Abstract
Aims
The CYP3A metric 4β‐hydroxycholesterol (4βOHC) has been shown to correlate with tacrolimus steady‐state apparent oral clearance (CL/F). Recently, pretransplant 4βOHC was shown not to predict tacrolimus CL/F after transplantation in a cohort of renal recipients (n = 79). The goal of the current study was determine whether these findings could be validated in a substantially larger cohort.
Methods
In a retrospective analysis of 279 renal recipients, tacrolimus trough concentrations (C0), daily dose, haematocrit and other relevant covariates were registered every day for the first 14 days after transplantation. 4βOHC and cholesterol were quantified on plasma collected immediately pretransplant using liquid chromatography tandem‐mass spectrometry. Patients were genotyped for CYP3A5*1 and CYP3A4*22.
Results
A total of 3551 tacrolimus C0 concentrations were registered. In a linear mixed model for the 14‐day period, determinants of tacrolimus C0 were CYP3A5 genotype, haematocrit, age and weight (overall R 2 = 0.179). Determinants of daily dose were CYP3A5 genotype, age, methylprednisolone dose, tacrolimus formulation, ALT and estimated glomerular filtration rate (overall R 2 = 0.242). Considering each of the first 5 days separately, 4βOHC had a limited effect on tacrolimus C0 on day 3 only (−1.00 ng ml−1 per ln, P = 0.035) but not on any other day, and no effect on dose or C0/dose. During the first 5 days, haematocrit and age, which were previously established as determinants of tacrolimus disposition under steady‐state conditions, never explained more than 17.7% of between‐subject variability in tacrolimus C0/dose.
Conclusions
The CYP3A metric 4βOHC cannot be used to predict tacrolimus dose requirements in the first days after transplantation.
Keywords: 4β‐hydroxycholesterol, CYP3A4, CYP3A5, kidney transplantation, tacrolimus
What is Already Known about this Subject
Tacrolimus, a substrate for cytochrome P450 enzymes 3A4 and 3A5, is characterized by high pharmacokinetic variability.
The endogenous CYP3A metric 4β‐hydroxycholesterol (4βOHC) has been shown to correlate with steady‐state tacrolimus apparent oral clearance (CL/F) in renal transplant recipients beyond the third month after transplantation.
However, a recent study found no relationship between pretransplant 4βOHC and tacrolimus CL/F in the first week after transplantation.
What this Study Adds
This retrospective cohort analysis of 279 renal recipients confirms that there is no significant relationship between 4βOHC and tacrolimus dose or (dose‐corrected) trough concentrations on any of the first 14 days after transplantation.
4βOHC cannot be used to optimize the initial tacrolimus dosing strategy in the context of renal transplantation.
This could be related to the intrinsic limitations of 4βOHC as a CYP3A metric and to a high degree of largely unexplained between‐ and within subject variability in tacrolimus exposure in the first days after transplantation, much of which could be independent of CYP3A4.
Tables of Links
| TARGETS | |
|---|---|
| Transporters 2 | Enzymes 3 |
| ABCB1 (P‐gp) | CYP3A5 |
| CYP3A4 |
| LIGANDS | |
|---|---|
| Midazolam | Tacrolimus |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Tacrolimus is the cornerstone of immunosuppressive therapy in solid organ transplantation, but is characterized by a narrow therapeutic window and high within‐ and between‐subject pharmacokinetic variability, necessitating therapeutic drug monitoring (TDM). Variability in the expression and activity of the tacrolimus metabolizing enzymes cytochrome P450 (CYP) 3A4 and 3A5 contributes significantly to the variability in tacrolimus exposure. CYP3A5 activity is only meaningful in patients possessing at least one CYP3A5*1 allele (CYP3A5 expressers), which have 50–100% higher apparent clearance (CL/F) of tacrolimus compared with CYP3A5*3/*3 patients (CYP3A5 non‐expressers) 4, 5. In spite of these significant between‐subject differences in clearance, initial tacrolimus dosing after transplantation is typically based only on total body weight, followed by dose adjustments based on trough concentration monitoring. A recent prospective trial in which patients were randomized to receive a starting dose of tacrolimus based on weight or on CYP3A5 genotype showed no difference in achievement of tacrolimus target concentrations early after transplantation, or on clinical outcomes 6. This may be related to the fact that there is considerable variability in tacrolimus exposure that is related to clinical parameters (particularly haematocrit) and CYP3A4 activity 7, 8. The problem is that CYP3A4 activity cannot easily be predicted from genetic polymorphisms as, individually, these generally have only limited functional effects 4. A possible exception is the CYP3A4*22 polymorphism, which is associated with 30–40% lower tacrolimus dose requirements 9, 10. An alternative approach, therefore, could be quantification of combined in vivo intestinal and hepatic CYP3A4 activity using a functional drug probe, midazolam being the current gold standard 11. We have previously demonstrated that tacrolimus CL/F can be predicted using a combination of CYP3A5 genotype, CYP3A4 activity (assessed using midazolam CL/F) and haematocrit 7. As it is impractical to perform a full 8‐h midazolam area under the curve (AUC) immediately prior to transplantation, endogenous CYP3A4 metrics such as 4β‐hydroxycholesterol (4βOHC) are an attractive alternative in this setting. 4βOHC is a metabolite of CYP3A4‐mediated cholesterol metabolism 12 with a very long elimination half‐life (~17 days), stable plasma concentrations and low intra‐individual variation (4.8–13.2%) 13, 14. We have demonstrated that a predictive model based on weight‐ and cholesterol‐corrected 4βOHC (4βOHC/C/W) performed similarly to a model based on midazolam in predicting steady‐state tacrolimus CL/F in renal recipients 3 months or more after transplantation 15. Very recently, however, Storset et al. observed no relationship between pretransplant 4βOHC and tacrolimus CL/Fplasma during the first days and weeks after transplantation 16. That study included a relatively low number of patients (n = 79) and its external validity may be somewhat limited by the fact that target C0 values were low by international standards. The goal of the current study was to determine whether pretransplant 4βOHC, alone or combined with genetic information, could be used to predict tacrolimus dose requirements and exposure immediately after transplantation in a large cohort of renal recipients with relatively high tacrolimus target concentrations.
