Abstract
Aims
Topical tranexamic acid (TXA) is used in patients undergoing total knee arthroplasty to reduce perioperative blood loss. However, the optimal dosing regimen remains undetermined. The aim of the present study was to quantitatively evaluate the effect of topical TXA on the reduction of postoperative drainage, and identify the dosing regimen factors affecting the efficacy of topical TXA.
Methods
Model‐based meta‐analysis was used to evaluate the efficacy of topical TXA and the dosing regimen factors influencing clinical efficacy. Data from a systemic literature search was identified and used to build a time‐effect model for placebo and TXA in treating perioperative blood loss.
Results
Fourteen studies containing 16 TXA–control groups of drainage volume data were included for MBMA modelling. The model described the postoperative drainage‐time profiles adequately. According to the model estimation, TXA can finally reduce the postoperative drainage by about 41.7%, and 10.9 h was needed to reach 50% of the maximal drainage volume. Covariate analysis indicated that both dose and contact time alone did not correlate well with clinical efficacy. However, when considered together, they can dramatically improve fitting of the data. Simulation showed that increasing dose or contact time extensively would produce a plateau‐like effect: 2–3 g TXA with contact time of 1–2 h would yield >60% reduction in the drainage volume.
Conclusions
Dose and contact time together determined the efficacy of TXA. Extensively large dose or long contact time seems unnecessary. These findings may further guide the clinical practice on the topical TXA regimen optimization.
Keywords: dosing regimen, haemostasis, model‐based meta‐analysis, total knee arthroplasty, tranexamic acid
What is Already Known about this Subject
Topical tranexamic acid (TXA) can significantly reduce perioperative blood loss and decrease the need for blood transfusion in patients undergoing total knee arthroplasty.
There has been debate on the optimal dosing regimen of topical TXA for preventing perioperative blood loss in total knee arthroplasty.
What this Study Adds
Effect of topical TXA on postoperative bleeding was adequately evaluated by a model‐based meta‐analysis approach.
The model revealed that both dose and contact time were critical for the efficacy of topical TXA and increasing either of them extensively would produce a plateau‐like effect that is far from being desirable.
Introduction
Total knee arthroplasty (TKA) is one of the most effective treatment options for end stage knee disease, which is becoming more and more common with the aging global population. However, this procedure is frequently associated with a very high perioperative blood loss of >1 l, resulting in a prevalent need of blood transfusion, delayed rehabilitation and other serious complications including infections and pulmonary embolism 1.
Tranexamic acid (TXA) is an antifibrinolytic haemostatic that interacts directly with the lysine binding site of plasminogen, preventing the access of plasmin to fibrin, thereby limiting fibrin degradation 2. Intravenous and intra‐articular administration of TXA has been demonstrated as a cost‐effective method to reduce total blood loss and transfusion requirements in TKA 3, 4, 5. Although frequently used, intravenous TXA has the potential to increase the risk of thromboembolism, and thus limiting its clinical use 6. By contrast, topical administration of TXA, remaining predominantly at the bleeding site with little systemic absorption, may be considered to have a better efficacy and safety profile than the intravenously dosed drug.
A variety of dosing regimens have recently been used for topical TXA, but the optimal one has yet to be determined. Although several meta‐analyses have been performed on different regimens of topical TXA, suggesting that they are effective on reducing perioperative blood loss and blood transfusions 7, 8, there is still a lack of information as to which regimen is better. There are several limitations to these analyses. First, the efficacy data obtained at different time points were pooled. Second, only a small number (<10) of studies were included. In addition, blood loss reduction after topical TXA dosing usually has a large variability among different trials. The variability induced by different dosing regimens and trials cannot be separated by conventional meta‐analysis and thus provides little help in identifying the optimal dosing regimens of topical TXA.
Model‐based meta‐analysis (MBMA), however, can be used to quantify not only the effects of treatment, but also the influence of different dosing regimens on the clinical responses 9, 10. By integrating multilevel information of dosing regimens, pharmacokinetic data and efficacy results obtained from different trials, MBMA increases the ability to detect small but clinically significant effects, and thus could effectively evaluate the comparative efficacy of different regimens 11. To date, no quantitative relationship using summary‐level efficacy data across trials has been established between different topical TXA dosing regimens and clinical efficacy end points. Therefore, it is of value to explore this relationship based on literature data regarding TXA topical administration in order to adequately select dosing regimens in clinical practice.
