Abstract
Aims
To describe psychotropic polypharmacy in Australia between 2006 and 2015.
Methods
We used pharmaceutical claims from a national 10% sample of people with complete dispensing histories to estimate the annual prevalence of the combined use (overlap of >60 days exposure) of ≥2 psychotropics overall and within the same class or subclass (class and subclass polypharmacy). We also estimated the proportion of polypharmacy episodes involving one, two, three and four or more unique prescribers.
Results
The prevalence of class polypharmacy between 2006 and 2015 in people dispensed specific psychotropic classes was 5.9–7.3% for antipsychotics, 2.1–3.7% for antidepressants and 4.3–2.9% for benzodiazepines. The prevalence of antipsychotic polypharmacy was higher than expected given the prevalence of antipsychotic exposure and combinations of sedating agents were notably common. Overall, 26.7% of polypharmacy episodes involved multiple prescribers but having multiple prescribers occurred more frequently for class and subclass polypharmacy and people with four or more concomitant psychotropics.
Discussion
Psychotropic polypharmacy is common, despite limited evidence of risks and benefits. Increases in polypharmacy with multiple prescribers may be due to poor communication with patients and between health care professionals.
Keywords: antidepressant, antipsychotic, benzodiazepine, polypharmacy, psychotropic
What is Already Known about this Subject
There is a paucity of contemporary studies measuring psychotropic polypharmacy at the population level.
Previous studies of elderly people have established an association between polypharmacy involving any medicine and multiple prescribers but this has not been documented for psychotropic polypharmacy.
What this Study Adds
Despite limited evidence of risks and benefits, psychotropic polypharmacy remains common.
Combinations of sedating agents as well as antipsychotics with other antipsychotics occur frequently.
Potentially inappropriate prescribing such as combinations of psychotropic medicines from the same class or subclass and the use of four or more psychotropics concomitantly tends to involve more prescribers.
Introduction
Psychotropic polypharmacy refers to combination therapy with two or more psychotropic medicines 1. In some circumstances, psychotropic polypharmacy may be clinically justified, such as when psychotropics from different pharmacological classes are combined to treat multiple co‐occurring psychiatric illnesses 2. However, psychotropic medicines are prescribed in combination most frequently because of perceived inadequate response to monotherapy 1 despite evidence that the clinical assessment of response to monotherapy is often inadequate 3, 4. This frequently results in attempts to augment treatment response by adding psychotropic medicines progressively from the same or different pharmacological classes 5. Polypharmacy may also occur when patients are under the care of multiple prescribers 6 who adopt different practices and have different views about polypharmacy 7; patients with multiple prescribers are at an increased risk of adverse drug reactions and medication interactions arising through gaps in communication 8.
Our overall aim is to investigate psychotropic polypharmacy in Australia between 2006 and 2015. Specifically, we quantify the annual prevalence of any, class and subclass polypharmacy; identify psychotropics that are over‐represented in polypharmacy relative to their overall use; and the relationship between polypharmacy and the number of prescribers.
Methods
Setting and data source
Australia has a publicly funded universal healthcare system entitling all citizens and permanent residents to subsidized prescribed medicines via the Pharmaceutical Benefits Scheme (PBS). We used PBS dispensing records from 1 January 2006 to 31 December 2015 from a 10% random sample of PBS‐eligible persons 9. The 10% PBS sample is a standardized dataset provided by the Department of Human Services for analytical use and the patient population is selected based on the last digit of each individual's randomly assigned unique identifier. The sample only includes dispensing records of PBS‐subsidized medicines; it does not capture PBS medicines priced below the PBS co‐payment threshold or those dispensed privately in the community.
Study population
We restricted our analyses to people aged ≥18 years for all or part of the study period and for whom we had a complete PBS dispensing history. PBS‐eligible patients pay a copayment according to their beneficiary status. Concessional beneficiaries (patients eligible for government entitlements, including those aged ≥65 years, low income earners and people with disabilities) have a lower co‐payment threshold than general beneficiaries (all other patients).
As the cost of many medicines falls below the general beneficiary co‐payment threshold but above the concessional beneficiary co‐payment threshold (and so are not recorded for general beneficiaries), we restricted our analyses to individuals with concessional beneficiary status for the entire study period.