Methods
Study population
This was a single‐centre, retrospective analysis. Inclusion criteria were (1) age ≥ 18 years, (2) recipient of a single kidney allograft between 1 January 2004 and 31 October 2014, (3) treated with the combination of oral tacrolimus (once daily Advagraf® or twice daily Prograft®, Astellas Pharma Europe, Staines, UK), mycophenolic acid (MMF; either mycophenolate mofetil [Cellcept®, Roche, Basel, Switzerland] or mycophenolate sodium [Myfortic®, Novartis, Basel, Switzerland]), and methylprednisolone (Medrol®, Pfizer, New York, NY, USA), (4) availability of DNA for genotyping, and (5) availability of a plasma sample collected within 24 h before transplantation for quantification of 4βOHC. Exclusion criteria were (1) use of moderately potent or potent CYP3A4 inhibiting or inducing medication immediately before or in the first 14 days after transplantation, (2) admission to an intensive care unit in the first 14 days after transplantation, and (3) death or graft loss in the first 14 days after transplantation. The standard tacrolimus loading dose was 0.2 mg kg−1 (single dose of twice daily formulation) administered 2 h before transplantation (the day of transplantation is referred to as ‘day 0’). After this point, tacrolimus dose was adjusted to maintain C0 of 12–15 ng ml−1 throughout the first month. Tacrolimus twice daily was administered at 8 h and 20 h and usually switched to tacrolimus once daily between days 5 and 14. During days 0–14, MMF dose was 2000 mg (or 1440 mg of mycophenolate sodium) daily. Methylprednisolone was dosed at 500 mg IV on day 0, 40 mg IV on day 1 and 16 mg orally on day 2, followed by standardized tapering to 4 mg by month 3. Because a few patients were included in clinical trials requiring a starting dose of tacrolimus other than 0.2 mg kg−1, only patients who received 0.15–0.25 mg kg−1 tacrolimus on day 0 were included in the analysis. For days 1–14 and month 3, the following parameters (where available) were retrieved from electronic patient records for every individual day: tacrolimus C0, dose and formulation, methylprednisolone dose, total body weight, haematocrit, ALT (as proxy for liver dysfunction) and serum creatinine. Fat‐free mass (FFM) was estimated from weight, height, age and gender 17. Delayed graft function was defined as the need for dialysis in the first 7 days after transplantation. Estimated glomerular filtration rate (eGFR) was calculated using the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) formula. This study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the University Hospitals Leuven, Faculty of Medicine, KU Leuven, Belgium (S53364). All study participants provided written informed consent.
Genotyping
Genomic DNA was isolated from whole blood samples using a salting out procedure 18 . The quantity and quality of genomic DNA were verified with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) before being assayed on an OpenArray platform (Life Technologies, Carlsbad, California, USA). Participants were genotyped for single nucleotide polymorphisms (SNPs) in genes CYP3A5 (CYP3A5*1; rs776746) and CYP3A4 (CYP3A4*22; rs35599367). Hardy–Weinberg equilibrium was assessed using Haploview 19.
Quantification of tacrolimus and 4β‐hydroxycholesterol
Pretransplant blood samples (for quantification of 4βOHC and genotyping) were collected in 4‐ml EDTA tubes when patients presented to the hospital for transplantation, i.e. a few hours before the actual surgery. After transplantation, whole‐blood samples (for quantification of tacrolimus trough concentration) were collected in 4‐ml EDTA tubes between 6 and 8 a.m., prior to intake of the morning dose of TAC, on every day for the first week after transplantation. During the second week, tacrolimus trough concentrations were measured 4–7 times (unless the patient was discharged earlier). In case of tacrolimus concentrations <5 ng ml−1 and >25 ng ml−1, medical records were manually checked to exclude errors (e.g. ingestion of tacrolimus immediately prior to quantification). Dose‐corrected trough concentrations (C0/dose) were determined by dividing C0 by the total dose of tacrolimus ingested on the prior day. 4βOHC and cholesterol were quantified on EDTA‐plasma using a liquid chromatography tandem‐mass spectrometry platform, as previously described 20. Accuracy and precision were 0.6–1.5% and 8.3–13.4% for 4βOHC, and 5.2–9.4% and 8.5–11.2% for cholesterol. Tacrolimus C0 concentrations were determined on the microparticulate enzyme immunoassay platform used by the central hospital laboratory at the time. Up to 16 December 2010 this was Dimension RxL (Siemens, Munich, Germany; analytical sensitivity 1.2 μg l−1; coefficient of variation [CV] 2.9–6.8%; applies to 52 trough concentrations or 1.5% of total). From 17 December 2010 onwards this was Architect i2000sr (Abbott Laboratories, Illinois, USA; analytical sensitivity ≤1.5 μg l−1; CV 8%; applies to 3499 trough concentrations or 98.5% of total).