The present study used the MBMA method to evaluate quantitatively the effect of topical TXA on the reduction of postoperative drainage, and identify the important influencing factors in the topical dosing regimen on the efficacy of TXA, which would provide useful information for further clinical practice on dosing regimen optimization.
Methods
Data extraction
A thorough literature search of published studies on intra‐articular administration of TXA for haemostasis after TKA was performed using the online PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/), Cochrane database (http://www.cochranelibrary.com/) and the following keywords: tranexamic acid, intra‐articular, topical, total knee arthroplasty and total knee replacement. Inclusion criteria were: (i) randomized, placebo‐controlled clinical trial; (ii) dosing consists of a single intra‐articular administration of TXA; (iii) drainage volume and recording time points available in tables and/or graphs; (iv) details of TXA dosing regimen and drug contact time provided in the report. Patient treatment information and arithmetic mean of the observed drainage volume were extracted from the published studies qualifying all the inclusion criteria.
Model development
It was hypothesized that bleeding rate after operation would decrease over time in both placebo and TXA group. Therefore, the relationship of drainage volume profiles with time for both treatment groups were described using sigmoid Emax models as the basic model described below:
| (1) |
where
| (2) |
| (3) |
| (4) |
In equation (1), Dri,j is the observed mean volume of the accumulated drainage in the ith TXA‐control group at time j, Drplacebo,i,j (also called the fundamental drainage) represents the predicted mean volume of the accumulated drainage when placebo was used in the ith group at time j. Etxa,i,j is the predicted mean effect of TXA in the ith group at time j expressed as decreased drainage volume. In the placebo group, Etxa,i,j is equal to 0 and consequently Dri,j is equal to Drplacebo,i,j. Ni,j is the sample size in the ith group at time j, and εi,j is the residual error in the ith group at time j, assumed to be normally distributed with a mean of 0 and variance of σ2/Ni,j. εi,j is weighted by the sample size. In equations (2), (3) and (4), Drmax‐placebo is the maximal volume of the accumulated drainage when placebo is used, ET50‐placebo is the time to achieve 50% of Drmax‐placebo, γ is the hill coefficient. E%txa represents the percentage reduction by TXA in the postoperative drainage compared with Drplacebo,i,j, and it also represents the efficacy of TXA when eliminating the effects of other factors on the postoperative drainage in addition to TXA. In this way, the intertrial variability of the drainage volume in the control group was adjusted so the E%txa value of different dosing regimens in each trial could be directly compared. Since E%txa can only be between 0 and 1, we developed a transfer equation equation (4) with parameter E%trans to control the range of E%txa. Tj is the time j, η1,i represents the intertrial variability of Drmax‐placebo and η2,i is the intertrial variability of E%trans. η1,i and η2,i are assumed to be independent with each other and normally distributed with a mean of 0 and variance of ω1 2 and ω2 2.
Covariate selection
Effect of dose and contact time on E%txa was firstly tested separately, considering that the aim of the current investigation is to look into the influence of both factors on clinical efficacy of TXA. Additionally, it might also be the case that for TXA to exert its pharmacological effect, a certain amount of drug needs to be applied for a long enough period of time, and therefore the effect of dose and contact time on E%txa were tested as a combinational covariate as shown in equation (5).
| (5) |
The parameters in equation (5) are as follows: E%txa represents the efficacy of TXA in the form of percentage reduction; Dose represents the dose of TXA; Con.T represents the topical contact time with the drug. Under the cases where TXA was injected via the drainage tube, which was subsequently clamped, contact time equals clamping time. k represents the maximal effect coefficient of the E%txa and was set to be 1; D50 and T50 are the dose and the contact time required to achieve 50% of the maximal dose‐dependent and contact time‐dependent effect, respectively. A difference in objective function value (OFV) of 6.63 (χ2, α = 0.01, d.f. = 1) was considered statistically significant in the covariate model building process.