Medicines of interest
Medicines belonging to Anatomical Therapeutic Chemical Classification (ATC) classes NO5 (psycholeptics) and NO6 (psychoanaleptics) that were PBS‐subsidized in Australia were reclassified into clinically meaningful therapeutic classes and subclasses according to the Australian Medicines Handbook 10 (Table 1; see Table S1 for details of all ATC codes). We did not include anticonvulsant mood stabilizers in this analysis as their use in mental illness cannot be differentiated from use in epilepsy, nor did we include modafinil or medications used to treat dementia.
Table 1.
Classification of psychotropics by class and subclass
| Class | Subclass |
|---|---|
| Antipsychotic | Typical agents |
| Atypical agents | |
| Antidepressant | Tricyclic antidepressants |
| Selective serotonin reuptake inhibitors | |
| Serotonin noradrenaline reuptake inhibitors and noradrenaline reuptake inhibitors | |
| Noradrenergic and specific serotonergic antidepressants | |
| Monoamine oxidase inhibitors | |
| Benzodiazepine | Anxiolytic |
| Hypnotic | |
| Lithium | Lithium |
| Attention‐deficit hyperactivity disorder medication | Psychostimulants |
| Noradrenaline reuptake inhibitor [atomoxetine] |
Measurements
PBS dispensing claims lack detailed information on the length of treatment following medicine dispensing so we estimated the period of exposure from the date of dispensing for each medicine. The estimated period of exposure (EPE) was based on the number of days in which 75% of people received a subsequent dispensing of the same medicine (P75). This definition has been applied previously to psychotropics and accounts for variability in dispensing due to adherence, dose changes and seasonality 11, 12. We calculated EPE separately for each psychotropic medicine and excluded time intervals between dispensings that were longer than 180 days. Medication exposure started on the date of dispensing and ended after one EPE for that medicine if there were no further dispensings of the same medicine within this period. We performed sensitivity analyses using P90 (the number of days in which 90% of people received a subsequent dispensing of the same medicine) to determine the impact of EPE on the prevalence of polypharmacy in 2015 and the number of prescribers involved in each episode of polypharmacy across the entire study period.
Polypharmacy
Definitions of polypharmacy measures used previously when measuring this practice in routinely collected medicines data vary from an overlap in the treatment period of at least two medicines that is as short as a single day 13 to longer periods such as >14, >60 and >90 days 14, 15. In clinical practice, the importance of overlap duration may also depend on the psychotropic combinations of interest and clinical context. We therefore used >60 days overlap for our primary analysis and >14 days for our secondary analysis. The former definition may misclassify shorter periods of polypharmacy as monotherapy while the latter may misclassify switching between medicines as polypharmacy.
One approach to defining inappropriate polypharmacy is to identify instances in which medications that are used for the same indication or have similar mechanisms of action are combined. As such we have stratified our analysis into three levels according to prior convention, representing potentially increasing irrationality of prescribing 1.
Any polypharmacy is defined as concomitant use of any psychotropic medicines.
Class polypharmacy is the concomitant use of medicines of the same class, representing medicines used for the same indication or that have similar mechanisms of action.
Subclass polypharmacy is the concomitant use of medicines of the same subclass that have the same indication and similar mechanism of action.
We defined specific episodes of polypharmacy by examining the temporal relationship between individual courses of therapy. In this study, a new polypharmacy episode occurred when we observed an overlap in exposure of at least two medicines for >60 (or 14) days or when another medicine was dispensed and added to an existing episode of polypharmacy. We classified these episodes as any, class or subclass polypharmacy depending on the medicines involved. An episode of polypharmacy ended when the treatment course for at least one of the medicines involved in the episode ceased. If the episode involved only two drugs then the polypharmacy episode was over. If more than two medicines were involved in the episode we considered the remaining combination a new episode of polypharmacy, except where the combination had been previously observed as part of the same course of treatment.