Statistical analysis
Data are expressed as mean ± standard deviation except when stated otherwise. Distribution of continuous data was evaluated according to Shapiro–Wilk. The following values were not normally distributed and log‐transformed for analysis: tacrolimus C0/D, 4βOHC and 4βOHC/C. Predictors of tacrolimus C0 and C0/dose throughout the first 14 days were determined by constructing linear mixed models with random slopes, random intercepts and a first‐order autoregressive covariance structure. Calculation of estimates was based on restricted maximum likelihoods. The following variables were considered as possible predictors of tacrolimus C0, C0/dose and dose; CYP3A4 and CYP3A5 genotype; recipient age, weight and FFM; time‐varying haematocrit, eGFR, ALT, steroid dose and tacrolimus formulation; time after transplantation; delayed graft function; pretransplant 4βOHC and 4βOHC/C. We only included terms which were statistically significant using F‐test and improved the model according to Akaike's information criterion (AIC). Pretransplant predictors of tacrolimus C0, dose or C0/dose at specific time points (e.g. day 3) were determined by multivariable analysis of variance (ANOVA) using stepwise selection of predictors (without prior univariable selection). For this analysis, only the biomarker of interest (4βOHC) and established determinants of tacrolimus C0/dose (CYP3A4 and CYP3A5 genotype, haematocrit, age, weight) were entered into the model. Consequently, this model was run for each individual day without correction for multiple testing. All reported R 2 values are semipartial. A two sided P‐value < 0.05 was considered statistically significant. IBM SPSS Statistics version 22 was used for all statistical analyses. Graphpad Prism version 6 was used for generation of figures.
Results
Study population, genetics and 4βOHC
After exclusion of 13 patients who had stayed in an ICU, died or lost their graft in the first 14 days after transplantation, a total of 279 patients contributed 3551 tacrolimus trough concentrations (mean 12.7 per patient) over the 14‐day study period. For 260 patients (93%), an additional trough concentration at 3 months was also available. Baseline characteristics of the study population are presented in Table 1. Out of 33 CYP3A5 expressers, seven were homozygous (CYP3A5*1/*1). There were no homozygous CYP3A4*22 carriers, nor were there patients who were both CYP3A5 expresser and CYP3A4*22 carrier. Consequently, patients who were both CYP3A4*1/*1 and CYP3A5*3/*3 are simply referred to as CYP3A4*1/*1. 4βOHC(/C) was not correlated with weight, FFM or BMI (Pearson r < 0.10 and P > 0.10 for all). Furthermore, 4βOHC concentration was not different across genotype groups (median [interquartile range] was 38.9 [24.4–65.9] ng ml−1, 34.4 [24.0–48.0] ng ml−1 and 30.4 [18.9–54.2] ng ml−1 in CYP3A5 expressers, CYP3A4*1/*1 patients and CYP3A4*22 carriers, respectively; P = 0.283 for between‐group difference). These 4βOHC concentrations are broadly comparable to previously reported values in the pretransplant setting 16 and in stable renal recipients at least 3 months after transplantation 15, 21. Mean haematocrit dropped from 36 ± 5% on day 0 to 30 ± 4% on day 1, but subsequently remained stable until day 14 (Supporting Information Figure S1).
Table 1.
Baseline patient characteristics
| Variable | Value | Range |
|---|---|---|
| Age (years) | 53 ± 13 | 19–75 |
| Gender (male/female) | 177/102 | |
| Weight (kg) | 73.4 ± 15.2 | 33.5–115.5 |
| BMI (kg m −2 ) | 25.1 ± 4.5 | 11.9–41.7 |
| Fat‐free mass (kg) | 49.3 ± 8.7 | 27.6–70.6 |
| Diabetes mellitus | 56 (20%) | |
| Tacrolimus dose on day 0 (mg kg −1 ) | 0.20 ± 0.01 | 0.16–0.22 |
| Induction with basiliximab | 75 (27%) | |
| 4βOHC (ng ml −1 ) a | 35.4 [23.7–48.7] | 3.3–679.0 |
| 4βOHC/C (molar ratio × 10 4 ) a | 0.25 [0.18–0.34] | 0.03–5.11 |
| Delayed graft function | 61 (22%) | |
| CYP3A5 expresser | 33 (12%) | |
| CYP3A4*22 carrier | 19 (7%) |
Median [interquartile range]
4βOHC, 4β‐hydroxycholesterol; 4βOHC/C, 4β‐hydroxycholesterol/cholesterol;
Weight, BMI and fat‐free mass refer to measurement on day 0 (the day of transplantation)
Tacrolimus dosing and exposure
Figure 1 shows the evolution of tacrolimus C0, dose and C0/dose over the study period, stratified by CYP3A4/5 genotype. Mean values for these parameters and between‐genotype comparisons on individual days are provided in Supporting Information Tables S1–S3. Tacrolimus C0 was significantly lower in CYP3A5 expressers compared with CYP3A4*1/*1 patients during the first 6 days, but was not significantly higher in CYP3A4*22 carriers compared with CYP3A4*1/*1 patients. Tacrolimus dose was significantly higher in CYP3A5 expressers compared with CYP3A4*1/*1 patients from day 1 onwards (mean difference on day 7: 7.5 mg (83%), P < 0.001) and lower in CYP3A4*22 carriers compared with CYP3A4*1/*1 patients from day 6 onwards (mean difference on day 7: 2.1 mg (23%), P = 0.04).
Figure 1.