Model validation
The accuracy of the model fit was evaluated by graphic assessment. Monte Carlo simulations were performed 1000 times to predict 90% confidence intervals of the postoperative drainage in control and TXA group. Simulation‐estimation exercise was conducted 100 times to validate the robustness of the present model.
Software
The model estimation and simulation were performed using NONMEM 7 (Level 3.0, ICON Development Solutions, USA). Diagnostic graphs and visual predictive check were performed using the R software (version 3.0.1, The R Foundation of Statistical Computing).
Results
Characteristics of the selected studies
Literature search identified 49 studies for consideration of inclusion in the analysis, and 35 were excluded based on our inclusion criteria. From the 14 studies included in the analysis, there were in total 63 mean drainage volume data from TXA group and 50 from placebo group. A total of 16 TXA‐control groups were included for model building (Figure 1, Table 1).
Figure 1.
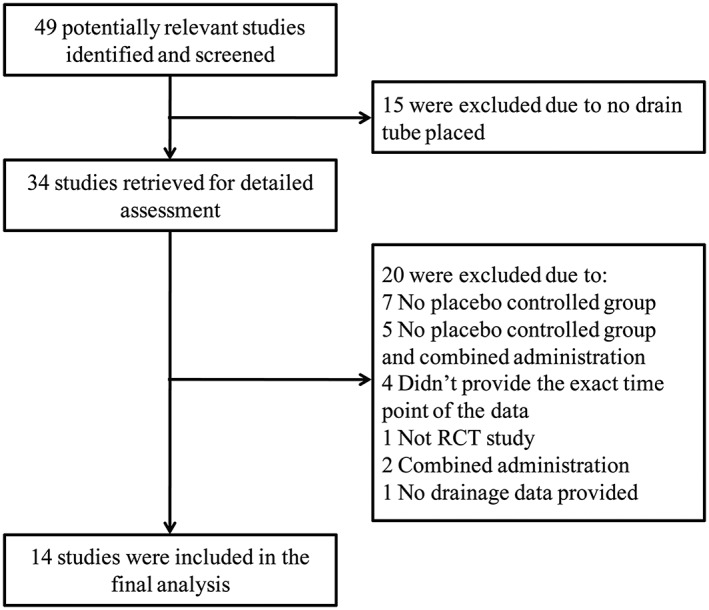
Flowchart for screening relevant articles
Table 1.
Summary of included placebo‐controlled trials of topical tranexamic acid (TXA)
| Source | Number of subjects (TXA/control) | Dosing regimen [dose (g), contact time (h)] | Time points (h) | TXA‐control group number |
|---|---|---|---|---|
| K. Ishida et al. 17 | 50/50 | 2, 0.5 | 1,3,6,12,24,48 | 1 |
| P. Sangasoongsong et al. 18 | 45/45 | 0.5, 2 | 1,2,4,6,8,10,12,16,20,24,36,48 | 2 |
| P. Sangasoongsong et al. 19 | 24/24 | 0.25, 2 | 1,2,4,6,8,10,12,16,20,24,36,48 | 3 |
| S. Roy et al. 20 | 25/25 | 0.5, 1 | 2,4,6,48 | 4 |
| P. Sangasoongsong et al. 18 | 45/45 | 0.25, 2 | 1,2,4,6,8,10,12,16,20,24,36,48 | 5 |
| R. Maniar et al. 13 | 40/40 | 3, 0.083 | 24,48 | 6 |
| G. Digas et al. 21 | 30/30 | 2, 3 | 24 | 7 |
| S. Alshryda et al. 22 | 79/78 | 1, 0.5 | 48 | 8 |
| H. Mutsuzaki et al. 23 | 70/70 | 1, 1 | 48 | 9 |
| X. Aguilera et al. 24 | 50/50 | 1, 1 | 24 | 10 |
| L. Zhaohui et al. 25 | 43/47 | 0.75, 4 | 2,8,20 | 11 |
| P. Antinolfi et al. 26 | 20/20 | 0.5, 0.667 | 12,24,36,48 | 12 |
| M. Sarzaeem et al. 14 | 50/50 | 1.5, 1 | 48 | 13 |
| M. Sarzaeem et al. 14 | 50/50 | 3, 0.083 | 48 | 14 |
| S. Lin et al. 27 | 40/40 | 1, 1 | 24 | 15 |
| W. Guowei et al. 28 | 50/50 | 1,4 | 48 | 16 |
The included studies all passed the Joanna Briggs critical appraisal checklist for observational studies and were published between 2011 and 2015 in some professional journals, such as The Journal of Arthroplasty and Journal of Bone and Joint Surgery. Taken together, the studies included in the analysis comprised a population of 1326 patients, with individual patient population of each study ranging from 40 to 157 (median: 86). Study duration ranged between 20 h and 48 h, with a median of 48 h. Details of the including studies are shown in Table 1.