Statistical analysis
First, we calculated the annual prevalence of psychotropic medicine use and psychotropic polypharmacy, expressed as the proportion of all people in the study population (concessional beneficiaries) that year. In all subsequent analyses we calculated our estimates using only people dispensed psychotropics as the denominator. For the prevalence of specific class and subclass polypharmacy we used the number of people dispensed at least one psychotropic from that class or subclass in a given year as the denominator. For 2015, we plotted the number of people with at least one dispensing of a specific medicine against the number of people who experienced a polypharmacy episode containing that medicine. We fitted a regression line with 95% confidence intervals for all medicines. This line was weighted by 1/Y2 , where Y was the number of people who experienced a polypharmacy episode containing a medicine, as there was greater dispersion of data points from the regression line with greater values of Y. If medicines were combined randomly the medicine should lie within the 95% confidence interval of the regression line. We also ranked the top 10 pairwise polypharmacy combinations and report the number of people exposed to these specific combinations in 2015. Finally, we described the proportion of polypharmacy episodes involving one, two, three and four or more unique prescribers across the entire study period for each level of polypharmacy.
The New South Wales Population and Health Services Research Ethics Committee granted ethics approval for this study (approval number 2013/11/494).
Results
Between 2006 and 2015, 51% (or 269 327) of the 519 999 people in our study population were dispensed at least one psychotropic medicine. Of these, 28.5% were aged 18–49 years, 27.4% were aged 50–69 years and 44.0% were aged 70 years and over; 60.6% were female (see Figure S2 for detailed age and sex information).
The proportion of the study population receiving at least one psychotropic in 2006 was 34.8% and 36.2% in 2015. Based on our primary definition of polypharmacy (overlap of >60 days), the prevalence of any psychotropic polypharmacy in the study population was 4.8% in 2006 and 5.2% in 2015. Using our secondary definition of polypharmacy (overlap of >14 days) the prevalence of any polypharmacy was 11.1% in 2006 and 12.0% in 2015.
For all remaining analyses, our denominator was people in the study population dispensed psychotropic medicines. Annual polypharmacy prevalence estimates were approximately 2‐fold higher using our secondary compared with primary polypharmacy definition. Hereafter, we refer only to the analysis based on our primary definition in the text of the results section but include the results based on both definitions in all tables and figures.
Among those dispensed a psychotropic medicine, the prevalence of any psychotropic polypharmacy was 13.8% in 2006 and 14.8% in 2015. In our analysis of class polypharmacy, expressed as a proportion of people dispensed the corresponding class, we observed antipsychotic polypharmacy to be 5.9% in 2006 and 7.3% in 2015; this class had the highest prevalence of polypharmacy for the entire study period (Figure 1a). Antidepressant polypharmacy was 2.1% in 2006 and 3.7% in 2015 where as benzodiazepine polypharmacy was 4.3% in 2006 and 2.9% in 2015 (Figure 1a). Class polypharmacy with attention‐deficit hyperactivity disorder medications was negligible throughout the study period and so is not discussed further.
Figure 1.
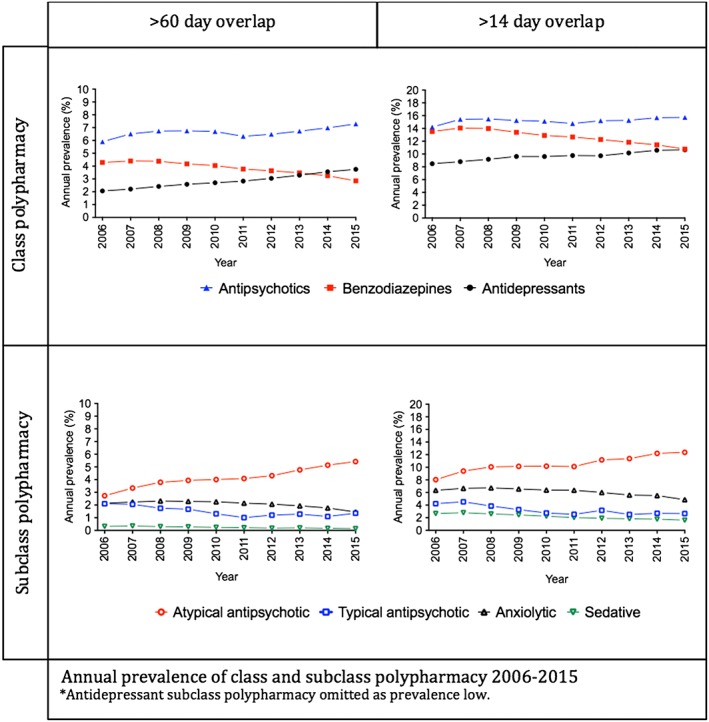
Annual prevalence of class and subclass polypharmacy 2006–2015
Subclass polypharmacy was consistently highest for atypical antipsychotics; in 2006, 2.7% of people dispensed an atypical agent experienced atypical subclass polypharmacy, doubling to 5.4% in 2015 (Figure 1b). Typical antipsychotics and anxiolytic benzodiazepines had a similar prevalence of subclass polypharmacy (2.1% in 2006 and 1.4% in 2015, and 2.1% in 2006 and 1.5% in 2015, respectively; Figure 1b). Sedative benzodiazepines consistently had the lowest prevalence of subclass polypharmacy at around 0.2% (Figure 1b).