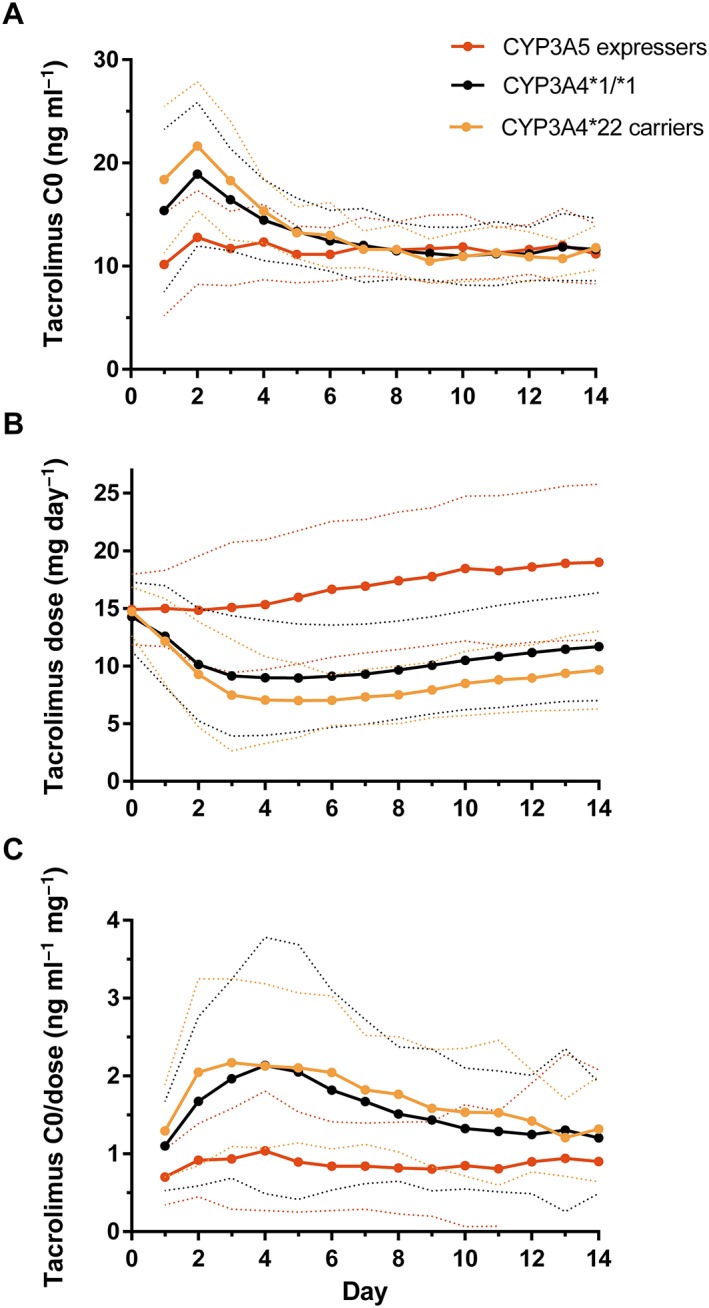
Evolution of tacrolimus C0 (A), dose (B) and C0/dose (C). The solid and dotted lines represent the mean and 95% confidence interval, respectively
Figure 2 plots the distribution of observed tacrolimus C0 on days 1–7 stratified by broad categories of exposure, i.e. underdosed (C0 < 5 ng ml−1), somewhat underdosed (5–7.9 ng ml−1), acceptable (8–15 ng ml−1), overdosed (15.1–25 ng ml−1) and severely overdosed (>25 ng ml−1). By these definitions, 94.7% of CYP3A4*22 carriers and 72.2% of CYP3A4*1/*1 patients were overdosed by day 2 using a 0.2 mg kg−1 starting dose of TAC. Frank underexposure occurred mainly in CYP3A5 expressers (four out of five patients with tacrolimus C0 < 5 ng ml−1 on day 1 were CYP3A5 expressers), and only during the first 2 days.
Figure 2.
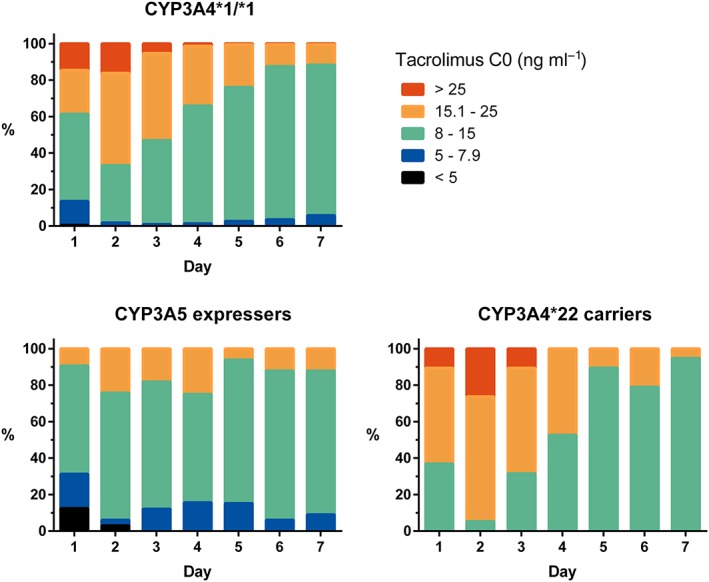
Distribution of tacrolimus C0 over the first 7 days, by category of exposure
Determinants of tacrolimus exposure
Determinants of tacrolimus C0 and C0/dose throughout the 14‐day study period in linear mixed model analysis are shown in Table 2. Determinants of C0 included CYP3A5 genotype, age, weight and haematocrit; overall R 2 was 0.179. Determinants of C0/dose included CYP3A5 genotype, haematocrit, age, methylprednisolone dose, tacrolimus formulation, ALT and eGFR; overall R 2 was 0.231. Determinants of tacrolimus dose were very similar to those of dose and are presented in Supporting Information Table S4. The effects of variables other than CYP3A5 genotype on C0/dose were quantitatively limited (e.g. increase in age of 30 years was predicted to increase C0/dose by 0.54 ng ml−1 mg−1).