Model building and validation
The OFV values of the three models tested are listed in Table 2. Based on these values and goodness‐of‐fit plots, the nonlinear mixed effects Emax model was selected as the basic model to go forward for covariate analysis.
Table 2.
Models evaluated and their objective function values. The final model is indicated by a bold italic font
| Model | Attributes | OFV |
|---|---|---|
| E max + linear mixed effects (lme) model | Interstudy/interarm variance + Additive error | 1223.387 |
| E max + nonlinear mixed effects (nlme) model | Interstudy variance + Additive error | 1113.138 |
| E max + nlme model (BASE MODEL) | Additive error | 1114.638 |
| Exponential + nlme model | Additive error | 1167.391 |
| Covariate model | BASE MODEL + Dose + Contact time | 1103.740 |
Typical value of E%txa estimated in the basic model was 41.7%. However, there was a lack of statistically significant correlation between the estimated E%txa value and the dose of TXA or the contact time (Figure 2C). To explore whether pharmacological effect of TXA is dependent on a combination of both drug amount and application time, dose and contact time were introduced into the model simultaneously for covariate search. As shown in Table 2, the OFV value was significantly decreased after introducing this combinational covariate.
Figure 2.
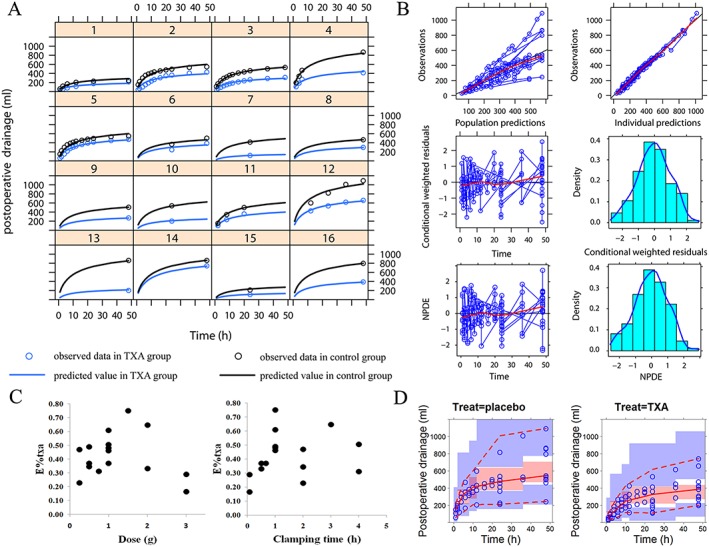
Model evaluation. (A) Time course of the postoperative drainage volume. The line represents the predicted value and the circle represents the observed value. The black represents the control group and the blue represents the tranexamic acid (TXA) group. (B) The goodness‐of‐fit plot. The upper left is the plot of population study prediction (PRED) vs. mean observation (DV). The upper right is the plot of individual study prediction (IPRED) vs. mean observation (DV). The middle left is the plot of conditional weighted residuals (CWRES) vs. time. The middle right is the plot of Density vs. CWRES. The lower left is the plot of normalized prediction distribution errors (NPDE) vs. time. The lower right is the plot of density vs. NPDE. (C) The correlation between the estimated E%txa and dose of TXA (left) and the correlation between the estimated E%txa and contact time (right). (D) Visual predictive check (VPC) of the model. The left is the VPC plot for control group and the right is the VPC plot for TXA group
The parameters estimated in the final model are shown in Table 3. The typical value obtained for Drmax‐placebo was 767 ml and 10.9 h were needed to reach 50% of Drmax‐placebo. Meanwhile, the typical value of D50 and T50 were 0.554 and 0.279 respectively. After the model establishment, we simulated the profiles of E%txa changing with TXA dose and contact time, and compared with the estimated E%txa,i value in the basic model (Figure 4A).