As a proportion of people dispensed the corresponding antidepressant subclass, tricyclic antidepressants and serotonin noradrenaline reuptake inhibitor and noradrenaline reuptake inhibitor subclass polypharmacy was around 0.2%, and noradrenergic and specific serotonergic antidepressant subclass polypharmacy was around 0.1% for the study period.
In 2015, the number of people dispensed a specific medicine was generally correlated with the number of people experiencing polypharmacy with that medicine (Figure 2, Figure S1). However, people exposed to combinations including antipsychotics, attention‐deficit hyperactivity disorder medications (with haloperidol and dexamphetamine being a notable exceptions) and lithium were more numerous than expected relative to their overall use. Conversely people exposed to combinations including benzodiazepines (with the exception of alprazolam) and most antidepressants were less numerous than expected.
Figure 2.
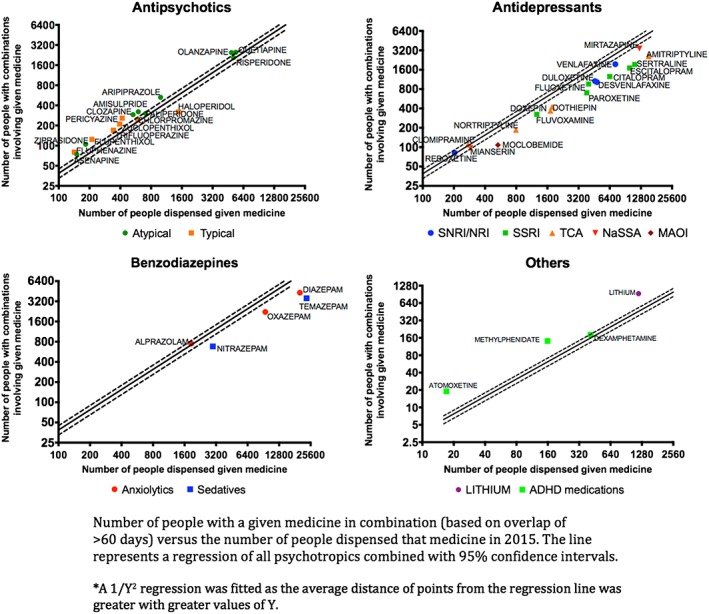
Number of people with a given medicine in combination (based on overlap of >60 days) vs. the number of people dispensed that medicine in 2015. The line represents a regression of all psychotropics combined with 95% confidence intervals
In 2015, all top 10 polypharmacy combinations based on the frequency of people exposed to given combinations included only two medicines (Table S3). At all levels of polypharmacy, combinations involving benzodiazepines (diazepam in particular) were common. These combinations most frequently included an antidepressant or other sedating medicines, such as antipsychotics, sedating antidepressants or another benzodiazepine. Combinations of atypical antipsychotics (especially involving quetiapine) were frequent within subclass polypharmacy.
Overall, we found 26.7% of polypharmacy episodes involved multiple prescribers, generally increasing from any, class to subclass polypharmacy and as the number of concomitant medicines increased (Table 2, Table S2). When three or four or more concomitant psychotropics were prescribed, potentially inappropriate polypharmacy increased (from any to class to subclass), as more prescribers were involved.