Table 2.
Determinants of tacrolimus C0 and C0/dose on days 1–14 in multivariable linear mixed model
| Determinant | Estimated effect | 95% CI | P |
|---|---|---|---|
| Model for tacrolimus C0 (ng ml −1 ) | |||
| CYP3A5 expresser | −1.739 | −2.517 – [−0.962] | <0.001 |
| Haematocrit (%) | 0.249 | 0.194–0.303 | <0.001 |
| Age (years) | 0.027 | 0.009–0.045 | 0.004 |
| Weight (kg) | 0.021 | 0.004–0.037 | 0.015 |
| Days after transplantation | −0.367 | −0.423 – [−0.311] | <0.001 |
| Model for tacrolimus C0/dose (ng ml −1 mg −1 ) | |||
| CYP3A5 expresser | −0.675 | −0.938 – [−0.412] | <0.001 |
| Haematocrit (%) | 0.017 | 0.005–0.030 | 0.006 |
| Age (years) | 0.018 | 0.011–0.024 | <0.001 |
| Methylprednisolone dose (mg) | −0.001 | −0.003 – [−0.001] | 0.007 |
| Tacrolimus formulation (twice daily) | 0.162 | 0.057–0.267 | 0.003 |
| ALT (IU ml −1 ) | 0.003 | −0.006 – [−0.001] | 0.004 |
| eGFR (ml min −1 1.73m −2 ) | 0.011 | 0.008–0.014 | <0.001 |
| Days after transplantation | −0.016 | −0.030 – [−0.002] | 0.025 |
Final R 2 was 0.179 for C0 model and 0.231 for C0/dose model.
ALT, serum alanine aminotransferase; eGFR, estimated glomerular filtration rate
Next, separate linear regression models were constructed for tacrolimus C0 on days 1–5 using only determinants that were available before transplantation (e.g. haematocrit and weight were not used as time‐varying variables, but only day 0 values were utilized). These are presented in Table 3. Haematocrit on day 0 had a clear effect on day 1 tacrolimus C0, but this effect was lost by day 3. Tacrolimus C0 on day 3, which is commonly used as the endpoint in clinical trials 6 was predicted by CYP3A5 genotype, age, weight and 4βOHC with overall R 2 = 0.158. However, 4βOHC explained only 1.1% of interpatient variability in tacrolimus C0 (P = 0.035) and was not a significant predictor on any other day. 4βOHC(/C) was not predictive of tacrolimus C0, C0/dose, dose or tacrolimus C0 category on any day. 4βOHC(/C) was also not predictive for tacrolimus C0 and C0/dose when the analyses where repeated after exclusion of genotype information and age (as this may affect CYP3A4 activity), and not predictive in the subgroup of CYP3A4*1/*1 patients (data not shown). Finally, 4βOHC(/C) did not affect the probability of a C0 > 25 ng ml−1 or <5 ng ml−1 on days 1 and 2 (P > 0.600 for all analyses).
Table 3.
Pretransplant determinants of tacrolimus C0 in the first 5 days after transplantation
| Determinants | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B value | P | R 2 | B value | P | R 2 | B value | P | R 2 | B value | P | R 2 | B value | P | R 2 | |
| CYP3A5 expresser | −5.04 | <0.001 | 0.043 | −6.26 | <0.001 | 0.084 | −4.82 | <0.001 | 0.095 | −2.25 | 0.002 | 0.032 | −2.29 | <0.001 | 0.052 |
| CYP3A4*22 carrier | 3.37 | 0.046 | 0.009 | — | — | — | — | — | — | — | — | — | — | — | — |
| Haematocrit (%) | 0.39 | <0.001 | 0.074 | 0.21 | 0.008 | 0.020 | — | — | — | — | — | — | — | — | — |
| Age (years) | 0.08 | 0.012 | 0.017 | 0.10 | <0.001 | 0.047 | 0.08 | <0.001 | 0.038 | 0.04 | 0.014 | 0.017 | — | — | — |
| Weight (kg) | 0.09 | 0.001 | 0.031 | 0.05 | 0.036 | 0.011 | 0.04 | 0.035 | 0.011 | — | — | — | — | — | — |
| 4βOHC (mg ml −1 ) | — | — | — | — | — | — | −1.00 | 0.035 | 0.011 | — | — | — | — | — | — |
| Overall R 2 | 0.174 | 0.162 | 0.158 | 0.049 | 0.052 | ||||||||||
Haematocrit and weight refer to the values on day 0.
When the analysis was repeated without considering CYP3A4*22 status, 4βOHC still was not a significant predictor of tacrolimus C0 on any day except day 3.