Table 3.
Estimated parameters in the final model
| Parameters | Value | RSE (%) | 95% CI |
|---|---|---|---|
| Dr max‐placebo (ml) | 767 | 15.5 | 534, 1000 |
| ET 50‐placebo (h) | 10.9 | 35.2 | 3.37, 18.4 |
| D 50 (g) | 0.554 | 24.9 | 0.284, 0.824 |
| T 50 (h) | 0.279 | 33.8 | 0.094, 0.464 |
| γ | 0.742 | 10.4 | 0.591, 0.893 |
| η 1 | 0.136 | 36.0 | 0.040, 0.232 |
| η 2 | 0.250 | 35.0 | 0.078, 0.422 |
| ε × 10 4 (ml) | 3.79 | 27.4 | 1.75, 5.83 |
CI, confidence interval; RSE, relative standard error
Model evaluation
As shown in Figure 2A, good agreement existed between predicted value of the final model and observed data from each individual trial. The large intertrial variability might have reflected the difference in operation procedures and operators. The goodness‐of‐fit plots shown in Figure 2B demonstrated that the data were fitted well by the final model. Specifically, there was good accordance between observed and population model‐predicted effects, and between observed and individual model‐predicted effects. The conditional weighted residuals and the normalized prediction distribution errors magnitude were small and randomly distributed around a straight line through 0, and located within ±3 from the centre.
Monte Carlo simulations (1000 times) showed that the 90% confidence interval of the 5%, median and 95% predicted percentiles covered the corresponding observed data, which indicated that the model had an adequate prediction capability (Figure 2D). The parameter estimations in simulation‐estimation exercise for 100 times were all successfully convergent and the estimated parameter values were randomly distributed around the typical value in the final model with the ratio located within 0.5–2 as shown in Figure 3.
Figure 3.
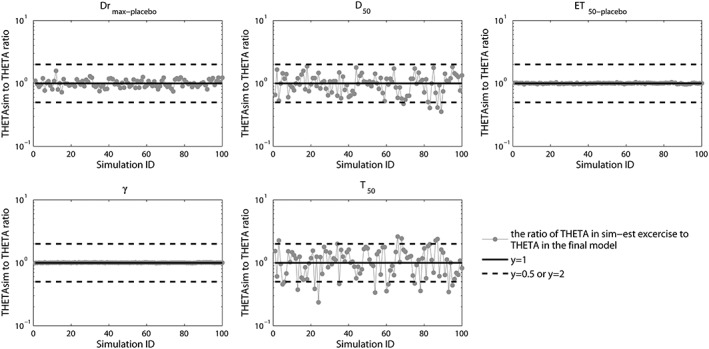
Ratio of the estimated parameter values in the simulation‐estimation exercise to the typical parameter values in the final model. The simulation‐estimation exercise was executed for 100 times. The control lines in the figure from top to bottom are for ratios of 2, 1 and 0.5 respectively
Influence of dose and contact time on the efficacy of TXA
As shown in Figure 2C, neither the dose nor the contact time alone significantly correlate with the efficacy of TXA (E%txa). However, the influence on the drug efficacy was prominent when both of contact time and dose are considered together. Influence of the dose and the contact time on E%txa could be described by the simulated curved surface in Figure 4A. Increasing of dose or contact time could both increase the drug effect. However, the increase induced by each single variable is moderate, as drug effect plateaued rapidly with the increasing of a single variable. For example, a very short contact time such as 5 min can only yield an efficacy <20%, even though the TXA dose is as high as 3 g (Figure 4B). A combination of both dose and contact time, by contrast, could exert the maximum pharmacological effect. As shown in Figure 4B, 2–3 g TXA with 1–2 h contact time would lead to >60% reduction in the postoperative drainage volume compared to the placebo group. Further increasing the dose or extending the contact time seems unnecessary.