Table 2.
Proportion of polypharmacy episodes (based on overlap of >60 days) with a given number of prescribers
| Number of prescribers involved (%) | ||||||
|---|---|---|---|---|---|---|
| Number of psychotropics | Level of polypharmacy | Number of polypharmacy episodes | 1 | ≥2 | ≥3 | ≥4 |
| 2 | Any | 283 713 | 76.0 | 24.0 | ‐ | ‐ |
| Class | 106 575 | 76.1 | 23.9 | ‐ | ‐ | |
| Subclass | 30 754 | 69.4 | 30.6 | ‐ | ‐ | |
| 3 | Any | 48 907 | 62.4 | 37.6 | 5.8 | ‐ |
| Class | 4992 | 55.6 | 44.4 | 8.9 | ‐ | |
| Subclass | 1038 | 49.4 | 50.6 | 11.6 | ‐ | |
| ≥4 a | Any | 10 081 | 51.4 | 48.6 | 13.8 | 2.6 |
| Class | 315 | 49.5 | 50.5 | 22.5 | ‐ | |
| Any polypharmacy overall | 342 701 | 73.3 | 26.7 | 1.2 | 0.1 | |
Subclass polypharmacy with four or more medicines was negligible
Sensitivity analyses
When we used a definition of >60 days overlap, there was up to approximately a 2‐fold difference in the prevalence of any, class and subclass polypharmacy in 2015 between P75 and P90 measures (Table S4). This was particularly pronounced for benzodiazepines. However, when we used a definition of >14 days, there was little difference in the prevalence of polypharmacy between P75 and P90 measures. The prescriber analysis was robust to changes in EPE (Table S5).
Discussion
Between 2006 and 2015, there were minimal changes in the annual prevalence of psychotropic use and psychotropic polypharmacy in our study population. In general, the frequency of use of psychotropics in combination was associated with the frequency of their use overall 16. However, the prevalence of combinations of sedating psychotropics, including benzodiazepines, antipsychotics and sedating antidepressants were higher than expected given their prevalence of use overall. Multiple prescribers were involved in approximately one quarter of all polypharmacy episodes, and more commonly within class and subclass polypharmacy and people with four or more concomitant psychotropics.
Antipsychotic class polypharmacy was consistently the highest of any class and accounted for almost 10% of people dispensed an antipsychotic in 2015. Most studies worldwide indicate that between 10% and 30% of people taking antipsychotics are exposed to antipsychotic polypharmacy 17. The prevalence of antipsychotic polypharmacy was mostly attributable to atypical subclass polypharmacy. Antipsychotics were also disproportionately seen in combination relative to their use. This may reflect the ongoing acceptance of atypical antipsychotics (especially quetiapine), either in combination with each other or other psychotropics to treat of a variety of nonpsychotic conditions including depression, anxiety and sleep disturbance 18.
While there are no other comparable contemporary studies, the prevalence of antidepressant and benzodiazepine class polypharmacy is consistent with trends observed in previous studies 13, 19, 20, 21. Antidepressant and antipsychotic polypharmacy may be related to the high proportion of non‐ or partial responders to these medicines, leading to attempts to augment treatment. Up to half of all patients with depression or schizophrenia fail to achieve remission with antidepressant or antipsychotic monotherapy 4, 22. The relatively long latency periods to clinical effect of antidepressants and antipsychotics and the desire to alleviate suffering rapidly may also compel clinicians to add in medicines without an adequate trial of monotherapy 3, 4. In another study, being stuck in a switch from one antipsychotic to another was quoted as the reason for 39% of antipsychotic polypharmacy 5. The evidence for combining antidepressant with antidepressants and antipsychotics with antipsychotics is conflicted, with potential for significant harm 4, 23, 24. This conflict is reflected in disparities between international guidelines. Numerous successful strategies to reduce antipsychotic polypharmacy have been explored 25.
Combinations of multiple sedating medicines, especially diazepam, dominated psychotropic polypharmacy. At the subclass level, combinations of atypical antipsychotics most commonly involved the sedating antipsychotic quetiapine, as previously documented 17. These combinations are putatively driven by the need for sedation and relief from anxiety and distress. However, combinations of sedatives are accompanied by greater risks of over sedation and adverse events, especially in older people 26.