4βOHC is log‐transformed
Pretransplant (i.e. potentially available before transplantation) determinants of tacrolimus C0/dose and dose on days 1–5 are shown in Supporting Information Tables S5 and S6. By day 5, only CYP3A5 genotype and age were predictive of C0/dose (B = −1.149, P < 0.001, R 2 = 0.161 and B = 0.031, P < 0.001, R 2 = 0.097, respectively). The effect of pretransplant haematocrit on C0/dose diminished progressively over the first 3 days and was lost by day 4. Time‐dependent determinants of tacrolimus C0/dose (i.e. using haematocrit and weight values for each specific day) for days 1–5 and day 90 are shown in Supporting Information Table S7. The determinants and total R 2 remained similar from day 6 to day 14 and are not shown. Because patients are not in steady state on days 1–5, C0/dose does not accurately reflect clearance. Even on day 90, steady state was not guaranteed because of the retrospective nature of the data (factors such as noncompliance and diarrhoea were not systematically registered). Therefore, the analysis for tacrolimus C0/dose was repeated on a previously published cohort of renal recipients, all of whom underwent 8‐hour steady‐state AUC for tacrolimus and midazolam a minimum of 90 days after transplantation as part of a prospective study 15. We only considered patients treated with the twice daily formulation of tacrolimus and randomly selected patients from that cohort to match the CYP3A5 genotype distribution of the current cohort (total of 94 renal recipients, including 12 (12.8%) CYP3A5 expressers and 9 (9.6%) CYP3A4*22 carriers). In this ‘steady‐state’ cohort, CYP3A5 genotype, midazolam CL/F, haematocrit and age together explained 46.8% of between‐subject variability in tacrolimus C0/dose (Supporting Information Table S8). Figure 3 shows the relative contribution of CYP3A5 genotype, haematocrit and age to tacrolimus C0/dose variability on days 1–5 and 90 in the current cohort as well as the added value of quantifying CYP3A4 activity (using midazolam) in the steady‐state cohort. It is likely that the predictive value of age on days 1–5 was partly due to age‐related decline in CYP3A4 activity (i.e. age was a proxy for CYP3A4 activity), as age and midazolam CL/F were negatively correlated in the steady‐state cohort (r = −0.269, P = 0.009).
Figure 3.
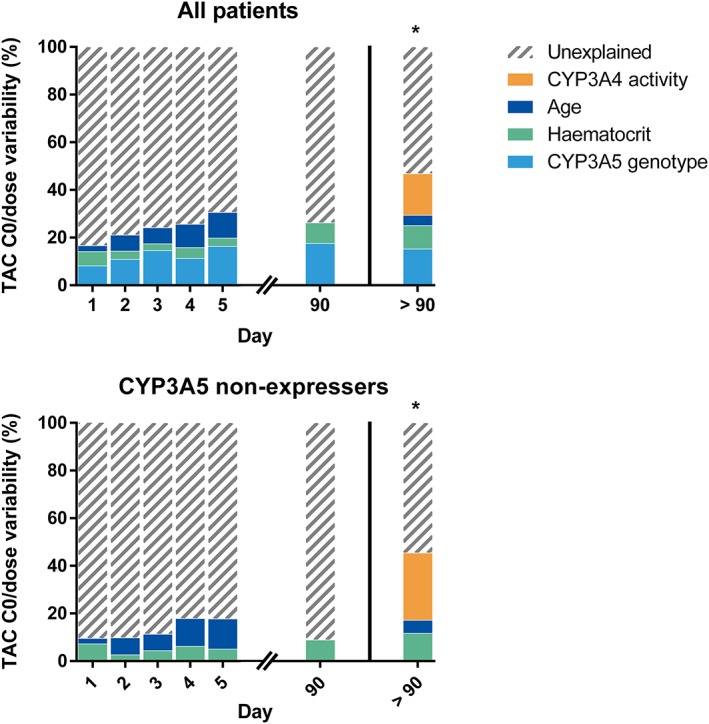
Determinants of between‐subject variability in tacrolimus (TAC) C0/dose. The Y‐axis plots R 2 values (expressed as %) in multivariable linear models for each day separately in the current cohort. The right‐hand panel (*) shows the relative contribution of these factors in explaining tacrolimus C0/dose variability in a historical cohort of 94 renal recipients who were known to be in steady state and >90 days after transplantation (see text for details). CYP3A4 activity refers to midazolam apparent oral clearance
Discussion
In this analysis of 279 renal recipients, the only major determinant of tacrolimus dose and absolute or dose‐corrected tacrolimus trough concentrations in the first 14 days after transplantation was CYP3A5 genotype. The effect of haematocrit was intermediate and the effects of age and weight were minor. This study, although retrospective and not a formal modelling effort, suggests that the usefulness of haematocrit, age and even weight to determine the correct starting dose of tacrolimus may be limited. However, haematocrit and FFM have previously been shown to be useful in a computerized dosing algorithm to optimize tacrolimus dose adjustments early after transplantation, which is a separate issue 22. The CYP3A metric 4βOHC determined immediately prior to transplantation could not be related to dose requirement or (dose‐corrected) tacrolimus C0 during the first 14 days.
We have previously shown that in vivo CYP3A4 activity (assessed using midazolam) does not affect steady‐state tacrolimus CL/F in CYP3A5 expressers 7, 15. The potential benefit of using a pretransplant CYP3A4 metric for tacrolimus dose optimization would therefore likely be limited to CYP3A5 non‐expressers. This also implies that the relative value of CYP3A5 genotyping and a CYP3A4 metric depend on the genetics of the transplant population. Prospective genotyping for CYP3A5 would be most informative in a population in which the allelic frequency of CYP3A5*1 approaches 50% (e.g. African Americans 23). The fact that the allelic frequency of CYP3A5*1 is only 10–20% in European transplant programmes may be one of the reasons why CYP3A5 genotype‐based dosing did not improve the number of patients having therapeutic tacrolimus exposure early after transplantation in a Dutch centre 6. Conversely, the potential added value of a CYP3A4 metric should be highest in such Western populations consisting mainly of CYP3A5 non‐expressers. However, we observed no relationship between 4βOHC and tacrolimus C0(/dose) even though only 12% of patients in the current study were CYP3A5 expressers, in which CYP3A4 activity explains up to 30% of steady‐state tacrolimus C0/dose (Figure 3) 7.