Figure 4.
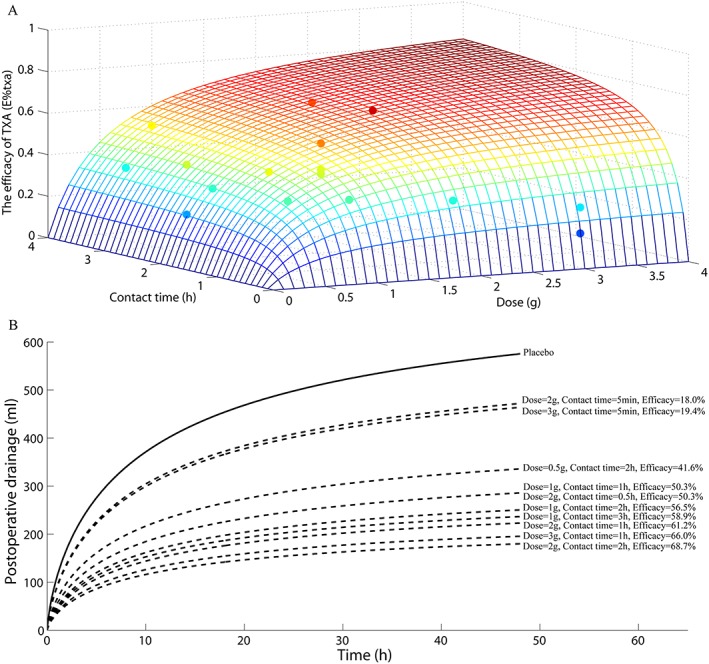
Influence of the dose and the contact time on the efficacy of tranexamic acid (TXA). (A) Simulation for the TXA efficacy profile changing with TXA dose and contact time. The simulation is according to the equation (5) in the final model and is shown as a wireframe surface with different colours. The surface was further compared with the estimated E%txa,i value in the basic model which were shown as the points with different colours. The colour is determined by the value of z axis (i.e. the efficacy of TXA) and is proportional to it. The surface overlaps the point if there is a coincidence between point and surface with similar colour. (B) Simulation for the postoperative drainage‐time profiles under a series of dosing regimens using the typical parameter value in the final model. The black line is for the placebo group and the black dotted line is for the TXA group. Different dosing regimens and the corresponding efficacy value were marked on the left
Discussion
For TKA patients, TXA could significantly reduce perioperative blood loss, therefore decreasing the need for blood transfusion, accelerate postoperative recovery, and reduce the average duration of hospital stay. Topical use of TXA was considered superior to intravenous administration because of the maximization of drug effect at the site of action and the minimization of systemic drug exposure‐induced side effect. Recent meta‐analyses have shown that various topical dosing regimens were effective. They reported a wide range of 0.25–3 g in a single dose and of 5 min to 12 h for the drug contact time 12, 13, 14. However, the optimal regimen remains undetermined. The direction of the dosing regimen optimization is especially confusing.
The conventional meta‐analysis is limited by pooling of data at different time points and large intertrial variability (shown in Figure 2A) in the drainage volume of the placebo group across studies. Thus, although helpful in establishing clinical efficacy of this treatment, it cannot quantitatively identify a better dosing regimen. MBMA, using a mathematical model to describe the details of the efficacy data, could overcome the above disadvantages of meta‐analysis. In the present study using MBMA method, we successfully estimated the efficacy of TXA (i.e. E%txa), which could be directly compared between different trials with different dosing regimens, consequently, the better dosing regimen could be evaluated.
Both the dose and the contact time, when considered alone, did not correlate well with the efficacy of TXA. However, model fitting was significantly improved when dose and contact time are considered together as evidenced by a statistically significant drop in OFV. This implies that for TXA to exert its pharmacological effect, the drug need to be applied in enough quantity for a certain period of time. The extreme cases where high doses combined with short application time or low doses applied for extended period of time might be less optimal.