Almost three quarters of episodes of polypharmacy with two psychotropics involved only one prescriber. However, as the number of medicines in a polypharmacy episode increased, the number of prescribers involved also increased. This effect was particularly seen for class and subclass polypharmacy when there were four or more concomitant drugs dispensed, which is concerning as these combinations are less likely to be evidence based and carry greater risk of harm.
The trend for increasing numbers of prescribers with increasing numbers of concomitant medicines has been observed with polypharmacy involving a broader range of prescribed medicines in older people and is associated with an increased risk of adverse drug reactions 8. While having two prescribers may be acceptable, such as a psychiatrist and a general practitioner working in partnership, it is difficult to account for three or more prescribers in most cases. This raises concerns about lack of communication between prescribers, diffusion of responsibility for adverse drug reaction monitoring and the question of whether the polypharmacy was intended. One study found dilution of responsibility with multiple prescribers as a barrier to de‐prescribing, particularly when GPs were coprescribing with a specialist 27. Enhancing communication between prescribers with programs such as electronic real‐time prescription tracking and national electronic heath records may help to address this contributor to polypharmacy.
Strengths and limitations
The advantages of using routinely collected prescribed medicines data are that they are timely, large volume, and represent real‐world clinical practice. However, our study is subject to a number of limitations. There is an underlying assumption that individuals are taking the medicines they are dispensed. We also do not have access to detailed clinical information and so cannot definitively determine prescribing appropriateness. Only subsidized medicines are captured within this study. This may be an issue for benzodiazepines in particular, as private prescribing may account for up to 10% of all dispensings; therefore, our findings may underestimate the true prevalence of psychotropic polypharmacy 16. While the restriction of the study population to concessional beneficiaries accounts for under–co‐payment prescribing, it may limit the generalizability of the study to populations other than the concessional population. However, this population is likely to be the same as other subsidized populations worldwide such as the US Medicare and Medicaid 14 and similar Canadian populations 26. Psychotropic polypharmacy is potentially even more concerning in our study population as this is the group that is likely to experience the most harm from this practice.
These data lack important information on duration of therapy, and medication exposure here is defined by the EPE. This is a population‐based approximation of the actual periods of exposure following a single dispensing of a medicine. While these measures have not been validated in our study population, sensitivity analyses show that polypharmacy prevalence is particularly sensitive to changes in EPE for benzodiazepines when a >60‐day overlap definition is used but not a >14‐day definition. This may be because medications from this class are more likely to be used intermittently. The prescriber analysis was robust to changes in EPE. Varying definitions of polypharmacy using >14‐day and >60‐day overlap results in up to 2‐fold differences in any, class and subclass polypharmacy but little difference to trends over time. There is a need to unify definitions of polypharmacy within dispensing claims data for studies to be directly comparable.
Conclusion
While not all psychotropic polypharmacy is inappropriate and clinical factors are necessary to evaluate appropriateness fully 2, there is frequently a lack of safety and effectiveness evidence to support many of the medicine combinations identified in this study. Psychotropic polypharmacy remained common over the study period. Combinations with sedating agents were frequent and higher numbers of prescribers were associated with more potentially inappropriate prescribing. Psychotropics are a clinically valuable class of medicines when used judiciously, but there remains concern about the relatively frequent use of combinations of psychotropics where there is evidence of harm or unclear evidence of benefit. Political will, economic investment, and physician and patient engagement are required to implement policies that address the underlying determinants of these practices where there is evidence of harm. Psychotropic combinations for which evidence is lacking pose a challenge for clinicians who are often presented with distressed patients experiencing treatment‐refractory disease. Until evidence is generated to fill this gap, clinicians are left with guidelines based on expert consensus and their individual clinical judgement. When possible, best practice would be to communicate uncertainty to the patient to facilitate collaborative and informed treatment decisions.
Competing Interests
There are no competing interests to declare.
This research was supported by the National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Medicines and Ageing (APP1060407) and funding from the University of Sydney BMRI SPARC Implementation Scheme (2015–2016). J.B. and B.D. are funded by NHMRC Postgraduate awards and A.N. by an Endeavour Postgraduate Award. We thank the Department of Human Services for providing the data.