There could be a number of reasons for the lack of an association between pretransplant 4βOHC and posttransplant tacrolimus dose requirements. Firstly, 4βOHC is a suboptimal CYP3A metric that correlates only moderately well with midazolam 15, 24, 25, 26. Secondly, patients undergo profound physiological changes during the first days after transplantation, including rapid resolution of uraemia, a sharp drop in haematocrit on the day of transplantation and gradual recovery of intestinal motility and function. Much time‐related (intra‐individual) variability in tacrolimus CL/F during this period is therefore likely to be unrelated to CYP3A4 activity. For example, resolution of uraemia is likely to affect tacrolimus disposition more than it does 4βOHC, because uraemia seems to have much more profound effects on the function of transporters (such as the P‐glycoprotein pump for which tacrolimus is a substrate) than on CYP3A4 27. Thirdly, 4βOHC has previously been shown to be related to steady‐state tacrolimus CL/F, but tacrolimus is not in steady state during the first days after transplantation, when most over‐ and underexposure occurred. In summary, there may not only be issues with the intrinsic value of 4βOHC as a CYP3A metric but also with a high degree of variability in tacrolimus disposition immediately after transplantation that may not be related to CYP3A4, but rather to factors such as drug distribution, resolution of uraemia and intestinal motility. Whether pretransplant midazolam CL/F would have value in optimizing initial tacrolimus dosing remains to be established but, apart from being impractical (it would be as cumbersome as administering a pretransplant test dose of tacrolimus and be less informative), the abovementioned factors may pose limits on the practical use of any CYP3A4 metric.
In a recent study of 79 renal recipients, Storset et al. observed no correlation between pretransplant 4βOHC and tacrolimus CL/Fplasma on days 2 and 7, week 4 or week 8 after transplantation 16, indicating that pretransplant 4βOHC is not a predictor of posttransplant tacrolimus clearance. The current study confirms these findings in a significantly larger cohort subject to higher tacrolimus target concentrations. We note that conversion between tacrolimus CL/Fplasma (the parameter used in the study by Storset et al.) and CL/Fwhole‐blood (used in clinical practice) requires haematocrit to be known. Therefore, a model that predicts CL/Fplasma on day 7 cannot be used to calculate the optimal tacrolimus dose to achieve a specific C0 unless assumptions are made about what the haematocrit will be at that future point in time.
Strengths of the current study include large sample size, high frequency of tacrolimus C0 measurements throughout the 14‐day study period (almost daily) and determination of 4βOHC as well as 4βOHC/C. Limitations include the use of trough concentrations rather than full concentration–time profiles and the retrospective nature of the cohort. Specifically, centre‐specific target trough levels for tacrolimus did not change throughout the study period, but may have been modified in specific patients depending on the clinical context.
In conclusion, CYP3A5 genotype, haematocrit and age, previously established as determinants of tacrolimus disposition under steady‐state conditions, were also related to tacrolimus exposure in the first days after transplantation. However, most between‐subject variability in tacrolimus exposure remained unexplained. The CYP3A metric 4βOHC cannot be used to predict tacrolimus dose requirements in the first days after transplantation.
Competing Interests
The analytic part of this research was supported by the grant 03IPT612X (Inno‐Profile) from the German Federal Ministry for Education and Research. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
We thank A. Herelixka for managing the clinical database. We also thank H. de Loor, T. Coopmans and M. Dekens of the laboratory of Nephrology for their technical assistance.
Contributors
T.V. and D.K. designed the study and wrote the manuscript; M.H., P.A. and S.O. revised the manuscript; M.H. performed the 4βOHC quantification; T.V. performed statistical analyses.
Supporting information
Figure S1 Evolution of haematocrit over time. Solid line represents the mean; dotted line represents 95% confidence interval
Table S1 Tacrolimus dose (mg kg−1) on individual days
Table S2 Tacrolimus C0 (ng ml−1) on individual days
Table S3 Tacrolimus C0/dose (ng ml−1 mg−1) on individual days
Table S4 Determinants of tacrolimus dose (mg day−1) on days 1–14 in multivariable linear mixed model
Table S5 Pretransplant determinants of tacrolimus C0/dose in the first 5 days after transplantation
Table S6 Pretransplant determinants of tacrolimus dose (mg) in the first 5 days after transplantation
Table S7 Time‐dependent determinants of tacrolimus C0/dose
Table S8 Determinants of tacrolimus C0/dose in additional steady‐state cohort (see panel * in Figure 3)
Vanhove, T. , Hasan, M. , Annaert, P. , Oswald, S. , and Kuypers, D. R. J. (2017) Pretransplant 4β‐hydroxycholesterol does not predict tacrolimus exposure or dose requirements during the first days after kidney transplantation. Br J Clin Pharmacol, 83: 2406–2415. doi: 10.1111/bcp.13343.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 2015; 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part II. Clin Pharmacokinet 2010; 49: 207–221. [DOI] [PubMed] [Google Scholar]
- 5. de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DRJ. Impact of CYP3A5 genotype on tacrolimus versus midazolam clearance in renal transplant recipients: new insights in CYP3A5‐mediated drug metabolism. Pharmacogenomics 2013; 14: 1467–1480. [DOI] [PubMed] [Google Scholar]