The influence of the dose and the contact time on the efficacy of TXA as simulated in Figure 4A showed a saturated effect. According to parameters estimated in the final model shown in Table 3, T50 is less than D50, which means extending the contact time is more likely to induce the plateau effect than increasing the dose. Previous studies 15, 16 reported that a longer clamping time would cause intra‐articular haematomas and increase the risk of postoperative infection. Considering the present results, a shorter contact time and a larger dose of TXA are recommended as the reasonable dosing regimen. According to the simulation results, we can predict that dosing 2–3 g TXA with 1–2 h contact time would yield >60% reduction in the drainage volume and further increasing the dose or contact time would increase the risk of adverse effect, which is far from being desirable.
Although a large intertrial variability was observed in the dataset included for analysis, the interstudy variance was not added into the final model because data fitting was not further improved by adding interstudy variance. This might have been due to the fact that the intertrial variability of the postoperative drainage volume (i.e. Dr) was already explained by the interstudy variance of parameter Drplacebo (i.e. η1), which accounts for a large proportion of the total variability of Dr. The fundamental drainage (i.e. Drplacebo) in each trial is determined by a combination of factors including different doctors/nurses, medical equipment and operation procedures. Since the focus of the model was the drainage reduction percentage as the efficacy of TXA, it is not only difficult but also unnecessary to characterize the variability of Drplacebo further using more covariate parameters.
In addition to the postoperative drainage, postoperative haemoglobin (Hb) level is also an important end point of the TXA effect. However, until now, only a few papers have provided the complete time cause of Hb level after the operation. Further clinical study is needed to investigate the effect of TXA on the Hb level. Further clinical studies could also add additional merit to the optimized dosing regimen provided in the present study, which is based on modelling and simulation methods.
The present study uses the MBMA method to quantify the effect of topical TXA on the postoperative drainage and to analyse the important dosing regimen factors that significantly influence the efficacy of TXA. These findings would further guide the clinical practice on the topical TXA regimen optimization.
Competing Interests
There are no competing interests to declare.
This work was supported by research grants from National Natural Science Foundation of China (No. 81 603 184), Natural Science Foundation of Jiangsu Province of China (No. BK20160124) and the Key Research and Development program of Jiangsu Province of China (No. BE2016608).
Xu, R. , Shi, D. , Ge, W. , and Jiang, Q. (2017) Quantitative efficacy of topical administration of tranexamic acid on postoperative bleeding in total knee arthroplasty. Br J Clin Pharmacol, 83: 2485–2493. doi: 10.1111/bcp.13374.
Contributor Information
Weihong Ge, Email: 6221230@sina.com.
Qing Jiang, Email: qingj@nju.edu.cn.
References
- 1. Rosencher N, Kerkkamp HE, Macheras G, Munuera LM, Menichella G, Barton DM, et al Orthopedic surgery transfusion hemoglobin European overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion 2003; 43: 459–469. [DOI] [PubMed] [Google Scholar]
- 2. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 2012; 72: 585–617. [DOI] [PubMed] [Google Scholar]
- 3. Tengborn L, Blomback M, Berntorp E. Tranexamic acid‐‐an old drug still going strong and making a revival. Thromb Res 2015; 135: 231–242. [DOI] [PubMed] [Google Scholar]
- 4. Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg 2015; 23: 732–740. [DOI] [PubMed] [Google Scholar]
- 5. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta‐analysis. J Bone Joint Surg Am 2012; 94: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 6. Xie J, Ma J, Kang P, Zhou Z, Shen B, Yang J, et al Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases. Thromb Res 2015; 136: 234–238. [DOI] [PubMed] [Google Scholar]
- 7. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta‐analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J 2014; 96‐B: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 8. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta‐analysis. Knee 2013; 20: 300–309. [DOI] [PubMed] [Google Scholar]