Supporting information
Table S1 List of included medicines, Anatomical Therapeutic Chemical Classification codes, estimated periods of exposure (EPE) and dates that medicines fell under co‐payment † (dating back to 1 January 2000)
Table S2 Proportion of polypharmacy episodes (based on overlap of >14 days) with a given number of prescribers
Table S3 Top 10 pairwise polypharmacy combinations in 2015 at level of any, class and subclass polypharmacy by number of people receiving this combination at least once
Table S4 Number of individuals experiencing polypharmacy as a proportion of those dispensed any psychotropic for different polypharmacy definitions in 2015; a comparison between P75 and P90
Table S5 Number of individuals experiencing polypharmacy as a proportion of those dispensed any psychotropic for different polypharmacy definitions in all study years; a comparison between P75 and P90
Figure S1 Number of people with a given medicine in combination (based on overlap of >14 days) vs. the number of people dispensed that medicine in 2015. The line represents a regression of all psychotropics combined with 95% confidence intervals
Figure S2 Comparisons of age and sex strata for the long‐term concessional population (concessional), the population dispensed at least one psychotropic (any psychotropic) and the population with any polypharmacy (polypharmacy) using P75 and a >60‐day overlap in exposure to define polypharmacy
Brett, J. , Daniels, B. , Karanges, E. A. , Buckley, N. A. , Schneider, C. , Nassir, A. , McLachlan, A. J. , and Pearson, S.‐A. (2017) Psychotropic polypharmacy in Australia, 2006 to 2015: a descriptive cohort study. Br J Clin Pharmacol, 83: 2581–2588. doi: 10.1111/bcp.13369.
References
- 1. National Association of State Mental Health Program Directors . Technical report on psychiatric polypharmacy. Alexandria, Virginia; 2001.
- 2. Kingsbury SJ, Yi D, Simpson GM. Psychopharmacology: rational and irrational polypharmacy. Psychiatr Serv 2001; 52: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 3. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand 2002; 106: 321–322. [DOI] [PubMed] [Google Scholar]
- 4. Keks NA, Burrows GD, Copolov DL, Newton R. Beyond the evidence: is there a place for antidepressant combinations in the pharmacotherapy of depression? Med J Aust 2007; 186: 142. [DOI] [PubMed] [Google Scholar]
- 5. Sernyak MJ, Rosenheck R. Clinicians' reasons for antipsychotic coprescribing. J Clin Psychiatry 2004; 65: 1597–1600. [DOI] [PubMed] [Google Scholar]
- 6. McManus P, Mant A, Mitchell P, Birkett D, Dudley J. Co‐prescribing of SSRIs and TCAs in Australia: how often does it occur and who is doing it? Br J Clin Pharmacol 2001; 51: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baandrup L, Allerup P, Nordentoft M, Lublin H, Glenthoj BY. Exploring regional variation in antipsychotic coprescribing practice: a Danish questionnaire survey. J Clin Psychiatry 2010; 71: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 8. Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother 2007; 5: 31–39. [DOI] [PubMed] [Google Scholar]
- 9. Mellish L, Karanges EA, Litchfield MJ, Schaffer AL, Blanch B, Daniels BJ, et al The Australian pharmaceutical benefits scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd. 2016.
