organ specific MCMs**+