- 9. Li H, Xu J, Fan X. Target‐mediated pharmacokinetic/pharmacodynamic model based meta‐analysis and dosing regimen optimization of a long‐acting release formulation of exenatide in patients with type 2 diabetes mellitus. J Pharmacol Sci 2015; 127: 170–180. [DOI] [PubMed] [Google Scholar]
- 10. Li L, Lv Y, Xu L, Zheng Q. Quantitative efficacy of soy isoflavones on menopausal hot flashes. Br J Clin Pharmacol 2015; 79: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mould DR. Model‐based meta‐analysis: an important tool for making quantitative decisions during drug development. Clin Pharmacol Ther 2012; 92: 283–286. [DOI] [PubMed] [Google Scholar]
- 12. Sa‐ngasoongsong P, Chanplakorn P, Wongsak S, Uthadorn K, Panpikoon T, Jittorntam P, et al An in vivo study of low‐dose intra‐articular tranexamic acid application with prolonged clamping drain method in Total knee replacement: clinical efficacy and safety. Biomed Res Int 2015; 2015: 164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 2012; 470: 2605–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarzaeem MM, Razi M, Kazemian G, Moghaddam ME, Rasi AM, Karimi M. Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty 2014; 29: 1521–1524. [DOI] [PubMed] [Google Scholar]
- 15. Minnema B, Vearncombe M, Augustin A, Gollish J, Simor AE. Risk factors for surgical‐site infection following primary total knee arthroplasty. Infect Control Hosp Epidemiol 2004; 25: 477–480. [DOI] [PubMed] [Google Scholar]
- 16. Kang Y, Zhang ZJ, Fu M, Xu DL, Sheng PY, Liao WM. Blood transfusion and drainage catheter clamping are associated with ecchymosis formation at the surgical site after total knee arthroplasty: an analysis of 102 unilateral cases. Eur J Orthop Surg Traumatol 2013; 23: 219–224. [DOI] [PubMed] [Google Scholar]
- 17. Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, et al Intra‐articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 2011; 35: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sa‐Ngasoongsong P, Wongsak S, Chanplakorn P, Woratanarat P, Wechmongkolgorn S, Wibulpolprasert B, et al Efficacy of low‐dose intra‐articular tranexamic acid in total knee replacement; a prospective triple‐blinded randomized controlled trial. BMC Musculoskelet Disord 2013; 14: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sa‐Ngasoongsong P, Channoom T, Kawinwonggowit V, Woratanarat P, Chanplakorn P, Wibulpolprasert B, et al Postoperative blood loss reduction in computer‐assisted surgery total knee replacement by low dose intra‐articular tranexamic acid injection together with 2‐hour clamp drain: a prospective triple‐blinded randomized controlled trial. Orthop Rev 2011; 3: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra‐articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2012; 20: 2494–2501. [DOI] [PubMed] [Google Scholar]
- 21. Digas G, Koutsogiannis I, Meletiadis G, Antonopoulou E, Karamoulas V, Bikos C. Intra‐articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol 2015; 25: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 22. Alshryda S, Mason J, Vaghela M, Sarda P, Nargol A, Maheswaran S, et al Topical (intra‐articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX‐K). J Bone Joint Surg Am 2013; 95: 1961–1968. [DOI] [PubMed] [Google Scholar]
- 23. Mutsuzaki H, Ikeda K. Intra‐articular injection of tranexamic acid via a drain plus drain‐clamping to reduce blood loss in cementless total knee arthroplasty. J Orthop Surg Res 2012; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aguilera X, Martinez‐Zapata MJ, Hinarejos P, Jordan M, Leal J, Gonzalez JC, et al Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg 2015; 135: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 25. Zhaohui L, Wanshou G, Qidong Z, Guangduo Z. Topical hemostatic procedures control blood loss in bilateral cemented single‐stage total knee arthroplasty. J Orthop Sci 2014; 19: 948–953. [DOI] [PubMed] [Google Scholar]
- 26. Antinolfi P, Innocenti B, Caraffa A, Peretti G, Cerulli G. Post‐operative blood loss in total knee arthroplasty: knee flexion versus pharmacological techniques. Knee Surg Sports Traumatol Arthrosc 2014; 22: 2756–2762. [DOI] [PubMed] [Google Scholar]
- 27. Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015; 30: 776–780. [DOI] [PubMed] [Google Scholar]
- 28. Wang G, Wang D, Wang B, Lin Y, Sun S. Efficacy and safety evaluation of intra‐articular injection of tranexamic acid in total knee arthroplasty operation with temporarily drainage close. Int J Clin Exp Med 2015; 8: 14328–14334. [PMC free article] [PubMed] [Google Scholar]