- 11. Pratt NL, Ramsay EN, Ellett LMK, Nguyen TA, Barratt JD, Roughead EE. Association between use of multiple psychoactive medicines and hospitalization for falls: retrospective analysis of a large healthcare claim database. Drug Saf 2014; 37: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pottegård A, Hallas J. Assigning exposure duration to single prescriptions by use of the waiting time distribution. Pharmacoepidemiol Drug Saf 2013; 22: 803–809. [DOI] [PubMed] [Google Scholar]
- 13. De las Cuevas C, Sanz EJ. Polypharmacy in psychiatric practice in the Canary Islands. BMC Psychiatry 2004; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leckman‐Westin E, Kealey E, Gupta N, Chen Q, Gerhard T, Crystal S, et al Validation of a claims‐based antipsychotic polypharmacy measure. Pharmacoepidemiol Drug Saf 2014; 23: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tapp A, Wood AE, Secrest L, Erdmann J, Cubberley L, Kilzieh N. Combination antipsychotic therapy in clinical practice. Psychiatr Serv 2003; 65: 1597–600. [DOI] [PubMed] [Google Scholar]
- 16. Stephenson CP, Karanges E, McGregor IS. Trends in the utilisation of psychotropic medications in Australia from 2000 to 2011. Aust N Z J Psychiatry 2013; 47: 74–87. [DOI] [PubMed] [Google Scholar]
- 17. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long‐standing clinical practice. Psychiatr Clin N Am 2012; 35: 661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKean A, Monasterio E. Off‐label use of atypical antipsychotics. CNS Drugs 2012; 26: 383–390. [DOI] [PubMed] [Google Scholar]
- 19. Roughead EE, McDermott B, Gilbert AL. Antidepressants: prevalence of duplicate therapy and avoidable drug interactions in Australian veterans. Aust N Z J Psychiatry 2007; 41: 366–370. [DOI] [PubMed] [Google Scholar]
- 20. Mojtabai R, Olfson M. National trends in psychotropic medication polypharmacy in office‐based psychiatry. Arch Gen Psychiatry 2010; 67: 26–36. [DOI] [PubMed] [Google Scholar]
- 21. Johnell K, Fastbom J. The use of benzodiazpines and related drugs amongst older people in Sweden: associated factors and concomitant use of other psychotropics. Int J Geriatr Psychiatry 2009; 24: 731–738. [DOI] [PubMed] [Google Scholar]
- 22. Correll CU, Shaikh L, Gallego JA, Nachbar J, Olshanskiy V, Kishimoto T, et al Antipsychotic polypharmacy: a survey study of prescriber attitudes, knowledge and behavior. Schizophr Res 2011; 131: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Correll CU, Rummel‐Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta‐analysis of randomized controlled trials. Schizophr Bull 2009; 35: 443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallego JA, Nielsen J, De Hert M, Kane JM, Correll CU. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf 2012; 11: 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tani H, Uchida H, Suzuki T, Fujii Y, Mimura M. Interventions to reduce antipsychotic polypharmacy: a systematic review. Schizophr Res 2013; 143: 215–220. [DOI] [PubMed] [Google Scholar]
- 26. Vasudev A, Shariff SZ, Liu K, Burhan AM, Herrmann N, Leonard S, et al Trends in psychotropic dispensing among older adults with dementia living in long‐term care facilities: 2004–2013. Am J Geriatr Psychiatry 2015; 23: 1259–1269. [DOI] [PubMed] [Google Scholar]
- 27. Kouladjian L, Gnjidic D, Reeve E, Chen TF, Hilmer SN. Passing the Buck health care Practitioners' perspectives on Deprescribing anticholinergic and sedative medications in older adults. Ann Pharmacother 2016; 50: 625–636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of included medicines, Anatomical Therapeutic Chemical Classification codes, estimated periods of exposure (EPE) and dates that medicines fell under co‐payment † (dating back to 1 January 2000)
Table S2 Proportion of polypharmacy episodes (based on overlap of >14 days) with a given number of prescribers
Table S3 Top 10 pairwise polypharmacy combinations in 2015 at level of any, class and subclass polypharmacy by number of people receiving this combination at least once
Table S4 Number of individuals experiencing polypharmacy as a proportion of those dispensed any psychotropic for different polypharmacy definitions in 2015; a comparison between P75 and P90
Table S5 Number of individuals experiencing polypharmacy as a proportion of those dispensed any psychotropic for different polypharmacy definitions in all study years; a comparison between P75 and P90
Figure S1 Number of people with a given medicine in combination (based on overlap of >14 days) vs. the number of people dispensed that medicine in 2015. The line represents a regression of all psychotropics combined with 95% confidence intervals
Figure S2 Comparisons of age and sex strata for the long‐term concessional population (concessional), the population dispensed at least one psychotropic (any psychotropic) and the population with any polypharmacy (polypharmacy) using P75 and a >60‐day overlap in exposure to define polypharmacy