- 6. Shuker N, Bouamar R, van Schaik RHNN, Clahsen‐van Groningen MC, Damman J, Baan CC, et al. A randomized controlled trial comparing the efficacy of CYP3A5 genotype‐based with bodyweight‐based tacrolimus dosing after living donor kidney transplantation. Am J Transplant 2016; 16: 2085–2096. [DOI] [PubMed] [Google Scholar]
- 7. de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther 2012; 92: 366–375. [DOI] [PubMed] [Google Scholar]
- 8. Vanhove T, Annaert P, Kuypers DRJ. Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev 2016; 2532: 1–25. [DOI] [PubMed] [Google Scholar]
- 9. Elens L, van Schaik RH, Panin N, de Meyer M, Wallemacq P, Lison D, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors' dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 2011; 12: 1383–1396. [DOI] [PubMed] [Google Scholar]
- 10. Pallet N, Jannot A‐S, El Bahri M, Etienne I, Buchler M, de Ligny BH, et al. Kidney transplant recipients carrying the CYP3A4*22 allelic variant have reduced tacrolimus clearance and often reach supratherapeutic tacrolimus concentrations. Am J Transplant 2015; 15: 800–805. [DOI] [PubMed] [Google Scholar]
- 11. Thummel KE, O'Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, et al. Oral first‐pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A‐mediated metabolism. Clin Pharmacol Ther 1996; 59: 491–502. [DOI] [PubMed] [Google Scholar]
- 12. Bodin K, Bretillon L, Aden Y, Bertilsson L, Broome U, Einarsson C, et al. Antiepileptic drugs increase plasma levels of 4beta‐hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J Biol Chem 2001; 276: 38685–38689. [DOI] [PubMed] [Google Scholar]
- 13. Diczfalusy U, Kanebratt KP, Bredberg E, Andersson TB, Bottiger Y, Bertilsson L. 4beta‐hydroxycholesterol as an endogenous marker for CYP3A4/5 activity: stability and half‐life of elimination after induction with rifampicin. Br J Clin Pharmacol 2009; 67: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bodin K, Andersson U, Rystedt E, Ellis E, Norlin M, Pikuleva I, et al. Metabolism of 4 beta‐hydroxycholesterol in humans. J Biol Chem 2002; 277: 31534–31540. [DOI] [PubMed] [Google Scholar]
- 15. Vanhove T, de Jonge H, de Loor H, Annaert P, Diczfalusy U, Kuypers DRJ. Comparative performance of oral midazolam clearance and plasma 4beta‐hydroxycholesterol to explain interindividual variability in tacrolimus clearance. Br J Clin Pharmacol 2016; 82: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storset E, Hole K, Midtvedt K, Bergan S, Molden E, Asberg A. The CYP3A biomarker 4beta‐hydroxycholesterol does not improve tacrolimus dose predictions early after kidney transplantation. Br J Clin Pharmacol 2017; 83: 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Storset E, von During ME, Godang K, Bergan S, Midtvedt K, Asberg A. Prediction of fat‐free mass in kidney transplant recipients. Ther Drug Monit 2016; 38: 439–446. [DOI] [PubMed] [Google Scholar]
- 18. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 20. Hasan M, Siegmund W, Oswald S. Rapid LC‐MS/MS method for the determination of 4‐hydroxycholesterol/cholesterol ratio in serum as endogenous biomarker for CYP3A activity in human and foals. J Chromatogr B, Anal Technol Biomed life Sci 2016; 1033–1034: 193–199. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki Y, Itoh H, Sato F, Kawasaki K, Sato Y, Fujioka T, et al. Significant increase in plasma 4beta‐hydroxycholesterol concentration in patients after kidney transplantation. J Lipid Res 2013; 54: 2568–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Storset E, Asberg A, Skauby M, Neely M, Bergan S, Bremer S, et al. Improved tacrolimus target concentration achievement using computerized dosing in renal transplant recipients – a prospective, randomized study. Transplantation 2015; 99: 2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International HapMap Project [Internet] . Available from: http://hapmap.ncbi.nlm.nih.gov (last accessed 15 June 2015).
- 24. Bjorkhem‐Bergman L, Backstrom T, Nylen H, Ronquist‐Nii Y, Bredberg E, Andersson TB, et al. Comparison of endogenous 4beta‐hydroxycholesterol with midazolam as markers for CYP3A4 induction by rifampicin. Drug Metab Dispos 2013; 41: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 25. Woolsey SJ, Beaton MD, Choi Y‐H, Dresser GK, Gryn SE, Kim RB, et al. Relationships between endogenous plasma biomarkers of constitutive cytochrome P450 3A activity and single‐time‐point oral midazolam microdose phenotype in healthy subjects. Basic Clin Pharmacol Toxicol 2016; 118: 284–291. [DOI] [PubMed] [Google Scholar]
- 26. Vanhove T, Annaert P, Kuypers DRJ. Response to: ‘Bodyweight‐adjustments introduce significant correlations between CYP3A metrics and tacrolimus clearance’. Br J Clin Pharmacol 2017; 83: 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, et al. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol 2009; 20: 2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Evolution of haematocrit over time. Solid line represents the mean; dotted line represents 95% confidence interval
Table S1 Tacrolimus dose (mg kg−1) on individual days
Table S2 Tacrolimus C0 (ng ml−1) on individual days
Table S3 Tacrolimus C0/dose (ng ml−1 mg−1) on individual days
Table S4 Determinants of tacrolimus dose (mg day−1) on days 1–14 in multivariable linear mixed model
Table S5 Pretransplant determinants of tacrolimus C0/dose in the first 5 days after transplantation
Table S6 Pretransplant determinants of tacrolimus dose (mg) in the first 5 days after transplantation
Table S7 Time‐dependent determinants of tacrolimus C0/dose
Table S8 Determinants of tacrolimus C0/dose in additional steady‐state cohort (see panel * in Figure 3)


